Abstract
Background
Hepatocellular carcinoma (HCC) is a common type of liver cancer, with a high mortality rate. Hepatocellular carcinoma is a type of liver cancer that can be effectively managed through early detection and accurate diagnosis, followed by a personalized treatment plan that may include surgical resection, liver transplantation, minimally-invasive techniques, immunotherapy, or targeted therapy depending on the stage and severity of the cancer.
Main body of the abstract
This paper discusses recent advances in the early detection, management, and prevention of HCC. The use of newer imaging techniques, such as Magnetic resonance imaging (MRI) and contrast-enhanced ultrasound, along with image segmentation technology and deep learning models, have greatly enhanced the accuracy of HCC detection and diagnosis. Minimally-invasive techniques, such as thermal ablation and radiofrequency ablation, have allowed for more precise and targeted destruction of tumors, while Nanoparticles, immunotherapy and targeted therapy have shown promise in the management of advanced stage HCC. The use of Artificial intelligence (AI) and machine learning has revolutionized HCC research, aiding in the identification of high-risk patients and predicting outcomes. Lifestyle modifications, such as weight management, alcohol avoidance, and hepatitis B vaccinations, can play a critical role in preventing HCC development.
Short conclusion
Recent advances in early detection, management, and prevention of HCC have shown promise in improving patient outcomes. The use of newer imaging techniques, minimally-invasive techniques, immunotherapy, targeted therapy, and AI and machine learning have greatly enhanced HCC research and management, while lifestyle modifications can play a critical role in prevention. However, further research is required to fully understand the potential benefits of nanoparticles, traditional Chinese medicine and herbal medicines in HCC treatment.
Graphical Abstract

Highlights
• Early detection and diagnosis: The advent of newer imaging techniques, such as MRI, contrast-enhanced ultrasound, and image segmentation technology, has allowed for earlier and more accurate detection of HCC, with higher sensitivity and specificity than previous techniques. Advances in radiomics and deep learning models have greatly enhanced the accuracy of HCC diagnosis.
• AI and machine learning: The use of AI and machine learning has revolutionized HCC research, with greater accuracy and an ability to analyze large volumes of data. AI-based predictive models have been shown to be helpful in identifying high-risk patients and predicting outcomes.
• Management: While surgical resection and liver transplantation remain effective treatments for early-stage HCC, advances in minimally-invasive techniques, such as thermal ablation and radiofrequency ablation, have allowed for more precise and targeted destruction of tumors. Additionally, nanoparticles, immunotherapy and targeted therapy have shown significant promise in the management of advanced stage HCC.
• Prevention: Lifestyle modifications, such as weight management and alcohol avoidance, along with hepatitis B vaccinations, can play a critical role in preventing HCC development.
• Traditional Chinese medicine and herbal medicines in HCC treatment require further investigation.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a major cause of cancer-related deaths worldwide. Liver fibrosis arises from chronic inflammatory damage and results in excessive accumulation of extracellular matrix proteins, leading to architectural distortion and impaired liver function. Advanced fibrosis progresses to cirrhosis and liver failure, major causes of morbidity and mortality for which liver transplantation is the only definitive treatment currently [1]. Early detection of HCC is essential for better patient outcomes. Screening high-risk groups such as those with chronic hepatitis B or C virus infection is recommended. Recent research has investigated the use of non-invasive biomarkers such as alpha-fetoprotein (AFP), des-gamma-carboxy prothrombin (DCP), and glypican-3 (GPC3), combined with imaging techniques such as ultrasound and magnetic resonance imaging (MRI) for early HCC detection [2]. A combination of these biomarkers with MRI has been shown to improve diagnosis sensitivity and specificity. Additionally, deep machine learning has been used to predict tumor recurrence on baseline MRI in patients with early-stage HCC [3]. Accurate diagnosis of HCC is critical for determining the appropriate treatment strategy. Contrast-enhanced ultrasound (CEUS) is a promising imaging technique for HCC diagnosis, providing real-time imaging of the liver vasculature and has been found to be more sensitive and specific than conventional ultrasound for HCC detection [4]. Artificial intelligence (AI) algorithms for HCC diagnosis have also gained attention. An AI algorithm based on radiomics features has been shown to improve the accuracy of HCC diagnosis compared to traditional imaging methods [5]. The von Willebrand factor ( vWF) levels have been found to be useful not only in predicting the risk of HCC in patients with cirrhosis but also in predicting the likelihood of complications following HCC resection and response to systemic therapies. The formation of portal microthrombi induced by vWF has been suggested to play a role in the development of acute liver failure progression and non-cirrhotic portal hypertension. The potential of drugs such as non-selective beta-blockers, statins, anticoagulants, and non-absorbable antibiotics to modulate vWF levels [6]. Recent advances in these areas have improved HCC management. For example, radiomics models based on multi-sequence MRI have shown promise in predicting PD-1/PD-L1 expression in HCC, which could help guide immunotherapy treatment decisions. Additionally, artificial intelligence (AI) has the potential to play a significant role in the detection and implementation of biomarkers for HCC [7].
HCC management depends on the stage of the disease and the patient's liver function. Early-stage HCC can be treated with surgical resection, liver transplantation, or local ablative therapies, such as radiofrequency ablation (RFA) or microwave ablation (MWA). Systemic therapies, including targeted therapy, immunotherapy, and chemotherapy, are recommended in advanced HCC. Pembrolizumab and lenvatinib have been found to improve overall survival in patients with advanced HCC compared to chemotherapy [8]. Immunotherapy in combination with local ablative therapies has also shown promising results. Recent evidence suggests that a combination of radiotherapy, chemotherapy, anti-angiogenic agents, and immune checkpoint inhibitors (ICI) can effectively address the unmet medical needs of patients with HCC. Furthermore, immunotherapies such as adoptive cellular therapy, cancer vaccines, and cytokines have shown promising results in enhancing the immune system's ability to eliminate tumor cells. This approach has the potential to greatly improve patient outcomes in the treatment of HCC [9]. Primary prevention of HCC can be achieved through vaccination against hepatitis B virus (HBV) and early treatment of chronic HBV or hepatitis C virus (HCV) infection. Secondary prevention involves surveillance for HCC in high-risk populations, such as patients with cirrhosis. Chemoprevention agents such as aspirin, metformin, and statins have been studied for their potential in reducing the risk of HCC development. In addition, a preoperative prediction model for macrotrabecular-massive HCC based on contrast-enhanced CT and clinical characteristics has been developed and could aid in treatment decision-making [10].
Our review provides a timely, comprehensive, and rigorous synthesis of the latest impactful data spanning HCC detection, diagnosis, treatment, and prevention. We focus specifically on human studies with direct patient relevance to inform management decisions. This sets us apart from prior reports dominated by preclinical findings lacking immediate bedside applicability. By bridging the gap between bench and bedside, our objectives are to guide clinician practice, shape future research by identifying unmet needs, and ultimately, improve patient outcomes through data-driven care and health policies. The exponential growth in HCC research warrants updated practice guidance to ensure widespread adoption of beneficial technologies. Our review distills actionable insights from high-quality data to provide stakeholders across the HCC landscape with current, authoritative, and practical recommendations to advance the standard-of-care.
Early detection of hepatocellular carcinoma
Early detection of hepatocellular carcinoma (HCC) is crucial for improving patient outcomes. However, there is currently no consensus on the optimal screening method for HCC. Some studies have suggested that ultrasound (US) is the most cost-effective and widely used screening tool for early detection of HCC in high-risk populations, such as patients with cirrhosis [11]. However, US has limitations in terms of accuracy and sensitivity. Therefore, novel biomarkers and imaging techniques are being explored to improve early detection and diagnosis of HCC.
Screening modalities and novel biomarkers
Several biomarkers, including alpha-fetoprotein (AFP), des-gamma-carboxy prothrombin (DCP), and glypican-3 (GPC3), have been investigated for their potential as diagnostic and prognostic markers for HCC. Recent studies have explored the use of novel biomarkers, such as microRNAs and long non-coding RNAs, for more accurate and early detection of HCC [12]. The development of targeted medicine and bioengineering has significantly advanced personalized treatment for hepatocellular carcinoma. By utilizing biomarkers, genetic testing, nanotechnology, and drug delivery systems, clinicians can tailor treatment plans to individual patients, resulting in improved outcomes. However, there are still several challenges that need to be addressed in this field, such as the need for more accurate diagnostic tools and the development of new targeted therapies for HCC [13].
Screening modalities
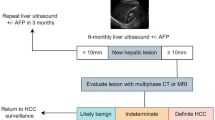
Several screening modalities have been investigated for HCC, including ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and serum biomarkers such as alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP). The American Association for the Study of Liver Diseases (AASLD) recommends ultrasound with or without AFP every 6 months for HCC screening in high-risk populations. A study showed that the combination of ultrasound and AFP with a machine learning algorithm could improve the sensitivity and specificity of HCC detection [14]. Gadolinium-based contrast agents (GBCAs) and chitosan-based nanoparticles can improve the sensitivity and specificity of hepatobiliary magnetic resonance imaging (MRI) [15]. CEUS LI-RADS and DEB-TACE loaded with raltitrexed are effective for the diagnosis and treatment of HCC [16].
Novel Biomarkers for HCC Screening
Several novel biomarkers for HCC screening have been identified, which may improve the early detection of HCC. The plasma glypican-3 (GPC3) could be used as a diagnostic biomarker for early-stage HCC [17]. Serum hepatocyte growth factor (HGF) has various levels in liver diseases, but it has exhibited a negative correlation with albumin concentration and prothrombin time in cirrhotic patients [18]. In addition, studies showed that circulating tumor DNA (ctDNA) analysis could detect HCC with a sensitivity of 77.0% and a specificity of 98.0% [19]. Radiomics models based on multisequence MRI and deep machine learning can predict PD-1/PD-L1 expression and tumor recurrence on baseline MRI in patients with early-stage HCC [20]. AI algorithms and machine learning models can aid in the diagnosis of HCC and enhance the quality of simulation, navigation, and outcome prediction for hepatectomy [21].
Screening interval
The optimal screening interval for HCC is a matter of debate. The AASLD recommends screening every 6 months in high-risk populations. However, some studies have suggested that longer screening intervals may be appropriate for patients with compensated cirrhosis and low HCC risk [22]. Studies showed that screening every 12 months was non-inferior to screening every 6 months in terms of overall survival in patients with compensated cirrhosis and low HCC risk [23]. Screening intervals for HCC depend on the patient's risk factors and disease stage [24].
Limitations of biomarkers for hepatocellular carcinoma (HCC)
Several challenges remain in the use of novel serum biomarkers for HCC, including the need for validation in larger patient cohorts, the lack of standardized cutoff values, and the potential for false-positive and false-negative results. One major limitation of novel serum biomarkers for HCC is the lack of standardized validation studies [25]. Although many studies have identified promising biomarkers, there is often a lack of validation in larger cohorts or clinical trials, which limits their clinical utility. Further validation studies are necessary to confirm the clinical utility of these biomarkers and translate these findings into effective strategies for HCC diagnosis, treatment, and management.
Another limitation of novel serum biomarkers for HCC is their sensitivity and specificity [26]. While many biomarkers show promise in early detection and prognosis prediction of HCC, they often lack specificity and may also be elevated in other conditions, leading to false positives. Improving the sensitivity and specificity of these novel biomarkers is critical to ensure accurate diagnosis and prognosis prediction. Moreover, the heterogeneity of HCC poses a challenge to the use of novel serum biomarkers [27]. Effective biomarkers need to accurately reflect the diverse etiologies and molecular subtypes of HCC to ensure accurate patient stratification and treatment selection. The identification of effective biomarkers for personalized treatment of HCC is crucial to improve patient outcomes. Lastly, the cost-effectiveness of novel serum biomarkers for HCC is a significant concern [28]. Developing cost-effective and accessible approaches for HCC diagnosis and management is essential for improving outcomes, especially in low-resource settings. Innovative approaches that are both effective and affordable are needed to address this challenge [29].
Imaging techniques
Advanced imaging techniques, such as magnetic resonance imaging (MRI) and computed tomography (CT), have been used for the early detection and diagnosis of HCC. Multisequence MRI and three-dimensional multifrequency magnetic resonance elastography have shown promise in improving preoperative assessment and predicting PD-1/PD-L1 expression in HCC [3, 4]. Contrast-enhanced CT has also been used to develop preoperative prediction models for macrotrabecular-massive HCC [8]. Moreover, deep machine learning models based on baseline MRI have been shown to accurately predict tumor recurrence in patients with early-stage HCC [3].
Contrast-Enhanced Ultrasound (CEUS) of Hepatocellular Carcinoma
Contrast-enhanced ultrasound (CEUS) is a non-invasive imaging modality that has shown promise in HCC detection, characterization, and surveillance. CEUS uses a microbubble contrast agent to enhance the vascular structures of the liver and is particularly useful in patients with liver cirrhosis, where other imaging modalities may not be reliable.
CEUS for HCC Characterization
Recent studies have demonstrated the utility of CEUS in the characterization of HCC. CEUS has been shown to have high sensitivity and specificity in the detection of small HCC nodules, particularly in patients with cirrhosis [30]. In addition, CEUS can provide real-time visualization of the tumor vascularization pattern, which can aid in the differentiation of HCC from other liver lesions [31]. CEUS has also been shown to be useful in the assessment of treatment response after locoregional therapy for HCC [32].
CEUS for HCC surveillance
CEUS has emerged as a promising tool for HCC surveillance in high-risk patients, particularly those with cirrhosis. Studies have shown that CEUS has high sensitivity and specificity in the detection of HCC nodules < 2 cm in size [33]. In addition, CEUS has been shown to be more sensitive than computed tomography (CT) or magnetic resonance imaging (MRI) in detecting small HCC nodules, particularly during early stages [34]. CEUS may also be useful in monitoring the progression of liver cirrhosis in high-risk patients, as it can detect early changes in liver perfusion that may indicate the development of HCC [35]. Recent advances in artificial intelligence (AI) and machine learning (ML) have shown promise in improving the accuracy and efficiency of CEUS in HCC detection and surveillance [36]. AI and ML algorithms can be used to analyze CEUS images and identify subtle changes in liver perfusion that may indicate the presence of HCC [37]. In addition, these technologies can be used to predict the risk of HCC recurrence after treatment and to develop personalized treatment plans for patients with HCC [38]. However, most current AI models are developed using retrospective training data from a single center, limiting their generalizability. To improve AI diagnosis accuracy in HCC, the use of big data and next-generation sequencing technology is recommended [39].
Multiparametric Magnetic Resonance Imaging (mpMRI)
Multiparametric Magnetic Resonance Imaging (mpMRI) has gained significant attention in the diagnosis and management of hepatocellular carcinoma (HCC), the most prevalent type of liver cancer [40]. Recent studies have focused on developing liver-specific contrast agents to enhance the accuracy of HCC detection and characterization. For instance, a liver-targeting MRI contrast agent based on galactose functionalized o-carboxymethyl chitosan has been proposed [41].
Additionally, the use of gadolinium-based contrast agents (GBCAs) in liver imaging has raised environmental and health concerns, leading to the exploration of their uniqueness and potential remediation strategies [42].
In terms of imaging modalities, Gadoxetate-enhanced MRI has been found to be more sensitive and accurate than CT in detecting small HCC lesions and predicting pathological tumor grade [43]. Contrast-enhanced ultrasound (CEUS) LI-RADS has also been evaluated for HCC diagnosis in individuals without LI-RADS-defined HCC risk factors, showing high sensitivity and specificity in detecting HCC lesions [44].
Overall, mpMRI and liver-specific contrast agents can improve the accuracy of HCC diagnosis and management. Chitosan-based nanoparticles and other HCC therapeutic agents also show potential for future research and development. GBCAs have environmental and health concerns, leading to exploration of their uniqueness and potential remediation strategies. Gadoxetate-enhanced MRI and CEUS LI-RADS have demonstrated high sensitivity and accuracy in HCC detection [45]. DEB-TACE and self-expandable metallic stent combined with 125I brachytherapy are additional options for HCC treatment [46].
Liquid biopsy
Liquid biopsy, which involves the analysis of circulating tumor cells, cell-free DNA, and exosomes, is being explored as a non-invasive method for early detection and diagnosis of HCC. Recent studies have shown promising results in the use of liquid biopsy for identifying biomarkers and monitoring treatment response in HCC patients [12]. Liquid biopsy has emerged as a promising diagnostic tool for HCC, offering several advantages over traditional tissue biopsy, including non-invasiveness, low risk of complications, and the ability to monitor disease progression and treatment response over time.
Circulating tumor cells (CTCs)
CTCs are tumor cells that are shed into the bloodstream by primary or metastatic tumors. Recent studies have evaluated the use of CTCs as liquid biopsy biomarkers for HCC diagnosis and monitoring. A study showed that the detection of CTCs in blood samples had a high diagnostic accuracy for HCC, with a sensitivity of 77.8% and a specificity of 96.9% [47]. In addition, CTCs can be used to monitor treatment response and detect disease recurrence, providing valuable information for patient management.
Circulating Tumor DNA (ctDNA)
Circulating tumor DNA (ctDNA) is DNA that is released into the bloodstream by tumor cells and can be detected in the serum or plasma of patients with cancer. Recent studies have evaluated the use of ctDNA for diagnostic and prognostic liquid biopsy biomarker for HCC. The ctDNA levels were significantly higher in patients with HCC than in healthy controls and that high ctDNA levels were associated with poor prognosis in patients with HCC [48].
Extracellular vesicles (EVs)
EVs are small membrane-bound vesicles that are released by cells, including tumor cells, into the bloodstream. Recent studies have evaluated the use of EVs as liquid biopsy biomarkers for HCC diagnosis and monitoring. The detection of EVs in blood samples had a high diagnostic accuracy for HCC, with a sensitivity of 92.3% and a specificity of 94.7% [49]. In addition, EVs can be used to monitor treatment response and detect disease recurrence, providing valuable information for patient management. In recent years, several studies have investigated new serum biomarkers for HCC using various approaches, including pyroptosis-related lncRNA pairs, midkine, and protein glycosylation alterations. Pyroptosis-related lncRNA pairs has investigated as potential prognostic markers for HCC [50]. Pyroptosis is a form of programmed cell death that plays a critical role in cancer development and progression. The study identified several pyroptosis-related lncRNA pairs that were significantly associated with HCC prognosis, highlighting their potential as novel serum biomarkers for HCC prognosis prediction [51].
The diagnostic accuracy of midkine has conducted as a serum biomarker for AFP-negative HCC. AFP-negative HCC is a particularly challenging subtype to diagnose, and there is a critical need for new biomarkers for early detection [52]. The study found that serum midkine has high diagnostic accuracy for detecting AFP-negative HCC, making it a potential biomarker for early diagnosis and treatment management [53]. Protein glycosylation is a post-translational modification that plays a critical role in cancer development and progression [54]. The study identified several glycoproteins that are differentially expressed in HCC patients, including alpha-fetoprotein, des-gamma-carboxy prothrombin, and glypican-3. These proteins could potentially serve as novel serum biomarkers for HCC diagnosis and prognosis prediction [18]. Novel serum has identified as autoantibody biomarkers for early esophageal squamous cell carcinoma (ESCC) and high-grade intraepithelial neoplasia (HGIN) detection [54]. Although ESCC is a different type of cancer, the study used a similar approach to identify novel serum biomarkers. The study found that several autoantibodies were significantly associated with ESCC and HGIN, highlighting their potential as novel serum biomarkers for cancer diagnosis [55]. The best diagnostic performance for HBV-associated HCC was observed when AFP and PIVKA were combined with PT and TP. On the other hand, the combination of PIVKA-II with GGT and ALB was effective in confirming the liver's functional capacity and forecasting the outcome of HCC [56].
The clinical application pattern of liquid biopsy in patients with hepatocellular carcinoma is presented In Fig. 1. The figure shows how tumor composition analyses, including circulating tumor cells, circulating tumor DNA, and exosomes, are released by tumors into the bloodstream. During various treatments such as surgery, transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), and targeting molecular treatment, liquid biopsy can be utilized to diagnose, monitor progress and prognosis of HCC patients. Next-generation sequencing (NGS) and minimal/molecular residual disease (MRD) can be used in conjunction with liquid biopsy to provide more accurate and detailed information [57].
In this context, the utilization of liquid biopsy in the clinical management of patients suffering from hepatocellular carcinoma is discussed [57]
Histopathology
Histopathological analysis of liver biopsy specimens remains the gold standard for the diagnosis of HCC. However, biopsy carries a risk of complications and may not always be feasible in certain patients. Biopsy of liver has practical role in estimation and diagnosis of fibrosis levels as well as assessment of another several diseases such as inflammation, steatosis and necrosis. Nevertheless, it is mostly a risk which causes suffering of severe pain and complication after operation which includes mortality in about 0.1% of cases in addition to long stay at hospital under observation in around 5% of patients [58]. Therefore, there is a growing interest in developing non-invasive methods for HCC diagnosis. Advanced imaging techniques and liquid biopsy are being explored as potential alternatives to liver biopsy for HCC diagnosis [4, 12].
Moreover, histopathological analysis remains important in the characterization of HCC and identification of prognostic biomarkers, such as tumor grade and vascular invasion [11]. An exceptional case of cirrhotomimetic hepatocellular carcinoma, which is a rare type of HCC that can mimic cirrhosis of the liver. The case highlights the need for clinicians to consider cirrhosis-mimicking tumors in the differential diagnosis of liver masses, particularly in patients with a history of chronic liver disease. The study emphasizes the importance of careful imaging and pathological evaluation to accurately diagnose and treat such cases. The findings of this case report can contribute to the understanding and management of cirrhotomimetic HCC and can help improve patient outcomes through early detection and appropriate treatment [59].
Artificial intelligence and machine learning models
Artificial intelligence (AI) has an increasingly important role in the detection and implementation of biomarkers for HCC detection and diagnosis [7]. Machine learning and deep learning algorithms have been applied to imaging data to develop predictive models for HCC recurrence and PD-1/PD-L1 expression [2, 3]. AI-based models could aid in the early detection and diagnosis of HCC. AI algorithms for HCC diagnosis use machine learning models to differentiate between HCC and non-HCC nodules. These algorithms have shown high accuracy rates in diagnosing HCC, and they can also help reduce the need for unnecessary biopsies.
AI algorithms for HCC characterization use radiomics and deep learning methods to analyze medical images and extract quantitative features that can help with tumor characterization. These algorithms help identify tumor characteristics, such as size, shape, and texture, that can help with treatment planning and monitoring [60]
AI algorithms for HCC prognosis use machine learning models to analyze clinical, pathological, and radiological data to predict patient outcomes. These algorithms can help identify patients with a higher risk of tumor recurrence or metastasis and help guide treatment decisions [61].
Recent studies have shown that AI algorithms can help improve the accuracy and efficiency of HCC diagnosis, characterization, and prognosis. AI algorithms can also help identify biomarkers for HCC and predict treatment response, which can help improve patient outcomes and survival rates [62]. In addition, AI algorithms can be used to predict patient survival and guide the selection of appropriate treatment options, such as liver transplantation or systemic therapy [63]. The use of AI in clinical decision-making for HCC is becoming more popular, especially in diagnosing HCC as illustrated in Fig. 2 [64]. In addition, AI algorithms can be used to predict patient survival and guide the selection of appropriate treatment options, such as liver transplantation or systemic therapy [39].
illustrates a conceptual diagram of how AI can be used to diagnose HCC [64]
Management of hepatocellular carcinoma (HCC)
HCC is a complex disease that requires a multidisciplinary approach to treatment. The selection of appropriate treatment modalities depends on several factors, including the stage of the disease, the underlying liver function, and the overall health of the patient.
Surgery
Surgical resection and liver transplantation are considered curative treatments for HCC, but they are only applicable to a subset of patients with early-stage disease and good liver function. Liver resection is recommended for patients with a single tumor less than 5 cm or up to three tumors each less than 3 cm in size, without evidence of vascular invasion or extrahepatic disease. Liver transplantation is recommended for patients with a single tumor less than 5 cm or up to three tumors each less than 3 cm in size, without evidence of vascular invasion or extrahepatic disease, and who meet the Milan criteria [65].
Liver transplantation (LT) is the most effective treatment for patients with hepatocellular carcinoma (HCC) and underlying liver cirrhosis. However, due to organ shortages, strict criteria must be used to select patients for LT. The use of clinical and molecular predictors can expand the criteria for patient selection and improve outcomes [66]. Nevertheless, when using the Milan selection criteria, the risk of HCC recurrence is still significant. The recurrence of HCC can be located in the liver or extrahepatic, and it is important to differentiate between recurrent HCC and other lesions using arterial phase enhancement. Early detection of HCC recurrence after LT is crucial for optimal management, and radiologists should be familiar with the spectrum of disease [1].
Ablation
Ablation, including radiofrequency ablation (RFA) and microwave ablation (MWA), is a minimally invasive treatment option for small HCCs that are not amenable to surgery or transplantation. RFA and MWA are safe and effective therapies for small HCCs, with similar long-term outcomes. RFA is preferred for tumors less than 3 cm, while MWA may be preferred for tumors larger than 3 cm or in locations where RFA may be technically challenging [67].
Systemic therapy
Systemic therapy, including tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), is used for advanced HCC or as adjuvant therapy after surgery or ablation. TKIs, such as sorafenib and lenvatinib, are recommended as first-line therapy for advanced HCC. ICIs, such as nivolumab and pembrolizumab, are approved for use in patients with advanced HCC who have progressed after treatment with TKIs. Recently, novel combination therapies, such as TKIs plus ICIs or TKIs plus anti-angiogenic agents, have shown promise for improving patient outcomes [68].
Locoregional therapy
Locoregional therapies, such as transarterial chemoembolization (TACE), yttrium-90 radioembolization (Y-90), and stereotactic body radiation therapy (SBRT), are used for intermediate-stage HCC or as bridging therapy before surgery or transplantation. TACE is the preferred locoregional therapy for intermediate-stage HCC and involves the injection of chemotherapy agents into the hepatic artery followed by embolization. Y-90 and SBRT are alternative locoregional therapies that may be used in selected patients [69]. Other HCC treatment options include drug-eluting bead transarterial chemoembolization (DEB-TACE) and self-expandable metallic stent combined with 125I brachytherapy.Other HCC treatment options include drug-eluting bead transarterial chemoembolization (DEB-TACE) and self-expandable metallic stent combined with 125I brachytherapy [70].
Effective role of Nanotechnology in HCC treatment
Nanotechnology has shown promise in the management of hepatocellular carcinoma (HCC), with the potential for targeted and controlled drug delivery, enhanced imaging, and improved diagnostic accuracy [71]. Nanoparticles can be engineered to selectively target HCC cells, delivering drugs directly to the tumor site while minimizing damage to healthy cells. Additionally, nanoparticles can be used as contrast agents in imaging techniques, allowing for earlier and more accurate detection of HCC. Furthermore, nanotechnology-based biosensors have the potential to detect HCC biomarkers in blood samples, providing a non-invasive and cost-effective diagnostic tool for HCC [72]. While further research is needed, nanotechnology has the potential to significantly improve the management of HCC. There are several recent examples of nanoparticle-based treatments for hepatocellular carcinoma (HCC), such as:
Liposomal doxorubicin (Doxil or Caelyx)
This is a chemotherapy drug that is encapsulated in a liposome nanoparticle. The nanoparticle helps to selectively deliver the drug to the tumor site and reduce toxicity to healthy cells [73].
Sorafenib-loaded nanoparticles
Sorafenib is a targeted therapy drug used in the treatment of HCC. Researchers have developed nanoparticles that can encapsulate sorafenib and deliver it directly to the tumor site, improving drug efficacy and reducing side effects [74].
Gold nanoparticles
Gold nanoparticles can be used as a photothermal therapy agent for HCC treatment. When exposed to near-infrared light, the gold nanoparticles generate heat, which can selectively kill cancer cells while sparing healthy cells [75].
Iron oxide nanoparticles
These nanoparticles can be used as contrast agents for magnetic resonance imaging (MRI) in the detection of HCC. Additionally, iron oxide nanoparticles can be used for photothermal ablation of HCC, where they generate heat in response to laser irradiation, leading to tumor cell death [76].
Polymeric nanoparticles
Polymer-based nanoparticles can also be used to deliver chemotherapy drugs to HCC cells. These nanoparticles are designed to release the drug slowly over time, increasing its effectiveness and reducing toxicity to healthy cells [77].
Carbon nanotubes
Carbon nanotubes have been investigated as a potential platform for targeted drug delivery in HCC treatment. Researchers have developed carbon nanotubes coated with a targeting agent that can selectively bind to HCC cells, delivering drugs directly to the tumor site [78].
Copper sulfide nanoparticles
Copper sulfide nanoparticles have been used in photothermal therapy for HCC treatment. Under near-infrared light, these nanoparticles generate heat, which can selectively kill cancer cells while sparing healthy cells [79].
Chitosan-based nanoparticles
Chitosan-based nanoparticles show promise as potential therapeutic agents for hepatocellular carcinoma. By encapsulating chemotherapy drugs inside chitosan nanoparticles, targeted delivery to tumor tissues can be achieved while reducing toxicity to healthy cells. Additionally, chitosan nanoparticles may serve as contrast agents to improve cancer imaging [80].
Limitations of Nanotechnology in HCC treatment
Firstly, the complex and dynamic tumor microenvironment and heterogeneity of HCC cells can hinder the selective targeting of nanoparticles to the tumor site. Additionally, the size, shape, surface charge, and stability of nanoparticles can affect their pharmacokinetics, biodistribution, and toxicity, which need to be carefully optimized. Secondly, the safety of nanoparticles in clinical settings needs to be thoroughly evaluated, as they can potentially cause toxicity, inflammation, immunogenicity, or other adverse effects [81]. Moreover, the long-term fate and toxicity of nanoparticles in the body, including their accumulation in organs and tissues, need to be better understood. Thirdly, the high cost and complexity of nanoparticle synthesis, characterization, and scale-up can limit their widespread adoption in clinical settings. Additionally, regulatory approval and intellectual property issues can also hinder the translation of nanoparticle-based treatments from bench to bedside. Lastly, the potential of nanoparticle-based treatments in HCC management needs to be evaluated in a clinical context, including their efficacy, safety, and cost-effectiveness compared to current treatments. Clinical trials are necessary to establish the optimal dose, schedule, and administration route of nanoparticle-based treatments, as well as their potential combination with other therapies [82].
Traditional Chinese and herbal medicine for HCC
Herbal medicines
Herbal medicines have been used for centuries in the treatment of various diseases, including cancer. In recent years, there has been increasing interest in the use of herbal medicines for the treatment of HCC [83, 84]. However, the efficacy and safety of these treatments are not well-established, and the use of herbal medicines should be approached with caution [85]. A study investigated the efficacy and safety of herbal medicine for the treatment of HCC. The study included 49 randomized controlled trials involving over 4,000 participants [86]. The results showed that some herbal medicines, such as Astragalus membranaceus, Curcumin, and Ganoderma lucidum, may improve survival and quality of life in HCC patients [87].
The studies of the using herbal medicines in combination with conventional therapies for the treatment of HCC, included included 22 randomized controlled trials involving over 2,000 participants showed that herbal medicines may have potential benefits when used in combination with conventional therapies, such as transarterial chemoembolization (TACE) or radiofrequency ablation (RFA), for HCC treatment [88]. The herbal extract Scutellaria barbata may inhibit the growth and proliferation of HCC cells and induce apoptosis. The study also found that the herbalextract may enhance the sensitivity of HCC cells to chemotherapy drugs, suggesting its potential as an adjuvant therapy for HCC [89, 90]. The herbal extract Silibinin may inhibit the growth and proliferation of HCC cells and induce apoptosis. The study also found that Silibinin may enhance the sensitivity of HCC cells to chemotherapy drugs and may have potential as a therapeutic agent for HCC [91, 92].
The studies of the effects of the herbal extract Coptis chinensis on HCC showed that Coptis chinensis may inhibit the growth and proliferation of HCC cells and induce apoptosis. The study also found that Coptis chinensis may inhibit the migration and invasion of HCC cells, indicating its potential as a therapeutic agent for HCC [93]. Several studies have explored the potential of traditional Chinese herbal medicines (TCHMs) in the treatment of HCC [94]. The protective effects of plant-derived natural products against HCC, including curcumin, resveratrol, and silymarin may have anti-tumor effects, as well as anti-inflammatory and immune-modulatory effects, which may contribute to their potential efficacy in the prevention and treatment of HCC [95]. Moreover, the potential of diarylheptanoids/sorafenib combination therapy in HCC may have a synergistic effect on the p53/MMP9 axis of action and improve outcomes for HCC patients [96].
Traditional Chinese medicine
Traditional Chinese medicine (TCM) has been used for centuries in the treatment of liver diseases, including HCC. TCM treatments for HCC include herbal medicines, acupuncture, and dietary therapies [97]. Many TCM treatments have been reported to have potential anti-cancer effects, and some have been shown to improve the quality of life and prolong survival in patients with HCC [98]. Several traditional Chinese treatments have been studied for their potential effects on HCC. The study included 49 randomized controlled trials involving over 4,000 participants. The results showed that TCM, either alone or in combination with conventional therapy, may improve overall survival, progression-free survival, and objective response rate in HCC patients [99]. Randomized controlled trials involving over 1,200 participants showed that the use of TCM in combination with TACE may improve overall survival, disease control rate, and quality of life in HCC patients [100].
A recent clinical trial used of the TCM formula Huachansu injection in combination with TACE for the treatment of HCC showed that the combination therapy may improve overall survival, disease-free survival, and objective response rate in HCC patients, with no significant increase in adverse events [101]. Another clinical trial of the TCM formula Jianpi Jiedu decoction in combination with TACE for the treatment of HCC showed that the combination therapy may improve overall survival, progression-free survival, and objective response rate in HCC patients, with no significant increase in adverse events [102].
Also, Shengjing Capsule may improve overall survival and quality of life in HCC patients, with no significant increase in adverse events [103]. The natural products can provide an effective and promising alternative for the treatment of liver cancer, considering the limitations of conventional therapies [104]. In addition, the dietary fiber intake may offer some protective benefits against hepatocellular carcinoma in their meta-analysis of observational studies [105]. These studies suggest that natural products and dietary interventions may provide effective strategies for the prevention and treatment of liver cancer [106]. The combination of modern medicine and TCHMs in the treatment of HCC may play potential roles in enhancing the efficacy of conventional treatments and reducing their side effects as shown in Fig. 3 [107].
Advancements in the integration of modern medicine and traditional Chinese medicine for the treatment of hepatocellular carcinoma have been made [107]
Limitations of herbal and traditional medicine for HCC
While some herbal medicines have shown promise in preclinical studies, their efficacy and safety in human trials are not well-established. Some of the limitations of herbal treatment for HCC include:
Lack of Standardization
Herbal medicines are complex mixtures of plant-derived compounds, and the composition can vary widely depending on factors such as the plant source, growing conditions, and processing methods. This lack of standardization can make it difficult to determine the optimal dose and duration of treatment [108].
Limited clinical data
There is a lack of high-quality clinical data on the efficacy and safety of herbal medicines for HCC. Many of the studies conducted to date have been small and poorly designed, making it difficult to draw meaningful conclusions [109].
Interactions with conventional treatments
Herbal medicines can interact with conventional treatments, such as chemotherapy and radiation therapy, which can lead to adverse effects [110]. Patients should inform their healthcare providers of any herbal treatments they are using to avoid potential interactions [111].
Safety concerns
Some herbal medicines may have toxic effects on the liver, which can be particularly concerning for patients with HCC, as the liver is already compromised [112]. Patients should be aware of the potential risks associated with herbal treatments and should only use them under the supervision of a qualified healthcare provider [113].
The prevention of Hepatocellular Carcinoma (HCC)
Preventing Hepatocellular Carcinoma (HCC): Current Strategies and Emerging Evidence.
Hepatocellular carcinoma (HCC) is a major global health issue, with high morbidity and mortality rates. While HCC treatment options have improved, prevention remains the most effective strategy for reducing the incidence of this deadly disease. In recent years, several prevention strategies have emerged, including vaccination, lifestyle modifications, and pharmacological interventions [114].
Vaccination
Vaccination against hepatitis B virus (HBV) is one of the most effective prevention strategies for HCC. A large-scale study showed that universal HBV vaccination programs have greatly reduced the incidence of HCC in Taiwan, with the incidence decreasing by 70% in the vaccinated population compared to the unvaccinated population [115]. Prophylactic vaccination against viral-associated cancers like HPV and HBV has already proven highly successful, lending credibility to the premise of precancer immunization for non-viral tumors. However, over 150 human cancer types lack a viral etiology [116]. Vaccination against hepatitis C virus (HCV) is also effective in preventing HCC, and the development of direct-acting antivirals has made HCV elimination a realistic goal [117].
Lifestyle modifications
Lifestyle modifications, such as maintaining a healthy weight, avoiding alcohol and tobacco use, and increasing physical activity, have also been shown to reduce the risk of HCC. A recent study found that a healthy lifestyle score was associated with a lower risk of HCC, with each point increase in the score corresponding to a 13% reduction in HCC risk (105). Emerging evidence also suggests that dietary modifications, such as increasing the intake of coffee, tea, and soy products, may have a protective effect against HCC [118].
Pharmacological interventions
Pharmacological interventions have also been investigated for the prevention of HCC. Aspirin has been shown to reduce the risk of HCC in patients with chronic liver disease, with long-term use associated with a 30% reduction in HCC risk [119]. Statins, commonly used for the treatment of hyperlipidemia, have also been associated with a reduced risk of HCC in observational studies [120]. Emerging evidence also suggests that metformin, a medication used for the treatment of type 2 diabetes, may have a protective effect against HCC [121].
Gut microbiota
Probiotics and Gut microbiota are live microorganisms that confer health benefits to the host by improving the gut microbiome's composition and function [122]. Gut microbiota may play a role in the development of HCC, with dysbiosis and inflammation in the gut associated with an increased risk of HCC. Probiotics and prebiotics have shown promise in modulating the gut microbiota to prevent HCC [123]. Dysbiosis and inflammation in the gut microbiota have been associated with an increased risk of developing hepatocellular carcinoma (HCC). Modulating the gut microbiota composition through the use of probiotics and prebiotics may help prevent HCC by reducing dysbiosis, inflammation, and subsequent liver damage. More research is warranted to further understand the precise mechanisms linking the gut microbiota to HCC and to evaluate the efficacy of microbiota-targeted interventions.
Conclusions
In conclusion, the recent advances in HCC management and prevention provide hope for improved patient outcomes and reduced burden on healthcare systems. The use of non-invasive biomarkers, advanced imaging techniques, AI algorithms, and nanotechnology-based treatments hold promise in improving the accuracy of HCC diagnosis, personalized treatment, and reducing the toxicity of current treatments. Additionally, lifestyle modifications and early detection and treatment of chronic hepatitis B or C virus infection are essential in reducing the risk of HCC development. However, further research and optimization are needed to fully harness the potential of these developments in clinical settings.
While progress has been made in HCC management, the potential of traditional Chinese medicine and herbal medicines in HCC treatment requires further investigation. Moreover, ongoing research is necessary to identify new biomarkers, therapeutic targets, and treatment strategies to further improve patient outcomes and reduce the burden of HCC. Therefore, collaboration among researchers, clinicians, and patients is essential to continue advancing our understanding of HCC and developing effective and personalized treatment strategies. The summary Table 1 highlighting the key advances in hepatocellular carcinoma (HCC) detection, diagnosis, treatment, and prevention covered in this review:
Availability of data and materials
All data are available and sharing is available as well as publication.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- LT:
-
Liver transplantation
- AI:
-
Artificial intelligence
- MRI:
-
Magnetic resonance imaging
- US:
-
Ultrasound
- CT:
-
Computed tomography
- AFP:
-
Alpha-fetoprotein
- TACE:
-
Transarterial chemoembolization
- TARE:
-
Transarterial radioembolization
- RFA:
-
Radiofrequency ablation
- MWA:
-
Microwave ablation
- PD-1:
-
Programmed cell death protein 1
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- EASL:
-
European Association for the Study of the Liver
- AASLD:
-
American Association for the Study of Liver Diseases
- BCLC:
-
Barcelona Clinic Liver Cancer
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- TTP:
-
Time to progression
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- ORR:
-
Overall response rate
- DCR:
-
Disease control rate
- HAI:
-
Hepatic arterial infusion
- IRE:
-
Irreversible electroporation
- GPC3:
-
Glypican-3
- TLR:
-
Toll-like receptor
- HIFU:
-
High-intensity focused ultrasound
- NASH:
-
Non-alcoholic steatohepatitis
- NAFLD:
-
Non-alcoholic fatty liver disease
- VEGF:
-
Vascular endothelial growth factor
- PDGF:
-
Platelet-derived growth factor
- HCC-MF:
-
Hepatocellular carcinoma with macrovascular invasion
- HCC-CP:
-
Hepatocellular carcinoma with extrahepatic metastasis
- PFS:
-
Progression-free survival
- CBCT:
-
Cone-beam computed tomography
- CTC:
-
Circulating tumor cell
- WES:
-
Whole-exome sequencing
- WGS:
-
Whole-genome sequencing
- NGS:
-
Next-generation sequencing
- TIL:
-
Tumor-infiltrating lymphocyte
- IHC:
-
Immunohistochemistry
- MSI-H:
-
High microsatellite instability
- MSS:
-
Microsatellite stable
- DDR:
-
DNA damage response
- EGFR:
-
Epidermal growth factor receptor
- TGF-β:
-
Transforming growth factor beta
- PDL1:
-
Programmed death-ligand 1
- ASO:
-
Antisense oligonucleotide
- siRNA:
-
Small interfering RNA
- miRNA:
-
MicroRNA
- LCLC:
-
Large cell lung carcinoma
- SIRT:
-
Selective internal radiation therapy
- SPECT:
-
Single-photon emission computed tomography
- PET:
-
Positron emission tomography
- TCR:
-
T-cell receptor
- CAR-T:
-
Chimeric antigen receptor T-cell
- SLN:
-
Sentinel lymph node
- TIL:
-
Tumor-infiltrating lymphocyte
- OS rate:
-
Overall survival rate
- DFS rate:
-
Disease-free survival rate
- TCM:
-
Traditional Chinese medicine
References
Addissouky TA, Ali MMA, El Tantawy El Sayed I, Wang Y, El Baz A, Elarabany N et al (2023) Preclinical Promise and Clinical Challenges for Innovative Therapies Targeting Liver Fibrogenesis. Arch Gastroenterol Res 4(1):14–23. https://doi.org/10.33696/Gastroenterology.4.044
Gong X, Liu N, Tao Y, Li L, Li Z, Yang L, Zhang X (2023) Radiomics models based on multisequence MRI for predicting PD-1/PD-L1 expression in hepatocellular carcinoma. Sci Rep 13(1):1–13. https://doi.org/10.1038/s41598-023-34763-y
Kucukkaya AS, Zeevi T, Chai NX, Raju R, Haider SP, Elbanan M, Lin M, Onofrey J, Nowak M, Cooper K, Thomas E, Santana J, Gebauer B, Mulligan D, Staib L, Batra R, Chapiro J (2023) Predicting tumor recurrence on baseline MR imaging in patients with early-stage hepatocellular carcinoma using deep machine learning. Sci Rep 13(1):1–9. https://doi.org/10.1038/s41598-023-34439-7
Liu G, Ma D, Wang H, Zhou J, Shen Z, Yang Y, Chen Y, Sack I, Guo J, Li R, Yan F (2023) Three-dimensional multifrequency magnetic resonance elastography improves preoperative assessment of proliferative hepatocellular carcinoma. Insights Imaging 14(1):1–13. https://doi.org/10.1186/s13244-023-01427-4
Mamatha Bhat, Madhumitha Rabindranath, Beatriz Sordi Chara,Douglas A. Simonetto. (2023)Artificial intelligence, machine learning, and deep learning in liver transplantation, J Hepatol, https://doi.org/10.1016/j.jhep.2023.01.006
Elhence A (2023) Von Willebrand Factor as a biomarker for liver disease – an update. J Clin Exp Hepatol. https://doi.org/10.1016/j.jceh.2023.05.016
Mansur A, Vrionis A, Charles JP, Hancel K, Panagides JC, Moloudi F, Iqbal S, Daye D (2023) The Role of Artificial Intelligence in the Detection and Implementation of Biomarkers for Hepatocellular Carcinoma: Outlook and Opportunities. Cancers 15(11):2928. https://doi.org/10.3390/cancers15112928
He, C., Zhang, W., Zhao, Y., Li, J., Wang, Y., Yao, W., Wang, N., Ding, W., Wei, X., Yang, R., & Jiang, X. (2023). Preoperative prediction model for macrotrabecular-massive hepatocellular carcinoma based on contrast-enhanced CT and clinical characteristics: A retrospective study. Front Oncol, 13. https://doi.org/10.3389/fonc.2023.1124069
Santagata S, Rea G, Castaldo D et al (2023) Hepatocellular carcinoma (HCC) tumor microenvironment is more suppressive than colorectal cancer liver metastasis (CRLM) tumor microenvironment. Hepatol Int. https://doi.org/10.1007/s12072-023-10537-6
Li, J., Xuan, S., Dong, P., Xiang, Z., Gao, C., Li, M., Huang, L., & Wu, J. (2023). Immunotherapy of hepatocellular carcinoma: Recent progress and new strategy. Front Immunol, 14. https://doi.org/10.3389/fimmu.2023.1192506
Jose A, Bavetta MG, Martinelli E, Bronte F, Giunta EF, Manu KA (2022) Hepatocellular Carcinoma: Current Therapeutic Algorithm for Localized and Advanced Disease. J Oncol 2022:3817724. https://doi.org/10.1155/2022/3817724
Gabbia D, De Martin S (2023) Toward a New Era in the Management of Hepatocellular Carcinoma: Novel Perspectives on Therapeutic Options and Biomarkers. Int J Mol Sci 24(10):9018. https://doi.org/10.3390/ijms24109018
Sun, H., Yang, H., & Mao, Y. (2023). Personalized treatment for hepatocellular carcinoma in the era of targeted medicine and bioengineering. Front Pharmacol, 14. https://doi.org/10.3389/fphar.2023.1150151
Pan A, Truong TN, Su YH, Dao DY (2023) Circulating Biomarkers for the Early Diagnosis and Management of Hepatocellular Carcinoma with Potential Application in Resource-Limited Settings. Diagnostics (Basel) 13(4):676. https://doi.org/10.3390/diagnostics13040676.PMID:36832164;PMCID:PMC9954913
Govindan B, Sabri MA, Hai A, Banat F, Haija MA (2023) A Review of Advanced Multifunctional Magnetic Nanostructures for Cancer Diagnosis and Therapy Integrated into an Artificial Intelligence Approach. Pharmaceutics 15:868. https://doi.org/10.3390/pharmaceutics15030868
Gibson EA, Goldman RE, Culp WTN (2022) Comparative Oncology: Management of Hepatic Neoplasia in Humans and Dogs. Vet Sci 9(9):489. https://doi.org/10.3390/vetsci9090489. (PMID:36136704;PMCID:PMC9505178)
Su TH, Wu CH, Liu TH, Ho CM, Liu CJ (2023) Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A Taiwan perspective. Clin Mol Hepatol. 29(2):230–241. https://doi.org/10.3350/cmh.2022.0421. (Epub 2023 Jan 30. PMID: 36710607; PMCID: PMC10121301)
Addissouky TA, Wang Y, Megahed FAK et al (2021) Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egypt Liver Journal 11:86. https://doi.org/10.1186/s43066-021-00156-x
Kopystecka A, Patryn R, Leśniewska M, Budzyńska J, Kozioł I (2023) The Use of ctDNA in the Diagnosis and Monitoring of Hepatocellular Carcinoma—Literature Review. Int J Mol Sci 24:9342. https://doi.org/10.3390/ijms24119342
Bai, W., Qiu, Q., & Zhang, J. (2023). Molecular and functional imaging in cancer-targeted therapy: Current applications and future directions. Signal Transduction and Targeted Therapy, 8. https://doi.org/10.1038/s41392-023-01366-y
Huang, Jl., Sun, Y., Wu, Zh. et al. Differential diagnosis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on spatial and channel attention mechanisms. J Cancer Res Clin Oncol (2023). https://doi.org/10.1007/s00432-023-04935-4
Elbalka SS, Abdallah A, Metwally IH (2023) Hepatocellular carcinoma associated other primaries: common types and prognosis. Egypt Liver J 13:5. https://doi.org/10.1186/s43066-023-00241-3
Hydes, Theresa J., Daniel J. Cuthbertson, Daniel H. Palmer, Omar Elshaarawy, Philip J. Johnson, Rashika Fernando, Timothy J. Cross. 2023. "Ultrasonography in surveillance for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease" Hepatoma Res. 9: 12. https://doi.org/10.20517/2394-5079.2022.97
Innes H, Nahon P (2023) Statistical perspectives on using hepatocellular carcinoma risk models to inform surveillance decisions. J Hepatol. https://doi.org/10.1016/j.jhep.2023.05.005
Shi Q, Yuan X, Xue C, Gu X, Li L (2023) Establishment and Validation of a Novel Risk Score for Hepatocellular Carcinoma Based on Bile Acid and Bile Salt Metabolism-Related Genes. Int J Mol Sci 24(10):8597. https://doi.org/10.3390/ijms24108597
McMahon B et al (2023) Opportunities to address gaps in early detection and improve outcomes of liver cancer. JNCI Cancer Spectr 7(3):pkad034. https://doi.org/10.1093/jncics/pkad034
Chen, Z., Xing, J., Zheng, C., Zhu, Q., He, P., Zhou, D., Li, X., Li, Y., Qi, S., Ouyang, Q., Zhang, B., Xie, Y., Ren, J., Cao, B., Zhu, S., & Huang, J. (2023). Identification of novel serum autoantibody biomarkers for early esophageal squamous cell carcinoma and high-grade intraepithelial neoplasia detection. Front Oncol, 13. https://doi.org/10.3389/fonc.2023.1161489
Parikh ND, Tayob N, Singal AG (2023) Blood-based biomarkers for hepatocellular carcinoma screening: Approaching the end of the ultrasound era? J Hepatol 78(1):207–216. https://doi.org/10.1016/j.jhep.2022.08.036. (Epub 2022 Sep 8. PMID: 36089157; PMCID: PMC10229257)
Oikawa T, Yamada K, Tsubota A, Saeki C, Tago N, Nakagawa C, Ueda K, Kamioka H, Taniai T, Haruki K, Nakano M, Torisu Y, Ikegami T, Yoshida K, Saruta M (2023) Protein Kinase C Delta Is a Novel Biomarker for Hepatocellular Carcinoma. Gastro Hep Advances 2(1):83–95. https://doi.org/10.1016/j.gastha.2022.07.020
Ainora, M. E., Cerrito, L., Liguori, A., Mignini, I., Luca, A. D., Galasso, L., Garcovich, M., Riccardi, L., Ponziani, F., Santopaolo, F., Pompili, M., Gasbarrini, A., & Zocco, M. A. (2023). Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors. International Journal of Molecular Sciences, 24(10). https://doi.org/10.3390/ijms24108548
Huang, W., Wen, R., Wu, Y., Lin, P., Guo, D., Peng, Y., Liu, D., Mou, M., Chen, F., Huang, F., Yang, H., & He, Y. (2023). Can modifications of LR-M criteria improve the diagnostic performance of contrast-enhanced ultrasound LI-RADS for small hepatic lesions up to 3 cm? Journal of Ultrasound in Medicine. Advance online publication. https://doi.org/10.1002/jum.16267
Sinagra L, Orlandi R, Caspanello T, Troisi A, Iannelli NM, Vallesi E, Pettina G, Bargellini P, De Majo M, Boiti C, Cristarella S, Quartuccio M, Polisca A (2023) Contrast-Enhanced Ultrasonography (CEUS) in Imaging of the Reproductive System in Dogs: A Literature Review. Animals 13(10):1615. https://doi.org/10.3390/ani13101615
Zhang W, Ni T, Tang W, Yang G (2023) The Role of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Tuberous Vas Deferens Tuberculosis and Metastatic Inguinal Lymph Nodes. Diagnostics 13(10):1762. https://doi.org/10.3390/diagnostics13101762
Liao, W., Que, Q., Wen, R., Lin, P., Chen, Y., Pang, J., Guo, D., Wen, D., Yang, H., & He, Y. (2023). Comparison of the feasibility and diagnostic performance of ACR CEUS LI-RADS and a modified CEUS LI-RADS for HCC in examinations using Sonazoid. Journal of Ultrasound in Medicine. Advance online publication. https://doi.org/10.1002/jum.16282
Bortot B, Mangogna A, Di Lorenzo G et al (2023) Image-guided cancer surgery: a narrative review on imaging modalities and emerging nanotechnology strategies. J Nanobiotechnol 21:155. https://doi.org/10.1186/s12951-023-01926-y
Juang, E. K., De Koninck, L. H., Vuong, K. S., Gnanaskandan, A., Hsiao, C.-T., & Averkiou, M. A. (2023). Controlled hyperthermia with high-intensity focused ultrasound and ultrasound contrast agent microbubbles in porcine liver. Ultrasound in Medicine and Biology. Advance online publication. https://doi.org/10.1016/j.ultrasmedbio.2023.04.015
Martin, Marisa, Anum Aslam, Eman Mubarak, Cate Hofley, Kayli Lala, Sandeep Arora, David C. Madoff, Elainea Smith, Dawn Owen, Ahmed Gabr, Charles Kim, Neehar Parikh, Erica Stein, Benjamin Mervak, Kimberly Shampain, Mishal Mendiratta-Lala. 2023. "Imaging after liver-directed therapy: evidenced-based update of the LI-RADS treatment response algorithm" Hepatoma Research. 9: 21. https://doi.org/10.20517/2394-5079.2022.95
Granata, V., Fusco, R., Villanacci, A., Grassi, F., Grassi, R., Stefano, F. D., Petrone, A., Fusco, N., & Ianniello, S. (2023). Qualitative and semi-quantitative ultrasound assessment in delta and Omicron Covid-19 patients: Data from high volume reference center. Infectious Agents and Cancer, 18. https://doi.org/10.1186/s13027-023-00515-w
Shen, X., Wu, J., Su, J., Yao, Z., Huang, W., Zhang, L., Jiang, Y., Yu, W., & Li, Z. (2023). Revisiting artificial intelligence diagnosis of hepatocellular carcinoma with DIKWH framework. Front Genet, 14. https://doi.org/10.3389/fgene.2023.1004481
Tao YY, Shi Y, Gong XQ, Li L, Li ZM, Yang L, Zhang XM (2023) Radiomic Analysis Based on Magnetic Resonance Imaging for Predicting PD-L2 Expression in Hepatocellular Carcinoma. Cancers (Basel) 15(2):365. https://doi.org/10.3390/cancers15020365. (PMID:36672315;PMCID:PMC9856314)
Jafernik K, Ładniak A, Blicharska E, Czarnek K, Ekiert H, Wiącek AE, Szopa A (2023) Chitosan-Based Nanoparticles as Effective Drug Delivery Systems-A review. Molecules 28(4):1963. https://doi.org/10.3390/molecules28041963. (PMID:36838951;PMCID:PMC9959713)
Loevner LA, Kolumban B, Hutóczki G, Dziadziuszko K, Bereczki D, Bago A, Pichiecchio A (2023) Efficacy and Safety of Gadopiclenol for Contrast-Enhanced MRI of the Central Nervous System: The PICTURE Randomized Clinical Trial. Invest Radiol. 58(5):307–313. https://doi.org/10.1097/RLI.0000000000000944. (Epub 2022 Dec 19. PMID: 36729404; PMCID: PMC10090311)
Jhaveri KS, Babaei Jandaghi A, Bhayana R, Elbanna KY, Espin-Garcia O, Fischer SE, Ghanekar A, Sapisochin G (2023) Prospective evaluation of Gadoxetate-enhanced magnetic resonance imaging and computed tomography for hepatocellular carcinoma detection and transplant eligibility assessment with explant histopathology correlation. Cancer Imaging 23(1):22. https://doi.org/10.1186/s40644-023-00532-3. (PMID:36841796;PMCID:PMC9960413)
Parra NS, Ross HM, Khan A, Wu M, Goldberg R, Shah L, Mukhtar S, Beiriger J, Gerber A (2023) Advancements in the Diagnosis of Hepatocellular Carcinoma. Int J Transl Med 3(1):51–65. https://doi.org/10.3390/ijtm3010005
Zhong Y, Jin C, Chen J, Zhu D, Zhu L (2023) Role of Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma. J Clin Transl Hepatol 11(2):480–489
Wang J, Xu H, Wang Y, Feng L, Yi F (2023) Efficacy and Safety of Drug-Eluting Bead TACE in the Treatment of Primary or Secondary Liver Cancer. Can J Gastroenterol Hepatol 2023:5492931. https://doi.org/10.1155/2023/5492931
Pan A, TN Truong, Su YH, Dao DY (2023) Circulating Biomarkers for the Early Diagnosis and Management of Hepatocellular Carcinoma with Potential Application in Resource-Limited Settings. Diagnostics 13(4):676. https://doi.org/10.3390/diagnostics13040676
Ma S, Zhou M, Xu Y et al (2023) Clinical application and detection techniques of liquid biopsy in gastric cancer. Mol Cancer 22:7. https://doi.org/10.1186/s12943-023-01715-z
Logozzi M, Orefice NS, Di Raimo R, Mizzoni D, Fais S (2023) The Importance of Detecting, Quantifying, and Characterizing Exosomes as a New Diagnostic/Prognostic Approach for Tumor Patients. Cancers 15(11):2878. https://doi.org/10.3390/cancers15112878
Chen X, Yang M, Wang L et al (2023) Identification and in vitro and in vivo validation of the key role of GSDME in pyroptosis-related genes signature in hepatocellular carcinoma. BMC Cancer 23:411. https://doi.org/10.1186/s12885-023-10850-1
Wang W, Zhou Q, Lan L, Xu X (2023) PANoptosis-related prognostic signature predicts overall survival of cutaneous melanoma and provides insights into immune infiltration landscape. Sci Rep 13(1):1–15. https://doi.org/10.1038/s41598-023-35462-4
Devan AR, Nair B, Aryan MK, Liju VB, Koshy JJ, Mathew B, Valsan A, Kim H, Nath LR (2023) Decoding Immune Signature to Detect the Risk for Early-Stage HCC Recurrence. Cancers 15(10):2729. https://doi.org/10.3390/cancers15102729
Wang Y, Chen H (2023) Protein glycosylation alterations in hepatocellular carcinoma: function and clinical implications. Oncogene. https://doi.org/10.1038/s41388-023-02702-w
Xie, X., Kong, S., & Cao, W. (2023). Targeting protein glycosylation to regulate inflammation in the respiratory tract: Novel diagnostic and therapeutic candidates for chronic respiratory diseases. Front Immunol, 14. https://doi.org/10.3389/fimmu.2023.1168023
Song R, Liu F, Ping Y et al (2023) Potential non-invasive biomarkers in tumor immune checkpoint inhibitor therapy: response and prognosis prediction. Biomark Res 11:57. https://doi.org/10.1186/s40364-023-00498-1
An S, Zhan X, Liu M, Li L, Wu J (2023) Diagnostic and Prognostic Nomograms for Hepatocellular Carcinoma Based on PIVKA-II and Serum Biomarkers. Diagnostics 13(8):1442. https://doi.org/10.3390/diagnostics13081442
Yang J-C, Hu J-J, Li Y-X, Luo W, Liu J-Z, Ye D-W (2022) Clinical Applications of Liquid Biopsy in Hepatocellular Carcinoma. Front Oncol 12:781820. https://doi.org/10.3389/fonc.2022.781820
Addissouky, T. A.; El Agroudy, A, E; El Sayed,I,E; El, E.; Eltorgman, A. A; Ibrahim, I, E. Efficiency of Alternative Markers to Assess Liver Fibrosis Levels in Viral Hepatitis B Patients. 2019, 30 (2). https://doi.org/10.35841/biomedicalresearch.30-19-107
Aby, E. S., Lou, S. M., Amin, K., & Leventhal, T. M. (2023). An exceptional finding in an explanted liver: A case report of cirrhotomimetic hepatocellular carcinoma. Frontiers in Gastroenterology, 2. https://doi.org/10.3389/fgstr.2023.1181037
Feng S, Wang J, Wang L, Qiu Q, Chen D, Su H, et al. Current Status and Analysis of Machine Learning in Hepatocellular Carcinoma. J Clin Transl Hepatol. Published online: May 17, 2023. https://doi.org/10.14218/JCTH.2022.00077S
Shinkawa, Hiroji, Takeaki Ishizawa. 2023. "Artificial intelligence-based technology for enhancing the quality of simulation, navigation, and outcome prediction for hepatectomy" Artificial Intelligence Surgery. 3, no.2: 69–79. https://doi.org/10.20517/ais.2022.37
Agarwal S, Yadav AS, Dinesh V, Vatsav KSS, Prakash KSS, Jaiswal S (2023) By artificial intelligence algorithms and machine learning models to diagnosis cancer. Materials Today: Proc 80:2969–2975. https://doi.org/10.1016/j.matpr.2021.07.088
Bakrania A, Joshi N, Zhao X, Zheng G, Bhat M (2023) Artificial intelligence in liver cancers: Decoding the impact of machine learning models in clinical diagnosis of primary liver cancers and liver cancer metastases. Pharmacol Res 189:106706. https://doi.org/10.1016/j.phrs.2023.106706
Xu, Flora Wen Xin, Sarah S Tang, Hann Natalie Soh, Ning Qi Pang, Glenn Kunnath Bonney. 2023. "Augmenting care in hepatocellular carcinoma with artificial intelligence" Artificial Intelligence Surgery. 3, no.1: 48–63. https://doi.org/10.20517/ais.2022.33
Otto CC, Wang G, Mantas A et al (2023) Time to surgery is not an oncological risk factor in HCC patients undergoing liver resection. Langenbecks Arch Surg 408:187. https://doi.org/10.1007/s00423-023-02922-4
Mamone G, Caruso S, Milazzo M, Porrello G, Di Piazza A, Gentile G, Carollo V, Crinò F, Marrone G, Sparacia G, Maruzzelli L, Miraglia R, Gruttadauria S (2023) Imaging of hepatocellular carcinoma recurrence after liver transplantation. Insights Imaging 14(1):1–13. https://doi.org/10.1186/s13244-023-01425-6
Zhou Y, Yuan K, Yang Y, Shan X, Ji Z, Zhou D, Ouyang J, Wang Z, Zhang Q, Zhou J, Li Q (2023) Predictive Factors of Treatment Outcomes After Percutaneous Radiofrequency Ablation of Hepatocellular Carcinoma in the Hepatocaval Confluence: A Propensity Score Matching Analysis. Acad Radiol. https://doi.org/10.1016/j.acra.2023.03.043
Pan, Y., Zhu, X., Liu, J., Zhong, J., Zhang, W., Shen, S., Jin, R., Liu, H., Ye, F., Hu, K., Xu, D., Zhang, Y., Chen, Z., Xing, B., Zhou, L., Chen, Y., Zeng, Y., Liang, X., Kuang, M., . . . Xu, L. (2023). Systemic therapy with or without transcatheter intra-arterial therapies for unresectable hepatocellular carcinoma: A real-world, multi-center study. Front Immunol, 14. https://doi.org/10.3389/fimmu.2023.1138355
Auer TA, Collettini F, Segger L, Pelzer U, Mohr R, Krenzien F, Gebauer B, Geisel D, Hosse C, Schöning W, Fehrenbach U (2023) Interventional Treatment Strategies in Intrahepatic Cholangiocarcinoma and Perspectives for Combined Hepatocellular-Cholangiocarcinoma. Cancers 15(9):2655. https://doi.org/10.3390/cancers15092655
Huang Z, Zhou PP, Li SS et al (2023) CEUS LI-RADS for diagnosis of hepatocellular carcinoma in individuals without LI-RADS-defined hepatocellular carcinoma risk factors. Cancer Imaging 23:24. https://doi.org/10.1186/s40644-023-00541-2
Xu M, Yang L, Lin Y et al (2022) Emerging nanobiotechnology for precise theranostics of hepatocellular carcinoma. J Nanobiotechnol 20:427. https://doi.org/10.1186/s12951-022-01615-2
Mansour, W., EL Fedawy, S., Atta, S. et al. Targeted therapy for HCC using dumbbell-like nanoparticles conjugated to monoclonal antibodies against VEGF and cancer stem cell receptors in mice. Cancer Nano 14, 14 (2023). https://doi.org/10.1186/s12645-023-00163-0
Fulton, M. D., & Najahi-Missaoui, W. (2023). Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int J Mol Sci 24(7). https://doi.org/10.3390/ijms24076615
Dahiya M, Awasthi R, Dua K, Dureja H (2023) Sorafenib tosylate loaded superparamagnetic nanoparticles: Development, optimization and cytotoxicity analysis on HepG2 human hepatocellular carcinoma cell line. J Drug Deliv Sci Technol 79:104044. https://doi.org/10.1016/j.jddst.2022.104044
Chavda VP, Balar PC, Patel SB (2023) Interventional nanotheranostics in hepatocellular carcinoma. Nanotheranostics 7(2):128–141. https://doi.org/10.7150/ntno.80120
Badawy MMM, Abdel-Hamid GR, Mohamed HE (2023) Antitumor Activity of Chitosan-Coated Iron Oxide Nanocomposite Against Hepatocellular Carcinoma in Animal Models. Biol Trace Elem Res 201(3):1274–1285. https://doi.org/10.1007/s12011-022-03221-7
Luo F, Yu Y, Li M et al (2022) Polymeric nanomedicines for the treatment of hepatic diseases. J Nanobiotechnol 20:488. https://doi.org/10.1186/s12951-022-01708-y
Ahmadian E, Janas D, Eftekhari A, Zare N (2022) Application of carbon nanotubes in sensing/monitoring of pancreas and liver cancer. Chemosphere 302:134826. https://doi.org/10.1016/j.chemosphere.2022.134826
Aishajiang, R., Liu, Z., Wang, T., Zhou, L., & Yu, D. (2023). Recent Advances in Cancer Therapeutic Copper-Based Nanomaterials for Antitumor Therapy. Molecules, 28(5). https://doi.org/10.3390/molecules28052303
Tian B, Hua S, Liu J (2023) Multi-functional chitosan-based nanoparticles for drug delivery: Recent advanced insight into cancer therapy. Carbohyd Polym 315:120972. https://doi.org/10.1016/j.carbpol.2023.120972
Sedighi M, Mahmoudi Z, Abbaszadeh S, Eskandari MR, Saeinasab M, Sefat F (2023) Nanomedicines for hepatocellular carcinoma therapy: Challenges and clinical applications. Materials Today Communications 34:105242. https://doi.org/10.1016/j.mtcomm.2022.105242
Li Y, Zou H, Zheng Z, Liu Z, Hu H, Wu W, Wang T (2023) Advances in the Study of Bioactive Nanoparticles for the Treatment of HCC and Its Postoperative Residual Cancer. Int J Nanomedicine 18:2721–2735. https://doi.org/10.2147/IJN.S399146
Foghis M, Bungau SG, Bungau AF, Vesa CM, Purza AL, Tarce AG, Tit DM, Pallag A, Behl T, Ul Hassan SS, Radu A (2023) Plants-based medicine implication in the evolution of chronic liver diseases. Biomed Pharmacother 158:114207. https://doi.org/10.1016/j.biopha.2022.114207
Marino P, Pepe G, Basilicata MG, Vestuto V, Marzocco S, Autore G, Procino A, Maria I, Manfra M, Campiglia P (2023) Potential Role of Natural Antioxidant Products in Oncological Diseases. Antioxidants 12(3):704. https://doi.org/10.3390/antiox12030704
Wang X-H, Duan W-B, Liang W, Li H, Xie X-Y, Li S-Q et al (2023) Efficacy of radiofrequency ablation following transarterial chemoembolisation combined with sorafenib for intermediate stage recurrent hepatocellular carcinoma: A retrospective, multicentre, cohort study. EClinicalMedicine 56:101816. https://doi.org/10.1016/j.eclinm.2022.101816
Lee, S.K., Yang, H., Kwon, J.H. et al. Chemoembolization combined radiofrequency ablation vs. chemoembolization alone for treatment of beyond the Milan criteria viable hepatocellular carcinoma (CERFA): study protocol for a randomized controlled trial. Trials 24, 234 (2023). https://doi.org/10.1186/s13063-023-07266-4
Tuli HS, Bhushan S, Kumar A, Aggarwal P, Sak K, Ramniwas S, Vashishth K, Behl T, Rana R, Haque S, Prieto MA (2023) Autophagy Induction by Scutellaria Flavones in Cancer: Recent Advances. Pharmaceuticals 16(2):302. https://doi.org/10.3390/ph16020302
Hao X, Feng P, Zhang Y, Wang F, Wang G, Fei H (2023) Scutebarbatine A induces ROS-mediated DNA damage and apoptosis in breast cancer cells by modulating MAPK and EGFR/Akt signaling pathway. Chem Biol Interact 378:110487. https://doi.org/10.1016/j.cbi.2023.110487
Shao H, Chen J, Li A et al (2023) Salvigenin Suppresses Hepatocellular Carcinoma Glycolysis and Chemoresistance Through Inactivating the PI3K/AKT/GSK-3β Pathway. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-023-04511-z
Hashemi M, Nadafzadeh N, Imani MH, Rajabi R, Ziaolhagh S, Bayanzadeh SD, Norouzi R et al (2023) Targeting and regulation of autophagy in hepatocellular carcinoma: revisiting the molecular interactions and mechanisms for new therapy approaches. Cell Commun Signal 21(1):32. https://doi.org/10.1186/s12964-023-01053-z. (PMID:36759819;PMCID:PMC9912665)
Koushki M, Farrokhi Yekta R, Amiri-Dashatan N (2023) Critical review of therapeutic potential of silymarin in cancer: A bioactive polyphenolic flavonoid. J Funct Foods 104:105502. https://doi.org/10.1016/j.jff.2023.105502
Zhao, W., Zheng, D., Yun-Zhi Tang, P., Li, M., Liu, X., Zhong, J., & Tang, J. Advances of antitumor drug discovery in traditional Chinese medicine and natural active products by using multi-active components combination. Medicinal Research Reviews. First published: 14 May 2023, https://doi.org/10.1002/med.21963
Zhou X, Zeng M, Huang F et al (2023) The potential role of plant secondary metabolites on antifungal and immunomodulatory effect. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-023-12601-5
Wang Y, Yuan A, Wu Y, Wu L, Zhang L (2023) Silymarin in cancer therapy: Mechanisms of action, protective roles in chemotherapy-induced toxicity, and nanoformulations. J Funct Foods 100:105384. https://doi.org/10.1016/j.jff.2022.105384
Hegde M, Girisa S, Chetty BB, Vishwa R, Kunnumakkara AB (2023) Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 8(12):10713–10746. https://doi.org/10.1021/acsomega.2c07326
Elmetwalli, A., Diab, T., Albalawi, A.N., et al. (2023). Diarylheptanoids/sorafenib as a potential anticancer combination against hepatocellular carcinoma: the p53/MMP9 axis of action. Naunyn-Schmiedeberg's Archives of Pharmacology, 1–13. https://doi.org/10.1007/s00210-023-02470-0
Li Ying, Zhou Pan, Zhu Lin-Yi, Hong Wan-Er, Wang De-He, Zhang Zhen-Jie, Chu Xi, Wang Yi-Qun, Shen Tian-Bai, Zhang Wei, "Treatment of Liver Fibrosis after Hepatitis B with TCM Combined with NAs Evaluated by Noninvasive Diagnostic Methods: A Retrospective Study", Evidence-Based Complementary and Alternative Medicine, vol. 2023, Article ID 5711151, 13 pages, 2023. https://doi.org/10.1155/2023/5711151
Li J, Yang F, Li J, Huang Y, Cheng Q, Zhang L (2023) Postoperative adjuvant therapy for hepatocellular carcinoma with microvascular invasion. World J Gastrointest Surg 15(1):19–31. https://doi.org/10.4240/wjgs.v15.i1.19
Duan R, Gong F, Wang Y et al (2023) Transarterial chemoembolization (TACE) plus tyrosine kinase inhibitors versus TACE in patients with hepatocellular carcinoma: a systematic review and meta-analysis. World J Surg Onc 21:120. https://doi.org/10.1186/s12957-023-02961-7
Soumoy L, Ghanem GE, Saussez S, Journe F (2022) Bufalin for an innovative therapeutic approach against cancer. Pharmacol Res 184:106442. https://doi.org/10.1016/j.phrs.2022.106442
Wei L, Wang Z, Jing N, Lu Y, Yang J, Xiao H, Guo H, Sun S, Li M, Zhao D, Li X, Qi W, Zhang Y (2022) Frontier progress of the combination of modern medicine and traditional Chinese medicine in the treatment of hepatocellular carcinoma. Chin Med 17(1):90. https://doi.org/10.1186/s13020-022-00645-0. (PMID:35907976;PMCID:PMC9338659)
Jiang Z, Dai C (2023) Potential Treatment Strategies for Hepatocellular Carcinoma Cell Sensitization to Sorafenib. J Hepatocell Carcinoma 10:257–266. https://doi.org/10.2147/JHC.S396231
Han, Y., Kim, H. I., & Park, J. (2023). The Role of Natural Products in the Improvement of Cancer-Associated Cachexia. Int J Mol Sci, 24(10). https://doi.org/10.3390/ijms24108772
Niero, M., Bartoli, G., Colle, P. D., Scarcella, M., & Zanetti, M. (2023). Impact of Dietary Fiber on Inflammation and Insulin Resistance in Older Patients: A Narrative Review. Nutrients, 15(10). https://doi.org/10.3390/nu15102365
Pan, L., Sui, J., Xu, Y., & Zhao, Q. (2023). Effect of Nut Consumption on Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Nutrients, 15(10). https://doi.org/10.3390/nu15102394
Vajdi M, Karimi A, Tousi AZ et al (2023) Association between plant-based diets and metabolic syndrome in obese adults from Iran: a cross-sectional study. BMC Endocr Disord 23:109. https://doi.org/10.1186/s12902-023-01358-7
Yang, J., Hu, J., Li, Y., Luo, W., Liu, J., & Ye, D. (2022). Clinical Applications of Liquid Biopsy in Hepatocellular Carcinoma. Front Oncol, 12. https://doi.org/10.3389/fonc.2022.781820
Dores, A. R., Peixoto, M., Castro, M., Sá, C., Carvalho, I. P., Martins, A., Maia, E., Praça, I., & Marques, A. (2023). Knowledge and Beliefs about Herb/Supplement Consumption and Herb/Supplement–Drug Interactions among the General Population, including Healthcare Professionals and Pharmacists: A Systematic Review and Guidelines for a Smart Decision System. Nutrients, 15(10). https://doi.org/10.3390/nu15102298
Wu, YY., Xu, YM. & Lau, A.T.Y. Epigenetic effects of herbal medicine. Clin Epigenet 15, 85 (2023). https://doi.org/10.1186/s13148-023-01481-1
Amini S, Bagherniya M, Butler AE, Askari G, Sahebkar A (2023) The effect of medicinal plants on cirrhosis: A systematic review of clinical trials. Advance online publication, Phytotherapy Research. https://doi.org/10.1002/ptr.7816
Yuan, J., Abdurahman, A., Cui, N., Hao, T., Zou, J., Liu, L., & Wu, Y. (2023). Adjuvant therapy with Huatan Sanjie Granules improves the prognosis of patients with primary liver cancer: A cohort study and the investigation of its mechanism of action based on network pharmacology. Front Pharmacol, 14. https://doi.org/10.3389/fphar.2023.1091177
Yeh T, Ho S, Hsu C, Wang J, Kao S, Su Y, Lin SJ, Liou H, Lin T (2023) Preoperative Use and Discontinuation of Traditional Chinese Herbal Medicine and Dietary Supplements in Taiwan: A Cross-Sectional Questionnaire Survey. Healthcare 11(11):1605. https://doi.org/10.3390/healthcare11111605
Ali, M., Din Wani, S. U., Salahuddin, M., K, M., Dey, T., Zargar, M. I., & Singh, J. (2023). Recent advance of herbal medicines in cancer- a molecular approach. Heliyon, 9(2). https://doi.org/10.1016/j.heliyon.2023.e13684
Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA et al (2023) AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 78(6):1922–1965. https://doi.org/10.1097/HEP.0000000000000466. (Epub 2023 May 22. Erratum in: Hepatology. 2023 Oct 16;: PMID: 37199193; PMCID: PMC10663390)
Innes, H., & Nahon, P. (2023). Statistical perspectives on using hepatocellular carcinoma risk models to inform surveillance decisions. J Hepatol. Advance online publication. https://doi.org/10.1016/j.jhep.2023.05.005
Addissouky TA, El Tantawy El Sayed I, Ali MMA, Wang Y, El Baz A, Khalil AA, et al (2023) Can Vaccines Stop Cancer Before It Starts? Assessing the Promise of Prophylactic Immunization Against High-Risk Preneoplastic Lesions. J Cell Immunol. 5(4):127–40. https://doi.org/10.33696/immunology.5.178.
Kamari N, Fateh HL, Darbandi M et al (2023) Fatty liver index relationship with biomarkers and lifestyle: result from RaNCD cohort study. BMC Gastroenterol 23:172. https://doi.org/10.1186/s12876-023-02785-5
Wai-Sun Wong V, Ekstedt M, Lai-Hung Wong G, Hagström H (2023) Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. https://doi.org/10.1016/j.jhep.2023.04.036
Ducreux M, Abou-Alfa GK, Bekaii-Saab T, Berlin J, Cervantes A, de Baere T et al (2023) The management of hepatocellular carcinoma: Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open 8(3):101567. https://doi.org/10.1016/j.esmoop.2023.101567
Mohammadnezhad G, Noqani H, Rostamian P et al (2023) Lenvatinib in the treatment of unresectable hepatocellular carcinoma: a systematic review of economic evaluations. Eur J Clin Pharmacol. https://doi.org/10.1007/s00228-023-03502-7
Brandi N, Renzulli M (2023) The Synergistic Effect of Interventional Locoregional Treatments and Immunotherapy for the Treatment of Hepatocellular Carcinoma. Int J Mol Sci 24(10):8598. https://doi.org/10.3390/ijms24108598
Addissouky TA, Wang Y, El Sayed IE et al (2023) Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef Univ J Basic Appl Sci 12:80. https://doi.org/10.1186/s43088-023-00417-1
Villarruel-Melquiades F, Mendoza-Garrido ME, García-Cuellar CM, Sánchez-Pérez Y, Pérez-Carreón JI, Camacho J (2023) Current and novel approaches in the pharmacological treatment of hepatocellular carcinoma. World J Gastroenterol 29(17):2571–2599. https://doi.org/10.3748/wjg.v29.i17.2571.PMID:37213397;PMCID:PMC10198058
Acknowledgements
The authors thank all the researchers, editors, reviewers, and the supported universities that have done great efforts on their studies. Moreover, we are grateful to the editors, reviewers, and reader of this journal.
Funding
Corresponding author supplied all study materials. There was no further funding for this study.
Author information
Authors and Affiliations
Contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript. Lastly, the authors reviewed and confirmed the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors hereby that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Addissouky, T.A., Sayed, I.E.T.E., Ali, M.M.A. et al. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egypt Liver Journal 14, 2 (2024). https://doi.org/10.1186/s43066-023-00306-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00306-3