Abstract
The eye is the organ in charge of vision and, given its properties, has become an excellent organ to test genetic therapies, including antisense oligonucleotide (AON) technology. In fact, the first AON receiving FDA and EMA approval was meant to treat an eye condition. Currently, dozens of clinical trials are being conducted for a variety of subtypes of inherited retinal disease. Although most of them are based on gene augmentation therapies, a phase 3 and two phase 1/2 clinical trials using AONs are ongoing. Since the retina is a layered structure of nondividing cells, obtaining human retinal tissue and expanding it in the lab is not possible, unless induced pluripotent stem cell technology is used. Mouse models have helped to elucidate the function of many genes, and the retinal structure is quite similar to that of humans. Thus, drug delivery to the mouse eye can provide valuable information for further optimization of therapies. In this chapter, the protocol for intravitreal injections of AONs is described in detail.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Keywords
- Retina
- Intravitreal injection
- Mouse
- Inherited retinal diseases
- Antisense oligonucleotide
- Drug delivery
- Intraocular injection
1 Introduction
The eye is the window to the world around us. Vision occurs thanks to a thin neuronal layer located at the back of the eye called the retina. When light enters the eye, the photosensitive cells (photoreceptors) capture the photons and convert them into chemical and electrical signals that travel to the brain, where the final image will be generated [1].
Inherited retinal diseases (IRDs) are monogenic disorders affecting approximately 1 in 3000 individuals worldwide [2]. So far, mutations in more than 250 genes have been associated with IRDs (RetNet: https://sph.uth.edu/retnet/). IRDs are highly heterogeneous, but in general, the first symptoms appear during the first decade of life, and the disease progresses with age, often leading to total blindness.
Although no cure is available for IRDs, the eye is at the forefront of the development of molecular therapies. Its accessibility, containment, immune-privileged status, together with the window of opportunity that IRDs present, make the eye a model organ for therapeutics. An example is Luxturna, an adeno-associated virus (AAV)-based therapy , which has become the first gene therapy treatment with market approval for an eye disease [3]. This achievement has sped up the development of many other potential treatments currently under investigation at both preclinical and clinical levels for a variety of retinal diseases. Another example of molecules that have shown promising results in humans is antisense oligonucleotides (AONs). These 18–21 nucleotide oligonucleotides are able to bind to the pre-mRNA and, among other functions, can modulate splicing or degrade RNA transcripts [4]. After the promising results obtained in a phase 1/2 clinical trial [5], a phase 2/3 clinical trial (NCT03913143) using AONs to correct a splicing defect in CEP290-associated IRD has recently started. Furthermore, two other phase 1/2 clinical trials have also commenced for specific mutations in the IRD-causing genes USH2A (NCT03780257) and RHO (NCT04123626).
When considering testing AON efficacy in the mouse eye, it is important to remember that AONs are sequence-specific molecules. In the past, we developed a humanized mouse model for the intronic variant c.2991+1655A>G in CEP290 [6]. This model contained a ~6 kb fragment of the human CEP290 including the intronic mutation, and the splicing defect associated with this mutation was only slightly recapitulated [6]. Despite, using a previously efficacious AON characterized in patient-derived cells [7], a single dose of 60 μg of AON was delivered to the retina intravitreally, and pseudoexon skipping was observed up to 30 days after injection [8].
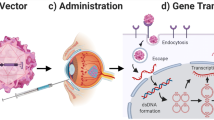
Delivery to the eye can be performed in multiple ways. The most common ones are intravitreal and subretinal injections. For AONs, intravitreal injections are a good choice since the entire retina can be targeted (Fig. 1). There are several ways to perform intravitreal injections, and in this chapter, the protocol we successfully use in our lab is described step by step. For this procedure, no sophisticated equipment is required.
2 Materials
2.1 For Injections
-
1.
Permission from the animal ethical committee and a safe place to work (see Note 1).
-
2.
Stereomicroscope.
-
3.
Balance to weigh the animals.
-
4.
Microdissection scissors (e.g., Vannas 8 cm str).
-
5.
Dumont #5 forceps.
-
6.
Dumont #5 curve 45-degree forceps.
-
7.
30G needles.
-
8.
25G needles.
-
9.
1-ml plastic syringes.
-
10.
10 μl Hamilton syringe without needle (e.g., 1701 RN or 701 RN).
-
11.
Hamilton needles: 33G, 51 mm, blunt end or 34G, 12.7 mm, blunt end (see Note 2).
-
12.
Sterile gauzes.
-
13.
Isoflurane or other types of anesthetics.
-
14.
In case of using isoflurane, a box to anaesthetize the animals, and corresponding tubes (including a stopcock to allow distribution of the isoflurane to the box, but also to the animal undergoing the procedure).
-
15.
Analgesic such as carprofen (stock 50 mg/ml).
-
16.
Tropicamide ophthalmic solution (see Note 3).
-
17.
Antibiotics, formulated as eye ointments (e.g., PRED-G, TobraDex, etc.).
-
18.
Heat pad to warm up the animals during the recovery time.
-
19.
1-ml tips.
-
20.
Ethanol 70%.
-
21.
Sterile deionized water (in a falcon tube).
-
22.
1.5-ml Eppendorf tubes.
-
23.
Bench diapers.
-
24.
The solution to resuspend the molecule of interest (in this case, sterile PBS 1× or saline commercial).
-
25.
The molecule of interest (in this case AONs) resuspended at the proper concentration in the appropriate solution (between 5 and 20 μg/μl).
-
26.
A colleague willing to help and that is allowed to work with animals (see Note 4).
-
27.
Mice to inject (see Note 5).
2.2 For Harvesting Eyes
-
1.
Dumont #5 forceps.
-
2.
2-ml Eppendorf tubes.
-
3.
Scissors.
-
4.
Small blades.
-
5.
OCT compound.
-
6.
Molds for cryoblocks.
-
7.
Liquid nitrogen.
-
8.
Polystyrene box.
-
9.
Isopentane.
-
10.
Aluminum boxes (to store the cryoblocks individually (14.5 mm) or in groups (29 mm)).
3 Methods
3.1 Before Starting
Prepare the selected spot with enough space to place a stereomicroscope, sit in a comfortable way, have safe connection to isoflurane (see Note 6), and have enough room to have all the necessary tools around. For administering AONs, a safety cabinet is not per se needed; however depending on the animal facility, the animal handling must be conducted inside the cabinet, meaning that the procedure is too (see Note 7). Last but not least, remember to sterilize the forceps and scissors before the procedure.
3.2 Preparation for the Procedure
Once at the location, prepare the place for the procedure:
-
1.
Cover the surface with the bench diapers.
-
2.
Place the microscope on top of it.
-
3.
Connect the box to the isoflurane using a tube with a stopcock and an extra exit tube.
-
4.
Place a 1-ml tip at the end of the extra tube (cut the 1 cm of the tip first) if performed in the cabinet, or use a safe tube connected to the aspirating system.
-
5.
Put the balance in a stable place.
-
6.
Wash the Hamilton syringes with deionized water (MQ), then ethanol 70%, and then water again.
-
7.
Prepare all the tools around the microscope: forceps, scissors, a 1-ml syringe with a 30G needle (for the procedure), the AONs, and antibiotics.
-
8.
Prepare the carprofen at the correct concentration (usually 1:100 in saline, to inject 10 μl for each gram of weight). Alternatively, carprofen can be added to the drinking water 24 h prior to the procedure in a 1:1000 dilution (stock 50 mg/ml). In this case, injection is not needed.
-
9.
Prepare a 1-ml syringe with a 33G needle (to inject the carprofen).
-
10.
Prepare the heat pad.
-
11.
Prepare the animals for the procedure.
3.3 The Procedure
-
1.
Take the first animal.
-
2.
Weight the animal and write down the weight in grams.
-
3.
Place the mouse in the box with the isoflurane at 4–5% for induction.
-
4.
Once the animal is asleep, transfer it under the stereomicroscope and place the extra tube in the mouth/nose of the mouse , using the 1-ml tip if working on the cabinet or the safe tube. Reduce the isoflurane to 2–2.5%.
-
5.
Tape the tip to the base of the microscope to avoid it moving and the mouse waking up during the procedure.
-
6.
Deliver the carprofen via subcutaneous injection (10 μl of a solution 0.5 mg/ml (dilution 1:100) for each gram of weight). Alternatively, carprofen can be added to the drinking water 24 h prior to the procedure (dilution 1:1000), in this case injection is not needed.
-
7.
Place the animal in a position such as the eye is clearly visible through the center of the stereomicroscope (see Note 8). In our lab, left eye is injected first by a right-handed person (see Notes 8 and 9).
-
8.
Prepare the volume to be injected in one of the Hamilton syringes. For adult mice, this is usually 2 μl. In this example, the left eye is injected first, thus the solution will be PBS 1×.
-
9.
With the forceps, take the conjunctiva below the upper eyelid. Pull it a bit to the outside, and with the surgery, scissors make a small cut to clearly see the eyeball toward the inner part. Make sure you do not cut the conjunctiva in the border, otherwise it will not be possible to pull it in the next steps (see Note 10). The cut should be done in the area behind the ora serrata, to make sure we inject in the retina.
-
10.
Leave the scissors and take the syringe with the 30G needle.
-
11.
While still holding the conjunctiva with the forceps, use the syringe with the 30G needle to make a tiny hole in the clean area (without any type of membrane) of the eyeball (Fig. 2a), by only using the tip of the needle (see Note 11).
-
12.
Leave the syringe and grab the Hamilton loaded with 2 μl of PBS 1×.
-
13.
Place the needle of the Hamilton in the hole previously made, for intravitreal injection, carefully and slowly placing the needle into the eye in the intravitreal space (Fig. 2b). Release slowly the content of the syringe with the help of a colleague (see Notes 12–14).
-
14.
Leave the needle a couple of seconds to avoid reflux.
-
15.
Slowly remove the needle from the eye (see Note 15).
-
16.
Release also the conjunctiva.
-
17.
Turn the animal and perform the same procedure (steps 7–15) on the right eye, but now injecting the AON instead of PBS 1× (see Note 16).
-
18.
Once both eyes have been injected, remove the mouse from the isoflurane.
-
19.
Place some antibiotic on top of each eye.
-
20.
Transfer the mouse to the cage located on top of the heat pad.
-
21.
Label the cage properly to avoid confusions (see Note 17).
-
22.
After a couple of minutes, the animal should wake up and start moving around.
-
23.
Clean with ethanol the forceps, scissors.
-
24.
Continue with the next animal following the same steps as described above (see Note 18).
-
25.
If multiple AONs are injected, clean the Hamilton syringe as described in step 6 of Subheading 3.2 and proceed with the same steps described in Subheading 3.3.
Schematic representation of the steps to perform an intravitreal injection in mice. (a) The 30G needle is used to make a small hole in the eye to be able to introduce the Hamilton needle with a blunt end in the intravitreal space. It is important to only perform the incision with the very tip part of the needle (marked with an asterisk). (b) Schematic representation of the intravitreal injection of the left eye. The figure was made with Biorender
3.4 After the Procedure
-
1.
Stop the isoflurane.
-
2.
Check that all animals are awake.
-
3.
Place them back in the corresponding rack and recheck again that labels are correctly placed.
-
4.
Clean the needles first with MQ water (to remove any kind of possible tissue left), then with 70% ethanol, and then with MQ water again and allow to dry (see Note 19).
-
5.
Discard all the 30G and 33G needles used in the proper container.
-
6.
Clean the scissors and forceps with 70% ethanol and water.
-
7.
Discard the bench diaper, the tip, and syringes used to inject carprofen.
-
8.
Clean the working space.
3.5 Follow-Up
During the next 2 days, it is important to check the eyes of the animals on a daily basis in order to detect possible infections. In our hands, we have never observed any infection or complication derived from the injections (see Note 20).
3.6 Harvesting the Tissue and Read-Outs
Depending on the molecule injected and the purpose of the experiment, the harvesting time will vary. For instance, it is known that when injecting viruses such as adeno-associated viruses (AAVs), the highest expression is obtained after 3–4 weeks [9]. In contrast, the effect of an AON can be already detected a couple of days after injection [8]. Therefore, the timing on tissue collection will be based on the specific project and the expected efficacy of the therapeutic molecule.
The way to sacrifice the mice can also modify the read out. In our group, we sacrifice the animals by cervical dislocation to collect the samples as quickly as possible and avoid possible changes triggered by CO2 inhalation (the other type of euthanasia approved in our facility).
AON efficacy assessment can be done at RNA or protein level. For example, checking by RT-PCR if the splicing is redirected, or by Western blot whether protein levels were increased or decreased, depending on the purpose of the study. For that, the retina needs to be harvested, and this can be done easily following the instructions previously described elsewhere [10]. Once the tissue is collected, it can be processed according to standard protocols for RNA isolation and protein lysates [6].
Morphological studies can also be performed. These tests are important for example, to study if the newly created protein localizes at the proper place or if the AON itself is creating any toxic effect. For that, the entire eye will be collected and subsequently sectioned to analyze these aspects by conventional eosin/hematoxylin or toluidine-blue staining or immunohistochemical studies. In order to prepare the samples, the eye needs to be sectioned. Here, there are two options. (A) The eye is fixed and dissected, removing the cornea and lens, cryopreserved in sucrose, and embedded in OCT, as previously described elsewhere [10], or (B) embedded directly in OCT. In general terms, fixed eyes will give better morphology than non-fixed. However, in our experience, often primary antibodies work only in unfixed tissue (especially those that are home-made). Therefore, choosing the best protocol to follow will depend on the reagents that will be employed for the different read-outs. Since option A has already been described in detail previously [10], option B is explained below:
-
1.
Add OCT to the cryomold (see Note 21).
-
2.
Enucleate the eye using the forceps.
-
3.
Pull the eye from the animal.
-
4.
Place the eye in the OCT with the cornea looking to the right side (right eye) or left side (left eye) (Fig. 3, see Note 22).
-
5.
Make sure the eye is entirely submerged in the OCT.
-
6.
Align the ora serrata parallel to the border of the cryomold.
-
7.
Add isopentane in an aluminum box.
-
8.
Place the aluminum box with isopentane in a polystyrene box.
-
9.
Place the mold on top of the aluminum box, making sure the lower part is in contact with the isopentane.
-
10.
Add some liquid nitrogen in the polystyrene box.
-
11.
Allow the sample to freeze slowly.
-
12.
Once completely frozen, unmold and store for sectioning in an aluminum box properly labeled at −80 °C until sectioning (see Note 23).
Schematic representation on how to place the right eye in the cryomold. The figure shows the different areas of the eye in the position of the mouse . When placing the eye in the mold, we put the dorsal (D) and the ventral (V) areas as indicated. Nasal (N) area will be at the bottom of the mold and the temporal (T) at the top. Arrows indicate the side of the mold used as reference to place the ora serrata of the eye in parallel. This is the common procedure we use in our lab, but the position of the eye can be done in different ways. Also the eye can be marked with a dye or by carefully burning the cornea at a specific position to recognize the orientation. The figure was made with Biorender
4 Notes
-
1.
Before starting the procedure, please make sure that permission by the corresponding ethical committee of the research institute has been granted. Subsequently, find a location where the injections can be performed in the most comfortable way, and adhering to the rules for animal experimentation and the animal facility.
-
2.
Depending on the skills of the person, the stereomicroscope (space left to perform the injection), the angle, etc., you can use long or short needles. Long needles allow more flexibility in terms of space (the Hamilton does not interfere with the stereomicroscope); however, small movements are more noticeable. Also, a second person needs to help by pressing the syringe to deliver the volume. In our experience, for adult mice long needles are better.
-
3.
Tropicamide ophthalmic solution can be used to relax the musculature of the eye and therefore dilate the pupils by applying one drop on the eye, and after 1 or 2 min, dry it with a gauze. It is not per se needed, but it might help.
-
4.
During the injection procedure, a third hand is needed to press the syringe and release the content. This colleague can also help with the preparations, for instance, while one person injects, the other can already weigh and prepare the next animal, in order to optimize time as much as possible.
-
5.
It is recommended to start with pigmented animals. This will allow to perform the procedure easily since all the membranes and the eyeball will be completely distinguishable. Having acquired more practice, albino animals can also be injected. If this is not possible, practicing with albino mice can be done using some type of dye that will allow to observe where the liquid is being injected.
-
6.
Isoflurane is the method we use in the lab. Animals fall asleep and recover fast. However, other types of analgesia are also possible if they are accepted by the ethics committee and animal facility.
-
7.
In general, animal facilities are relatively far away from the regular labs; therefore, if the animal facility does not have a stock of materials, such as needles, syringes, tubes, tips, etc., take more materials than needed just in case.
-
8.
Depending on the person, the right or left eye might be easier to inject. Based on our experience, left-handed persons find it easier to inject the left eye, while the opposite is observed for right-handed persons.
-
9.
One of the benefits of working in the eye field is that each animal is its own control. To avoid confusion and perform the procedure in a systematic way, we deliver the control solution to the left eye, and the AON molecule to the right eye. However, it may happen that both eyes might be injected with the same molecule. This will be determined based on the hypothesis and study design .
-
10.
It is really important to have a small area without any membrane, otherwise future steps will be complicated. Multiple cuts might be needed.
-
11.
It is very important to make the hole with the very beginning of the tip of the needle; otherwise, the hole might be too big, or the needle might penetrate into the eye, damaging the retina (Fig. 2a).
-
12.
Sometimes a possibility is to find the lens carefully and go back 1–2 mm, in that way you know you are in intravitreal space.
-
13.
While releasing, hold the syringe in a way that it will not move. Practice first with your colleague and make sure you both adapt to each other. Be clear with the person assisting you by using a specific quote such as “inject,” “now,” etc. It is very important that the person pressing is also aware of doing it gently and slowly to avoid moving, or even pushing the syringe deeper.
-
14.
When injecting, you should see that the solution gets into the eye.
-
15.
When removing the needle there can be some reflux. This happens often, and it is more obvious when injecting 3 μl. With 2 μl it can be observed depending on the size of the eye and with 1 μl it is rare. However, with 1 μl it can happen that the molecules do not reach the entire retina. Removing the needle slowly after a couple of seconds (instead of immediately) after injection also reduces the amount of reflux.
-
16.
To avoid having to clean the Hamilton syringe and needle between left and right eye, the use of two syringes, one for the PBS 1×, and the other for the AON, is recommended. This will ensure no traces of AON in the control eye and will contribute to reduce the overall intervention time.
-
17.
When injecting the mice, make sure that the accompanying labels are moved, and the treatment is clearly stated in the label. Usually, it is also needed to identify individual animals. This can be done with earmarks or any other system used at the facility.
-
18.
The 30G and 33G needles often get less sharp after a couple of injections. So replace them every time you feel they are not as sharp as they used to.
-
19.
When cleaned directly with ethanol, the salts tend to precipitate, and the needle can get clogged. Therefore, the extra step with MQ water after ethanol is to remove the possible salts that could precipitate and therefore cause malfunction of the Hamilton syringe or needle.
-
20.
We have not observed any discomfort or side effect caused by the procedure. However, some spontaneous casualties may occur due to aging, spontaneous tumors, or fights between animals. This highly depends on the age of the animals at the moment of injection, and the period of time they need to be in the experiment until analysis .
-
21.
Make sure that no bubbles are present. If so, remove them since they can affect the sectioning procedure.
-
22.
This is the way we do it to differentiate left and right eyes but also knowing the orientation of the eye. Alternatively, the eye can be marked to identify the orientation after sectioning.
-
23.
A couple of hours before sectioning, we usually move the cryoblocks to −20 °C.
References
Kolb H (2003) How the retina works. Am Sci 91(1):28–35. citeulike-article-id:1560678
Vazquez-Dominguez I, Garanto A, Collin RWJ (2019) Molecular therapies for inherited retinal diseases-current standing, opportunities and challenges. Genes (Basel) 10(9):654. https://doi.org/10.3390/genes10090654
Keeler AM, Flotte TR (2019) Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol 6(1):601–621. https://doi.org/10.1146/annurev-virology-092818-015530
Hammond SM, Wood MJ (2011) Genetic therapies for RNA mis-splicing diseases. Trends Genet 27(5):196–205. https://doi.org/10.1016/j.tig.2011.02.004
Cideciyan AV, Jacobson SG, Ho A, Drack A, Pfeifer W, Charng J, Taiel M, Schwartz M, Biasutto P, de Wit W, Cheetham ME, Adamson P, Rodman D, Nerinckx F, De Zaeytijd J, van Cauwenbergh C, Leroy BP, Russel S (2018) Intravitreal antisense oligonucleotide therapy (QR-110) for the Treatment of Leber Congenital Amaurosis due to Photoreceptor Cilium Defect in Subjects with the CEP290 p.Cys998X Mutation. Paper presented at the RD2018 XVIIIth international symposium on retinal degeneration, Killarney, Ireland
Garanto A, van Beersum SE, Peters TA, Roepman R, Cremers FP, Collin RW (2013) Unexpected CEP290 mRNA splicing in a humanized knock-in mouse model for Leber congenital amaurosis. PLoS One 8(11):e79369. https://doi.org/10.1371/journal.pone.0079369
Collin RW, den Hollander AI, van der Velde-Visser SD, Bennicelli J, Bennett J, Cremers FP (2012) Antisense oligonucleotide (AON)-based therapy for Leber congenital amaurosis caused by a frequent mutation in CEP290. Mol Ther Nucleic Acids 1:e14. https://doi.org/10.1038/mtna.2012.3
Garanto A, Chung DC, Duijkers L, Corral-Serrano JC, Messchaert M, Xiao R, Bennett J, Vandenberghe LH, Collin RW (2016) In vitro and in vivo rescue of aberrant splicing in CEP290-associated LCA by antisense oligonucleotide delivery. Hum Mol Genet 25(12):2552–2563. https://doi.org/10.1093/hmg/ddw118
Surace EM, Auricchio A (2008) Versatility of AAV vectors for retinal gene transfer. Vis Res 48(3):353–359. https://doi.org/10.1016/j.visres.2007.07.027
Toulis V, Garanto A, Marfany G (2016) Combining zebrafish and mouse models to test the function of deubiquitinating enzyme (Dubs) genes in development: role of USP45 in the retina. Methods Mol Biol 1449:85–101. https://doi.org/10.1007/978-1-4939-3756-1_3
Acknowledgments
The author wants to acknowledge Prof. Rob W.J. Collin and Dr. Irene Vázquez Domínguez for critical review of this chapter. The group is financially supported by the Foundation Fighting Blindness (PPA-0517-0717-RAD), the Curing Retinal Blindness Foundation, as well as the Landelijke Stichting voor Blinden en Slechtzienden and Stichting Oogfonds via Uitzicht 2019-17, together with Stichting Blindenhulp, Rotterdamse Stichting Blindenbelangen, and Dowilwo. The funding organizations had no role in the design or conduct of this research and provided unrestricted grants. All figures were made with BioRender.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Garanto, A. (2022). Delivery of Antisense Oligonucleotides to the Mouse Retina. In: Arechavala-Gomeza, V., Garanto, A. (eds) Antisense RNA Design, Delivery, and Analysis. Methods in Molecular Biology, vol 2434. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2010-6_22
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2010-6_22
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2009-0
Online ISBN: 978-1-0716-2010-6
eBook Packages: Springer Protocols