Abstract
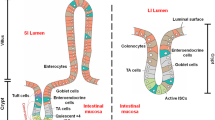
Increasing ethical and biological concerns require a paradigm shift toward animal-free testing strategies for drug testing and hazard assessments. To this end, the application of bioprinting technology in the field of biomedicine is driving a rapid progress in tissue engineering. In particular, standardized and reproducible in vitro models produced by three-dimensional (3D) bioprinting technique represent a possible alternative to animal models, enabling in vitro studies relevant to in vivo conditions. The innovative approach of 3D bioprinting allows a spatially controlled deposition of cells and biomaterial in a layer-by-layer fashion providing a platform for engineering reproducible models. However, despite the promising and revolutionizing character of 3D bioprinting technology, standardized protocols providing detailed instructions are lacking. Here, we provide a protocol for the automatized printing of simple alveolar, bronchial, and intestine epithelial cell layers as the basis for more complex respiratory and gastrointestinal tissue models. Such systems will be useful for high-throughput toxicity screening and drug efficacy evaluation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Stern ST, McNeil SE (2008) Nanotechnology safety concerns revisited. Toxicol Sci 101:4–21. https://doi.org/10.1093/toxsci/kfm169
Hartung T, Rovida C (2009) Chemical regulators have overreached. Nature 460:1080–1081. https://doi.org/10.1038/4601080a
Shukla SJ, Huang R, Austin CP, Xia M (2010) Foundation review: the future of toxicity testing: a focus on in vitro methods using a quantitative high-throughput screening platform. Drug Discov Today 15:997–1007. https://doi.org/10.1016/j.drudis.2010.07.007
Astashkina A, Mann B, Grainger DW (2012) A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther 134:82–106. https://doi.org/10.1016/j.pharmthera.2012.01.001
Xia M, Huang R, Witt KL et al (2008) Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect 116:284–291. https://doi.org/10.1289/ehp.10727
OECD (2015) Test No. 439: In vitro skin irritation: reconstructed human epidermis test method. OECD Publishing, Paris
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773–785. https://doi.org/10.1038/nbt.2958
Wüst S, Godla ME, Müller R, Hofmann S (2014) Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater 10:630–640. https://doi.org/10.1016/J.ACTBIO.2013.10.016
Pati F, Jang J, Ha D-H et al (2014) Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5:3935. https://doi.org/10.1038/ncomms4935
Horváth L, Umehara Y, Jud C et al (2015) Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep 5:7974. https://doi.org/10.1038/srep07974
Lieber M, Todaro G, Smith B et al (1976) A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 17:62–70. https://doi.org/10.1002/ijc.2910170110
Shapiro DL, Nardone LL, Rooney SA et al (1978) Phospholipid biosynthesis and secretion by a cell line (A549) which resembles type II alveolar epithelial cells. Biochim Biophys Acta 530:197–207. https://doi.org/10.1016/0005-2760(78)90005-X
Foster KA, Oster CG, Mayer MM et al (1998) Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp Cell Res 243:359–366. https://doi.org/10.1006/EXCR.1998.4172
Foldbjerg R, Dang DA, Autrup H (2011) Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol 85:743–750. https://doi.org/10.1007/s00204-010-0545-5
Forbes B, Ehrhardt C (2005) Human respiratory epithelial cell culture for drug delivery applications. Eur J Pharm Biopharm 60:193–205. https://doi.org/10.1016/J.EJPB.2005.02.010
Lehmann A, Brandenberger C, Blank F et al (2010) A 3D model of the human epithelial airway barrier. In: Maguire D, Novik E (eds) Methods in bioengineering: Alternative technologies to animal testing. Artech House, London, pp S35–S36
Wan H, Winton HL, Soeller C, et al (2000) Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o-. Eur Respir J 15:1058–1068
Cozens AL, Yezzi MJ, Kunzelmann K et al (1994) CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 10:38–47. https://doi.org/10.1165/ajrcmb.10.1.7507342
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749
Sambuy Y, De Angelis I, Ranaldi G et al (2005) The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol 21:1–26. https://doi.org/10.1007/s10565-005-0085-6
Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 175:880–885. https://doi.org/10.1016/0006-291X(91)91647-U
Fratzl P (2008) Collagen: structure and mechanics, an introduction. In: Collagen. Springer US, Boston, MA, pp 1–13
Legrand C, Bour JM, Jacob C et al (1992) Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J Biotechnol 25:231–243. https://doi.org/10.1016/0168-1656(92)90158-6
Balda MS, Whitney JA, Flores C et al (1996) Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134:1031–1049. https://doi.org/10.1083/JCB.134.4.1031
Velegol D, Lanni F (2001) Cell traction forces on soft biomaterials. I. Microrheology of type I collagen gels. Biophys J 81:1786–1792. https://doi.org/10.1016/S0006-3495(01)75829-8
Williams BR, Gelman RA, Poppke DC, Piez KA (1978) Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem 253:6578–6585
Acknowledgments
This work was supported by the Swiss National Science Foundation (grant number CRSII5_171037), the Run4Science grant, and the Adolphe Merkle Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Estermann, M., Bisig, C., Septiadi, D., Petri-Fink, A., Rothen-Rutishauser, B. (2020). Bioprinting for Human Respiratory and Gastrointestinal In Vitro Models. In: Crook, J.M. (eds) 3D Bioprinting. Methods in Molecular Biology, vol 2140. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0520-2_13
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0520-2_13
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0519-6
Online ISBN: 978-1-0716-0520-2
eBook Packages: Springer Protocols