Abstract
Fluorinated compounds feature favorable toxicity profile and can be used as a contrast agent for magnetic resonance imaging and spectroscopy. Fluorine nucleus from fluorinated compounds exhibit well-known advantages of being a high signal nucleus with a natural abundance of its stable isotope, a convenient gyromagnetic ratio close to that of protons, and a unique spectral signature with no detectable background at clinical field strengths. Perfluorocarbon core nanoparticles (PFC NP) are a class of clinically approved emulsion agents recently applied in vivo for ligand-targeted molecular imaging. The objective of this chapter is to outline a multinuclear 1H/19F MRI protocol for functional kidney imaging in rodents for mapping of renal blood volume and oxygenation (pO2) in renal disease models.
This chapter is based upon work from the COST Action PARENCHIMA, a community-driven network funded by the European Cooperation in Science and Technology (COST) program of the European Union, which aims to improve the reproducibility and standardization of renal MRI biomarkers. This experimental protocol chapter is complemented by a separate chapter describing the basic concept of functional imaging using fluorine (19F) MR methods.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
In healthy kidneys, the countercurrent design of blood vessels and tubules maintains the necessary osmolar gradient to permit efficient urinary concentration, yet the system operates under conditions of a relatively hypoxic environment in some regions that requires extensive extraction of oxygen from flowing blood [1, 2]. During acute and chronic kidney injury, ischemia challenges effective O2 delivery to the capillary beds of the renal tubules where extraction of extra oxygen is marginal, resulting in heightened susceptibility to ischemic damage that may culminate in renal failure [3]. Besides the traditional biochemical metrics for assessing renal failure (e.g., blood urea nitrogen, creatinine), 1H/19F imaging offers a multiparametric quantifiable approach to functional imaging in kidney injury that avoids the use of potentially nephrotoxic gadolinium (Gd) based contrast agents [3].
Perfluorocarbon core nanoparticles (PFC NP) are a class of emulsion agents clinically approved as blood substitutes that have been applied recently for in vivo for targeted molecular imaging and noninvasive oxygen tension (pO2) assessment [1,2,3]. As compared with standard iodinated or Gd contrast agents, PFC NP are biologically inert and exhibit a good safety profile with no renal toxicity reported in both animals and human [3]. Because PFC NP (nominal size 200–250 nm) are not cleared through glomerular filtration, they do not increase kidney workload, which is a major risk factor for kidney failure with the use of other agents that undergo renal clearance [3]. For imaging purposes, the unique 19F MR signal emanating from the fluorine core of PFC NP can be measured directly in vivo in quite sparse concentrations (e.g., picomolar voxel amounts) with no background signal, as contrasted with other paramagnetic or superparamagnetic moieties that exert indirect contrast effects on circulating protons [4,5,6]. PFC NP have been demonstrated as effective functional probes for regional blood volume as the detected 19F signal intensity directly reflects the actual quantity of PFC NP within a voxel. Furthermore, for pO2 measurements, the 19F longitudinal relaxation rate (R1) responds directly to local oxygen content in a linear manner [1, 2].
Herein we describe 1H/19F MRI for monitoring of renal perfusion in the kidney of rodents in a step-by-step experimental protocol. The rationale for choosing acquisition parameters is described in generic terms, together with specific parameter examples.
This experimental protocol chapter is complemented by a separate chapter describing the basic concept of functional imaging using fluorine (19F) MR methods, which is part of this book.
This chapter is part of the book Pohlmann A, Niendorf T (eds) (2020) Preclinical MRI of the Kidney—Methods and Protocols. Springer, New York.
2 Materials
2.1 Animals
This experimental protocol is applicable to mice and rats.
2.2 Lab Equipment
Enumerated below are the contrast agents and equipment components needed:
-
1.
Perfluorocarbon Nanoparticle Preparation: PFC NP (~200–250 nm) can be formulated with a variety of PFC that are liquid at body temperature. Here we describe either perfluorooctylbromide (PFOB) or perfluoro-15-crown-5-ether (CE). The PFOB or CE emulsions are composed of 20% or 40% (v/v) of PFOB or CE, 2.0% (w/v) of a surfactant commixture, and 1.7% (w/v) glycerin in water (see Note 1).
-
2.
Gases: O2, N2, and compressed air, as well as a gas-mixing system to achieve required changes in the oxygen fraction of inspired gas mixture (FiO2). In addition to room air, the following gas mixtures are required during the experiment: 10% O2–90% N2 for hypoxia and 100% O2 for hyperoxia.
-
3.
Anesthesia: An MRI-compatible slow infusion system is needed for anesthesia with ketamine–xylazine (e.g., syringe pump). Isoflurane is not recommended for 19F imaging as it could interfere with the detected 19F MRI signal from PFC NP.
-
4.
Small Animal Monitoring System: An MRI-compatible small animal physiological monitoring system (e.g., SAI Inc., USA) is needed for real time respiratory monitoring and respiratory triggering and/or gating. Respiratory gating is recommended during 19F MRI acquisition because it reduces motion artifact and preserves 19F signal intensity in the final images, which could otherwise be degraded by motion.
-
5.
Small Animal Holder: a dedicated customer built small animal holder is needed to accommodate animal imaging and for maintenance of body temperature at 37 °C. A warm water blanket or warm air blower can be used for maintaining body temperature of animals (see Note 2).
-
6.
Animal Surgery Suite: A fully equipped animal surgery suite is needed for preparing animal models of renal injury (see Note 3).
2.3 MRI Hardware
The technique described in this chapter is tailored for an 11.7 T MR system (e.g., Varian, USA) but advice for adaptation to other field strengths is given where necessary. In general, the MRI scanner should be equipped with a wide band spectrometer and a wide band RF power amplifier. The scanner needs to be functional at both 1H and 19F frequencies.
-
1.
1H/19F dual-tuned Coils: use actively decoupled coil pair for renal imaging applications. Such coils can be custom-built as described in our prior publications [7] or obtained from commercial coil manufacturers (see Note 4).
-
2.
Transmit Coils: use a volume transmit coil to generate a homogeneous B1 field that covers both kidneys. Tune and match to the 19F frequency.
-
3.
Receive Coils: Place a surface receive coil on the dorsal side of the animal to achieve high sensitivity to 19F signal from the two kidneys. Tune and match to the 19F frequency.
2.4 MRI Techniques
-
1.
1H MRI: a gradient echo sequence is employed to locate two imaging slices with 2 mm thickness, positioned 1–2 mm apart, and centered respectively at the left and right kidney.
-
2.
1H MRI: a multiecho gradient-echo blood oxygenation level dependent (BOLD ) scan can be performed. For an example of parameters used for a specific setting (see Note 5).
-
3.
19F MRI: For spin density 19F MRI, TR is set to 4 s. For T1-weighted MRI, TR is set to two respiration periods, or ~300 ms. All other imaging parameters are identical in these two sets of 19F MRI (see Note 6)
-
4.
19F MRI Oxygenation Measurement: For PFC NP dependent oxygenation measurements in fast flowing blood of major blood vessels, a Blood Flow-Enhanced-Saturation-Recovery (BESR) sequence [8] can be utilized to minimize in-flow effects on the image (see Note 7).
3 Methods
3.1 PFC Nanoparticle Formulation
-
1.
Prepare PFC NP emulsifying 40% (v/v) of CE (Exfluor Research Corp, Round Rock, TX), 2.0% (w/v) of a surfactant commixture, and 1.7% (w/v) glycerin, in distilled water using a microfluidic apparatus (e.g., benchtop LV1 fluidizer, Microfluidics Corporation, Westwood, MA) (see Note 8).
-
2.
Determine size and charge with a submicron particle analyzer (e.g., Zetasizer, Malvern, UK) [9, 10].
3.2 PFC NP Injection and Imaging
-
1.
Inject PFC NP for functional perfusion imaging to quantify blood volume and oxygenation.
-
2.
Prepare an external reference for 19F signal quantification. Place the external reference close to the animal within the field of view (see Note 9).
-
3.
Perform 1H and 19F imaging 10 min to 1 h after NP injection. A shorter circulation time is preferred to maximize the intravascular 19F signal (see Note 10).
3.3 Coil Sensitivity Profiling for Quantification of 19F Signal Intensity
-
1.
Acquire an RF coil sensitivity map using a saline phantom positioned at the selected imaging location to correct for the signal bias created by surface coil receive profile inhomogeneity. The saline phantom is prepared to cover the entire region of imaging FOV (see Note 11).
-
2.
Compensate for the spatial inhomogeneity of the RF coil sensitivity profile by multiplying the correction factor obtained from the phantom image (see Note 12) with the in vivo measured image signal intensity at the corresponding locations.
3.4 Calibration of 19F R1 vs. pO2 Curve In Vitro
-
1.
Perform an in vitro calibration of 19F relaxation time (R1) as a function of pO2 using at least three different O2 concentrations (e.g., 0%, 21%, 100% O2 balanced with N2) by bubbling gas mixtures into a PFC emulsion sample for 30 min at 37 °C (Fig. 1).
-
2.
Perform 19F T1 measurements with inversion recovery spectroscopy at TR > 10 s, and at least ten different inversion delays (TI) ranging from 3 ms to 5 s. Use exponential fitting to extract R1 from T1 measurements and linear fitting to calibrate the relationship between 19F R1 and pO2.
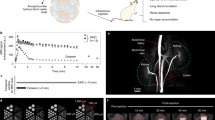
In vitro calibration shows the 19F R1 (=1/T1) of PFC NP as a function of pO2. Data were collected at 11.7 T using a PFC NP phantom. Temperature of the PFC NP phantom was maintained at 37 °C. The correlation coefficient R > 0.99 (From Hu et al. [1])
3.5 Quantification of 19F Based pO2 Measurement In Vivo
-
1.
Create two sets of images, one using a short TR and one using a long TR, for T1 mapping (see Note 13).
-
2.
Determine the actual TR using the external 19F standard (see Note 14).
-
3.
After determining the actual short TR, perform voxel-wise T1 mapping of the kidney using the standard saturation recovery signal model:
$$ S={S}_0\left(1-{e}^{\frac{TR}{T_1}}\right). $$(1) -
4.
Translate T1 values into corresponding pO2 levels according to the predetermined calibration curve.
4 Notes
-
1.
The CE signal intensity is effectively greater for narrow band reception as all fluorine atoms contribute to a single spectral peak, as compared to PFOB where chemical shifts apportion the signal into several peaks (Fig. 2). Suppliers of PFC include Exfluor Research Corporation (Round Rock, TX). The clearance half-life for PFOB is ~3–5 h depending on species and whether the PFC contrast material is molecularly targeted or not [11]. The CE materials exhibit a longer tissue residence time in bloodstream than PFOB compounds, which makes them problematic for clinical use or repeat studies. For the present protocol, no targeting ligands are attached to the surface of the PFC NP to avoid changing the pharmacokinetics of the contrast agent.
-
2.
Potential MRI in-flow artifacts may be present on 1H but not 19F MR images if a warm water blanket is used. Special attention should be given when choosing the animal holder and the MRI coils as reduced animal motion during lengthy MRI acquisitions will improve image quality.
-
3.
In general, ischemic renal injury can be produced unilaterally or bilaterally by exposing the kidneys either through the abdomen or retroperitoneally, and then clamping the renal artery for a selected interval [1, 2]. The animal surgery suite does not need to be located in the MRI facility but should be close enough for imaging a short time after surgery and later for tissue harvest after imaging is completed if indicated.
-
4.
Despite being slightly off-resonance for 1H imaging, the 19F coil pair should be sufficient to generate high quality anatomic images within <1 min scan time. The size of the transmit and receive coils should be designed to match the animal size, so that different coil sizes will be needed for imaging mice and rats. For imaging at field strengths lower than 11.7 T, it is recommended to use double tuned receive coils that simultaneously function at 1H and 19F frequency to assure maximal detection efficiency while avoiding potential image mismatches between 1H and 19F images that may occur as a result of manual coil retuning.
-
5.
The imaging parameters of BOLD at 4.7 T are: TR = 100 ms, TE = 1.96 ms with 1.75 ms increment for every echo, flip angle = 10°, field of view = 26 × 26 mm2, voxel size = 0.2 × 0.2 × 2 mm3. Field of view and voxel size should be adjusted based on animal size to achieve optimal image quality.
-
6.
ETL = 4, TE = 11.5 ms, receiver bandwidth = 20 kHz, voxel size = 0.4 × 0.4 × 2 mm3, and the field of view is identical to that for 1H BOLD imaging.
-
7.
For an example of parameters used at 4.7 T, the imaging parameters of BESR sequence are: TR = 2.5 s; TE = 2.2 ms; number of points along recovery curve = 8; number of averages = 16; in plane resolution = 1.5 mm × 0.75 mm; slice thickness = 2 mm
-
8.
PFC NP can be colabeled with Alexa Fluor 594 for ex vivo fluorescence microscopy examination.
-
9.
As reference, a tube of another PFC NP formulation (e.g., perfluorodecalin) at a similar PFC concentration to that administered in vivo for the imaging studies can be used.
-
10.
In healthy tissues, PFC NP are generally constrained to the intravascular space because of their large particle size (200–250 nm), which ensures that the measured 19F signal intensity correlates linearly with intravascular blood volume. One has to also consider that during injury, PFC NP will escape from the circulation and will get trapped in tissue compartments. In this case the total fluorine signal would comprise both stationary (i.e., trapped in tissue) and flowing (i.e., in blood) PFC NP. Employ spin labeling [12] to eliminate the signal from trapped PFC NP, leaving only the signal from the flowing blood compartment. Apply diffusion gradients to suppress the signal from flowing blood [13] and acquire distinct signal from either compartment. For O2 measurements, a mixture of signals could occur where trapped PFC NP exist under hypoxic conditions and the flowing PFC NP under more normoxic conditions. In this case the 19F relaxation times would represent an average of the two situations.
-
11.
At 11.7 T, imaging parameters are as follows: TR = 100 ms, TE = 1.96 ms, and flip angle = 10°. For other field strengths, minimize TE and select similar TR and flip angle.
-
12.
For each voxel location, the phantom image defines a correction factor matrix R(x,y,z) that is the inverse of local signal intensity I(x,y,z): R(x,y,z) = 1/I(x,y,z).
-
13.
In vivo quantification of renal T1 and pO2 is based on the general assumption that image signal intensity in a fast spin echo sequence follows a saturation-recovery process (Fig. 3). Because short TR or T1-weighed 19F MRI is gated with respiration, the actual short TR can vary from animal to animal.
-
14.
Given that the 19F T1 of PFC standard is 0.9–1 s at room air oxygen tension, the signal acquired with a long TR = 4 s, that is, >3 × T1, should approximate the 19F spin-density signal of PFC NP at room temperature (Fig. 4).
(a, b) Representative renal blood volume and pO2 mapping of healthy kidneys determined by quantitative 19F MRI. (c) Representative 1H T2* (BOLD) mapping of healthy kidneys. (d–f) Quantification of functional indexes in different anatomical regions. *P < 0.05 compared with cortex (From Hu et al. [1])
Imaging of improved renal medullary perfusion after antithrombin PFC NP treatments. Here the thrombin inhibitor PPACK (phenylalanine-proline-arginine-chloromethylketone) was conjugated to the PFC NP. Mice were pretreated with PFOB PPACK NP, plain PFOB NP, or saline before ischemia induction. At 3 h after reperfusion, a single dose of CE NP was injected i.v. for 19F MRI. The 19F signal from circulating CE NP of uninjured kidney illustrates normal kidney perfusion (a). In contrast, all injured kidneys exhibited reduced 19F signal in the medulla (b–d) reflecting the severity of regional nonreperfusion. Compared to kidneys pretreated with saline or plain NP (b, c), the improved medullary perfusion in PPACK NP treated kidneys is visually appreciable (d). Using in vivo 19F MRI data, the ratio of nonperfused kidney area after reperfusion was determined. The PPACK NP treated kidneys (n = 4) developed less regional nonreperfusion than did saline (n = 6) or plain NP (n = 6) treated kidneys. *p < 0.05 compared to saline or plain NP treated kidneys. Note that the arrow in d indicates the partial overlay of 19F signal from the adjacent spleen (From Chen et al. [2])
References
Hu LZ et al (2014) Assessing intrarenal nonperfusion and vascular leakage in acute kidney injury with multinuclear H-1/F-19 MRI and perfluorocarbon nanoparticles. Magn Reson Med 71(6):2186–2196
Chen JJ et al (2015) Antithrombin nanoparticles improve kidney reperfusion and protect kidney function after ischemia-reperfusion injury. Am J Physiol Renal Physiol 308(7):F765–F773
Chen JJ et al (2013) Perfluorocarbon nanoparticles for physiological and molecular imaging and therapy. Adv Chronic Kidney Dis 20(6):466–478
Goette MJ et al (2015) Balanced UTE-SSFP for 19F MR imaging of complex spectra. Magn Reson Med 74(2):537–543
Goette MJ et al (2015) Improved quantitative 19 F MR molecular imaging with flip angle calibration and B1-mapping compensation. J Magn Reson Imaging 42(2):488–494
Palekar RU et al (2015) Quantifying progression and regression of thrombotic risk in experimental atherosclerosis. FASEB J 29(7):3100–3109
Hu LZ et al (2011) A generalized strategy for designing (19)F/(1)H dual-frequency MRI coil for small animal imaging at 4.7 Tesla. J Magn Reson Imaging 34(1):245–252
Hu LZ et al (2013) Rapid quantification of oxygen tension in blood flow with a fluorine nanoparticle reporter and a novel blood flow-enhanced-saturation-recovery sequence. Magn Reson Med 70(1):176–183
Winter PM et al (2003) Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res 63(18):5838–5843
Winter PM et al (2003) Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation 108(18):2270–2274
Kaneda MM et al (2009) Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng 37(10):1922–1933
Wu WC et al (2011) Renal perfusion 3-T MR imaging: a comparative study of arterial spin labeling and dynamic contrast-enhanced techniques. Radiology 261(3):845–853
Ichikawa S et al (2013) Intravoxel incoherent motion imaging of the kidney: alterations in diffusion and perfusion in patients with renal dysfunction. Magn Reson Imaging 31(3):414–417
Acknowledgments
This chapter is based upon work from COST Action PARENCHIMA, supported by European Cooperation in Science and Technology (COST). COST (www.cost.eu) is a funding agency for research and innovation networks. COST Actions help connect research initiatives across Europe and enable scientists to enrich their ideas by sharing them with their peers. This boosts their research, career, and innovation.
PARENCHIMA (renalmri.org) is a community-driven Action in the COST program of the European Union, which unites more than 200 experts in renal MRI from 30 countries with the aim to improve the reproducibility and standardization of renal MRI biomarkers.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this protocol
Cite this protocol
Hu, L., Pan, H., Wickline, S.A. (2021). Fluorine (19F) MRI to Measure Renal Oxygen Tension and Blood Volume: Experimental Protocol. In: Pohlmann, A., Niendorf, T. (eds) Preclinical MRI of the Kidney. Methods in Molecular Biology, vol 2216. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0978-1_31
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0978-1_31
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0977-4
Online ISBN: 978-1-0716-0978-1
eBook Packages: Springer Protocols