Abstract
The genodermatosis dystrophic epidermolysis bullosa (DEB) is caused by mutations in the COL7A1 gene which encodes type VII collagen (C7). In the cutaneous basement membrane zone, C7 secures attachment of the epidermal basal keratinocyte to the papillary dermis by means of anchoring fibril formation. The complete absence of these anchoring fibrils leads to severe blistering of skin and mucosa upon the slightest friction and early mortality. To date, although preclinical advances toward therapy are promising, treatment for the disease is merely symptomatic. Therefore, research into novel therapeutics is warranted.
Antisense oligonucleotide (ASO)-mediated exon skipping is such a therapy . Clinical examination of naturally occurring exon skipping suggested that this mechanism could most likely benefit the most severely affected patients. The severe form of DEB is caused by biallelic null mutations. Exon skipping aims to bind an ASO to the mutated exon of the pre-mRNA in the cell nucleus. Thereby, the ASO inhibits the recognition of the mutated exon by the splicing machinery, and as a result, the mutated exon is spliced out from the mRNA with its surrounding introns, i.e., it is skipped. Here, we describe in vitro methods to evaluate ASO-mediated exon skipping in a preclinical setting.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
In this chapter, we describe the evaluation of ASO-mediated exon skipping as a therapeutic approach for DEB in a preclinical in vitro setting. DEB is caused by mutations in the COL7A1 gene which encodes type VII collagen (C7) [1]. DEB is a rare disease affecting 1–9 in every one million births, worldwide. The disease is characterized by severe blistering of skin and mucosae. DEB can be inherited both dominantly and recessively, and the severity of the disease strongly depends on the quantity and functionality of the C7 protein present at the cutaneous basement membrane zone. The most severe recessive form of DEB (RDEB-gen sev) is caused by biallelic null mutations and the complete absence of C7 in the skin. Previously, we have shown that for RDEB-gen sev caused by biallelic null mutations, exon skipping is anticipated to be clinically beneficial [2].
Exon skipping relies on specifically designed ASOs that bind to the pre-mRNA in the cell nucleus. When bound, these ASOs inhibit the recognition of the mutated exon by the splicing machinery through steric hindrance [3]. As a result, the mutated exon is spliced out (skipped) of the mRNA together with its surrounding introns. If the skipped exon is in frame, the reading frame of the transcript is maintained and produces a slightly shorter but functional protein [4].
Exon skipping affects the pre-mRNA; therefore, it is essential for the ASO to pass the cell membrane and the nuclear envelope. The commonly used 2′-O-methyl phosphorothioate (2OMePS) and 2′-methoxyethyl phosphorothioate (2MOE) ASOs are negatively charged and therefore not able to easily pass the cell membrane in cell cultures. Therefore, active transfer across the cell membrane is essential. Cationic lipid transfection is such a way of active transfer and widely used to achieve efficient uptake by in vitro cultured cells. Widely studied cells of the skin are dermal fibroblasts and epidermal keratinocytes. Here, we describe the in vitro evaluation of distribution and activity of ASOs in cultured fibroblasts and keratinocytes, as C7 is expressed by both the cell types. However, cationic lipid transfection of fibroblasts and keratinocytes can be used to evaluate the activity of antisense RNA for many diseases, as they express many proteins.
2 Materials
2.1 Cell Culture
-
1.
Trypsin/EDTA (2.5% trypsin/0.2% EDTA).
-
2.
Dispase II (2.4 U/mL).
-
3.
Penicillin/Streptomycin (100 U/mL and 100 μg/mL, respectively).
-
4.
Saline solution: 0.9% NaCl in dH2O sterilized through 0.22-μm filter.
-
5.
Antisense oligonucleotides: 50 μM stock solution in dH2O (final concentration depends on experimental setup).
-
6.
Fibroblast medium: DMEM 4.5 g/L glucose, l-glutamate, 10% fetal bovine serum (FBS), 1× penicillin/streptomycin.
-
7.
Phosphate-buffered saline (PBS).
-
8.
Fibroblast transfection agent: Polyethylenimine (PEI) 1 mg/mL.
-
9.
Keratinocyte medium: CellnTec Prime (CnT-PR).
-
10.
HEPES-buffered saline solution (HBSS).
-
11.
Keratinocyte transfection medium: Opti-MEM.
-
12.
Keratinocyte transfection agent: Lipofectamine-2000.
3 Methods
3.1 Isolation and Culture of Epidermal Keratinocytes and Dermal Fibroblasts
Full-thickness skin biopsies (4–6 mm) or larger skin tissue (1–2 cm) are used to isolate cells.
-
1.
On day 1, incubate the tissue at room temperature overnight in 2× penicillin/streptomycin solution protected from light.
-
2.
On day 2, using tweezers and a scalpel, scrape off excess fatty tissue from the dermal side of the tissue.
-
3.
Place the tissue in a 100-mm petri dish, floating with the dermal side down in 10 mL Dispase II and incubated overnight at 4 °C.
-
4.
On day 3, separate the epidermis from the dermis as a sheet using tweezers. After separation , place the dermis into a glass petri dish and set aside. Transfer the epidermal sheets into a clean 100-mm petri dish containing 10 mL trypsin/EDTA and incubate for 10 min at 37 °C.
-
5.
During the 10 min incubation time, cut the dermis into small pieces of around 1–2 mm in size, using two scalpels in a “scissor” fashion on the glass surface of the petri dishes. Transfer the tissue fragments onto the bottom of the well of a six-well plate and add complete fibroblast medium dropwise onto the tissue and refresh every other day. Within 2 weeks, fibroblasts should be growing out from the tissue. When proliferation of these fibroblasts starts to stagnate due to confluency, remove the tissue remnants, and harvest and passage the cells.
-
6.
After 10 min trypsinization of the epidermal sheets, pipette the trypsin/EDTA solution up and down repeatedly to dissociate the sheets into individual cells.
-
7.
Transfer the cell suspension into a 15-mL tube containing 500 μL FBS to inactivate trypsinization.
-
8.
Spin the cells down by centrifugation for 10 min at 200 × g.
-
9.
Discard the supernatant and resuspend the pellet in Cnt-PR medium.
-
10.
Seed the cells into the culture vessel. Usually, keratinocytes isolated from one 6 mm biopsy are seeded in two 35-mm petri dishes . Refresh the medium three times a week and harvest and passage the cells once they reach 75–90% confluence.
3.2 Transfection of Primary Fibroblasts and Keratinocytes
In this protocol, we describe the transfection of cells with an ASO in a well of a 12-well plate at a concentration of 250 nM, as an example. This is the final concentration of ASOs in the wells after the transfection. All concentrations can be adjusted according to the needs of the individual experiments.
3.2.1 Fibroblasts
Fibroblasts are cultured and transfected in normal fibroblast medium (see Subheading 2.1, step 6)
-
1.
Seed the fibroblasts in 12-well plates at a density at which the cells reach 70–80% confluence within 24–48 h (see Note 1), depending on proliferation rate.
-
2.
When the cells reach 70–80% confluence, wash the cells three times with PBS and carefully remove the PBS with a pipet or vacuum aspiration system.
-
3.
Add 900 μL fresh medium and place the plate back in the incubator (see Note 2).
-
4.
To prepare the lipid-ASO complexes, for each transfected well, pipette 91.5 μL sterile saline solution in a sterile Eppendorf cup.
-
5.
Add 5 μL of ASO, and 3.5 μL PEI and immediately vortex for 10 s (see Note 3).
-
6.
Incubate the saline-ASO-PEI solution at room temperature for 10 min.
-
7.
After incubation, add the transfection mix dropwise to the well.
-
8.
Shake the plate in a north to south and east to west motion and place in the incubator (see Note 4).
-
9.
After 5–6 h, gently remove the transfection medium, wash twice with PBS, and add 1 mL fresh medium and place back into the incubator (see Note 5).
-
10.
After 48–72 h, analyze the cells for exon skipping at RNA or protein level.
-
11.
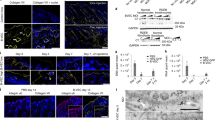
As a positive control, a fluorescently labeled nonspecific AON is used. In case exon skipping exerts its effect in the nuclei, localization in nuclei corresponds to transfection efficiency (Fig. 1).
Microscopy image of cells transfected with fluorescently labeled AON. Left: Fibroblasts transfected with a fluorescently labeled (green) AON using polyethylenimine. Right: Keratinocytes transfected with the same fluorescently labeled AON. A transfection efficiency of more than 95% is observed in both fibroblasts and keratinocytes, as shown by the green signal in the nuclei
3.2.2 Keratinocytes
Keratinocytes are cultured in CnT-PR serum-free low-calcium keratinocyte medium and transfected in Opti-MEM medium.
-
1.
Seed keratinocytes in 12-well plates at a density at which the cells reach 70–80% confluence within 24–48 h.
-
2.
When the cells reach 70–80% confluence, wash the cells three times with HBSS and add 900 μL Opti-MEM to the well and place back in the incubator.
-
3.
In an 1.5-mL tube, add 45 μL Opti-MEM, then add 5 μL ASO and gently mix by pipetting in and out.
-
4.
In a second 1.5-mL tube, add 48 μL Opti-MEM and 2 μL Lipofectamine-2000 and gently mix by pipetting in and out and incubate at room temperature for 5 min.
-
5.
Add the Lipofectamin-2000 solution to the ASO solution and gently mix by pipetting up and down.
-
6.
Incubate at room temperature for 30 min.
-
7.
Add the lipid-ASO complexes dropwise to the wells and place back in the incubator.
-
8.
After 6 h of incubation, remove the medium and gently wash the cells twice with HBSS. Add fresh keratinocyte medium and place the plate in the incubator.
-
9.
After 24–72 h cells can be analyzed for exon skipping and protein expression.
4 Notes
-
1.
For both fibroblasts and keratinocytes: usually between 0.5 and 1.5 × 105 of primary cultured cells is sufficient depending on passage and viability.
-
2.
Do not pipet the PBS, HBSS, or medium directly onto the cells. Instead, gently pipet the liquid against the side wall of the well to prevent unnecessary stress to the cells.
-
3.
It is essential to pipet in the order saline, ASO, PEI. Do not vortex longer than 10 s.
-
4.
During incubation, prevent the solution from agitating. When pipetting the solution from the Eppendorf tube onto the wells, do not pipet repeatedly up and down, as this might disrupt the lipid-ASO complexes. Additionally, when placing the plate back into the incubator, prevent swirling motions, as this will concentrate the AON-transfection reagent complexes in the middle of the wells.
-
5.
Transfection using cationic lipids induces, to some extent, cell death and will have an effect on the cell membrane as the lipids bind and pass them. Therefore, when washing cells and refreshing media, gently and smoothly pipet via the side of the well.
References
Has C, Bauer JW, Bodemer C, Bolling MC, Bruckner-Tuderman L, Diem A, Fine JD, Heagerty A, Hovnanian A, Marinkovich MP, Martinez AE, McGrath JA, Moss C, Murrell DF, Palisson F, Schwieger-Briel A, Sprecher E, Tamai K, Uitto J, Woodley DT, Zambruno G, Mellerio JE (2020) Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol 183(4):614–627. https://doi.org/10.1111/bjd.18921
Bremer J, van der Heijden EH, Eichhorn DS, Meijer R, Lemmink HH, Scheffer H, Sinke RJ, Jonkman MF, Pasmooij AMG, Van den Akker PC (2019) Natural exon skipping sets the stage for exon skipping as therapy for dystrophic epidermolysis bullosa. Mol Ther Nucleic Acids 18:465–475
Aartsma-Rus A, van Vliet L, Hirschi M, Janson AA, Heemskerk H, de Winter CL, de Kimpe S, van Deutekom JC, t Hoen PA, van Ommen GJ (2009) Guidelines for antisense oligonucleotide design and insight into splice-modulating mechanisms. Mol Ther 17(3):548–553. https://doi.org/10.1038/mt.2008.205
Bornert O, Kühl T, Bremer J, van den Akker PC, Pasmooij AM, Nyström A (2016) Analysis of the functional consequences of targeted exon deletion in COL7A1 reveals prospects for dystrophic epidermolysis bullosa therapy. Mol Ther 24(7):1302–1311. https://doi.org/10.1038/mt.2016.92
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Bremer, J., van den Akker, P.C. (2022). In Vitro Models for the Evaluation of Antisense Oligonucleotides in Skin. In: Arechavala-Gomeza, V., Garanto, A. (eds) Antisense RNA Design, Delivery, and Analysis. Methods in Molecular Biology, vol 2434. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2010-6_11
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2010-6_11
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2009-0
Online ISBN: 978-1-0716-2010-6
eBook Packages: Springer Protocols