Abstract
Background
Ankylosing spondylitis (AS) is one of inflammatory rheumatic diseases which result in wide range of manifestations on the musculoskeletal system and axial joint specifically. Endothelial cell migration and proliferation, as well as subsequent neoangiogenesis and remodelling in autoimmune disorders, are pathogenic mechanisms that are fundamental to inflammation activation and angiogenesis. The development of advanced lesions is thought to involve vascular proliferation as well as vascular endothelial growth factor (VEGF), which serves a regulatory role. It was found that AS patients had increased serum levels of VEGF, which were linked to the disease activity.
Aim of the work
The purpose of this study is to measure serum VEGF levels in Egyptian AS patients and assess their relation to disease-related variables, including radiographic findings.
Results
VEGF serum levels showed a highly significant positive correlation with Bath Ankylosing Spondylitis Functional Index (BASFI) and modified Stroke Ankylosing Spondylitis Spinal Score (MSASS) (p < 0.001); also, there was a significant correlation between the VEGF values and the Ankylosing Spondylitis Disease Activity Index (ASDAS) and the New York x-ray sacroiliac score.
Conclusions
These findings and data illustrate the strong relationship between ASDAS and VEGF and the radiographic score in AS patients. ASDAS combined with VEGF not only is considered a tool for determining the level of disease activity only but also is considered as an indicator for the assessment of the syndesmophytes formation, which performs a crucial role in the prognosis and outcome in AS patients.
Similar content being viewed by others
Background
Ankylosing spondylitis (AS) is one of inflammatory rheumatic diseases which result in wide range of manifestations on the musculoskeletal system and axial joint specifically. Patients experience new bone and cartilage production, which is followed by calcification, which causes spine ankylosis, syndesmophytes, and enthesophytes. This process’ mechanism is still unknown. One theory to account for the creation of new bones in AS is angiogenesis. Sacroiliitis and enthesitis, two other AS symptoms, as well as new bone growth all require angiogenesis. VEGF is a key regulator of this process [1].
A key component of pathogenic processes, such as endothelial cell migration, proliferation, subsequent neoangiogenesis, and remodelling in autoimmune disorders, is angiogenesis, a hallmark of inflammatory activation. Rheumatic disorders’ inherent inflammation may promote the expression of VEGF. Angiogenesis could be used as a possible target for treatment of inflammatory joint disease. VEGF takes part in nearly every stage of angiogenesis. Recent research has revealed that VEGF, due to its function in angiogenesis, greatly contributes to the pathophysiology of numerous conditions, including autoimmune diseases [2], considered as a marker in disease activity assessment and treatment following. VEGF contributes to various aspects of the pathogenesis of joint damage in rheumatic diseases including angiogenesis, synovitis, inflammatory, osteoclast differentiation, and cartilage degradation. Interestingly, VEGF reveals the association with the mechanism of pain in osteoarthritis (OA), while in rheumatoid arthritis (RA) it regulates the migration, proliferation of endothelial cells, prevents the synoviocyte apoptosis, regulates osteoclast differentiation, and induces RANKL secretion. It promotes endothelial dysfunction in systemic lupus erythematosus (SLE), and in systemic sclerosis (SSC), its high levels are usually associated with microangiopathy, and it is considered as a biomarker for interstitial lung involvement [2].
Vascular proliferation and VEGF, which play a regulatory role, are thought to contribute to the emergence of advanced lesions. VEGF levels were greater in the serum of those with AS, which were linked to the disease activity (ASDAS, C-reactive protein). It was also proven that the serum level of VEGF may predict how radiographic progression would develop [3]. AS disease state appears to be linked to increased plasma levels of VEGF, whether this is due to inflammation or an actual angiogenic pathomechanism [4].
Because biological treatments such as tumor necrosis factor-alpha (TNF-α) inhibitors have become available, there has been a notable advancement in the management of AS. Additionally, to their effect on the removal of abnormalities in magnetic resonance (such as enlargement of vertebral corners and bone marrow edema), the treatment appears to reduce pain and inflammatory markers in serum [5]. Currently, it is unclear if TNF-α inhibitors affect the radiographic progression in AS patients. However, it is noted that patients who received anti-TNF-α medication experienced a significant drop in VEGF levels, a key indicator of radiographic development [3, 6].
This study’s objective is to measure the serum levels of VEGF in Egyptian patients with AS and assess their relation to disease-related parameters including radiographic outcomes.
Methods
Seventy adult Egyptian patients with confirmed AS, as determined by the modified New York criteria for AS, participated in this case–control study [7] and were chosen from a rheumatology clinic; their ages ranged from 20 to 60 years, and seventy healthy individuals of the same sex and age were taken as a control group.
Exclusion criteria
Patients with any associated rheumatic diseases, patients with cancers and diabetic patients
Patients’ group was subjected to comprehensive clinical evaluation and complete medical history, after informed consent, with the following data to be recorded: age, sex, hip involvement, peripheral arthritis, eye involvement, and treatment type, and AS disease activity was graded with the ASDAS (Ankylosing Spondylitis Disease Activity Index) [8]. And Bath Ankylosing Spondylitis Functional Index (BASFI) were calculated to assess functional limitations [9], and radiological investigations such as plain x-ray of both sacroiliac joints anteroposterior view and sacroiliac grading were performed using New York criteria [10]. Plain x-ray of cervical and lumbar spines lateral view, while using modified Stroke Ankylosing Spondylitis Spinal Score (MSASS) for spines affection grading [11].
Laboratory investigations were as follows: complete blood picture (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), hemoglobin A1c (HBA1C), and human leukocyte antigen B27 (HLA-B27).
Patients and control groups were subjected to vascular endothelial growth factor A level (VEGF-A) assessment. After informed consent, peripheral venous blood was drawn from all patients and controls and collected in dry tubes containing no anticoagulant for serum separation. All samples were centrifuged for 20 min at a speed of roughly 2000–3000 rpm after being allowed to clot at room temperature for 10–20 min. After separation, the serum samples were used immediately. Investigators assessing VEGF serum levels were blinded to the serum of 70 AS patients and 70 healthy controls. In line with the manufacturer’s instructions, an enzyme-linked immunosorbent assay (ELISA) was employed to gauge the serum concentration of VEGF-A.
Statistical analysis
The mean, standard deviation, Student t-test, chi-square, and ROC curve were used in the statistical presentation and analysis of the current study — analysis of variance (ANOVA) tests, linear correlation coefficient, and receiver operating characteristic (ROC) curve analysis for sensitivity, specificity, and cut-off value by SPSS V20. Variables were presented as frequencies and percentages, mean ± standard deviation, and range. A comparison was done using chi-square and Mann–Whitney U-tests. P-value 0.05 was considered significant.
Results
This case–control study was executed on 70 adult Egyptian patients with definite ankylosing spondylitis who were receiving treatment and 70 age- and sex-matched healthy controls as shown in Table 1. Regarding the sociodemographic data of the studied patients’ group were illustrated in Table 2. As regards the clinical data of our patients, the disease duration of AS patients ranged from 1 to 20 years with a mean of 6.029 ± 4.403 years. The mean of the morning stiffness duration was 1.29 ± 0.4 h. Eighteen patients had peripheral arthritis (25.71%), and eight had extraarticular manifestation (11.43%). HLA-B27 was positive in 16 patients. The treatment received by all patients was shown in Table 2 as well.
Ankylosing Spondylitis Disease Activity Score (ASDAS) ranged from 0.5 to 3.9 with a mean of 2.18 ± 0.94. BASFI ranged from 3 to 9.5 with mean ± SD 5.64 ± 1.85 (Table 2).
The laboratory investigation revealed that ESR ranged from 9 to 46 mm/h, and CRP titer ranged from 2–15 mg/dl. The VEGF in the studied patients ranged from 210 to 780 ng/l (Table 2).
Regarding the radiological evaluation of the studied patients, New York x-ray sacroiliac joint score ranged from 1 to 3, and the mean was 1.9 ± 0.7. MSASS score ranged from 12 to 66 with a mean of 31.86 ± 15.15 (Table 2).
A comparison between the AS group and the healthy control group was done as regards the VEGF values as shown in Table 3 and Fig. 1. The VEGF values were significantly higher in AS group than in the healthy control group (p-value < 0.001).
There was no statistically significant difference (p-value ≥ 0.05) in VEGF serum levels in different patient’s subsets as regards sex, smoking, clinical parameters (comorbidity, peripheral arthritis, extraarticular manifestations), and HLAB27 as shown in Table 4.
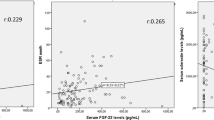
Correlations were done between the VEGF values, and the age of studied patients, the disease duration, morning stiffness duration, ESR, and CRP showed no statistically significant correlation between them (P ≥ 0.05); at the same time, we found a highly significant positive correlation with BASFI and MSASS score (P < 0.001); also, there was a significant correlation between the VEGF values and the ASDAS and the New York x-ray sacroiliac score (P = 0.002), and these correlations were shown in Table 5 and Figs. 2, 3, 4 and 5.
ROC curve analysis indicated to diagnose AS patients by using the VEGF cut-off value of > 140 ng/l, the sensitivity was 100% (95% CI 78.2–100.0), and specificity was 100% (95% CI 32.3–83.7), as shown in Table 6 and Fig. 6.
Discussion
Ankylosing spondylitis, a member of the spondyloarthropathy family, is a systemic autoimmune rheumatic disorder. Delay in its diagnosis and management leads to increasing morbidity and mortality [12]. Estimation of the incidence and prevalence of AS ranged widely from 0.05 to 1.4/10,000 person per year and from 0.1% to 1.4. This is caused by an increase of the awareness about the clinical features of AS [13, 14].
A growing number of investigations have been performed to identify the cause of AS [15,16,17]. Disequilibrium between Th17, Th1, and Th2 encourages the idea that AS is caused by a disruption in the harmony between the innate immune system and the acquired immune system [5, 17]. Axial spondyloarthropathy (SpA) is characterized by new bone development and structural degeneration in the SI joints and spine as a result of inflammation [18]. It has been hypothesized that granulation tissue containing osteoblasts replaces the subchondral bone marrow, which encourages new bone formation and results in intra-articular ankyloses of the facet joints, the growth of syndesmophytes, and finally ankyloses in the spine [19, 20].
Several biomarkers were studied in AS pathogenesis [20] (matrix metalloproteinase-3 MMP-3, bone morphogenetic protein 2, procollagen type 2 N-propeptide, and VEGF), have a role in the pathogenesis of AS, and increase bone formation. The production of new bones, particularly endochondral ossification and syndesmophyte formation, depends on VEGF, a signal protein that is essential for angiogenesis [21].
This study was carried out to assess the VEGF titer and its relation to the different parameters of activity in Egyptian AS patients. Considering the findings of the recent study, elevated VEGF levels in serum seem to be related to the illness status of AS. VEGF levels ranged from 210 to 780 ng/l, which are substantially greater than the values that were found in the healthy controls, in accordance with Wang et al. [21], Zhan et al., [22] Lin et al., [23] and Przepiera-Bedzak et al. [24].
The serum VEGF values of more than 140 ng/l could discriminate between the AS patients from the healthy controls. Poddubnyy et al. [25] reported that VEGF equal to or more than 600 pg/ml (ng/l) is the cut-off value which is specific for the radiographic progression in axial SPA.
Smoking has a crucial role in AS pathogenesis, and it is connected to increased disease activity [26]. There is a previous result confirms the association between high VEGF in AS patients and smoking [27]. In the current study, values of VEGF were numerically higher in smokers than nonsmokers; however, there was a nonsignificant statistical association between them that could be clarified by the small sample size of the subjects under study.
In the AS group under study, VEGF was found to be strongly linked with disease activity and functional impairment; this is consistent with the finding demonstrated by Pedersen et al. [27], Bhuvanesh M. [28], Sakellariou G. T. [29], Sakalyte R. [30] and Poddubnyy D. [25]. Also, the VEGF was found positively correlated with the radiological scores (New York x-ray sacroiliac and MSASS) in the studied patients which goes in line with Prajzlerová et al. [31] and Poddubnyy [25].
However, serum VEGF levels in the studied group were not significantly correlated with the inflammatory markers ESR and CRP titer; this may be explained by the finding of Lories et al. [32] who declared that ankylosing spondylitis (AS) is inconsistent with rheumatoid arthritis (RA). The presence of inflammation is not related to the radiographic progression, and the two processes are not relied on each other [21, 33]. Also, Maksymoych et al. [34] reported that MRI study for AS patients shows a continuum of the new bone formation irrespective of the inflammatory state of the studied patients at a vertebral corner following the administration of TNFi [35]. These findings authenticate that VEGF is comparable to ASDAS, which is more specific than ESR and CRP to assess the disease activity, especially in patients receiving anti-TNFi [36].
The VEGF values in the studied AS group were independent on the disease duration, morning stiffness duration, the presence of peripheral arthritis, extraarticular manifestations, and comorbidity; this is in accordance with Zhan et al. [22], Braun et al. [5], and Poddubnyy et al. [25]. Contrary to the current research, other studies found a strong association between the VEGF and the clinical parameters of axial SPA in AS patients [27,28,29,30]. This confirms VEGF-differentiated nature which necessitates partnership with other clinical and laboratory factors for faster and more accurate diagnosis of AS.
Limitations of this study
The relatively small presented number of patients could be one of the limitations of this study as larger scale can reveal new findings. We did not measure a follow-up level to correlate the disease progression parameters with the base and follow-up level.
Conclusion
These findings and data illustrate the strong relationship between ASDAS and VEGF and the radiographic score in AS patients. The ASDAS combined with VEGF not only is considered only a tool for the evaluation of illness activity but also it is considered as an indicator for the radiographic progression, which plays a vital role in the prognosis and outcome in AS patients and indicates the important role of anti-VEGF in the treatment of AS patients in the future plans.
Availability of data and materials
The data of the current study are available from the corresponding author on reasonable request.
Abbreviations
- AS:
-
Ankylosing spondyloarthritis
- ASDAS:
-
Ankylosing Spondylitis Disease Activity Score
- BASFI:
-
Bath Ankylosing Spondylitis Functional Index
- CBC:
-
Complete blood picture
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- HLA B-27:
-
Human leukocyte antigen B27
- MMPs:
-
Matrix metalloproteinases
- MSASS:
-
Modified Stoke Ankylosing Spondylitis Spine Score
- RA:
-
Rheumatoid arthritis
- SLE:
-
Systemic lupus erythematosus
- SPA:
-
Spondyloarthropathy
- SSC:
-
Systemic sclerosis
- Th:
-
T helper
- TNF-α:
-
Tumor necrosis factor-alpha
- VEGF:
-
Vascular endothelial growth factor
References
Yu T, Zhang J, Zhu W, Wang X et al (2021) Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification. Bone Research 9(1):1–2
Le THV, Kwon SM (2021) Vascular endothelial growth factor biology and its potential as a therapeutic target in rheumatic diseases. Int J Mol Sci 22(10):5387
Tošovský M, Bradna P, Andrýs C et al (2014) (2014): The VEGF and BMP-2 levels in patients with ankylosing spondylitis and the relationship to treatment with tumour necrosis factor alpha inhibitors. Acta Medica (Hradec Kralove) 57(2):56–61. https://doi.org/10.14712/18059694.2014.40. (PMID: 25257151)
Goldberger C, Dulak J, Duftner C, Weidinger F et al (2002) (2002): Vascular endothelial growth factor (VEGF) in ankylosing spondylitis–a pilot study. Wien Med Wochenschr 152(9–10):223–225
Braun J, Baraliakos X (2011) Imaging of axial spondyloarthritis including ankylosing spondylitis. Ann Rheum Dis 70(Suppl 1):i97-103
Zong HX, Xu SQ, Tong H, Wang XR, Pan MJ, Teng YZ (2019) Effect of anti-tumor necrosis factor a treatment on radiographic progression in patient with ankylosing spondylitis: a systematic review and meta-analysis. Mod Rheumatol 29(3):503–509. https://doi.org/10.1080/14397595.2018.1525017. (Epub 2019 Jan 3 PMID: 30220240)
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984 Apr;27(4):361–8. doi: https://doi.org/10.1002/art.1780270401. PMID: 6231933.
Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, van der Linden S, van der Heijde D, For the Assessment of Spondyloarthritis International Society (2009) Development of an ASAS-endorsed disease activity score
Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, Jenkinson T (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21(12):2281–2285
Dale K (1979) Radiographic gradings of sacroiliitis in Bechterew’s syndrome and allied disorders. Scand J Rheumatol Suppl 32:92–97
Creemers MC, Franssen MJ, van’t Hof MA. et al (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 64:127–129
Burgos-Varga R, Wei JC-C, Rahman MU et al (2016) The prevalence and clinical characteristics of nonradiographic axial spondyloarthritis among patients with inflammatory back pain in rheumatology practices: a multinational, multicenter study. Arthritis Res Ther 18(1):132
Crossfield SS, Marzo-Ortega H, Kingsbury SR et al (2021) Changes in ankylosing spondylitis incidence, prevalence and time to diagnosis over two decades. RMD Open 7(3):e001888
Wei JC, Chen HH, Hsieh TY et al (2020) Clinical practice recommendations for the use of imaging in the diagnosis and management of axial spondyloarthritis in Taiwan. Int J Rheum Dis 23(1):24–36
Huang CH, Wei JC, Chang WC et al (2014) Higher expression of whole blood microRNA-21 in patients with ankylosing spondylitis associated with programmed cell death 4 mRNA expression and collagen cross-linked C-telopeptide concentration. J Rheumatol 41(6):1104–1111. https://doi.org/10.3899/jrheum.130515
Chen C-W, Wei JC-C, Gu J (2021) Editorial: advances in pathogenesis, etiology, and therapies for ankylosing spondylitis. Front Immunol 12:822582
Dulic S, Vasarhelyi Z, Bajnok A et al (2018) The impact of anti-TNF therapy on CD4+ and CD8+ cell subsets in ankylosing spondylitis. Pathobiol 85(3):201–210
Bleil J, Maier R, Hempfing A et al (2016) Granulation tissue eroding the subchondral bone also promotes new bone formation in ankylosing spondylitis. Arthritis Rheumatol 68:2456–2465
Poddubnyy D, Protopopov M, Haibel H et al (2016) High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 75:2114–2118
Rademacher J, Siderius M, Gellert L et al (2022) Baseline serum biomarkers of inflammation, bone turnover and adipokines predict spinal radiographic progression in ankylosing spondylitis patients on TNF inhibitor therapy. InSeminars in Arthritis and Rheumatism 53:151974
Wang M, Zhou X, Zhang H et al (2016) Associations of the VEGF level, VEGF rs2010963 G/C gene polymorphism and ankylosing spondylitis risk in a Chinese Han population. Immunol Lett 179:56–60
Zhan H, Li H, Liu C et al (2021) Association of circulating vascular endothelial growth factor levels with autoimmune diseases: a systematic review and meta-analysis Front. Immunol 12:674343
Lin TT, Lu J, Qi CY et al (2015) Elevated serum level of IL-27 and VEGF in patients with ankylosing spondylitis and associate with disease activity. Clin Exp Med 15:227–231
Przepiera-Bedzak H, Fischer K, Brzosko M (2016) Serum VEGF, EGF, basic FGF and acidic FGF levels and their association with disease activity and extra-articular symptoms in ankylosing spondylitis. Pol. Arch. Intern Med 126:290–292
Poddubnyy D, Conrad K, Haibel H et al (2014) Elevated serum level of the vascular endothelial growth factor predicts radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis 73(12):2137–2143
Farouk HM, Abdel-Rahman MA, Hassan RM (2021) Relationship between smoking, clinical, inflammatory, and radiographic parameters in patients with ankylosing spondylitis. Egyptian Rheumatology and Rehabilitation 48(1):1
Pedersen SJ, Sørensen IJ, Garnero P, et al. (2011): ASDAS, BASDAI and different treatment responses and their relation to biomarkers of inflammation, cartilage and bone turnover in patients with axial spondyloarthritis treated with TNFα inhibitors. Ann Rheum Dis; 70:1375 81
Bhuvanesh M, Balaji C, Saranya C et al (2018) Serum levels of tumor necrosis factor alpha and vascular endothelial growth factor as markers of disease activity in patients with axial spondyloarthritis. Indian Journal of Rheumatology 13(1):9–13
Sakellariou GT, Iliopoulos A, Konsta M et al (2017) Serum levels of Dkk-1, sclerostin and VEGF in patients with ankylosing spondylitis and their association with smoking, and clinical, inflammatory and radiographic parameters. Joint Bone Spine 84(3):309–315
Sakalyte R, Bagdonaite L, Stropuviene S et al (2022) VEGF profile in early undifferentiated arthritis cohort. Medicina 58(6):833
Prajzlerová K, Grobelná K, Pavelka K et al (2016) An update on biomarkers in axial spondyloarthritis. Autoimmun Rev 15(6):501–509
Lories RJ, Derese I, de Bari C et al (2007) (2007): Evidence for uncoupling of inflammation and joint remodeling in a mouse model of spondylarthritis. Arthritis Rheum 56:489–497
Maksymowych WP (2009) What do biomarkers tell us about the pathogenesis of ankylosing spondylitis? Arthritis Res Ther 11(1):1–2
Maksymowych WP, Chiowchanwisawakit P, Clare T et al (2009) Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 60(1):93–102
Drouart M, Saas P, Billot M et al (2003) High serum vascular endothelial growth factor correlates with disease activity of spondylarthropathies. Clin Exp Immunol 132(1):158–162
Llop M, Moreno M, Navarro-Compán V et al (2022) Sustained low disease activity measured by ASDAS slow radiographic spinal progression in axial spondyloarthritis patients treated with TNF-inhibitors: data from REGISPONSERBIO. Arthritis Res Ther 24(1):1–9
Acknowledgements
To all patients included in this study for their cooperation, also department’s nurses and workers who assisted in the study processing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
All authors were involved in concept, design, data collection, analysis, and drafting the manuscript equally. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Faculty of Medicine Ain Shams University Research Ethics Committee (FMASU REC) approved this research on 11/9/2022 (reference number: R 129/2022). This research was conducted according to the standard of the Declaration of Helsinki, and all participants signed written informed consent and is organized and operated according to guidelines of the International Council on Harmonization (ICH).
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. The authors declare that this paper nor part of it has not been published or under publication elsewhere.
Competing interests
The only conflict of interest is that Dr. Salwa Galal is an associate editor in the Egyptian Rheumatology and Rehabilitation journal. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galal, S., Hassan, R.M. & Labib, H.S.A. Association of vascular endothelial growth factor serum levels with ankylosing spondylitis in Egyptian patients. Egypt Rheumatol Rehabil 50, 13 (2023). https://doi.org/10.1186/s43166-023-00179-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-023-00179-9