Abstract
A noninvasive, robust, and reproducible method to measure renal perfusion is important to understand the physiology of kidney. Arterial spin labeling (ASL) MRI technique labels the endogenous blood water as freely diffusible tracers to measure perfusion quantitatively without relying on exogenous contrast agent. Therefore, it alleviates the safety concern involving gadolinium chelates. To obtain quantitative tissue perfusion information is particularly relevant for multisite and longitudinal imaging of living subjects.
This chapter is based upon work from the PARENCHIMA COST Action, a community-driven network funded by the European Cooperation in Science and Technology (COST) program of the European Union, which aims to improve the reproducibility and standardization of renal MRI biomarkers. This experimental protocol chapter is complemented by two separate chapters describing the basic concept and data analysis.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
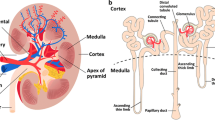
Arterial spin labeling (ASL) is a magnetic resonance imaging (MRI) method for measuring tissue perfusion [1, 2]. The term perfusion refers to the delivery of blood to capillary beds, and is quantified by the amount of blood delivered to the tissue per unit time, per unit volume or mass of tissue. The quantification of renal tissue perfusion is essential because it determines the rate of nutrients (e.g., oxygenation and glucose) to the renal tissue, and the rate of clearance of waste products.
The principle of ASL-MRI is to label the arterial blood as an endogenous diffusible tracer. Before the blood flows into the target tissue, the blood proton spins are “labeled” (tagged) by inverting the longitudinal magnetization using radiofrequency (RF) pulses. The labeled blood then flows into the kidney tissue, resembling direct exogenous contrast more than MRI contrast agents that act on relaxation times. The labeled blood, however, loses its contrast on its way to kidney tissue within a few seconds due to the T1 relaxation of blood. This makes ASL only suitable for probing renal perfusion, but not later processes in the kidney such as glomerular filtration. On the other hand, the intrinsic signals from the static kidney tissue have to be eliminated. For this, a control image without labeling arterial blood is acquired, and subtraction of the two images with and without labeling would result in an image enhanced with only the labeled arterial blood.
The most frequently used ASL technique for kidney imaging is flow-sensitive alternating inversion recovery (FAIR) which uses an inversion pulse for spin labeling [3]. Its principle is illustrated in the chapter by Ku M-C et al. “Noninvasive Renal Perfusion Measurement Using Arterial Spin Labeling (ASL) MRI: Basic Concept.” In this technique, two acquisitions with different inversions are alternatingly applied: one acquisition with selective inversion of a slab that is slightly larger than the imaging slice (no labeling of in-flowing blood) and a second acquisition with global inversion of all blood within the RF coil (nonselective inversion). Subtraction of the image with global inversion from the image with spatially selective inversion results in a perfusion-weighted image, as the difference between the two images is caused by the noninverted blood spins moving from outside the selective inversion slab into the imaging plane.
Here we describe ASL-MRI using FAIR method for monitoring of the renal blood flow in the kidney of rodents in a step-by-step experimental protocol. The rationale for the chosen acquisition parameters is given in generic terms, together with specific parameter examples.
This experimental protocol chapter is complemented by two separate chapters describing the basic concept and data analysis, which are part of this book.
This chapter is part of the book Pohlmann A, Niendorf T (eds) (2020) Preclinical MRI of the Kidney—Methods and Protocols. Springer, New York.
2 Materials
2.1 Animals
This experimental protocol is tailored to mice (e.g., wild type C57BL/6 or disease model in immune-deficient nude mice) with a body mass of 20–40 g. Some advice for adaptation to other rodents such as rats is given as Notes when necessary.
2.2 Lab Equipment
-
1.
Anesthesia: For an in-depth description and discussion of anesthesia please refer to the chapter by Kaucsar T et al. “Preparation and Monitoring of Small Animals in Renal MRI.” Typically 0.5–1.5% isoflurane is used for anesthesia administered to the mice using an anesthetic gas vaporizer (Dräger Vapor® FMI Föhr Medical Instruments GmbH, Seeheim-Ober Beerbach, Germany).
-
2.
Gases: O2, N2 and compressed air, as well as a gas-mixing system or general inhalation anesthesia equipment, including an anesthetic vaporizer, a flow meter and an induction chamber.
2.3 MRI Hardware
The general hardware requirements for renal 1H MRI on mice and rats are described in the chapter by Ramos Delgado P et al. “Hardware Considerations for Preclinical Magnetic Resonance of the Kidney.” The technique described in this chapter was tailored for a 7 T MR system (Biospec 70/20, Bruker Biospin, Ettlingen, Germany) but advice for adaptation to other field strengths is given where necessary. No special or additional hardware is required, except for:
-
1.
A physiological monitoring system that can track the respiration, and is connected to the MR system such that it can be used to trigger the image acquisition. Typically, the MR-compatible rodent monitoring and gating system (Small Animal Instruments, New York, USA) equipped with an air-pillow to monitor breath rate can be used.
-
2.
Mouse cradle and RF-antenna (see Note 1): The 2 × 2 cardiac coil array (Bruker), originally designed for mouse heart is integrated into corresponding animal cradle tips. The combination of receive-only coil array and a circularly polarized volume transmitter coil (Bruker) will improve signal to noise ratio (SNR). Alternatively, a single-loop surface coil could be used for receiving provided that a volume coil is used for transmit in any case.
2.4 MRI Sequences
-
1.
Sequence type: For the choice of type of sequence to use for renal ASL in rodents please refer to the chapter by Ku M-C et al. “Noninvasive Renal Perfusion Measurement Using Arterial Spin Labeling (ASL) MRI: Basic Concept.” A 2D sequence composed by FAIR for labeling and EPI for image acquisition is described in this chapter. This is a standard sequence on Bruker MRI systems, where it is called “FAIR-EPI.” To minimize the susceptibility artifact and geometric distortion in the abdomen, spin-echo EPI or RARE acquisition is desirable as alternative (“FAIR-RARE”).
-
2.
Echo time (TE): To preserve signal from T2/T2* decay, a minimum TE should always be used (~10 ms) to maximize SNR.
Avoid crusher gradients: Although they reduce flow-related artifacts, they require prolongation of the minimum TE. They would reduce SNR and introduce more T2 (T2*) contrast into the ASL image. Crusher gradients also remove potentially important information, such as the presence of delayed or collateral flow.
-
3.
Repetition time (TR): There are two considerations on choosing appropriate TR. First, to allow substantial relaxation of labeled spins between acquisitions, TR should be long enough (~10,000 ms). Since T1 time is field dependent, TR as well. The long TR (~10,000 ms) is recommended for a 7 T system. For other field strength this value need to be justified. Second, if respiratory induced artifact is severe and the respiratory trigger is needed, a long TR that is at least five times greater than the tissue T1 (e.g., 10 s) can be used to minimize the variable T1-weighting due to irregular respiration rate. If the respiratory trigger is not needed, a shorter TR that has good signal-to-noise per time (SNR/t) efficiency could be used. TR will be limited by the maximum inversion time (TI) and the number of acquired slices.
-
4.
TR mode: TR must allow tag washout and refreshing, TE should be short to minimize T2 contamination.
-
5.
Repetition spacing: Choose “Const_Rep” to use the same TR.
-
6.
FAIR Experiment Mode: Choose “Interleaved (TI loop outside)” to measure control-label pairs, followed by different TI.
-
7.
Inversion mode (TI Setting): Set for “User” TIR.
-
8.
Inversion time (TI): TI should be long enough to permit tagging to leave tagging region and ensure tag exchange with tissue water (i.e., the arterial arrival time), but short enough to reduce loss of tag. TI should be increased whenever slow arterial flows (i.e., long arterial arrival time) are expected. For example, in the case of poor cardiac output. Shorter TI values are recommended in case of more rapid circulation times (this depends on the anesthesia level). Unless the arterial arrival time is known, measurement with multiple TI is recommended, e.g., from 25, 50, 100, 150, 200, 300, 500, 1000, 1500, 2000, to 5000 ms. The arrival time increases with the selective inversion slab thickness. For single slice FAIR, the arrival time is typically very short (see Note 2).
-
9.
Inversion pulse: For inversion, an adiabatic full passage frequency-selective inversion pulse (e.g., hyperbolic secant-shaped pulse) should be used with a high bandwidth of 5190 Hz. A calculated adiabatic pulse can be used as well.
-
10.
Inversion slab thickness: This depends on the imaging slice thickness. Usually at least 2.5 times of slice package thickness. For example, the slice package margin should be at least 1.5 mm resulting in a 5 mm inversion slice when you use 2 mm slice thickness.
-
11.
Acquisition bandwidth (BW): Use a high BW (e.g., 200 kHz) for EPI readout to shorten TE. Keep an eye on the SNR while modifying BW. SNR decreases with increasing BW. Low SNR may be balanced out with more averages or repetitions. For scanning with a short TE, an SNR of at least 60 is recommended (see Note 3).
-
12.
Fat saturation: Turn on. Important to avoid fat signal overlaying the kidney due to chemical shift.
-
13.
Geometry: this is a tricky part. To cover the whole kidney in one slice, an oblique coronal slice will be needed. It is important to avoid the selective inversion slab crossing the feeding arteries (aorta) as this will reduce the labeled arterial blood spins. Use a rectangular field of view (FOV) and use a frequency encoding in H-F direction to avoid aliasing (see Note 4).
-
14.
Respiration trigger: Turn on (per repetition). This is essential when respiration is very irregular or motion artifact is induced in multishot imaging such as RARE.
-
15.
Number of slice and thickness: Slice thickness is typically no less than 1 mm in mice or rats due to the low SNR of ASL . The number of slices and thickness determines the selective inversion slab thickness. It should be noted that ASL using FAIR is not suitable for multislice imaging per se, because the labeled blood magnetization decreases with increasing inversion slab thickness. This is due to relaxation during transit, but also to the excitation pulses related to imaging readout in the various slices. Therefore, usually no more than three slices are recommended. Besides, ASL has generally low SNR due to a relatively small fractional magnetization of available labeled arterial blood (1–5%). Therefore, rather thick imaging slices (≥1 mm) are needed to reduce noise.
-
16.
T1 map: A T1 map is useful to improve the quantification accuracy as it provides a measured tissue T1 (which is different in medulla and cortex) and inversion efficiency. It can be adapted from the ASL sequence by selecting the “nonselective inversion” mode in “FAIR Experiment.” Set up at least six TI in logarithmic order (e.g., 10, 50, 200, 1000, 2000, 5000, 9000 ms) with long constant TR (e.g., 10 s). A few (e.g., 3) data averaging will be needed to increase SNR.
3 Methods
3.1 Animal Preparations and Initial Setups for Imaging
-
1.
Anesthetize the animal with isoflurane in an induction box (approximately 3–3.5% isoflurane for induction with air and oxygen mixed at a 3:1 ratio) and then transfer the animal to the scanner (see Note 5).
-
2.
Set up the temperature monitoring (rectal probe) and respiratory monitoring (balloon on chest) unit. Ophthalmic ointment should be applied to the eyes of the animal.
-
3.
If isoflurane is used, adjust anesthesia level (e.g., 1.5–2%) to maintain regular respiration rate around 90 breaths per minute (bpm) in mouse or 60 bpm in rat during the scan. The level of isoflurane may need to be adjusted regularly to maintain a rather constant respiration rate (see Note 6).
-
4.
Carefully position the RF-antenna to be near the kidney and place it to the magnet isocenter based on the initial anatomical imaging.
-
5.
Perform coil tuning/matching and global shimming (see Note 7).
-
6.
Acquire kidney anatomical imaging (i.e., routine respiratory triggered T2-weighted turbo spin echo sequences in axial and coronal planes) as described in the chapter by Pohlmann A et al. “Essential Practical Steps for MRI of the Kidney in Experimental Research” (see Note 8).
-
7.
In the case of use a sequence with FAIR for labeling and EPI for image readout (FAIR-EPI), please perform the trajectory adjustment (see Note 9).
3.2 Renal Perfusion Imaging
3.2.1 Baseline Condition (Healthy Animal)
-
1.
Load the ASL (FAIR-EPI) sequence (see Note 10), adapt the slice orientation to provide either a coronal or axial view with respect to the kidney (in scanner coordinates this is double-oblique). Adjust the geometry of a flow saturation slice onto aorta and perpendicular to the ASL imaging slices (Fig. 1) (see Note 11).
-
2.
Acquire a pilot scan to evaluate slice location and image quality by setting repetition and average to 1. Fine tune the slice position based on the pilot scan to maximize the coverage of kidney while avoiding the selective inversion slab (imaging slab) to cross the feeding arteries (Fig. 2).
-
3.
Perform subtraction between label and control images to evaluate the level of perfusion signal.
-
4.
Acquire ASL scans with multiple repetitions. Increase repetition if SNR is not optimal (see Note 12).
-
5.
Inspect the acquired image time series. If motion artifacts are severe, use respiratory trigger and then repeat scans (see Note 13).
-
6.
When use respiration triggering: in the monitoring unit set the trigger delay so that the trigger starts at the beginning of the expiratory plateau (no chest motion) and the duration such that it covers the entire expiratory phase, that is, until just before the next inhalation starts.
-
7.
Clone (duplicate) the ASL scan and set all the optimized parameters. Run the sequence for ASL scanning.
-
8.
Load the T1-mapping (IR-EPI) scan using the same slice geometry and resolution.
Anatomical images with 3 planes (coronal, axial and sagittal views of mouse kidneys). Imaging is performed in a central coronal plane, adjusted to the long axis of the kidneys. The green box outlines slice position for a saturation slice. The yellow box outlines the imaging slice for a coronal view. The red dotted box outlines the inversion slab. Please note that the inversion slab has to be thicker than the imaging slice
3.2.2 Hypoxia/Hyperoxia/Hypercapnia for Benchmarking (Optional)
-
1.
Duplicate the ASL (FAIR-EPI) scan.
-
2.
Start of hypoxia: Change the gas flowing through the respiratory mask to 10% O2/90% N2.
-
3.
Exactly 5 min after the start of hypoxia run the ASL and T1-mapping scans.
-
4.
End of Hypoxia: Change the gas flowing through the respiratory mask back to air (21% O2).
-
5.
Similarly, hyperoxia and hypercapnia conditions could be assessed by switching to 100% O2 and 5% CO2 in air, respectively.
3.2.3 Perfusion Map
-
1.
The demonstration of a perfusion map that can be expected in physiological condition is shown in Fig. 3. Please note that the detailed protocol for the image analysis is given in the chapter by Chuang K-H et al. “Quantitative Analysis of Renal Perfusion by Arterial Spin Labeling.” Briefly, the perfusion-weighted signals at different TIs, ΔM(TI), can be fitted to a kinetic function by a nonlinear least square routine in Matlab (Mathworks, MA, USA) [4,5,6]:
$$ \Delta M\left(\mathrm{TI}\right)=2{M}_0\alpha f/\lambda \left[\frac{\exp \left(-\mathrm{TI}/{T}_{1\mathrm{app}}\right)-\exp \left(-\mathrm{TI}/{T}_{1\alpha}\right)}{\left(1/{T}_{1\alpha }-1/{T}_{1\mathrm{app}}\right)}\right], $$where 1/T1app = 1/T1 + f/λ, f is the perfusion (when lambda is in ml/100 g and T1 in minutes), T1a is the arterial blood T1, and λ is the blood/tissue partition coefficient. In this equation, three parameters can be derived from the additional T1 mapping: M0 represents the equilibrium magnetization, T1 the tissue longitudinal relaxation time, and α the inversion efficiency. They can be calculated by three-parameter curve fitting of the inversion recovery T1 mapping data. Please note that the above equation assumes minimal and negligible arterial transit time.
-
2.
In the Macro manager, choose Calculate Global T1 Map. Note that if there is a bug, you need to select each map twice in the macro.
-
3.
Step through the macro by first loading the ASL experiment.
-
4.
Before selecting Compute Perfusion Map, check the T1 of blood [7] (Fig. 3).
4 Notes
-
1.
The ASL technique is SNR limited. Multichannel array coils or cryoprobes are a better choice. Particularly, as FAIR requires a global inversion, a volume transmit coil is necessary. Using surface transmit/receive coil would lead to underestimation of perfusion. Parallel acceleration should be avoided due to its SNR penalty. For mice reduce the FOV to the body width and keep the matrix size the same as that used in rats. The relative resolution in resolving kidney is then comparable to that in rats. The SNR would also be similar because the smaller mouse RF coil gives better SNR, that is, mouse heart four-element surface coil vs rat heart four-element surface coil.
-
2.
For using CASL or pCASL instead of FAIR, depending on the field strength and expected flow velocities, the labeling duration and postlabeling delay should be adapted. Labeling durations of ≥1600 ms for (p)CASL with postlabeling delay < =500 ms are suitable.
-
3.
As the labeling effect of ASL on image contrast is weak, SNR considerations mandate acquisition of images that are of lower spatial resolution. For ASL in plane matrices are in the range of 64 × 64 to 128 × 128. To maintain acceptable SNR at reasonable imaging times (2–6 min), multiple signal averages/repetitions are required (N > =3).
-
4.
In CASL or pCASL but not for FAIR, the labeling plane should be perpendicular to the feeding artery. As the labeling plane in CASL is typically designed to be in parallel to the imaging plane, that would limit the images to be acquired in transverse planes so that labeling can be perpendicular to the aorta.
-
5.
The renal oxygen (O2) demand is associated primarily with renal tubular O2 consumption necessary for solute reabsorption. Increasing O2 delivery such as giving pure oxygen to the animals makes animal hyperoxia and leads to vasoconstriction which changes the basal physiology.
-
6.
The respiration rate must be monitored continuously throughout the entire experiment and if necessary adapt the TR accordingly to ensure full relaxation (~5 times of T1).
-
7.
Shimming is particularly important for nonselective (global) inversion, since adiabatic condition depends on B0 field homogeneity. If localized or high-order shim is used, the global field uniformity required for nonselective inversion (which is for labeling arterial spins) will be compromised and leads to inferior inversion (and spin labeling). Shimming should be performed on a global level to optimize uniformity over the whole body. Neither the default iterative shimming method nor the Mapshim technique is recommended.
-
8.
Example for a 25 g mouse at 7 T: Anatomical images can be acquired with routine respiratory-triggered T2-weighted turbo spin echo (RARE) sequences in axial and coronal planes. Additionally, bSSFP is a fast imaging that could be used for acquiring anatomical image with minimal motion artefact, with parameters such as TR = 3 ms, TE = 1.07 ms, flip angle of 70°, matrix = 128 × 128 and 16 averages (TA = 6.2 s for one slice without trigger).
-
9.
EPI sequence is highly depending on the gradient system therefore a good trajectory measurement is recommended.
-
10.
Example for a 25 g mouse at 7 T [4, 5, 8, 9]: a respiratory triggered, FAIR sequence with an EPI readout: TR = 16,000 ms; TE = 16.6 ms; TIR = 30, 100, 200, 300, 500, 700, 1000, 1200, 1500, 2000, 3000, 5000, 8000 ms; calculated inversion pulse; effective bandwidth 350 kHz; pulse bandwidth = 2000 Hz; FOV = 3.5 × 3.5 mm2, slice thickness = 2 mm, inversion slab = 5 mm, IR-spoiler duration = 10 ms, IR-Spoiler amplitude = 40%. As kidney has very high perfusion, the perfusion signal is expected to be high even with just a single pair of ASL scan. If perfusion signal is weak, the nonselective inversion may not be effective. Try increasing the bandwidth of the inversion pulse (i.e., from 2000 to 5000 Hz or higher). This will increase the RF power demand so be mindful about the safe limit of the RF coil and the amplifier.
-
11.
Optional: The flow saturation slice shown in Fig. 1 is a spatially selective saturation band applied to suppress unwanted flow artifacts from vessels entering a slice. This is not the most optimal way of suppressing when using FAIR, but it reduces flow artifacts significantly.
-
12.
Use repetition instead of averaging as repetition allows image registration in post-processing before averaging to minimize residual motion artefacts, whereas direct averaging on the scanner may lead to a blurred image in the presence of motion.
-
13.
If SE-EPI readout causes severe distortion, consider using RARE acquisition instead.
References
Williams DS, Detre JA, Leigh JS, Koretsky AP (1992) Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 89:212–216
Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R et al (1992) Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 89:5675–5679
Kim SG (1995) Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med 34:293–301
Hueper K, Gutberlet M, Rong S, Hartung D, Mengel M, Lu X, Haller H, Wacker F, Meier M, Gueler F (2014) Acute kidney injury: arterial spin labeling to monitor renal perfusion impairment in mice-comparison with histopathologic results and renal function. Radiology 270:117–124
Rajendran R, Lew SK, Yong CX, Tan J, Wang DJ, Chuang KH (2013) Quantitative mouse renal perfusion using arterial spin labeling. NMR Biomed 26:1225–1232
Pell GS, Thomas DL, Lythgoe MF, Calamante F, Howseman AM, Gadian DG, Ordidge RJ (1999) Implementation of quantitative FAIR perfusion imaging with a short repetition time in time-course studies. Magn Reson Med 41:829–840
Rane SD, Gore JC (2013) Measurement of T1 of human arterial and venous blood at 7T. Magn Reson Imaging 31:477–479
Hueper K, Schmidbauer M, Thorenz A, Brasen JH, Gutberlet M, Mengel M, Hartung D, Chen R, Meier M, Haller H, Wacker F, Rong S, Gueler F (2017) Longitudinal evaluation of perfusion changes in acute and chronic renal allograft rejection using arterial spin labeling in translational mouse models. J Magn Reson Imaging 46:1664–1672
Tewes S, Gueler F, Chen R, Gutberlet M, Jang MS, Meier M, Mengel M, Hartung D, Wacker F, Rong S, Hueper K (2017) Functional MRI for characterization of renal perfusion impairment and edema formation due to acute kidney injury in different mouse strains. PLoS One 12:e0173248
Acknowledgments
This chapter is based upon work from COST Action PARENCHIMA, supported by European Cooperation in Science and Technology (COST). COST (www.cost.eu) is a funding agency for research and innovation networks. COST Actions help connect research initiatives across Europe and enable scientists to enrich their ideas by sharing them with their peers. This boosts their research, career, and innovation.
PARENCHIMA (renalmri.org) is a community-driven Action in the COST program of the European Union, which unites more than 200 experts in renal MRI from 30 countries with the aim to improve the reproducibility and standardization of renal MRI biomarkers.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this protocol
Cite this protocol
Chuang, KH., Meier, M., Fernández-Seara, M.A., Kober, F., Ku, MC. (2021). Renal Blood Flow Using Arterial Spin Labeling (ASL) MRI: Experimental Protocol and Principles. In: Pohlmann, A., Niendorf, T. (eds) Preclinical MRI of the Kidney. Methods in Molecular Biology, vol 2216. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0978-1_26
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0978-1_26
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0977-4
Online ISBN: 978-1-0716-0978-1
eBook Packages: Springer Protocols