Abstract
Background
Helicobacter pylori (H. pylori) is a bacterial infection that is prevalent and affects more than half of the world's population, causing stomach disorders such as gastritis, peptic ulcer disease, and gastric cancer.
Main body
The diagnosis of H. pylori infection relies on invasive and non-invasive techniques emerging artificial intelligence, and antibiotic therapy is available, but antibiotic resistance is a growing concern. The development of a vaccine is crucial in preventing H. pylori-associated diseases, but it faces challenges due to the bacterium's variability and immune escape mechanisms. Despite the challenges, ongoing research into H. pylori's virulence factors and immune escape mechanisms, as well as the development of potential vaccine targets, provides hope for more effective management and prevention of H. pylori-associated diseases. Recent research on H. pylori's immune escape mechanisms and novel immune checkpoint inhibitors could also lead to biomarkers for early cancer detection. Therefore, experts have suggested a combination of traditional and herbal medicine with artificial intelligence to potentially eradicate H. pylori.
Short conclusion
H. pylori infection remains a significant global health problem, but ongoing research into its properties and advanced technologies in addition to the combination of traditional and herbal medicine with artificial intelligence may also lead to the eradication of H. pylori-associated diseases.
Graphical abstract

Similar content being viewed by others
1 Background
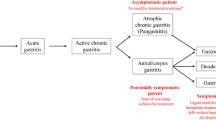
Helicobacter pylori (H. pylori) is a prevalent pathogen that infects half of the global population, causing various stomach disorders, including peptic ulcer disease, primary gastritis, and gastric cancer. It is a type of bacteria that has a spiral shape, does not require oxygen to grow, and is Gram-negative. It can only survive in very difficult growth conditions [1]. H. pylori is a widespread bacterial infection in humans, affecting about 58% of the global population. It is a well-known cause of gastritis and a type I carcinogen, with gastric cancer developing in up to 3% of patients with clinical symptoms of the infection. H. pylori infection is a major contributor to gastroduodenal diseases, particularly in countries where it is highly prevalent, and is typically acquired during childhood. It can lead to chronic gastritis and gastroduodenal ulcers and may increase the risk of stomach cancer or lymphoma, especially in children. Additionally, H. pylori infection may be linked to certain extra-intestinal diseases. Diagnosis relies on upper gastrointestinal endoscopy using biopsy-based diagnostic tests, with acceptable indications for endoscopies [2]. Over the last four decades, significant progress has been made in understanding the properties of H. pylori since its initial identification as a human pathogen. Although it was first linked to chronic gastritis and peptic ulcer disease, research has shown that H. pylori is the most potent risk factor for gastric cancer (GC), one of the deadliest forms of cancer worldwide. H. pylori is also linked to various extra-gastric conditions, including hematological, cardiovascular, respiratory, neurological, ophthalmic, metabolic syndrome-related disorders, and non-gastric neoplasms. The bacteria have been found in organs and tissues beyond the stomach, such as the nose and colonic polyps. H. pylori can reside in the nasal and oral cavities, potentially leading to sinus and nasal problems, including chronic rhinosinusitis and nasal polyps [3]. H. pylori is known to cause various disorders, including those related to the head and neck, atherosclerosis, lung, hepato-biliary, hematological, and intestinal diseases. Although primarily found in the stomach, studies have linked it to upper airway disorders. Gastroesophageal reflux patients may develop otitis media with effusion due to gastric acid's damaging effects on the Eustachian tube lining. However, recent studies have found no connection between H. pylori and otitis media with effusion, bilateral nasal polyposis, or chronic adenotonsillitis. The high prevalence of chronic inflammatory rhinosinusitis associated with nasal polyps is a significant public health issue with limited effective treatments available [4]. The development of gastric cancer associated with H. pylori infection can be broadly classified into different types based on its location, such as adenocarcinoma, lymphoma, carcinoid tumor, and leiomyosarcoma. Adenocarcinoma is the most common type of gastric cancer and can be further categorized into two types: intestinal and diffuse. The intestinal type is typically caused by an acute immune response triggered by H. pylori infection, which can lead to chronic inflammation, gastritis, and ulcers that may result in gastric perforation. Over time, the chronic inflammation may cause epithelial tissue to undergo metaplasia, or cell differentiation, leading to a loss of function and the formation of malignant neoplastic tissue caused by gene mutations. In contrast, genetic factors that affect the expression of intercellular adhesion proteins, such as E-cadherin, can cause the development of diffuse type adenocarcinoma, which disrupts the normal cell cycle and cell connections in gastric epithelial cells (Fig. 1) [5]. The primary treatment involves a triple combination of a proton pump inhibitor and two antibiotics, but eradication rates have decreased due to H. pylori developing resistance to antibiotics. An increased resistance rates to clarithromycin, metronidazole, levofloxacin, amoxicillin, and tetracycline, and MDR strains have been reported in China, indicating a global emergence of H. pylori antibiotic resistance. Recent data suggest that H. pylori resistance may be associated with dysbiosis caused by multi-drug use and cohabitation compositions. H. pylori resistance was linked to an increase in the diversity of the gastric microbiome composition, where non-H. pylori pathogens, particularly in triple-resistant strains, were more abundant. Clinicians should be careful when administering antibiotic combination treatments because drug resistance in the gut environment could affect the gastrointestinal niche and potentially induce drug resistance in other bacteria [6]. While antibiotic therapies are available to treat H. pylori infection, antibiotic resistance and the bacterium's high prevalence highlight the need for a protective vaccine. GC's bacterial etiology makes it a vaccine-preventable cancer. H. pylori has several virulence factors that mediate pathogenesis and could be potential vaccine targets to prevent the associated morbidity and mortality. However, vaccine development has encountered challenges due to the variability among H. pylori strains and the bacterium's immune escape mechanisms. Despite the lack of H. pylori vaccine, ongoing research is promising and may eventually lead to new ways to decrease the burden of H. pylori-associated diseases. Research into H. pylori's immune escape mechanisms and GC-induced expression of novel immune checkpoint inhibitors could yield candidate biomarkers to detect cancer progression in its early stages [7]. Several diagnostic methods are available to detect H. pylori, including invasive and non-invasive techniques. Histology was the first method, but non-invasive methods such as serology, urea breath test, and stool antigen tests have been developed. Invasive methods involve taking biopsies for histological examination and using various stains such as Giemsa, Warthin–Starry, acridine orange, Dieterle, H. pylori silver stain, Gimenez, McMullen, and immunostaining. Giemsa stain is frequently used as it is practical, easy to prepare, inexpensive, and has high sensitivity. Its specificity is 85.7%, better than H&E stains, with a 100% sensitivity in detecting H. pylori. PCR can also be used to detect H. pylori using samples from gastric juice, biopsy, dental plaque, saliva, stools, and even middle ear effusion [4]. Currently, there is no single treatment that can completely eliminate H. pylori, but a combination of an antiulcer medication and two antibiotics has shown success in many cases. Experts have proposed that a combination of traditional and herbal medicine, as well as the use of artificial intelligence, could potentially lead to the complete eradication of H. pylori. As a result, this review will examine recent advancements and research in preventing and managing H. pylori and their potential impact.
An overall description of how H. pylori infection is linked to the development of gastric cancer [5]
2 Novel diagnostic tools for H. pylori
Accurately diagnosing H. pylori infection is critical for effective management of the condition. There are currently a variety of diagnostic methods available that have high levels of sensitivity and specificity. These methods can be grouped into two categories: non-invasive and invasive. Non-invasive techniques include the urea breath test, stool antigen test, serological tests, and molecular tests, whereas invasive techniques require an endoscopy and consist of endoscopic imaging, histology determination, rapid urease testing, and culture and molecular tests [8].
2.1 Recent non-invasive techniques for H. pylori
There have been some recent advancements in non-invasive techniques for detecting H. pylori infection. These include:
2.1.1 Multiplex PCR
This is a new molecular diagnostic technique that can detect multiple bacterial pathogens, including H. pylori, in a single sample. It uses a single PCR to amplify and detect multiple target genes, providing a rapid and accurate diagnosis of H. pylori infection.
2.1.2 Fecal microbiota analysis
This novel method analyzes the gut microbiota, which refers to the collection of microorganisms inhabiting the digestive tract. Recent studies have suggested that changes in the gut microbiota may be linked to the development of H. pylori infection and other gastrointestinal disorders. Fecal microbiota analysis can provide valuable information about the composition and function of the gut microbiota, which may help diagnose and treat H. pylori infection.
2.1.3 Point-of-care testing
This new approach to testing for the bacteria H. pylori allows for faster diagnosis compared to traditional laboratory-based testing. H. pylori testing can now be performed at the point of care—meaning in the doctor's office or clinic—rather than having to send samples out to a diagnostic laboratory. This point-of-care testing is made possible by innovative rapid tests that can provide results within minutes or hours. Specifically, there are two main types of rapid tests for H. pylori suitable for point-of-care use. Rapid antigen tests detect proteins from the bacteria in biopsy or stool samples, providing results in just minutes. Molecular tests, such as loop-mediated isothermal amplification (LAMP) and PCR, detect H. pylori's genetic material and take about one hour to provide results.
2.1.4 Volatile organic compounds (VOCs) analysis
This is a new method that involves analyzing the VOCs in a patient's breath to detect the presence of H. pylori. H. pylori produces specific VOCs that can be detected using specialized equipment. This technique has shown promising results in early studies, but more research is needed to validate its accuracy and reliability.
2.1.5 Serological tests
Recent advances in serological testing have led to the development of new assays that can detect specific antibodies against H. pylori with high accuracy and sensitivity. These tests are based on advanced immunoassay techniques, such as enzyme-linked immunosorbent assay (ELISA) and chemiluminescence immunoassay (CLIA), and can provide rapid and reliable results. Recent studies have demonstrated that the combination of new criteria for gastric cancer risk classification, based on both pepsinogen (PG) values and H. pylori antibody, has been shown to be more effective in identifying individuals at high risk of the disease compared to the conventional criteria, thus reducing the possibility of missing truly high-risk subjects.
2.1.6 Smartphone-based diagnostics
This is a new approach to H. pylori testing that uses a smartphone app and a portable device to perform diagnostic tests. The device is connected to the smartphone, and the patient provides a sample of saliva or breath, which is analyzed using the device. The results are displayed on the smartphone app, allowing for rapid diagnosis and treatment of H. pylori infection.
2.1.7 Microbiome analysis
This is a new method of analyzing the gut microbiome, which is the collection of microorganisms that live in the digestive tract. Recent studies have suggested that changes in the gut microbiome may be linked to the development of H. pylori infection and other gastrointestinal disorders. Microbiome analysis can provide valuable information about the composition and function of the gut microbiome, which may help diagnose and treat H. pylori infection as shown in Fig. 2 which depicts the primary mechanisms that facilitate the connection between H. pylori and the gastric microbiota.
The primary mechanisms that underlie the connection between H. pylori and the microbiota within the stomach [16]
2.2 Recent invasive techniques of H. pylori
There have been some recent advancements in invasive techniques for diagnosing and treating H. pylori infection. These include:
2.2.1 Confocal laser endomicroscopy (CLE)
This is a new endoscopic technique that allows real-time microscopic examination of the gastrointestinal mucosa. CLE can be used to detect H. pylori infection and to evaluate the severity of gastritis and other gastrointestinal disorders. CLE can also be used to guide biopsies and to monitor the response to treatment.
2.2.2 Endoscopic submucosal dissection (ESD)
This is a new endoscopic technique that allows the removal of early-stage gastrointestinal tumors without the need for open surgery. ESD can also be used to remove H. pylori-infected gastric mucosa, which can help reduce the risk of developing gastric cancer.
2.2.3 Rapid urease test (RUT)
This is a widely used invasive technique for diagnosing H. pylori infection during endoscopy. Recent advancements in RUT technology have led to the development of new tests that provide rapid and accurate results.
2.2.4 Polymerase chain reaction (PCR)
This is a sensitive and specific molecular diagnostic technique that can detect H. pylori DNA in biopsy samples with high accuracy. Recent advancements in PCR technology have led to the development of new assays that can detect specific H. pylori strains and antibiotic resistance genes.
2.2.5 Helicobacter pylori culture
This is a traditional invasive technique for diagnosing H. pylori infection that involves culturing the bacteria from biopsy samples [21]. Recent advancements in culture techniques have led to the development of new media and methods that can improve the sensitivity and specificity of H. pylori culture [22]. Initially, the microbiology laboratory used Gram stained smears of fresh gastric biopsy smears as the first method for quickly detecting patients who were positive for C. pyloridis. To culture the bacteria, a microaerobic atmosphere was required, and researchers initially used Skirrow's medium. However, a modified version of Skirrow's medium was developed that incorporated in vitro antimicrobial susceptibility results. This modification involved substituting cefsulodin (5 mg/1) for polymyxin and adding amphotericin B (5 mg/l) to inhibit Candida spp., which is a common contaminant of the stomach. Dent's medium, which is a commercially available version of the modified medium, is still in use today and can be obtained from Oxoid [23].
3 Multi-drug resistant strains (MDRS) of H. pylori
Multi-drug resistant strains (MDRS) of H. pylori have become a growing concern in recent years due to the increasing prevalence of antibiotic-resistant strains. MDRS of H. pylori are strains that are resistant to multiple antibiotics, making them difficult to treat. The prevalence of MDRS of H. pylori is increasing worldwide, with some regions reporting rates as high as 50%. This is a major concern as it can lead to treatment failure and the development of more severe forms of gastritis and peptic ulcer disease. Accurate and timely diagnosis of antibiotic resistance is crucial for the effective treatment of H. pylori infection. Recent advancements in antibiotic resistance testing have led to the development of new molecular diagnostic techniques, such as PCR and whole-genome sequencing, which can provide rapid and accurate results [24]. Tailored therapy is a new approach to H. pylori treatment that involves testing for antibiotic resistance before selecting a treatment regimen. This approach can help reduce the risk of treatment failure and the development of MDRS. However, it may not be feasible in all settings due to costs and turnaround times [25].
There is a need for new antibiotics to treat MDRS of H. pylori. Recent studies have identified several potential new antibiotics, such as rifabutin, rifaximin, and sitafloxacin, that show promise in treating H. pylori infection, including MDRS. Combination therapy is another approach to treating MDRS of H. pylori. Recent studies have shown that combining different classes of antibiotics can improve treatment outcomes and reduce the risk of developing antibiotic resistance [26]. Recent studies have also shown that the use of probiotics may have potential in the treatment of H. pylori infection, including MDRS. Probiotics are live microorganisms that can confer health benefits when ingested. They have antibacterial activity against H. pylori and can help to restore the balance of gut microbiota, which may be disrupted by antibiotic treatment. The emergence of MDRS of H. pylori underscores the importance of appropriate antibiotic use and stewardship. Overuse and misuse of antibiotics can lead to the development of antibiotic resistance, which can have serious consequences for the treatment of infectious diseases. Healthcare providers should be judicious in their use of antibiotics and should follow established guidelines for the treatment of H. pylori infection [27].
In addition to antibiotic stewardship, other strategies for reducing the burden of MDRS of H. pylori include improved sanitation and hygiene, vaccination, and the development of new treatments and preventive measures. It is important for healthcare providers, researchers, and policymakers to work together to address this growing public health challenge and to help ensure the effective management of H. pylori infection, including MDRS [28].
4 Novel pharmaceutical treatment against H. pylori
Novel pharmaceutical treatments against H. pylori have been developed in recent years to address the growing concern of antibiotic resistance and treatment failure. There are several novel pharmaceutical treatments for H. pylori infection that have emerged in recent years. Some of these treatments include:
4.1 Hybrid therapy
This is a novel treatment regimen that combines two antibiotics (amoxicillin and clarithromycin) with a proton pump inhibitor (PPI) and a nitroimidazole (such as metronidazole or tinidazole) [29, 30]. Hybrid therapy has been shown to be highly effective in treating H. pylori infection, even in areas with high antibiotic resistance [31, 32].
4.2 Levofloxacin-based therapy
This is a newer treatment regimen that uses the antibiotic levofloxacin in combination with a PPI and amoxicillin [33]. Levofloxacin-based therapy has been shown to be effective in areas with high clarithromycin and metronidazole resistance [34].
4.3 Lactoferrin-based therapy
Lactoferrin is a protein that has antimicrobial activity against H. pylori. Recent studies have suggested that lactoferrin-based therapy may be effective in treating H. pylori infection, either alone or in combination with other antibiotics [35].
4.4 Bismuth-based therapies
Bismuth-based therapies have been used for the treatment of H. pylori infection for many years. Recent studies have shown that bismuth-based quadruple therapy, which includes bismuth, metronidazole, tetracycline, and a proton pump inhibitor (PPI), can achieve high eradication rates, even in areas with high rates of antibiotic resistance [36].
4.5 Non-bismuth-based quadruple therapy
Non-bismuth-based quadruple therapy, which includes a PPI, amoxicillin, clarithromycin, and nitroimidazole, has been proposed as an alternative to bismuth-based therapy. Recent studies have shown that non-bismuth-based quadruple therapy can achieve high eradication rates, even in areas with high rates of clarithromycin resistance [37].
4.6 Sequential therapy
Sequential therapy involves the use of two different antibiotics, followed by a PPI and another antibiotic, for the treatment of H. pylori infection. Recent studies have shown that sequential therapy can achieve high eradication rates and may be effective in areas with high rates of clarithromycin resistance [38].
4.7 Novel antibiotics
New antibiotics, such as rifabutin, rifaximin, sitafloxacin, and fidaxomicin, have shown promise in treating H. pylori infection, including MDRS [39]. These antibiotics offer new opportunities for effectively treating H. pylori infection and reducing the risk of antibiotic resistance [40].
4.8 New antibiotics to treat MDRS of H. pylori
The emergence of multi-drug resistant strains (MDRS) of H. pylori has become a major concern in recent years, and there is a need for new antibiotics to effectively treat these infections. Fortunately, recent advancements in antibiotic research have identified several new antibiotics that show promise in treating MDRS of H. pylori [41]. One of the most promising new antibiotics is rifabutin, which is a rifamycin antibiotic that has shown excellent activity against MDRS. In recent studies, rifabutin-based therapy has achieved high eradication rates in patients with MDRS, including those who have failed previous treatments [42]. Another new antibiotic, rifaximin, is a rifamycin that has been primarily used to treat gastrointestinal infections. However, recent studies have suggested that rifaximin may also be effective in treating H. pylori infection, including MDRS [43]. Sitafloxacin is a new fluoroquinolone antibiotic that has shown activity against MDRS of H. pylori. Recent studies have demonstrated that sitafloxacin-based therapy can achieve high eradication rates in patients with MDRS, including those who have failed previous treatments [44]. Nitazoxanide is an antiparasitic drug that has shown activity against H. pylori, including MDRS [45]. Recent studies have suggested that nitazoxanide-based therapy may be effective in treating H. pylori infection, either alone or in combination with other antibiotics [46]. Finally, fidaxomicin is a new macrocyclic antibiotic that has been approved for the treatment of Clostridioides difficile infection. Recent studies have suggested that fidaxomicin may have activity against H. pylori, including MDRS [47].
5 Nanotechnology against H. pylori infection
Nanotechnology has emerged as a promising field for the development of new treatments against H. pylori infection [48]. The current state of nanotechnology against H. pylori includes:
5.1 Nanoparticle-based therapies
Nanoparticles have been investigated for their potential to inhibit the growth of H. pylori. Metal nanoparticles, such as silver, copper, gold, titanium dioxide, magnesium oxide, zinc oxide, and selenium nanoparticles, have shown promising results in both in vitro and in vivo studies. Polymer-based nanoparticles, such as chitosan nanoparticles, have also been investigated for their potential as a drug delivery system for the treatment of H. pylori infection [49]. Functionalization of chitosan with the monosaccharide D-mannose has enhanced the targeting abilities of the nanoparticles [50]. Researchers have developed a new dual-action nanoparticle treatment that combines p-coumaric acid (p-CoA) and gallic acid (GA) to more effectively treat bacteria. These nanoparticles are loaded with the two antimicrobial compounds and also have magnetic and biosurfactant properties. The p-CoA and GA act together to inhibit bacterial growth and prevent biofilm formation. Biofilms are problematic as they allow bacteria to adhere to surfaces and evade antibiotics. By disrupting biofilm formation, this new therapy helps overcome bacterial resistance mechanisms. Additionally, the magnetic nanoparticle delivery system targets and accumulates the antimicrobial agents at infected sites [51].
5.2 Nanobiosensors
Nanobiosensors have been developed for the rapid and sensitive detection of H. pylori. These biosensors can detect H. pylori in biological samples, such as saliva, blood, and stool, and can provide results within minutes [52].
5.3 Nanoemulsions
Nanoemulsions have been developed as a potential treatment for H. pylori infection. These emulsions consist of oil droplets dispersed in water and can be used to deliver antimicrobial agents directly to the site of infection [53].
5.4 Nanopore sequencing
Nanopore sequencing has been investigated for the rapid and accurate detection of H. pylori [54]. This technology uses a nanopore to sequence DNA, allowing for the detection of H. pylori and its antibiotic resistance genes [55].
5.5 Nanocarriers
Nanocarriers have been developed as a potential drug delivery system for the treatment of H. pylori infection [56]. These carriers can protect drugs from degradation and can deliver them directly to the site of infection, increasing their efficacy and reducing their side effects [57].
5.6 Nanostructured surfaces
Recent studies have explored the use of nanostructured surfaces to inhibit the adhesion of H. pylori to gastric epithelial cells. These surfaces consist of nanoparticles that are coated onto a substrate to create a nanostructured surface. The nanostructured surface reduces the surface area available for bacterial adhesion and can prevent the formation of biofilms [58].
5.7 Nanoparticle-based vaccines
Nanoparticle-based vaccines have been developed as a potential prophylactic treatment against H. pylori infection. These vaccines consist of nanoparticles that are coated with H. pylori antigens to stimulate an immune response. Recent studies have shown that nanoparticle-based vaccines can induce a strong immune response and may be effective in preventing H. pylori infection [59].
5.8 Nanoparticle-based imaging
Nanoparticle-based imaging has been investigated for the diagnosis of H. pylori infection. These imaging agents consist of nanoparticles that are coated with antibodies or peptides that target H. pylori. The imaging agents can be used for non-invasive imaging of H. pylori in vivo, allowing for the early detection of infection [60].
5.9 Nanoparticle-based drug conjugates
Nanoparticle-based drug conjugates have been developed as a potential treatment for H. pylori infection. These conjugates consist of nanoparticles that are coated with antimicrobial agents, such as antibiotics or probiotics. The nanoparticles protect the drugs from degradation and can deliver them directly to the site of infection, increasing their efficacy and reducing their side effects [61].
5.10 Lipid-based nanocarrier for drug delivery
Lipid-based carriers have gained considerable attention in targeted drug delivery, particularly for drugs with poor solubility. Nanostructured lipid carriers (NLCs), which are lipid nanoparticles composed of non-toxic surfactants and physiological lipids, are highly regarded for their low toxicity and excellent drug delivery capabilities. NLCs, distinguished by their solid lipid core and surfactant stabilization, provide enhanced stability, efficient drug entrapment, and high drug loading capacity compared to other lipid nanoparticles. These NLCs have shown promising therapeutic potential as antibacterial drug delivery systems, effectively targeting various microorganisms, including Staphylococcus aureus, Propionibacterium acnes, Pseudomonas aeruginosa, Escherichia coli, and Helicobacter pylori [62]. Also, nanostructured lipid carriers (NLCs) loaded with two drugs, hesperidin and clarithromycin at specific drug doses. The loaded NLCs were stable in water and simulated gastric fluids, biocompatible, and negatively charged. The in vitro experiments showed that these NLCs were effective in delivering the drugs to target H. pylori and exhibited prolonged release of both drugs for approximately 24 h [63].
6 Challenges and limitations of novel pharmaceuticals and nanotechnology
Novel pharmaceuticals and nanotechnology have been explored as potential treatment options for H. pylori, but they also face several challenges and limitations.
One of the challenges in developing novel pharmaceuticals for H. pylori is its ability to develop antibiotic resistance. H. pylori has been shown to develop resistance to commonly used antibiotics, such as clarithromycin and metronidazole, which can make treatment more difficult. This highlights the need for novel pharmaceuticals with different mechanisms of action that can overcome antibiotic resistance [64].
Nanotechnology-based approaches, such as using nanoparticles to deliver drugs specifically to the site of infection, have shown promise in treating H. pylori. However, there are also limitations to this approach [65]. The nanoparticles need to be stable, biocompatible, and specifically targeted to the site of infection. Additionally, there may be issues with toxicity and the potential for nanoparticles to accumulate in organs and tissues, which could cause unintended side effects. Another challenge in treating H. pylori is that the bacterium can exist in a biofilm, which can protect it from antibiotics and other treatments. This makes it difficult to completely eradicate the infection. Novel pharmaceuticals and nanotechnology-based approaches will need to be able to penetrate these biofilms to be effective [66].
7 Effective herbal and traditional medicine against H. pylori
The use of herbal and traditional medicine against H. pylori has shown promising results. Several studies have reported the efficacy of various plants and natural compounds in treating H. pylori infections, with fewer side effects than conventional antibiotics. One such study found that the use of garlic extract significantly reduced H. pylori colonization in the stomach. Garlic has been shown to have potent antimicrobial properties, and its active compounds, such as allicin, can inhibit H. pylori growth and reduce inflammation in the stomach. Another study investigated the use of honey as a potential treatment for H. pylori [67]. The study found that honey had antibacterial activity against H. pylori, and it could inhibit the growth and formation of H. pylori biofilms. Honey also has anti-inflammatory properties, which can help reduce the severity of gastritis and other gastrointestinal symptoms [68]. Other natural compounds, such as curcumin, green tea, and probiotics, have also been studied for their potential anti-H. pylori activity. These compounds have been shown to reduce H. pylori colonization and improve gastric health in animal and human studies [69]. Hesperetin demonstrated potent inhibition against H. pylori urease by effectively slowing down its activity. This inhibition was achieved through various mechanisms, including the establishment of hydrogen bonding interactions with specific residues in the active pocket and the formation of molecular interactions with the target proteins [70].
Mastic gum is a natural resin obtained from the stem and branches of the mastic tree (Pistacia lentiscus) [71]. It has been traditionally used for its medicinal properties, particularly for the treatment of gastrointestinal disorders. Recent studies have investigated the potential efficacy of mastic gum against H. pylori infections. Several in vitro studies have shown that mastic gum has antibacterial activity against H. pylori, inhibiting its growth and reducing its colonization in the stomach. Mastic gum has anti-inflammatory properties, which may help alleviate the symptoms of gastritis and other gastrointestinal disorders caused by H. pylori infections [72]. Mastic gum has antibacterial activity against H. pylori, and Artemisia has been traditionally used for the treatment of gastrointestinal disorders [73]. Pomegranate has antibacterial properties, and castor oil has been traditionally used as a laxative and for its anti-inflammatory effects. The mix of fig with olive is also known to have antioxidant and anti-inflammatory properties [74]. While these natural compounds may have individual benefits, there is currently limited research on their combined effects on H. pylori eradication. One study investigated the efficacy of a combination therapy consisting of mastic gum and fennel essential oil for the treatment of H. pylori infections and found that it was effective in eradicating the bacterium in 14 out of 19 patients (73.7%) [75]. However, it is important to note that natural compounds may interact with each other and with other medications, which could lead to adverse effects or reduce their efficacy. Patients should always consult with their healthcare provider before using any natural or herbal remedies for the treatment of H. pylori infections [76]. The combination of mastic gum, Artemisia, pomegranate, castor oil, and a mix of fig with olive has been proposed as a potential natural treatment for H. pylori infections. While there is some evidence to suggest that these natural compounds may have antibacterial and anti-inflammatory properties, there is currently limited research on the efficacy of this combination therapy for H. pylori eradication. Our suggestion is that the combination of mastic gum, Artemisia, pomegranate, castor oil, and a mix of fig with olive may have potential benefits for the treatment of H. pylori infections, further research is needed to establish its efficacy and safety [77].
One of the advantages of using herbal and traditional medicine against H. pylori is that they are generally safe and well tolerated, with fewer side effects than conventional antibiotics. However, it is important to note that these natural compounds may not be as potent as antibiotics and may require longer treatment durations or higher doses to achieve the desired effects. Furthermore, the use of herbal and traditional medicine may also provide a more sustainable and cost-effective approach to treating H. pylori infections, particularly in resource-limited settings. Many of these natural compounds are readily available and affordable, making them accessible to a wider population. It is also worth noting that the use of herbal and traditional medicine should not replace conventional antibiotics for the treatment of H. pylori infections. Instead, they may be used as adjunct therapies or as a complementary approach to conventional treatments. Additionally, patients should always consult with their healthcare provider before using any herbal or traditional medicine, as some natural compounds may interact with other medications or have adverse effects in certain individuals.
7.1 Challenges and limitations of herbal and traditional medicine in treating H. pylori
One of the main challenges is the lack of standardization and quality control of herbal and traditional medicine. Many natural compounds used for the treatment of H. pylori infections have not been fully characterized, and their efficacy and safety may vary depending on the source and preparation method. This highlights the need for standardized protocols for the production and quality control of herbal and traditional medicine [78]. Another challenge is the limited availability of clinical data on the efficacy and safety of herbal and traditional medicine for the treatment of H. pylori infections. While several studies have reported promising results, the sample sizes and study designs are often limited, and there is a lack of randomized controlled trials. This makes it difficult to establish the true efficacy and safety of these natural compounds. Additionally, the use of herbal and traditional medicine may not be suitable for all patients, particularly those with underlying medical conditions or who are taking other medications. Some natural compounds may interact with other medications or have adverse effects in certain individuals, which could lead to unintended health consequences [79].
8 The potential role of artificial intelligence on H. pylori
Artificial intelligence (AI) has emerged as a powerful tool for diagnosing and treating various diseases, including H. pylori infections. Recent updates on the potential role of AI in the management of H. pylori have provided valuable insights into the development of novel strategies for the detection and treatment of this bacterium. One potential application of AI in the management of H. pylori is in the development of algorithms for the analysis of endoscopic images to detect H. pylori-associated gastritis [80]. Several studies have reported the use of machine learning algorithms to analyze endoscopic images and accurately diagnose H. pylori infection with high sensitivity and specificity.
Another potential application of AI in the management of H. pylori is in the development of predictive models for the identification of patients at high risk of developing H. pylori-associated diseases, such as gastric cancer [80]. AI can be used to analyze endoscopic images and accurately diagnose H. pylori infections with high sensitivity and specificity. Several studies have reported the use of machine learning algorithms to analyze endoscopic images, which could lead to a more efficient and cost-effective method for the detection and diagnosis of H. pylori infections. AI can also be used in the development of personalized treatment plans for H. pylori infections. Machine learning algorithms can analyze patient data, such as medical history, laboratory results, and imaging studies, to develop individualized treatment plans based on the patient's unique characteristics and medical history [81]. AI can be used to accelerate drug discovery and development. Machine learning algorithms can analyze large datasets and identify potential drug candidates for the treatment of H. pylori infections. AI can also be applied in the area of nanotechnology, where it can be used to develop nano-based drug delivery systems for the targeted delivery of drugs to the site of H. pylori infection. AI can be used to analyze the efficacy and safety of natural compounds for the treatment of H. pylori infections. Machine learning algorithms can analyze large datasets and identify potential natural compounds with anti-H. pylori activity. This could lead to the development of new and effective treatment options based on natural compounds [82]. Finally, AI can be used for the management and prevention of H. pylori infections. Machine learning algorithms can analyze large datasets and identify risk factors associated with the development of H. pylori-associated diseases, such as gastric cancer. This could help healthcare providers identify and manage high-risk patients more effectively and prevent the development of H. pylori-associated diseases [83].
8.1 Challenges and Limitations of Artificial Intelligence in Managing H. Pylori
While artificial intelligence (AI) has the potential to revolutionize the management of H. pylori infections, there are also several challenges and limitations that need to be addressed.
One major challenge is the need for large datasets and high-quality data. Machine learning algorithms require large datasets to train and validate their models, and the quality of the data can affect the accuracy and reliability of the algorithms [84]. In the case of H. pylori infections, there is a lack of large, high-quality datasets that can be used for the development and validation of AI-based approaches. Another challenge is the ethical considerations surrounding the use of AI in healthcare [85]. The use of AI raises questions about patient privacy, data security, and the impact on healthcare professionals. It is important to establish ethical guidelines and frameworks to ensure that the use of AI in healthcare is safe, ethical, and beneficial to patients [86].
Furthermore, the use of AI in the management of H. pylori infections should not replace conventional approaches, but rather complement them. AI-based approaches should be used in conjunction with existing diagnostic and treatment methods to improve patient outcomes. Another limitation is the complexity of H. pylori infections. H. pylori is a highly adaptable bacterium that can evade the immune system and develop resistance to antibiotics. This complexity makes it challenging to develop effective AI-based approaches for the diagnosis and treatment of H. pylori infections. Finally, there is a lack of standardization and regulation of AI-based approaches. AI is a rapidly evolving field, and there is a need for standardized protocols and guidelines for the development and validation of AI-based approaches for the management of H. pylori infections [87].
8.2 Future and directions for Prevention and management of H. pylori infections
The diagram in Fig. 3 illustrates how the invasion of Helicobacter pylori in the human stomach can have a detrimental effect on the host's health, which can be exacerbated by particular dietary habits. The primary consequences include gastritis, which may progress to peptic ulcers and potentially gastric cancer, changes in the gut's microbiota, and inflammation. To evaluate the effectiveness of new treatments for eradicating Helicobacter pylori, animal and in vitro models are commonly employed [88].
The consequences of Helicobacter pylori infection in humans and the latest methods for its eradication [83]
8.3 Vaccination against H. pylori
Vaccination against H. pylori is an area of active research and development, with several promising approaches under investigation. Recent updates on the development and implementation of H. pylori vaccines highlight the potential for these vaccines to prevent H. pylori infections and associated diseases, including gastritis, peptic ulcers, and gastric cancer. One approach to H. pylori vaccination is the use of subunit vaccines, which contain specific antigens from H. pylori that can stimulate an immune response [89]. Clinical trials of subunit vaccines have shown promising results in preventing H. pylori infection and reducing the incidence of associated diseases. Another approach is the use of whole-cell vaccines, which contain inactivated or killed H. pylori bacteria. These vaccines can stimulate a broad immune response and have been shown to be effective in preventing H. pylori infection and reducing the incidence of associated diseases. Live attenuated vaccines, which contain weakened strains of H. pylori, are also under investigation. These vaccines can stimulate a strong immune response and have shown promising results in preventing H. pylori infection and reducing the incidence of associated diseases [90].
Recent updates on the development and implementation of H. pylori vaccines highlight the safety and efficacy of these vaccines in preventing H. pylori infection and reducing the incidence of associated diseases. Additionally, the development of H. pylori vaccines has the potential to significantly reduce the burden of H. pylori-associated diseases, making them an important area of research and development. However, there are also challenges associated with the development and implementation of H. pylori vaccines. One significant challenge is the high genetic diversity of H. pylori strains, which can make it difficult to develop a vaccine that is effective against all strains. Additionally, the development of H. pylori vaccines requires significant investment and resources, which may limit their availability and accessibility in some regions [91].
8.4 Probiotics against H. pylori
Probiotics are live microorganisms that confer health benefits to the host by improving the gut microbiome's composition and function. Recent updates on the role of probiotics in the prevention and management of H. pylori infections highlight their potential to reduce the incidence of H. pylori infection and alleviate H. pylori-associated symptoms. Studies have shown that certain strains of probiotics, such as Lactobacillus and Bifidobacterium [92], can reduce the incidence of H. pylori infection and alleviate H. pylori-associated symptoms, such as abdominal pain and bloating. Additionally, probiotics can modulate the immune system and improve the gut barrier function, which can reduce the risk of H. pylori infection and associated diseases [93].
Recent updates on the role of probiotics in the prevention and management of H. pylori infections highlight the potential for these interventions to be used in conjunction with other approaches, such as antibiotics and lifestyle modifications. Probiotics can improve the effectiveness of antibiotics in eradicating H. pylori bacteria and reducing the risk of antibiotic-associated side effects, such as diarrhea. However, there are also challenges associated with the use of probiotics in the prevention and management of H. pylori infections. One significant challenge is the lack of standardization in probiotic products, which can make it difficult to compare their efficacy and safety. Additionally, the optimal dose, duration, and strain of probiotics for the prevention and management of H. pylori infections are still being studied [94].
8.5 Antibiotic stewardship against H. pylori
Antibiotic stewardship is a critical component of the management of H. pylori infections, as overuse and misuse of antibiotics can lead to the development of antibiotic resistance, which can make treatment more difficult. Recent updates on the role of antibiotic stewardship in the management of H. pylori infections highlight the importance of using antibiotics judiciously and developing more targeted and effective antibiotic therapies [95]. One approach to antibiotic stewardship in the management of H. pylori infections is to use the minimum effective dose of antibiotics for the shortest duration possible. This can help reduce the risk of antibiotic resistance while still effectively eradicating the H. pylori bacteria. Another approach is to use targeted antibiotic therapy based on the patient's microbiome analysis data. By identifying the specific strains of H. pylori bacteria present in the patient's gut, healthcare providers can develop individualized treatment plans that target the specific strains of H. pylori present in the patient's gut, increasing the likelihood of successful treatment and reducing the risk of antibiotic resistance [96].
Recent updates on the role of antibiotic stewardship in the management of H. pylori infections also highlight the potential of alternative therapies, such as probiotics and herbal remedies, as an adjunct to antibiotic therapy [97]. By improving the gut microbiome's composition and function, alternative therapies can reduce the risk of H. pylori infection and associated diseases and improve the efficacy of antibiotic therapy [98]. However, there are also challenges associated with antibiotic stewardship in the management of H. pylori infections. One significant challenge is the increasing prevalence of antibiotic-resistant strains of H. pylori, which can limit the effectiveness of antibiotic therapy. Additionally, the optimal antibiotic regimen for the management of H. pylori infections is still being studied, and more research is needed to develop more targeted and effective antibiotic therapies [99].
8.6 Personalized medicine against H. pylori
Personalized medicine is an area of active research and development for the management of H. pylori infections, which can lead to various gastrointestinal diseases, including gastritis, peptic ulcers, and gastric cancer. Recent updates on the role of personalized medicine in the management of H. pylori infections highlight the potential for individualized treatment plans to improve patient outcomes. Personalized medicine for H. pylori infections involves tailoring the treatment to the patient's unique characteristics, such as their genetic makeup, medical history, and lifestyle factors. By taking a personalized approach, healthcare providers can develop more effective treatment plans that are tailored to the individual patient's needs. One approach to personalized medicine for H. pylori infections is the use of genetic testing to identify patients who are at increased risk of H. pylori-associated diseases, such as gastric cancer. By identifying these high-risk patients, healthcare providers can develop individualized treatment plans that aim to prevent the development of these diseases. Another approach is to use microbiome analysis to identify the specific strains of H. pylori bacteria present in the patient's gut. This information can be used to develop individualized treatment plans that target the specific strains of H. pylori present in the patient's gut, increasing the likelihood of successful treatment [100].
Recent updates on the role of personalized medicine in the management of H. pylori infections highlight the potential for individualized treatment plans to improve patient outcomes. By taking a personalized approach, healthcare providers can develop more effective treatment plans that are tailored to the individual patient's needs, leading to improved patient outcomes. However, there are also challenges associated with personalized medicine for the management of H. pylori infections. One significant challenge is the cost and availability of genetic testing and microbiome analysis, which may limit their use in some regions. Additionally, personalized medicine may not be effective in all cases and may need to be combined with other interventions, such as vaccination or lifestyle modifications [101].
8.7 Lifestyle against H. pylori
Lifestyle modifications have been shown to help prevent and manage H. pylori infections, which can lead to various gastrointestinal diseases, including gastritis, peptic ulcers, and gastric cancer. Recent updates on the role of lifestyle modifications in the prevention and management of H. pylori infections highlight their potential to improve patient outcomes.
Good hygiene practices, such as frequent hand washing, can help prevent the acquisition and transmission of H. pylori bacteria. Additionally, avoiding contaminated food and water, as well as avoiding smoking and excessive alcohol consumption, can reduce the risk of H. pylori infection and associated diseases. Adopting a healthy diet that is rich in fruits, vegetables, and fiber, as well as engaging in regular exercise, can also boost the immune system and reduce the risk of H. pylori-associated diseases [68]. Recent updates on the role of lifestyle modifications in the prevention and management of H. pylori infections highlight the importance of these interventions in improving patient outcomes [102]. Lifestyle modifications can reduce the risk of H. pylori infection and associated diseases, as well as improve the effectiveness of conventional treatments, such as antibiotics and proton pump inhibitors [103]. However, there are also challenges associated with lifestyle modifications for the prevention and management of H. pylori infections. One significant challenge is the difficulty in implementing and maintaining lifestyle modifications, particularly in populations with limited access to healthy food and safe water. Additionally, lifestyle modifications may not be sufficient to prevent or manage H. pylori infections in all cases and may need to be combined with other interventions, such as vaccination or personalized medicine [104].
9 Conclusion
Helicobacter pylori is a prevalent pathogen that infects half of the global population and is a well-known cause of gastritis and a type I carcinogen. Recent advancements in invasive techniques for diagnosing and treating H. pylori infection offer new opportunities for improving patient outcomes and reducing the risk of developing gastric cancer. Novel pharmaceutical treatments, including bismuth-based therapies, non-bismuth-based quadruple therapy, concomitant therapy, and sequential therapy, have shown promise in achieving high eradication rates, even in areas with high rates of antibiotic resistance. New antibiotics also offer promising new opportunities for treating H. pylori infection, including multi-drug resistant strains. Nanotechnology has shown great potential in the fight against H. pylori infection, with the development of nanoparticle-based therapies, nanobiosensors, nanoemulsions, nanopore sequencing, nanocarriers, nanostructured surfaces, nanoparticle-based vaccines, nanoparticle-based imaging, and nanoparticles-based drug and lipid conjugates opening up new avenues for diagnosis and treatment. Herbal and traditional medicine and lifestyle modifications also offer potential alternative treatment options, while personalized medicine and antibiotic stewardship can improve patient outcomes. Therefore, advanced technologies and innovative approaches, such as novel diagnostics, pharmaceuticals, nanotechnology, herbal medicine, AI, and future directions, have the potential to play significant roles in the eradication of H. pylori infection.
While these advancements offer promising solutions, challenges and limitations remain. Further research is needed to develop effective and safe treatments for H. pylori infections and optimize these technologies for clinical use, ensure their safety and efficacy, and address challenges such as limited resources, the emergence of multi-drug resistant strains, ethical considerations, and lack of standardization and regulation of AI-based approaches. However, continued efforts in prevention and management are crucial to reduce the burden of H. pylori infection and improve patient outcomes, and healthcare providers should stay up-to-date with the latest advancements in H. pylori diagnosis and treatment to provide the best possible care for their patients.
10 Recommendations
-
While several diagnostic methods are available to detect H. pylori, ongoing research should focus on developing more rapid and accurate diagnostic tools that can detect H. pylori in its early stages.
-
Antibiotic therapies are available to treat H. pylori infection, but given the high prevalence of the bacterium and its increasing resistance to antibiotics, alternative treatment options should be explored. Combination of traditional and herbal medicine could be a promising approach, and research should be conducted to identify effective herbal compounds that could be used in conjunction with antibiotics.
-
With H. pylori being a vaccine-preventable cancer, developing a protective vaccine could significantly decrease the burden of H. pylori-associated diseases. Ongoing research into H. pylori's immune escape mechanisms and potential vaccine targets should be a priority.
-
The use of artificial intelligence in H. pylori management is still in its early stages, but it holds promise in optimizing treatment strategies, predicting outcomes, and identifying novel drug targets. Researchers should continue to explore the use of AI in H. pylori management.
-
Public health campaigns should be developed to increase awareness of H. pylori infection, its associated diseases, and the importance of early detection and treatment. This should include educating people on the importance of good hygiene practices, such as hand washing and food safety, to prevent the spread of H. pylori.
Availability of data and materials
All data are publicly available for sharing and publication. The manuscript does not have any other associated data, and all necessary data have been declared within the original manuscript.
Abbreviations
- H. pylori :
-
Helicobacter pylori
- GC:
-
Gastric cancer
- Gram-negative:
-
A group of bacteria that do not retain the violet stain used in the Gram staining method
- PCR:
-
Polymerase chain reaction
- E-cadherin:
-
Epithelial cadherin, a protein that plays a crucial role in cell adhesion
- MDR:
-
Multi-drug-resistant
- H&E :
-
Hematoxylin and eosin, a commonly used stain in histology
- AI:
-
Artificial intelligence
- VOCs:
-
Volatile organic compounds
- ELISA:
-
Enzyme-linked immunosorbent assay
- CLIA:
-
Chemiluminescence immunoassay
- PG:
-
Pepsinogen
- CLE:
-
Confocal laser endomicroscopy
- ESD:
-
Endoscopic submucosal dissection
- RUT:
-
Rapid urease test
- PPI:
-
Proton pump inhibitor
- p-CoA:
-
P-coumaric acid
- GA:
-
Gallic acid
- NLCs:
-
Nanostructured lipid carriers
References
Li R, Xu J, Wang X, Liao L, Wei X, Xie P, Xu W, Xu Z, Xie S, Jiang Y, Huang L, Wang L, Huang G, Huang Y (2023) Therapeutic effect of demethylated hydroxylated phillygenin derivative on Helicobacter pylori infection. Front Microbiol 14:1071603. https://doi.org/10.3389/fmicb.2023.1071603
Nguyen TC, Tang NL, Le GK, Nguyen VT, Nguyen KH, Che TH, Phan VT, Nguyen NM, Truong DQ, Ngo XM, Nguyen HT, Robert A, Bontems P, Nguyen PN (2023) Helicobacter pylori Infection and peptic ulcer disease in symptomatic children in Southern Vietnam: a prospective multicenter study. Healthcare 11(11):1658. https://doi.org/10.3390/healthcare11111658
Doulberis M, Kountouras J, Stadler T, Meerwein C, Polyzos SA, Kulaksiz H, Chapman MH, Rogler G, Riva D, Linas I, Kavaliotis J, Kazakos E, Mouratidou M, Liatsos C, Papaefthymiou A (2023) Association between helicobacter pylori infection and nasal polyps: a systematic review and meta-analysis. Microorganisms 11(6):1581. https://doi.org/10.3390/microorganisms11061581
Aref ZF, Bazeed SES, Nafady A, Fahim DFM, Ghweil AA, Sayed MAA, Qubaisy HM, Khalefa M, Arafa UA, Badawy BS, Abdelmohsen AS, Hassan MH, Abdelmaksoud AA (2023) Possible role of helicobacter pylori in ear nose and throat diseases. Infect Drug Resist 16:3497–3509. https://doi.org/10.2147/IDR.S411867
Rêgo A, Guedes Silvestre GF, Albino SL, Pimentel MM, Silva Costa Cruz SB, Silva Wurzba SD, Rodrigues WF, Damasceno L, Cançado Castellano LR (2022) Flavonoids-rich plant extracts against helicobacter pylori infection as prevention to gastric cancer. Front Pharmacol. https://doi.org/10.3389/fphar.2022.951125
Dewayani A, Fauzia KA, Alfaray RI, Waskito LA, Doohan D, Rejeki PS, Alshawsh MA, Ayu Rezkitha YA, Yamaoka Y, Miftahussurur M (2023) Gastric microbiome changes in relation with Helicobacter pylori resistance. PLoS ONE. https://doi.org/10.1371/journal.pone.0284958
Reyes VE (2023) Helicobacter pylori and its role in gastric cancer. Microorganisms 11(5):1312. https://doi.org/10.3390/microorganisms11051312
Alfaro E, Sostres C, Lanas A (2023) Diagnosis and treatment of helicobacter pylori infection in real practice—new role of primary care services in antibiotic resistance era. Diagnostics 13(11):1918. https://doi.org/10.3390/diagnostics13111918
Kim I, Maeng LS, Kim JS et al (2023) Quantitative multiplex real-time polymerase chain reaction assay for the detection of Helicobacter pylori and clarithromycin resistance. BMC Microbiol 23:155. https://doi.org/10.1186/s12866-023-02868-z
Zaidi S, Ali K, Khan AU (2023) It’s all relative: analyzing microbiome compositions, its significance, pathogenesis and microbiota derived biofilms: challenges and opportunities for disease intervention. Arch Microbiol 205:257. https://doi.org/10.1007/s00203-023-03589-7
Rescalvo-Casas C, Hernando-Gozalo M, Pereda LS et al (2023) Comparison of chemiluminiscence versus lateral flow assay for the detection of Helicobacter pylori antigen in human fecal samples. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-023-04624-7
Xiao Y, Li H, Wang C, Pan S, He J, Liu A, Wang J, Sun P, Liu F, Lu G (2023) Room temperature wearable gas sensors for fabrication and applications. Adv Sens Res. https://doi.org/10.1002/adsr.202300035
Sasakabe T, Obata Y, Kawai S, Lin Y, Kikuchi S (2023) Comparison of gastric cancer risk classifications using conventional and new pepsinogen criteria. Gastroenterol Res Pract. https://doi.org/10.1155/2023/7646536
Fan L, Huang JJ, Liao J (2022) Competitive smartphone-based portable electrochemical aptasensor system based on an MXene/cDNA-MB probe for the determination of Microcystin-LR. Sens Actuators B Chem 369:132164. https://doi.org/10.1016/j.snb.2022.132164
Zou H, Lin C, Zan H, Hu Y, Xu X, Wang D, Wang Q, Xie Y, Zhou C (2022) A novel fluorescent aptasensor for ultrasensitive detection of Helicobacter pylori in stool samples based on catalytic hairpin assembly cascade hybridization chain reaction. Sens Actuators B Chem 368:132157. https://doi.org/10.1016/j.snb.2022.132157
Fiorani M, Tohumcu E, Del Vecchio LE, Porcari S, Cammarota G, Gasbarrini A, Ianiro G (2023) The influence of Helicobacter pylori on human gastric and gut microbiota. Antibiotics 12(4):765. https://doi.org/10.3390/antibiotics12040765
Han W, Kong R, Wang N, Bao W, Mao X, Lu J (2023) Confocal laser endomicroscopy for detection of early upper gastrointestinal cancer. Cancers. https://doi.org/10.3390/cancers15030776
Suzuki H, Nonaka S, Maetani I et al (2023) Clinical and endoscopic features of metachronous gastric cancer with possible lymph node metastasis after endoscopic submucosal dissection and Helicobacter pylori eradication. Gastric Cancer. https://doi.org/10.1007/s10120-023-01394-1
Lee H, Hwang HS, Chung JW, Kim KA, Kim ST (2023) Development of a new liquid type rapid urease test kit (Helicotest®): comparison with other commercial kits. Korean J Gastroenterol 81(5):209–215. https://doi.org/10.4166/kjg.2022.139
Asaad AM, El-Azab G, Abdelsameea E, Elbahr O, Kamal A, Abdel-Samiee M, Abdelfattah A, Abdallah H, Maher D, El-Refaie A, Ghanem SE, Ansari S, Awad SM (2023) Susceptibility patterns and virulence genotypes of Helicobacter pylori affecting eradication therapy outcomes among Egyptian patients with gastroduodenal diseases. World J Gastroenterol 29(19):2950–2960. https://doi.org/10.3748/wjg.v29.i19.2950
Miqueleiz A, Valdez VB, Alarcón T (2022) Are molecular methods helpful for the diagnosis of Helicobacter pylori infection and for the prediction of its antimicrobial resistance? Front Microbiol 13:962063. https://doi.org/10.3389/fmicb.2022.962063
Duquesne A, Falcón R, Galindo B, Feliciano O, Gutiérrez O, Baldoquín W, Fonseca MC, Llanes R, Sarmiento L (2023) Diagnostic testing accuracy for helicobacter pylori infection among adult patients with dyspepsia in Cuba’s primary care setting. Microorganisms 11(4):997. https://doi.org/10.3390/microorganisms11040997
McNulty M, C. A. (2023) The first 5 years of Helicobacter pylori research—with an emphasis on the United Kingdom. Helicobacter. https://doi.org/10.1111/hel.12982
Dascălu RI, Bolocan A, Păduaru DN, Constantinescu A, Mitache MM, Stoica AD, Andronic O (2023) Multidrug resistance in Helicobacter pylori infection. Front Microbiol 14:1128497. https://doi.org/10.3389/fmicb.2023.1128497
Karbalaei M, Keikha M, Abadi TB (2022) Prevalence of primary multidrug-resistant Helicobacter pylori in children: a systematic review and meta-analysis. Arch Med Res 53(6):634–640. https://doi.org/10.1016/j.arcmed.2022.08.010
Tang X, Wang Z, Shen Y et al (2022) Antibiotic resistance patterns of Helicobacter pylori strains isolated from the Tibet Autonomous Region, China. BMC Microbiol 22:196. https://doi.org/10.1186/s12866-022-02613-y
Sukri A, Hanafiah A, Yusoff H, Shamsul Nizam NA, Nameyrra Z, Wong Z, Raja Ali RA (2022) Multidrug-resistant helicobacter pylori strains: a five-year surveillance study and its genome characteristics. Antibiotics 11(10):1391. https://doi.org/10.3390/antibiotics11101391
Lin Y, Shao Y, Yan J, Ye G (2023) Antibiotic resistance in Helicobacter pylori: from potential biomolecular mechanisms to clinical practice. J Clin Lab Anal 37(7):e24885. https://doi.org/10.1002/jcla.24885
Shih A, Shie B, Hsu I (2022) Update on the first-line treatment of Helicobacter pylori infection in areas with high and low clarithromycin resistances. Ther Adv Gastroenterol. https://doi.org/10.1177/17562848221138168
Manfredi M, Gargano G, Gismondi P, Ferrari B, Iuliano S (2023) Therapeutic eradication choices in Helicobacter pylori infection in children. Ther Adv Gastroenterol. https://doi.org/10.1177/17562848231170052
Malfertheiner P, Megraud F, Rokkas T et al (2023) Empiric use of standard triple therapy in Helicobacter pylori eradication does not require readjustment in the clarithromycin resistance cut-off pointGut. https://doi.org/10.1136/gutjnl-2023-329712
Lee JY, Park KS (2016) Optimal first-line treatment for helicobacter pylori infection: recent strategies. Gastroenterol Res Pract 2016, Article ID 9086581. https://doi.org/10.1155/2016/9086581
Liou J-M, Jiang X-T, Chen C-C et al (2023) Second-line levofloxacin-based quadruple therapy versus bismuth-based quadruple therapy for Helicobacter pylori eradication and long-term changes to the gut microbiota and antibiotic resistome: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 8(3):228–241. https://doi.org/10.1016/S2468-1253(22)00384-3
Mansour-Ghanaei F, Masihipour B, Fathalipour M, Hassanipour S, Sokhanvar H, Mansour-Ghanaei A, Asgharnezhad M, Joukar F (2022) The efficacy, safety, and tolerability of levofloxacin quadruple therapy for helicobacter pylori eradication: a randomized, double-blind clinical trial. Evid Based Complement Altern Med 2022, Article ID 9794901. https://doi.org/10.1155/2022/9794901
Imoto I, Yasuma T, Oka S, Misaki M, Horiki N, Gabazza EC (2023) Antimicrobial effects of lactoferrin against Helicobacter pylori Infection. Pathogens 12(4):25. https://doi.org/10.3390/pathogens12040599
Cheng S, Li H, Luo J, Chi J, Zhao W, Lin J, Xu C (2023) Egg yolk antibody combined with bismuth-based quadruple therapy in Helicobacter pylori infection rescue treatment: a single-center, randomized, controlled study. Front Microbiol 14:1150129. https://doi.org/10.3389/fmicb.2023.1150129
Alfadhli A, Alboraie M, Afifi M, Dangi A (2022) A randomized clinical trial comparing triple therapy versus non-bismuth based quadruple therapy for the eradication of Helicobacter pylori in Kuwait. J Global Infect Dis 14(3):99–105. https://doi.org/10.4103/jgid.jgid_13_22
Galoș F, Boboc C, Ieșanu I, Anghel M, Ioan A, Iana E, Coșoreanu MT, Boboc AA (2023) Antibiotic resistance and therapeutic efficacy of Helicobacter pylori infection in pediatric patients—a tertiary center experience. Antibiotics. https://doi.org/10.3390/antibiotics12010146
Liang B, Yuan Y, Peng X, Liu X, Hu X, Xing D (2022) Current and future perspectives for Helicobacter pylori treatment and management: from antibiotics to probiotics. Front Cell Infect Microbiol 12:1042070. https://doi.org/10.3389/fcimb.2022.1042070
Godavarthy PK, Puli C (2023) From antibiotic resistance to antibiotic renaissance: a new era in helicobacter pylori treatment. Cureus. https://doi.org/10.7759/cureus.36041
Chen J, Guo Y, Huang Y, Ding Z, Wang J, Liang X, Xu P, Han Y, Lu H (2023) Rifabutin-containing triple therapy versus bismuth quadruple therapy for helicobacter pylori rescue treatment: a multicenter, randomized controlled trial. J Infect Dis. https://doi.org/10.1093/infdis/jiad114
Howden CW, Shah S, Pendse SN, Offman E, Almenoff JS, Sheldon KL (2020) Physiologically based pharmacokinetic modelling to predict Intragastric Rifabutin concentrations in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. https://doi.org/10.1111/apt.17526
Malfertheiner P, Camargo MC, Liou J, Peek R, Schulz C, Smith SI, Suerbaum S (2023) Helicobacter pylori infection. Nat Rev Dis Primers 9(1):1–24. https://doi.org/10.1038/s41572-023-00431-8
Liu L, Nahata MC (2023) Treatment of Helicobacter pylori infection in patients with penicillin allergy. Antibiotics. https://doi.org/10.3390/antibiotics12040737
Kim SE, Hwang JH (2022) Management of helicobacter pylori infection: a comparison between Korea and the United States. Gut Liver 16(4):503–514. https://doi.org/10.5009/gnl210224
Khan SA, Lee TK (2022) Investigations of nitazoxanide molecular targets and pathways for the treatment of hepatocellular carcinoma using network pharmacology and molecular docking. Front Pharmacol 13:968148. https://doi.org/10.3389/fphar.2022.968148
Alshrari AS, Hudu SA, Elmigdadi F, Imran M (2023) The urgent threat of clostridioides difficile infection: a glimpse of the drugs of the future, with related patents and prospects. Biomedicines. https://doi.org/10.3390/biomedicines11020426
Li P, Chen X, Shen Y, Li H, Zou Y, Yuan G, Hu P, Hu H (2019) Mucus penetration enhanced lipid polymer nanoparticles improve the eradication rate of Helicobacter pylori biofilm. J Control Release 300:52–63. https://doi.org/10.1016/j.jconrel.2019.02.039
Shu C, Xu Z, He C, Xu X, Zhou Y, Cai B, Zhu Y (2023) Application of biomaterials in the eradication of Helicobacter pylori: a bibliometric analysis and overview. Front Microbiol 14:1081271. https://doi.org/10.3389/fmicb.2023.1081271
Shabana S, Hamouda HI, Abdalla M, Sharaf MA, Chi Z, Liu C-G (2022) Multifunctional nanoparticles based on marine polysaccharides for apremilast delivery to inflammatory macrophages: preparation, targeting ability, and uptake mechanism. J Res 222:1709–1722. https://doi.org/10.1016/j.ijbiomac.2022.09.225
Sharaf M, Sewid AH, Hamouda HI, Elharrif MG, El-Demerdash AS, Alharthi A, Hashim N, Hamad AA, Selim S, Alkhalifah DHM, Hozzein WN, Abdalla M, Saber T (2022) Rhamnolipid-coated iron oxide nanoparticles as a novel multitarget candidate against major foodborne E. coli serotypes and methicillin-resistant S. aureus. Microbiol Spectr 10(4):e0025022. https://doi.org/10.1128/spectrum.00250-22
Elbehiry A, Marzouk E, Aldubaib M, Abalkhail A, Anagreyyah S, Anajirih N, Almuzaini AM, Rawway M, Alfadhel A, Draz A, Abu-Okail A (2023) Helicobacter pylori infection: current status and future prospects on diagnostic. Ther Control Chall Antibiot. https://doi.org/10.3390/antibiotics12020191
Luo Q, Liu N, Pu S, Zhuang Z, Gong H, Zhang D (2023) A review on the research progress on non-pharmacological therapy of Helicobacter pylori. Front Microbiol. https://doi.org/10.3389/fmicb.2023.1134254
Fauzia KA, Alfaray RI, Yamaoka Y (2023) Advantages of whole genome sequencing in mitigating the Helicobacter pylori antimicrobial resistance problem. Microorganisms 11(5):1239. https://doi.org/10.3390/microorganisms11051239
Wu X, Luo H, Ge C, Xu F, Deng X, Wiedmann M, Baker RC, Stevenson AE, Zhang G, Tang S (2023) Evaluation of multiplex nanopore sequencing for Salmonella serotype prediction and antimicrobial resistance gene and virulence gene detection. Front Microbiol 13:1073057. https://doi.org/10.3389/fmicb.2022.1073057
Asgari S, Nikkam N, Saniee P (2022) Metallic nanoparticles as promising tools to eradicate H. pylori: a comprehensive review on recent advancements. Talanta Open 6:100129. https://doi.org/10.1016/j.talo.2022.100129
Zafar H, Kiani MH, Raza F et al (2020) Design of enzyme decorated mucopermeating nanocarriers for eradication of H. pylori infection. J Nanopart Res 22:4. https://doi.org/10.1007/s11051-019-4719-7
Pinho AS, Seabra CL, Nunes C, Reis S, Martins L (2022) Helicobacter pylori biofilms are disrupted by nanostructured lipid carriers: a path to eradication? J Control Release 348:489–498. https://doi.org/10.1016/j.jconrel.2022.05.050
Sukri A, Hanafiah A, Patil S, Lopes BS (2023) The potential of alternative therapies and vaccine candidates against Helicobacter pylori. Pharmaceuticals 16(4):552. https://doi.org/10.3390/ph16040552
Yahya RHAM, Alzaid SZ, Al Abboud MA, Almuhayawi MS, Al Jaouni SK, Selim S, Ismail KS, Abdelghany TM (2022) Molecular docking and efficacy of Aloe vera gel based on chitosan nanoparticles against helicobacter pylori and its antioxidant and anti-inflammatory activities. Polymers 14(15):2994. https://doi.org/10.3390/polym14152994
Zhou Z, Kai M, Wang S, Wang D, Peng Y, Yu Y, Gao W, Zhang L (2020) Emerging nanoparticle designs against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol 25:e1881. https://doi.org/10.1002/wnan.1881
Sharaf M, Hamouda HI, Shabana S, Khan S, Arif M, Rozan HE, Abdalla M, Chi Z, Liu C (2021) Design of lipid-based nanocarrier for drug delivery has a double therapy for six common pathogens eradication. 625, 126662–126662. https://doi.org/10.1016/j.colsurfa.2021.126662
Sharaf MA, Arif M, Khan SA, Abdalla M, Samah Shabana CZ-M, Liu C-G (2021) Co-delivery of hesperidin and clarithromycin in a nanostructured lipid carrier for the eradication of Helicobacter pylori in vitro. 112, 104896–104896. https://doi.org/10.1016/j.bioorg.2021.104896
de Barros C, Portugal I, Batain F, Portella D, Severino P, Cardoso J, Arcuri P, Chaud M, Alves T (2022) Formulation, design and strategies for efficient nanotechnology-based nasal delivery systems. RPS Pharm Pharmacol Rep. https://doi.org/10.1093/rpsppr/rqac003
Jiang L, Ding L, Liu G (2023) Nanoparticle formulations for therapeutic delivery, pathogen imaging and theranostic applications in bacterial infections. Theranostics 13(5):1545–1570. https://doi.org/10.7150/thno.82790
Shen X, Zhang Y, Mao Q, Huang Z, Yan T, Lin T, Chen W, Wang Y, Cai X, Liang Y (2022) Peptide-polymer conjugates: a promising therapeutic solution for drug-resistant bacteria. Int J Polym Sci 2022:7610951. https://doi.org/10.1155/2022/7610951
Verma T, Aggarwal A, Dey P, Chauhan AK, Rashid S, Chen T, Sharma R (2023) Medicinal and therapeutic properties of garlic, garlic essential oil, and garlic-based snack food: an updated review. Front Nutr. https://doi.org/10.3389/fnut.2023.1120377
Habbash F, Alalwan TA, Perna S, Ahmed N, Sharif O, Sayyad AA, Gasparri C, Ferraris C, Rondanelli M (2022) Association between dietary habits and Helicobacter pylori infection among Bahraini adults. Nutrients 14(19):25. https://doi.org/10.3390/nu14194215
Lenka S, Bhuyan R (2022) Management of H. pylori induced pepticulcer—a phytotherapeutic approach. J Pure Appl Microbiol 16(3):1530–1537. https://doi.org/10.22207/JPAM.16.3.36
Sharaf MA, Arif M, Hamouda HI, Khan SA, Abdalla M, Shabana S, Rozan HE, Khan TU, Chi Z-M, Liu C-G (2022) Preparation, urease inhibition mechanisms, and anti-Helicobacter pylori activities of hesperetin-7-rhamnoglucoside. J Res 3:100103–100103. https://doi.org/10.1016/j.crmicr.2021.100103
Bebb JR, Atherton JC (2003) Mastic gum has no effect on Helicobacter pylori load in vivo. J Antimicrob Chemother 52(3):522–523. https://doi.org/10.1093/jac/dkg366
Fu H, Lai Y (2023) The role of helicobacter pylori neutrophil-activating protein in the pathogenesis of H. pylori and beyond: from a virulence factor to therapeutic targets and therapeutic agents. Int J Mol Sci 24(1):91. https://doi.org/10.3390/ijms24010091
Ahmad R, Alqathama A, Alam MM, Riaz M, Abdalla AN, Aldholmi MMH, Aljishi FS, Althomali EH, Alabdullah MM, Altaweel NH, Almubarak AF, Asghar SS (2023) Biological quality and phytochemical profiling of olive fruits using gas chromatography–mass spectrometry (GCMS) analysis. Chem Biol Technol Agric. https://doi.org/10.1186/s40538-023-00413-8
Bakry SM, AboulNaser AF, El Negoumy SIS, Kassem ME, Abdel-Sattar E, Meselhy MR (2023) Phenolic acids-rich fraction from Ficus drupacea leaves for the prevention and treatment of ethanol-induced gastric mucosal injury in rats. Inflammopharmacology 31(3):1423–1436. https://doi.org/10.1007/s10787-023-01158-4
Bakry SM, Naser AFA, Negoumy SIE et al (2023) Phenolic acids-rich fraction from Ficus drupacea leaves for the prevention and treatment of ethanol-induced gastric mucosal injury in rats. Inflammopharmacol 31:1423–1436. https://doi.org/10.1007/s10787-023-01158-4
Cabada-Aguirre P, López López AM, Ostos Mendoza KC, Garay Buenrostro KD, Luna-Vital DA, Mahady GB (2023) Mexican traditional medicines for women’s reproductive health. Sci Rep. https://doi.org/10.1038/s41598-023-29921-1
Akhter N, Alam M, Amin Khan MR, Sharmin S, Emon NU, Bakar Siddique MA, Hossain KH, Rahman MA (2023) Therapeutic potentials of Adenostemma lavenia (L.) O.Kuntze evidenced into an array of pharmacological effects and ligand-receptor interactions. Heliyon 9(4):e15541. https://doi.org/10.1016/j.heliyon.2023.e15541
Hasna B, Houari H, Koula D, Marina S, Emilia U, Assia B (2023) In vitro and in vivo study of combined effect of some Algerian medicinal plants and probiotics against Helicobacter pylori. Microorganisms 11(5):1242. https://doi.org/10.3390/microorganisms11051242
Shi Y, Ning J, Norbu K et al (2022) The tibetan medicine Zuozhu-Daxi can prevent Helicobacter pylori induced-gastric mucosa inflammation by inhibiting lipid metabolism. Chin Med 17:126. https://doi.org/10.1186/s13020-022-00682-9
Luo Q, Yang H, Hu B (2022) Application of artificial intelligence in the endoscopic diagnosis of early gastric cancer, atrophic gastritis, and Helicobacter pylori infection. J Dig Dis 23(12):666–674. https://doi.org/10.1111/1751-2980.13154
Yacob YM, Alquran H, Mustafa WA, Alsalatie M, Sakim HAM, Lola MS (2023) H. pylori related atrophic gastritis detection using enhanced convolution neural network (CNN) learner. Diagnostics 13(3):336. https://doi.org/10.3390/diagnostics13030336
Deng Y, Qin Y, Zhou Y, Liu H, Jiang Y, Liu P, Bao J (2022) Artificial intelligence applications in pathological diagnosis of gastric cancer. Heliyon. https://doi.org/10.1016/j.heliyon.2022.e12431
Yang H, Guan L, Hu B (2022) Detection and treatment of helicobacter pylori: problems and advances. Gastroenterol Res Pract 2022, Article ID 4710964. https://doi.org/10.1155/2022/4710964
Balderas D, Ponce P, Rojas M, Molina A (2023) Implications of artificial intelligence algorithms in the diagnosis and treatment of motor neuron diseases—a review. Life 13(4):1031. https://doi.org/10.3390/life13041031
Mărginean CO, Meliț LE, Săsăran MO (2022) Traditional and modern diagnostic approaches in diagnosing pediatric helicobacter pylori infection. Children. https://doi.org/10.3390/children9070994
Tran V, Saad T, Tesfaye M et al (2022) Helicobacter pylori (H. pylori) risk factor analysis and prevalence prediction: a machine learning-based approach. BMC Infect Dis 22:655. https://doi.org/10.1186/s12879-022-07625-7
Cao R, Tang L, Fang M, Zhong L, Wang S, Gong L, Li J, Dong D, Tian J (2022) Artificial intelligence in gastric cancer: applications and challenges. Gastroenterol Rep. https://doi.org/10.1093/gastro/goac064
Alvarez-Mercado I (2021) Impact of dietary patterns on H. pylori infection and the modulation of microbiota to counteract its effect. A narrative review. Pathogens 10(7):875. https://doi.org/10.3390/pathogens10070875
Katsande PM, Nguyen VD, Phuong Nguyen TL, Cuc Nguyen TK, Mills GD, Bailey DM, Christie G, Hong HA, Cutting SM (2023) Prophylactic immunization to Helicobacter pylori infection using spore vectored vaccines. Helicobacter. https://doi.org/10.1111/hel.12997
Li S, Zhao W, Xia L, Kong L, Yang L (2023) How long will it take to launch an effective Helicobacter pylori vaccine for humans? Infect Drug Resist 16:3787–3805. https://doi.org/10.2147/IDR.S412361
Chehelgerdi M, Heidarnia F, Dehkordi FB et al (2023) Immunoinformatic prediction of potential immunodominant epitopes from cagW in order to investigate protection against Helicobacter pylori infection based on experimental consequences. Funct Integr Genomics 23:107. https://doi.org/10.1007/s10142-023-01031-1
Mestre A, Narayanan RS, Rivas D, John J, Abdulqader MA, Khanna T, Chakinala RC, Gupta S (2022) Role of probiotics in the management of Helicobacter pylori. Cureus. https://doi.org/10.7759/cureus.26463
Yu Z, Cao M, Peng J et al (2023) Lacticaseibacillus casei T1 attenuates Helicobacter pylori-induced inflammation and gut microbiota disorders in mice. BMC Microbiol 23:39. https://doi.org/10.1186/s12866-023-02782-4
Zhou Y, Li T (2022) Effects of quadruple therapy combined with probiotics on helicobacter pylori-related peptic ulcer. Comput Math Methods Med 2022, Article ID 1221190. https://doi.org/10.1155/2022/1221190
Ayaş M, Gürol Y (2023) Antibiotic resistance of Helicobacter pylori in Turkey: A systematic review and meta-analysis. Microb Drug Resist 29(2):96–103. https://doi.org/10.1089/mdr.2022.0146
Aumpan N, Mahachai V (2023) Management of Helicobacter pylori infection. JGH Open 7(1):3–15. https://doi.org/10.1002/jgh3.12843
Le Thi TG, Werkstetter K, Kotilea K et al (2022) Management of Helicobacter pylori infection in paediatric patients in Europe: results from the EuroPedHp Registry. Infection. https://doi.org/10.1007/s15010-022-01948-y
Shrestha AB, Pokharel P, Sapkota UH, Shrestha S, Mohamed SA, Khanal S, Jha SK, Mohanty A, Padhi BK, Asija A, Sedhai YR, Rijal R, Singh K, Chattu VK, J., A., Barboza, J. J., & Sah, R. (2023) Drug resistance patterns of commonly used antibiotics for the treatment of helicobacter pylori infection among South Asian countries: a systematic review and meta-analysis. Trop Med Infect Dis 8(3):172. https://doi.org/10.3390/tropicalmed8030172
Fukatsu M, Umemura T, Mizuno T, Ohguchi H, Iwatsu S, Matsumoto S, Yamada T, Takenaka H, Kuroiwa M (2021) Positive impacts of antimicrobial stewardship on initial eradication success and drug costs of treatment for clarithromycin-resistant Helicobacter pylori infections. Iryo Yakugaku (Jpn J Pharm Health Care Sci) 47(11):609–615. https://doi.org/10.5649/jjphcs.47.609
Mestrovic A, Perkovic N, Tonkic A, Sundov Z, Kumric M, Bozic J (2023) Personalized approach in eradication of Helicobacter pylori infection. Antibiotics. https://doi.org/10.3390/antibiotics12010007
Ma Q, Li H, Liao J, Cai Z, Zhang B (2022) Tailored therapy for Helicobacter pylori eradication: a systematic review and meta-analysis. Front Pharmacol 13:908202. https://doi.org/10.3389/fphar.2022.908202
Kassid OM, Khalaf Raheem A, Shamikh Hassan A (2022) Prevalence and risk factors of helicobacter pylori infection in Misan, Iraq: a cross-sectional screening study using stool antigen test. J Med Chem Sci 5(7 (Special Issue)):1177–1182. https://doi.org/10.26655/JMCHEMSCI.2022.7.5
Birato YC, ArmandMasimango B, Katabana DM, Shindano TA (2023) Risk factors of Helicobacter pylori infection in Bukavu, Democratic Republic of the Congo: a case–control study. Ann Med Surg 85(4):727–731. https://doi.org/10.1097/MS9.0000000000000409
Bashir SK, Khan MB (2023) Overview of Helicobacter pylori infection, prevalence, risk factors, and its prevention. Adv Gut Microbiome Res. 2023, Article ID 9747027. https://doi.org/10.1155/2023/9747027
Acknowledgements
The authors thank all the researchers who have done great efforts on their studies. Moreover, we are grateful to the editors, reviewers, and reader of this journal.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Author information
Authors and Affiliations
Contributions
The authors completed the study protocol and were the primary organizers of data collection, as well as the draft and revision process of the manuscript. TAA wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol, and engaged in critical discussions of the draft manuscript. Lastly, the authors (TA, YW, IE, AE, AK) reviewed and confirmed the final version of the manuscript. In addition, MA reviewed the edited manuscript after incorporating the reviewers' comments. Furthermore, all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors hereby declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Addissouky, T.A., Wang, Y., El Sayed, I.E. et al. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef Univ J Basic Appl Sci 12, 80 (2023). https://doi.org/10.1186/s43088-023-00417-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00417-1