Abstract
Medical image analysis plays a pivotal role in the evaluation of diseases, including screening, surveillance, diagnosis, and prognosis. Liver is one of the major organs responsible for key functions of metabolism, protein and hormone synthesis, detoxification, and waste excretion. Patients with advanced liver disease and Hepatocellular Carcinoma (HCC) are often asymptomatic in the early stages; however delays in diagnosis and treatment can lead to increased rates of decompensated liver diseases, late-stage HCC, morbidity and mortality. Ultrasound (US) is commonly used imaging modality for diagnosis of chronic liver diseases that includes fibrosis, cirrhosis and portal hypertension. In this paper, we first provide an overview of various diagnostic methods for stages of liver diseases and discuss the role of Computer-Aided Diagnosis (CAD) systems in diagnosing liver diseases. Second, we review the utility of machine learning and deep learning approaches as diagnostic tools. Finally, we present the limitations of existing studies and outline future directions to further improve diagnostic accuracy, as well as reduce cost and subjectivity, while also improving workflow for the clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The delivery of quality healthcare is one of the primary agendas of every nation. Medical imaging includes techniques and processes designed to visualise body parts, tissue or organs for medical purposes, including both diagnostic and therapeutic. With recent advances in Artificial Intelligence (AI) and medical imaging technologies, biomedical image analysis has transformed clinical practice by providing improved insights into human anatomy and disease processes.
Liver disease progression can be characterised by histopathological and haemodynamic changes within the hepatic parenchyma, which correlates to signs found on imaging modalities. Liver fibrosis is the most common outcome of chronic liver injury. Persistent hepatic parenchymal damage results in activation of immune cells and synthesis of fibrotic extracellular matrix components leading to scar formation, which impairs cell function [1, 2]. Progressive liver fibrosis can lead to liver cirrhosis and related complications such as portal hypertension [3]. Portal hypertension in turn leads to multiple complications including splenomegaly, ascites, varices, hepatorenal syndrome and hepatic encephalopathy. Further, the process of chronic liver injury eventually leads to hepatocellular carcinoma (HCC), with cirrhosis being the main precursor of HCC [4]. Overall, one-third of cirrhotic patients will develop HCC during their lifetime. Risk factors for chronic liver disease and eventually liver cirrhosis include chronic infection with HBV or HCV, heavy alcohol intake, and metabolic liver disease [5].
According to World Health Organisation (WHO), HCC is the fourth-leading cause of cancer-related deaths in the world [6]. The prognosis of patients with this tumour remains poor, with a 5-year survival rate of 19% at time of diagnosis [7]. Unfortunately, this is because HCC is often diagnosed at its advanced stages due to the absence of symptoms in patients with early disease, and the poor adherence to surveillance in high-risk patients. The five-year survival rate for patients whose tumours are detected at an early stage and who receive treatment exceeds 70% [8]. Therefore, early diagnosis and staging of liver diseases plays a pivotal role in reducing HCC-related deaths, as well as reducing healthcare costs.
Many computational methods have been developed for the radiological diagnosis of chronic liver disease and HCC. Among the various options, Machine Learning (ML) and Deep Learning (DL) methods have received significant attention due to their outstanding performance on disease diagnosis and prognosis. In this review, we aim to perform a comprehensive analysis of various ML and DL methods for the diagnosis of chronic liver disease and HCC. We first provide an overview of methods in the ML pipeline including pre-processing, feature extraction, and learning algorithms. We then provide an overview of Convolutional Neural Networks (CNNs), which are specialised deep learning algorithms for processing 2D or 3D data. We discuss in detail the application of various methods for liver diseases such as fibrosis, cirrhosis, and HCC. We further outline limitations in current studies and provide research directions that need attention from the scientific community.
Radiological diagnosis of fibrosis and cirrhosis
Ultrasound (US) is typically the first-line radiological study obtained in patients suspected of having cirrhosis because it is readily available, non-invasive, well-tolerated, less expensive than its CT or MRI counterparts, provides real-time image acquisition and display, and does not expose patients to the adverse effects of intravenous contrast or radiation. Changes in tissue composition in a cirrhotic liver can be detected on gray-scale US. The ultrasonographic hallmarks of cirrhosis are a nodular or irregular surface, coarsened liver edge and increased echogenicity; in advanced disease, the gross liver appears atrophied and multi-nodular (with typically atrophy of right lobe and hypertrophy of caudate or left lobes) [9]. A prospective study of 100 patients with suspected cirrhosis who underwent liver biopsy showed that high-resolution US had 91% sensitivity and 94% specificity in detecting cirrhosis [10]. In another similar study, hepatic surface nodularity, especially detected by a linear probe, was shown to be the most direct sign of advanced fibrosis, with reported sensitivity and specificity of 54% and 95% respectively [11, 12]. However, the disadvantages of US in diagnosis of cirrhosis includes high operator dependency and effect on resolution due to presence of speckle noise and fat in obese patients [13].
Ultrasonography also detects portal hypertension, which is a predictive marker of poor outcomes in cirrhosis, with reverse portal flow in decompensated cirrhosis being a poor prognostic marker [14]. Recent studies have shown that HCC incidence increases in parallel to portal pressure. B-mode US signs suggestive of increased portal pressures include increased portal vein diameter, splenomegaly, ascites and presence of abnormal collateral route; US is able to detect the onset of these complications early. Doppler US has high specificity and moderate sensitivity for the diagnosis of clinically significant portal hypertension, but is limited in detecting slow blood flow and has reduced frame rate [15, 16].
Ultrasound-based elastography is a radiological technique that is used as an alternative to liver biopsy to stage the degree of liver fibrosis. Shear wave elastography and strain elastography are the two main techniques used to evaluate liver stiffness, by essentially measuring the hepatic tissue response after mechanical excitation [17]. The accuracy of elastography has primarily been investigated in patients with chronic HCV and HBV. Overall, it has an estimated sensitivity of 70% and specificity of 85% in diagnosing significant fibrosis (F greater than or equal to 2), and 87% and 91% respectively in cirrhosis (F4). A meta-analysis of 17 studies consisting of 7,058 patients has also shown that it can be used to predict complications in chronic liver disease patients, with baseline liver stiffness associated with risk of hepatic decompensation (relative risk [RR] 1.07, 95% CI 1.03\(-\)1.11), HCC development (RR 1.11, 95% CI 1.05\(-\)1.18), and death (RR 1.22, 95% CI 1.05\(-\)1.43) [18]. However, it can be limited in its use in some cases where other factors affect measured liver stiffness, including elevated central venous pressures in patients with severe cardio-respiratory disease, obesity and anatomic distortion.
Given the high sensitivity of ultrasound diagnosis of cirrhosis, CT or MRI is not typically required for diagnosis. However, [12] did show that early stages of liver parenchymal abnormalities and morphological changes in the liver on MRI and CT were predictive of cirrhosis by multivariate analysis (the diagnostic accuracy being 66.0%, 71.9% and 67.9% for US, CT and MRI respectively). Furthermore, physiological parameters have been identified from measurements on multiphase CT as markers of fibrosis - for example, changes in liver perfusion, arterial fraction and mean transit time of contrast do correlate well with severity of cirrhosis (by Child-Pugh classification) [19, 20]. These techniques are yet to be validated in multi-centre trials and remain investigated at this stage.
Diffusion-weighted and contrast-enhanced MRI are able to quantify fibrosis as MRI can detect restricted movement of water that occurs in the expansion of extracellular fluid space in liver fibrosis. [21] showed this had a sensitivity of 85% and specificity of 100% for diagnosis of cirrhosis; as well as sensitivity of 89% and specificity of 80% to stage the degree of cirrhosis [22]. However, despite its strengths, the use of CT and MRI are limited in the clinical setting because they subject patients to ionizing radiation and intravenous contrast material, can significantly increase the cost of the procedure, and MRI-based techniques in particular are subject to limited availability and degree of technical expertise [23].
Radiological diagnosis of HCC
Focal liver lesions seen on ultrasonography in both cirrhotic and non-cirrhotic patients are concerning for HCC. As per the European Association for the Study of the Liver (EASL) Clinical Practice Guidelines, HCC surveillance in at-risk patients consists of six-monthly abdominal ultrasounds. This time interval is based on the expected tumour growth rate supported by observational data, and therefore the interval is not shortened for people at higher risk of HCC. This surveillance has shown a reduction in disease-related mortality with a meta-analysis including 19 studies showing that ultrasound surveillance detected the majority of HCC tumours before they presented clinically, with a pooled sensitivity of 94% [24].
The detection of nodules in cirrhotic patients always warrants further diagnostic contrast-enhanced imaging because benign and malignant nodules are not able to be differentiated based on ultrasonographic appearance alone, with current guidelines recommending CT or MRI to further characterise lesions 1 cm or greater identified on surveillance US [25]. Also, US has a low sensitivity of 63% for detecting early-stage HCC, particularly in the instance of very coarse liver echotexture in advanced fibrosis; and therefore, the performance of US in identification of small nodules in these cases is highly dependent on operator expertise, patient factors (for example, obesity) and the quality of equipment. Reported specificity for detecting HCC with US at any stage is uniformly high at > 90% [24,25,26,27,28]. Ultrasound-based elastography has also been studied for the evaluation of focal liver lesions, but because of its limitations with restricted depth of penetration and inability to differentiate between stiffness of benign and malignant tissue, it is not recommended for this use.
Contrast-enhanced radiological diagnosis of HCC is based on vascular phases (that is, lesion appearance in the late arterial phase, portal venous phase, and the delayed phase). The typical hallmark of HCC is the combination of hypervascularity in the late arterial phase and washout on portal venous and/or delayed phases, which reflects the vascular derangement occurring during hepatocarcinogenesis [29]. Both CT and MRI are more sensitive than ultrasound for detecting HCC < 2 cm and thus more likely to identify candidates for liver transplantation therapy [30]. As expected, in most studies, MRI has higher sensitivity compared to CT in HCC diagnosis which does vary according to HCC size, with MRI performing better on smaller lesions (sensitivity of 48% and 62% for CT and MRI, respectively, in tumours smaller than 20 mm vs. 92% and 95% for CT and MRI, respectively, in tumours equal or larger than 20 mm) [31, 32]. CT or MRI can be considered when patient factors such as obesity, severe parenchymal heterogeneity from advanced cirrhosis, intestinal gas and chest wall deformity prevent adequate US assessment [24].
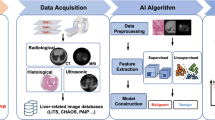
There is a considerable false positive rate with CT or MRI that triggers further cost-ineffective investigations [24]. These imaging modalities also involve the use of contrast and repeated surveillance would result in accumulated exposure to radiation; and incur higher costs. Therefore, CT and MRI are not recommended for routine surveillance. Contrast-enhanced ultrasound (CEUS) in the delayed phase can be used to detect HCC. A recent meta-analysis showed pooled sensitivity and specificity of CEUS for the diagnosis of HCC at 85% and 91% respectively, with these values being almost comparable with MRI and CT for HCC nodules larger than 2 cm. However its use in surveillance has not been validated and is therefore, currently not recommended. It is important to note that CEUS only allows for one or a limited number of identified nodules as it cannot image the entire liver during the multiple phases of contrast administration [27, 33]. In Fig. 1, the diagnostic workflow of HCC is shown.
Alfa faeto protein (AFP) as a tumour marker for HCC has insufficient sensitivity and specificity for tumour detection when used alone. This is because fluctuating levels of AFP in cirrhotic patients can not only indicate HCC development but may also reflect exacerbation of underlying liver disease or flares of HBV or HCV infection. Also, only a small proportion of tumours at an early stage (10-20%) present with elevated AFP. However, when combined with US surveillance, AFP serum levels’ sensitivity for diagnosing early-stage HCC is significantly higher, 45% when using US alone versus 63% when using US and AFP [34, 35]. The decision to perform a liver biopsy is made on a case-by-case basis. Generally, biopsy is indicated when the imaging-based diagnosis remains inconclusive, but the malignancy is considered probable. As per the EASL Clinical Practice Guidelines, in non-cirrhotic patients, imaging alone is not considered sufficient and tissue assessment is required to establish diagnosis [25]. In Table 1, typical radiological findings of cirrhosis, portal hypertension, and HCC are provided.
Artificial intelligence, machine learning, and deep learning
The term Artificial Intelligence (AI) is an umbrella term and refers to a suite of technologies in which computer systems are programmed to exhibit complex behaviour that would typically require intelligence in humans or animals [36]. The long overarching goal of AI is to enable machines to perform intellectual tasks such as decision making, problem solving, perception and understanding human communication, inspired by the human cognitive function.
Machine Learning (ML), a subset of AI, provides systems the ability to automatically learn and improve from experience without being explicitly programmed [37]. The conventional ML pipeline includes the steps of pre-processing, feature extraction, classification, and evaluation. As medical imaging datasets often have variations in characteristics such as contrast, resolution, orientation, side-markers, and noise, it is important to apply pre-processing techniques to improve the dataset quality. After data cleaning, a relevant region-of-interest (ROI) is selected using either fully-automatic segmentation, semi-automatic segmentation, or manual delineation by experts. After this step, salient features specific to the pattern of a particular medical condition are extracted, in a feature extraction step. Once features are extracted, ML algorithms are applied to map extracted features to the target task, such as classification. Overall, ML allows machines to learn from a set of data and subsequently make predictions on a new test data. Applications of ML in medical imaging date back to the early 1980 s when computer-aided detection (CADe) and computer-aided diagnosis (CADx) systems were developed [38]. These CAD(e/x) systems were based on a pre-defined set of explicit parameters, features, or rules developed from expert knowledge. However, one of the major limitations of classical ML systems is the need for handcrafted feature engineering, which is subjective, requires domain expertise and is time-consuming and often brittle.
Deep Learning (DL) [39], a subset of ML, uses multiple layers of neural networks to progressively extract higher-level features from the raw input, overcoming the limitations of hand-crafted feature engineering in classical ML systems. In DL, layers of neural networks are stacked in an hierarchy with increasing complexity and abstraction to obtain high-level representation of the raw data. DL-based models have demonstrated state-of-the-art performance on variety of tasks in various fields such as computer vision, natural language processing, speech, and medical imaging. The success of deep learning is attributed to the availability of large-scale annotated datasets, enhanced computing power with the rise of graphics processing units (GPUs), and novel algorithms and architectures.
In the following subsections, we provide an overview of traditional CAD systems using machine learning and deep learning for diagnosing liver diseases from US images.
Machine learning based CAD systems
Before the rise of deep learning, classical machine learning based Computer-Aided Detection and Diagnosis (CAD(e/x)) systems involved a pipeline of handcrafted feature extraction and a trainable classifier. The machine learning enabled CAD(e/x) systems assist radiologists in image interpretation, disease detection, segmentation of regions-of-interest (ROI) such as tumours, and statistical analysis of the extracted tumour. In addition to their use in diagnostics, CAD systems are integrated in the clinical workflow to triage and prioritise patients based on urgency, in turn maximising operational performance. A typical CAD system follows a standard pipeline consisting of the following four steps:
-
1.
Image pre-processing: Ultrasound (US) images are often of poor quality due to low contrast and presence of speckle noise during image acquisition. The goal of the pre-processing step is to reduce noise, enhance image quality, and standardise the data when it is acquired from multiple sources. Various denoising algorithms such as mean filter, median filter, bilateral filter, and Gaussian filters are applied to remove noise. In order to delineate certain regions, edges are also enhanced using unsharp masking and in the frequency domain. In order to improve contrast, methods such as histogram equalisation and more robust methods such as Contrast Limited Adaptive Histogram Equalisation (CLAHE) are applied. Finally, dataset is normalised using the mean and standard deviation of pixel values. In Table 2, an overview of various pre-processing methods in the reviewed studies to remove noise and improve the image quality is provided. The pre-processing is a critical step in obtaining consistent features and robust model performance.
-
2.
Image segmentation: The goal of segmentation is to define the region of interest (ROI) or the volume of interest (VOI) on medical images, that contain the area or volume of the given lesion or structure. Given that normal and abnormal anatomical structures alone do not form the complete image, it is important to segment the image into foreground and background so that ROI can be extracted. ROI selection helps to reduce the computational cost as computing features from ROI is more efficient compared to using the complete image. In the studies reviewed, a variety of segmentation methods are applied, ranging from fully-automatic segmentation algorithms, semi-automatic segmentation algorithms with seed provided by an expert, and manual segmentation for ROI selection. One of the popular semi-automatic methods is seeded region growing, in which an expert provides an initial seed point and the algorithm automatically finds the contour by growing over the ROI. Fully-automatic algorithms include active contour or snakes.
-
3.
Feature extraction and selection: The goal of the feature extraction step is to analyse selected ROI for special characteristics that can discriminate disease patterns from normal patterns. It extracts certain characteristic attributes and generates a set of meaningful descriptors from an image. The feature extraction step helps to obtain various quantitative measurements in selected ROI images which helps in decision making with respect to the pathology of a structure or tissue. Common visual features include colour, shape, and texture. Since medical images have homogeneous regions with little colour or intensity variations, shape and texture are more informative features. For US images, the most common characteristics include morphological features, gray-level features, and texture features. Feature extraction can be carried out in the spatial domain or frequency domain. In Table 3, an overview of various feature extraction algorithms used on most of the articles reviewed in this study is provided. After relevant features are extracted, a subset of the most relevant features are selected using feature selection algorithms. The feature selection step reduces the number of features by removing irrelevant and redundant features, and improves classification performance. The goal of feature selection is to reduce the dimensionality by removing less relevant features, in turn improving the classification accuracy. Common feature selection algorithms used in the reviewed studies include Principal Component Analysis (PCA), Analysis of Variance (ANOVA), and Locality Sensitive Discriminant Analysis (LSDA). In Table 4, an overview of various feature selection algorithms used in the reviewed studies is provided.
-
4.
Classification: Classification is the process of categorising items into pre-selected classes or categories of similar type. The items are categorised into classes based on a similarity defined by some distance measure. Most of the popular classification methods for CAD of liver diseases include Naive Bayes (NB), K-Nearest Neighbour (KNN), Support Vector Machine (SVM), Artificial Neural Network (ANN), and Discriminant Analysis. Ensemble learning methods such as Random Forests (RF) are also used to leverage the benefit of multiple classifiers and improving classification performance. Table 5 provides an overview of various machine learning classifiers used in the reviewed studies.
Deep learning
Deep learning (DL) [39] is a subset of machine learning where artificial neural networks, algorithms inspired by the human brain, learn from large amounts of data. DL uses multiple layers to represent data abstractions to build computational models. DL has shown high levels of performance on various complex tasks such as speech recognition [46], machine translation [47], object detection [48], caption generation [49], and visual question answering [50]. Some key deep learning algorithms include convolutional neural networks [51], recurrent neural networks [52], and generative adversarial networks [53]. In the following subsection we provide an overview of the building blocks of a typical convolutional neural network, which is one of the de-facto deep learning algorithms for processing 2D (images) or 3D (volumetric) data.
Convolutional neural networks
Convolutional Neural Networks (CNNs) are a special type of deep neural networks that are good at handling two-dimensional data such as images or three-dimensional data such as videos. CNNs have been successfully applied in medical imaging problems such as skin cancer, arrhythmia detection, fundus image segmentation, thoracic disease detection, and lung segmentation. CNNs consists of multiple layers stacked together which use local connections known as local receptive field and weight-sharing for better performance and efficiency. A typical CNN architecture consists of the following layers:
-
Convolutional layer: The convolutional layer is the core building block of a CNN which uses the convolution operation in place of general matrix multiplication. Its parameters consist of a set of learnable filters, also known as kernels. The main task of the convolutional layer is to detect features within local regions of the input image that are common throughout the dataset and map their appearance to a feature map. The output of each convolutional layer is fed to an activation function to introduce non-linearity. There are a number of activation functions available such as Rectified Linear Unit (ReLU), Sigmoid, etc.
-
Sub-sampling (Pooling) layer: In CNNs, the sequence of convolutional layer is followed by pooling layer which reduces the spatial size of the input and thus reduce the number of parameters of the network. A pooling layer takes each feature map output from the convolutional layer and down samples it. In other words, the pooling layer summarises a region of neurons in the convolution layer. The most common pooling techniques are max pooling and average pooling. Max pooling takes the largest value from a patch of the feature map, whereas average pooling takes the average of each patch for the feature map.
-
Activation function: The activation function refers to the features of activated neurons that can be retained and mapped out by a non-linear function, which can be used to solve non-linear problems. Common activation functions include sigmoid, tanh, ReLU, and Softmax. ReLU is one of the widely used activation function as it overcomes the vanishing gradient problem in deep neural networks.
-
Batch normalisation: Batch normalisation is used to address the issues related to internal covariance shift within feature maps. Internal covariance shift is a change in the distribution of hidden units’ values, which slows down the convergence and requires careful initialisation of parameters. Batch normalisation normalises the distribution of feature maps by setting them to zero mean and unit variance. It also makes the flow of gradients smooth and acts as a form of regularisation, helping the generalisation power of the network.
-
Dropout: Dropout is a regularisation techniques heavily used in convolutional neural networks. In dropout, some units or connections are randomly dropped (skipped) with a certain probability. Due to multiple connections, a neural network co-adapts by learning non-linear relations. Dropout helps to overcome this co-adaptation by randomly dropping some of the connections or units, preventing the network from overfitting on the training data.
-
Fully connected layer: In fully connected layers, each neuron from the previous layer is connected to every neuron in the next layer and every value contributes to predicting the class of the test sample. The output of the last fully connected layer is passed through an activation function, generally softmax, which outputs the class scores. Fully connected layers are mostly used at the end of the CNN for the classification task.
Representative convolutional neural networks
The history of convolutional neural networks dates back to LeNet-5 [54] which was proposed for digit recognition. Due to the lack of computational resources at that time, LeNet usage was limited. The LeNet-5 model uses tanh as a non-linear activation function followed by a pooling layer and three fully connected layers. It was the AlexNet [51] model, which made a major breakthrough by drastically reducing the top-5 error rate on the ImageNet challenge compared to the previous shallow networks. Since AlexNet, a series of CNN models have been proposed that have advanced the state of the art steadily on the ImageNet. In AlexNet, tanh is replaced by rectified linear units (ReLU) and the dropout technique is used to selectively ignore units to avoid overfitting of the model. In order to boost predictive performance, Visual Geometry Group at Oxford developed VGG-16 [55]. VGG increased the requirements of memory and computational power because of increased depth of the network with 16 layers combined with convolution and pooling layers. In order to limit memory requirements, various structural or topological decompositions were applied which led to more powerful models such as GoogleNet [56], and Residual Networks (ResNet) [57]. GoogleNet uses an Inception module which computes \(1 \times 1\) filters, \(3 \times 3\) filters and \(5 \times 5\) filters in parallel, but applies bottleneck \(1 \times 1\) filters to reduce the number of parameters. Further changes were made to the original Inception module by removing the \(5 \times 5\) filters and replacing them with two successive layers of \(3 \times 3\) filters, which is called Inception v2. Szegedy et al. [56] released the Inception v3 model where depth, width and number of features are increased systematically by increasing the feature maps before each pooling layer. ResNet was the first network having more than 100 layers using an idea similar to Inception v3. In ResNet, the output of two successive convolutional layers and input bypassing the two layers are combined, which act very similar to a Network-in-Network. ResNet further increases predictive performance by leveraging rich combinations of features, but keeping the computation low. In Table 6, a summary of some of the most common representative CNN models is provided.
CNN training strategies
In this section, we highlight various strategies which are helpful in training deep convolution neural networks and improving their performance.
-
Transfer learning: Transfer learning [59] refers to the ability to share and transfer knowledge from a source task to the target task. Convolutional neural networks learn features in an hierarchical manner, whereby early layers learn generic image features such as edges and corners, whereas later layers learn features specific to the dataset. Given that it is challenging to obtain large-scale annotated datasets in the medical domain due to cost and time constraints, transfer learning helps to leverage the learning of models trained on large-scale datasets such as the ImageNet [60].
-
Data augmentation: Current state-of-the-art CNNs need large-scale annotated data to train in a supervised manner. Given the complexity of CNN models, it is easy for them to overfit on small size medical imaging datasets. Data augmentation [61] is a technique to generate synthetic data, for example by applying different affine transformations such as rotation, scaling, translation, flipping, and adding noise. Data augmentation not only increases the dataset size during training, but also adds diversity to the data, making the model robust on unseen data.
Evaluation measures
For evaluating any model, precision, recall, F1-measure, and accuracy scores are computed using the confusion matrix.
-
True Positive (TP): If a person having Cirrhosis is detected as Cirrhosis
-
True Negative (TN): If a person not having Cirrhosis is correctly detected as non having Cirrhosis
-
False Positive (FP): If a healthy person is detected positive for having Cirrhosis
-
False Negative (FN): If a person having Cirrhosis is detected as a healthy one.
-
Precision calculates the fraction of correct positive detection of Cirrhosis.
-
Recall measures how good all the positives are, which depends on the percentage of total relevant cases correctly classified by the model. It is also called sensitivity.
-
F1-measure is the harmonic mean between precision and recall.
For a binary classification task, the confusion matrix is a \(2 \times 2\) table reporting four primary parameters known as False Positives (FP), False Negative (FN), True Positives (TP), and True Negatives (TN).
Receiver Operating Curve (ROC) is a 2D graphical plot between the True Positive Rate (Sensitivity) and the False Positive Rate (Specificity). The ROC represents the trade-off between sensitivity and specificity. The Area Under the ROC Curve (AUC) represents a measure of how well a model can discriminate between patients with liver diseases and a healthy group of individuals.
Contributions
This article aims to characterise diagnosis, staging, and surveillance of liver diseases using medical imaging, machine learning and deep learning techniques through a methodical review of the literature. We seek to answer the following research questions:
-
1.
Methods \(\longrightarrow\) What AI methods are being applied to the diagnosis and staging of liver diseases using ultrasound imaging?
-
2.
Datasets \(\longrightarrow\) What are different sources of publicly available datasets?
-
3.
Scope \(\longrightarrow\) What types of problems are addressed and solved using AI in diagnosing liver diseases?
-
4.
Performance \(\longrightarrow\) How well do AI techniques including machine learning and deep learning perform in terms of diagnostic accuracy?
To answer these questions and draw our insights, we methodically studied 77 articles from a variety of publication venues, mostly published between January 2010 to December 2021. There has been surveys related to liver diseases [62,63,64,65,66,67]. The survey by [62] mostly focused on diffuse liver diseases and cover only conventional CAD systems. [63] focused on radiographic features under different medical imaging modalities for diagnosing liver diseases. Similar to [62, 64, 65] focused on conventional ML pipeline for diagnosing liver lesions using US imaging. Although [66] provided details about both machine learning and deep learning models for diagnosing liver diseases using US imaging, they did not follow a systematic approach. In Table 7, current study is compared to the existing surveys. The current study provides a more detailed and systematic approach of the current state-of-the-art ML and DL approaches for diagnosing liver diseases. We also provide details about the datasets, methods of severity scoring, and professional societies guidelines. We close the review by discussing the limitations of existing studies and noting future research directions to further improve diagnostic performance, expediting clinical workflow, augmenting clinicians in their decision making, and reducing healthcare cost.
The review is structured as follows:
-
Sect. 7 provides search strategy in terms of selected databases, inclusion and exclusion criteria, and keywords related to search query.
-
Sect. 8 provides a systematic review of diagnosing liver diseases using ultrasound imaging.
-
Sect. 9 provides an overview of various public datasets for the diagnosis of liver diseases.
-
Sect. 10 provides current limitations and future research directions.
Search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [68] to perform our review.
Data sources and search queries
We conducted a comprehensive search to identify potentially all relevant publications on the application of AI including machine learning and deep learning to the diagnosis of liver diseases using medical imaging. The Web of Science, Scopus, IEEE Xplore, and the ACM digital library were queried for articles indexed from Jan 2010 up to 31 December 2021. We included articles written in English and excluded those in the form of editorial, erratum, letter, note, or comment. In Table 8, our inclusion and exclusion criteria are provided. We first identified keywords and their associations to form our search query. For ease of search, we divided our keywords based on four main concepts. The first concept refers to keywords related to liver diseases such as chronic liver disease(s), acute liver disease(s), liver lesion(s), nonalcoholic fatty liver disease, and hepatocellular carcinoma. The first row in Table 9 shows all keywords relevant to the first concept. The second concept relates to various tasks such as classification, detection, segmentation, and staging of liver diseases. The second row in Table 9 shows all keywords for tasks relevant to the second concept. The third concept relates to various imaging modalities by which liver diseases are diagnosed. These include ultrasound, contrast-enhanced ultrasound, computed tomography, and magnetic resonance imaging. The third row in Table 9 shows various keywords and their abbreviations for concepts related to imaging modalities. The fourth concept belongs to keywords related to computer applications such as computer-aided diagnosis, machine learning, and deep learning. For each concept we included associated keywords as well as their abbreviations to make the search criteria complete. The final query is the logical AND of all the four concepts. A complete picture of concepts,keywords related to each concept, and the final search query on databases is presented in Table 9.
Article selection
The search query retrieved in total 1,878 studies (Web of Science: 455, Scopus: 543, IEEE Xplore: 786, and ACM digital library: 94). We created an EndNote library for our screening process. We first used “Find Duplicates" function in EndNote to find any potential duplicate studies. The software highlighted 356 duplicate studies which we removed, with a total of 1,700 studies left. Given the limited functionality of the in-built EndNote function for finding duplicates, we then manually removed duplicates. After the manual duplicate removal step, we were left with a total of 1,494 studies. We started our first screening step based on article title and abstract. We found that there were many studies which are for other human organs but using one of the imaging modalities for diagnosis. After our first screening, we were left with 608 relevant studies. In the second screening step, we read the full-texts of articles and found studies on animals (3), other topic (30), review papers (42), and studies focusing on specific medical condition (9). After excluding all these studies, we were left with a total of 524 studies after our second screening. In the third screening step, we separated studies based on imaging modalities. Out of 524 studies, 243 studies belonged to CT, 81 studies to MRI, and 147 studies to US. However, 53 studies used liver biopsy, blood bio-marker, urine bio-marker, and diffraction enhanced imaging for diagnosing liver diseases, In the final stage of our screening, we reviewed 147 liver US studies and assessed the quality based on the questions in our quality assessment given in Table 10. After the final quality assessment, we were left with a total of 62 studies. During our detailed review, we also went through the references of these 62 studies and found a few relevant studies which our search query was unable to find, to obtain a total of 77 studies. We have reviewed the 77 studies in detail and provide a detailed discussion of the applications, methodology and results. In Fig. 2, the flowchart of our complete article selection process following the PRISMA guidelines is shown. Finally, we conducted a methodical review and qualitative analysis of the 77 studies in accordance with our inclusion and exclusion criteria. The article selection was performed by the first author (S.S.) and was agreed by all other authors of this article.
Due to the heterogeneity and multidisciplinary nature of the included studies, a formal meta-analysis is not possible. We did, however, visually determine overall performance by representing different performance metrics including sensitivity, specificity, F1-score, and receiver operating characteristic (ROC) curves, which are presented later.
Review of studies on the diagnosis and staging of liver diseases
In this section, we review selected studies based on the disease of interest. In Table 11, a summary on studies for fibrosis classification or staging is provided. Most of these studies extracted textural features and applied conventional machine learning algorithms for classification. A few of studies [69, 70] performed fusion of multiple ultrasound modalities to improve diagnostic performance on fibrosis staging. In Table 12, a summary of studies on cirrhosis classification is provided. The focus is on separating normal cases from Cirrhosis. In terms of methods, studies have applied both conventional machine learning and deep learning methods. Table 13 provides summary of studies focusing on nonalcoholic fatty liver disease diagnosis. Most of the studies applied a combination of texture feature extraction algorithms and conventional ML classifiers. Table 14 provides summary of classification of various chronic liver diseases. In this setup, studies focus on three class classification (Normal vs. NAFLD vs. Cirrhosis) or four class classification (Normal vs. Steatosis vs. Fibrosis vs. Cirrhosis). One study [71] performed six class classification with six labels namely, normal, steatosis, chronic hepatitis without cirrhosis, compensated cirrhosis, decompensated cirrhosis, and HCC. In Fig. 3, an year-wise number of studies is shown. The plot shows an increasing trend in the number of studies over years. In Fig. 4, we provide number of studies applying ML or DL methods for diagnosing liver diseases. The plot shows that machine learning was a de-facto choice before 2017. However, with the rise of deep learning, there has been a sharp increase in the number of studies using deep learning methods. In Fig. 5, the distribution of studies is given based on various applications. Of the 77 studies covered in this review, the distribution is as: Fibrosis classification (n=10), Cirrhosis classification (n=7), NAFLD (n=22), CLD (n=8), FLL (n=24), and HCC diagnosis and prognosis (n=6). Most of these studies focus on the classification problem considering one disease versus rest of the diseases.
The results of studies in Table 14 show that as the number of classes increase, the performance of models degrade. This is due to the correlation between diseases and overlapping biomarkers of diseases. In Table 15, a summary of studies focusing on different liver lesions and hepatocellular carcinoma is presented. A major observation is that most of the studies focused on small private (in-house) datasets containing only a few samples of liver lesions such as hemangioma (HEM) or metastases (MET). The studies provide quite high classification performance in terms of accuracy scores. However, accuracy is not a robust metric especially when working with imbalanced datasets. Finally, Table 16 provides summary of studies focusing on HCC prognosis. Most of these studies focused on patient survival analysis. Few of the recent studies showed that CNNs outperforms classical ML algorithms for HCC diagnosis and prognosis.
Public datasets and online initiatives for the diagnosis of liver diseases
Most of the reviewed studies in this article use private (in-house) datasets. Recently, the research community has felt the need to release benchmark datasets in the public domain so that computational methods can be fairly compared. The ImageNet dataset [60] has been one of the underlying factors in the success of deep learning in computer vision because it enabled targeted progression and objective comparison of methods proposed by the community around the world. With the same motivation, researchers in the field of biomedical image computing have started sharing their curated datasets publicly to advance research and foster fair comparison of methods. We provide an overview of various liver datasets below:
-
B-mode fatty liver ultrasound: [103] released a B-mode US dataset for the diagnosis of NAFLD steatosis assessment using ultrasound images. It contains 550 B-mode ultrasound scans and the corresponding liver biopsy results. The dataset was collected from 55 subjects admitted for bariatric surgery in the Department of Internal Medicine, Hypertension and Vascular Diseases, Medical University of Warsaw, Poland.
-
SYSU-CEUS: The SYSU-CEUS dataset [157] contains 353 CEUS videos of three types of focal liver lesions, namely, 186 instances of Hepatocelluar carcinoma (HCC), 109 instances of Hemangioma (HEM), and 58 instances of Focal Nodular Hyperplasia (FNH). Datasets specific to liver tumours have also been made available through participation in online challenges.
-
LiTS: The Liver Tumor Segmentation Challenge(LiTS) [158] dataset provides 201 contrast-enhanced 3D abdominal CT scans and segmentation labels for liver and tumour regions. Each slice of the volume has a resolution of 512 x 512 pixels. Out of 231 volumes, 131 carry their respective annotations whereas no ground-truth labels are provided for the test set containing 70 volumes. The in-plane resolution ranges from 0.60 mm to 0.98 mm, and the slice spacing from 0.45 mm to 5.0 mm.
-
SLIVER07: The Segmentation of the Liver 2007 (SLIVER07) [159] dataset is a part of the grand-challenge organised in conjunction with the MICCAI 2007 for liver tumor segmentation. The training data consists of 10 tumors from 4 patients with their ground-truth segmentations. For the test set, consisting of 10 tumors from 6 patients, the ground-truth was not made available to public by the task organisers. The dataset contains liver tumor CT images corresponding to portal phase of a standard four-phase contrast enhanced imaging protocol.
-
3D-IRCADb: The 3D Image Reconstruction for Comparison of Algorithm Database (3D-IRCADb) Footnote 1 consists of 3D CT scans of 10 men and 10 women having liver tumors in 15 of the cases. The anonymised patient image, labelled image corresponding to ROI segmented, and mask images are given in the DICOM format. The in-plane resolution ranges from 0.57 mm to 0.87 mm, and the slice spacing from 1.6 mm to 4.0 mm.
-
CHAOS: The Combined (CT-MR) Healthy Abdominal Organ Segmentation Challenge (CHAOS) [160] is an IEEE ISBI 2019 challenge dataset focused on segmentation of healthy abdominal organs from CT and/or MRI. The CHAOS dataset contains abdominal CT of 40 subjects having healthy liver. Each slice has a resolution of 512 x 512 pixels.
-
Multi-organ abdominal CT reference standard segmentation: The Multi-organ Abdominal CT Reference Standard Segmentation dataset [161] comprises 90 abdominal CT images delineating multiple organs such as spleen, left-kidney, gallbladder, esophagus, liver, stomach, pancreas, and duodenum. The abdominal CT images and some of the reference segmentations are from two datasets: The Cancer Image Archieve (TCIA) Pancreas-CT dataset [162] and the Beyond the Cranial Vault (BTCV) abdominal dataset [163]. The segmentation of various organs across these CT volumes was performed by two experienced undergraduate students and verified by a radiologist on a volumetric basis.
-
DeepLesion: The DeepLesion [164] dataset, released by the National Institute of Health (NIH) consists of more than 32,000 annotated lesions identified on CT images, collected from 4,400 unique patients. Each of the 2D CT scans is annotated with lesion type, bounding box, and metadata. Each images has a resolution of 512 x 512 pixels.
-
MIDAS: The MIDAS liver tumor dataset from the National Library of Medicine (NLM)’s Imaging Methods Assessment and Reporting project provides 4 liver tumors from 4 patients with five expert hand segmentations. The dataset was made available by Dr. Kevin Cleary at the Imaging Science and Information Systems, Georgetown University Medical Center.
-
CLUST: The Challenge on Liver Ultrasound Track (CLUST) [165] provides a dataset for automatic tracking of liver in ultrasound volumes. The dataset consists of 86 independent studies, with 64 (2D + t) and 22 (3D + t) studies. The dataset was split into training (40% of all sequences) and testing set (60%) from the complete dataset. Annotations were provided for the training set but no ground-truth provided for the test set.
Limitations and future directions
In this section, we outline limitations of existing studies based on our extensive literature review. We also propose future research directions to overcome these limitations.
Limitations
-
Focus on classification Most of the studies focused on the classification task, i.e., binary classification such as: Normal vs. Fatty, Normal vs. Fibrosis, Normal vs. Cirrhosis or multi-class classification such as: Normal vs. Fibrosis vs. Cirrhosis, Normal vs. Hepatocellular Carcinoma vs. Metastasis vs Hemangiomas. However, very little work has been done on disease progression and severity scoring for diseases.
-
Small in-house datasets Although there has been a lot of work on diagnosing liver diseases using various imaging modalities such as ultrasound, CT, and MRI, there are only a few publicly available datasets. Most studies in our literature review worked on in-house data which are often of small size. Also, these datasets suffer from a class-imbalance problem. As accuracy score is not a reliable metric on imbalanced datasets, the lack of publicly available benchmark datasets often limits the true assessment of proposed algorithms in these studies.
-
Classical CAD system still prevalent Currently, deep learning has shown tremendous performance improvement across various fields such as computer vision, natural language processing, robotics, and biomedical image processing. Specifically in biomedical image computing, deep learning has shown superior results on various tasks such as classification, segmentation, and tracking. Due to the lack of publicly available large-scale annotated datasets on liver US studies, the classical machine learning pipeline is still prominent in the community.
Future research direction
-
Need for multidisciplinary approach: The management of HCC encompasses multiple disciplines including hepatologists, diagnostic radiologists, pathologists, transplant surgeons, surgical oncologists, interventional radiologists, nurses, and palliative care professionals [166]. A study by [167] showed that the development of true multidisciplinary clinic with a dedicated tumour board review for HCC patients increased survival; due to improved staging and diagnostic accuracy, efficient treatment times and increased adherence to clinical diagnostic and therapeutic guidelines. Therefore, the AASLD recommends referring HCC patients to a centre with multidisciplinary clinic.
-
Make use of multi-modal data: Current state-of-the-art deep learning models when trained on multi-modal data such as B-mode images, Doppler images, contrast-enhanced ultrasound images, and SWE images could improve the early staging and diagnosis of HCC. Multi-modal data can provide complementary information, in turn helping models to improve.
-
Need for benchmark datasets: In order to push the community’s effort in improving diagnostic performance by proposing novel methods, there is a need to establish a benchmark environment with the release of a large-scale annotated dataset in the public domain. Similar to benchmark algorithms during challenges, the task organisers can release annotated training and validation data but not release test data labels. Once participants have fine-tuned their methods, they may submit predictions on the test set on the challenge evaluation server.
Conclusion
HCC-related morbidity and mortality continues to up-trend due to delays in diagnosis and treatment as early disease is often asymptomatic. Ultrasound is the recommended first-line imaging modality for diagnosis of chronic liver disease and to screen for HCC; however, contrast-enhanced studies are required to confirm HCC diagnosis. In this paper, we first provide an overview of current diagnostic methods for stages of liver disease. We then lay the foundation of methods such as Image pre-processing, feature extraction, and classification for classical machine learning algorithms and a brief overview of convolutional neural networks, which are specialised deep learning algorithms for processing 2D or 3D data. Then, we reviewed the use of these methods as diagnostic tools in chronic liver disease and HCC. We also discussed the studies reviewed in the survey. Finally, we provide future research directions in assisting diagnostic accuracy and efficiency in clinical workflow. We believe that by adapting AI technologies into medical radiology, diagnostic imaging tools have the potential to be implemented in first-line management of chronic liver disease and HCC.
References
Roderfeld M (2018) Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biology 68-69:452–462. SI : Fibrosis – Mechanisms and Translational Aspects
Friedman SL (2000) Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury *. Journal of Biological Chemistry 275(4):2247–2250
García-Pagán JC, Gracia-Sancho J, Bosch J (2012) Functional aspects on the pathophysiology of portal hypertension in cirrhosis. Journal of Hepatology 57(2):458–461
Röcken C, Carl-McGrath S (2001) Pathology and pathogenesis of hepatocellular carcinoma. Digestive Diseases 19(4):269–278
Wolf E, Rich NE, Marrero JA, et al (2021) Use of hepatocellular carcinoma surveillance in patients with cirrhosis: A systematic review and meta-analysis. Hepatology 73(2):713–725
Ferlay J, Soerjomataram I, Dikshit R, et al (2015) Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. International Journal of Cancer 136(5):E359–E386
Wang Cy, Li S (2019) Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: A single center 14 years experience from china. Medicine 98(4)
Siegel RL, Miller KD, Wagle NS, et al (2023) Cancer statistics, 2023. CA: A Cancer Journal for Clinicians 73(1):17–48
Di Lelio A, Cestari C, Lomazzi A, et al (1989) Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 172(2):389–392
Simonovský V (1999) The diagnosis of cirrhosis by high resolution ultrasound of the liver surface. The British Journal of Radiology 72(853):29–34
Colli A, Fraquelli M, Andreoletti M, et al (2003) Severe liver fibrosis or cirrhosis: Accuracy of us for detection–analysis of 300 cases. Radiology 227(1):89–94
Kudo M, Zheng RQ, Kim SR, et al (2008) Diagnostic accuracy of imaging for liver cirrhosis compared to histologically proven liver cirrhosis. Intervirology 51(suppl 1)(Suppl. 1):17–26
Heidelbaugh JJ, Bruderly M (2006) Cirrhosis and chronic liver failure: part i. diagnosis and evaluation. Am Fam Physician 74(5):756–762
Berzigotti A, Piscaglia F, , et al (09.02.2012) Ultrasound in portal hypertension – part 2 – and efsumb recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med 33(01):8–32. 8
Maruyama H, Yokosuka O (2017) Ultrasonography for noninvasive assessment of portal hypertension. Gut and liver 11(4):464–473
Ripoll C, Groszmann RJ, Garcia-Tsao G, et al (2009) Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. Journal of hepatology 50(5):923–928
Talwalkar JA, Kurtz DM, Schoenleber SJ, et al (2007) Ultrasound-based transient elastography for the detection of hepatic fibrosis: Systematic review and meta-analysis. Clinical Gastroenterology and Hepatology 5(10):1214–1220
Singh S, Fujii LL, Murad MH, et al (2013) Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: A systematic review and meta-analysis. Clinical Gastroenterology and Hepatology 11(12):1573–1584.e2
Zissen MH, Wang ZJ, Yee J, et al (2013) Contrast-enhanced ct quantification of the hepatic fractional extracellular space: Correlation with diffuse liver disease severity. American Journal of Roentgenology 201(6):1204–1210
Van Beers BE, Leconte I, Materne R, et al (2001) Hepatic perfusion parameters in chronic liver disease. American Journal of Roentgenology 176(3):667–673
Patel J, Sigmund EE, Rusinek H, et al (2010) Diagnosis of cirrhosis with intravoxel incoherent motion diffusion mri and dynamic contrast-enhanced mri alone and in combination: Preliminary experience. Journal of Magnetic Resonance Imaging 31(3):589–600
Taouli B, Tolia AJ, Losada M, et al (2007) Diffusion-weighted mri for quantification of liver fibrosis: Preliminary experience. American Journal of Roentgenology 189(4):799–806
Sharma S, Khalili K, Nguyen GC (2014) Non-invasive diagnosis of advanced fibrosis and cirrhosis. World journal of gastroenterology 20(45):16,820–16,830
Singal A, Volk ML, Waljee A, et al (2009) Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Alimentary Pharmacology & Therapeutics 30(1):37–47
Marrero JA, Kulik LM, Sirlin CB, et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology 68(2):723–750
Colli A, Fraquelli M, Casazza G, et al (2006) Accuracy of ultrasonography, spiral ct, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: A systematic review. Official journal of the American College of Gastroenterology 101(3)
Tzartzeva K, Obi J, Rich NE, et al (2018) Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology 154(6):1706–1718.e1
Yu NC, Chaudhari V, Raman SS, et al (2011) Ct and mri improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clinical Gastroenterology and Hepatology 9(2):161–167
Matsui O, Kobayashi S, Sanada J, et al (2011) Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted ct) with special reference to multi-step hepatocarcinogenesis. Abdominal Imaging 36(3):264–272
Chou R, Cuevas C, Fu R, et al (2015) Imaging techniques for the diagnosis of hepatocellular carcinoma. Annals of Internal Medicine 162(10):697–711
Lee YJ, Lee JM, Lee JS, et al (2015) Hepatocellular carcinoma: Diagnostic performance of multidetector ct and mr imaging–a systematic review and meta-analysis. Radiology 275(1):97–109
Zhang J, Yu Y, Li Y, et al (2017) Diagnostic value of contrast-enhanced ultrasound in hepatocellular carcinoma: a meta-analysis with evidence from 1998 to 2016. Oncotarget 8(43):75,418–75,426
Claudon M, Dietrich CF, Choi BI, et al (18.01.2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (ceus) in the liver – update 2012. Ultraschall Med 34(01):11–29. 11
Biselli M, Conti F, Gramenzi A, et al (2015) A new approach to the use of \(\alpha\)-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. British journal of cancer 112(1):69–76
Villanueva A, Minguez B, Forner A, et al (2010) Hepatocellular carcinoma: Novel molecular approaches for diagnosis, prognosis, and therapy. Annual Review of Medicine 61(1):317–328
Russell SJ, Norvig P (2009) Artificial Intelligence: a modern approach, 3rd edn. Pearson
Mitchell TM (1997) Machine Learning, 1st edn. McGraw-Hill, Inc., USA
Doi K (2007) Computer-aided diagnosis in medical imaging: Historical review, current status and future potential. Computerized Medical Imaging and Graphics 31(4):198–211
Goodfellow I, Bengio Y, Courville A (2016) Deep Learning. MIT Press, http://www.deeplearningbook.org
Jain AK (1989) Fundamentals of Digital Image Processing. Prentice-Hall, Inc., USA
Benesty J, Chen J, Huang Y (2010) Study of the widely linear wiener filter for noise reduction. In: 2010 IEEE International Conference on Acoustics, Speech and Signal Processing, pp 205–208
Tomasi C, Manduchi R (1998) Bilateral filtering for gray and color images. In: Sixth International Conference on Computer Vision (IEEE Cat. No.98CH36271), pp 839–846
Wang M, Zheng S, Li X, et al (2014) A new image denoising method based on gaussian filter. In: 2014 International Conference on Information Science, Electronics and Electrical Engineering, pp 163–167
Gonzalez RC, Woods RE (2006) Digital Image Processing (3rd Edition). Prentice-Hall, Inc., USA
Zuiderveld K (1994) Contrast Limited Adaptive Histogram Equalization, Academic Press Professional, Inc., USA, p 474–485
Yu D, Deng L (2014) Automatic Speech Recognition: A Deep Learning Approach. Springer Publishing Company, Incorporated
Bahdanau D, Cho K, Bengio Y (2015) Neural machine translation by jointly learning to align and translate. 3rd International Conference on Learning Representations, ICLR 2015 ; Conference date: 07-05-2015 Through 09-05-2015
Ren S, He K, Girshick R, et al (2015) Faster r-cnn: Towards real-time object detection with region proposal networks. In: Cortes C, Lawrence N, Lee D, et al (eds) Advances in Neural Information Processing Systems, vol 28. Curran Associates, Inc.
Karpathy A, Fei-Fei L (2017) Deep visual-semantic alignments for generating image descriptions. IEEE Trans Pattern Anal Mach Intell 39(4):664–676
Agrawal A, Lu J, Antol S, et al (2017) Vqa: Visual question answering. Int J Comput Vision 123(1):4–31
Krizhevsky A, Sutskever I, Hinton GE (2012) Imagenet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al (eds) Advances in Neural Information Processing Systems, vol 25. Curran Associates, Inc.
Hochreiter S, Schmidhuber J (1997) Long Short-Term Memory. Neural Computation 9(8):1735–1780
Goodfellow I, Pouget-Abadie J, Mirza M, et al (2014) Generative adversarial nets. In: Ghahramani Z, Welling M, Cortes C, et al (eds) Advances in Neural Information Processing Systems, vol 27. Curran Associates, Inc.
Lecun Y, Bottou L, Bengio Y, et al (1998) Gradient-based learning applied to document recognition. Proceedings of the IEEE 86(11):2278–2324
Simonyan K, Zisserman A (2015) Very deep convolutional networks for large-scale image recognition
Szegedy C, Liu W, Jia Y, et al (2015) Going deeper with convolutions. In: 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), pp 1–9
He K, Zhang X, Ren S, et al (2016) Deep residual learning for image recognition. In: 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). IEEE Computer Society, Los Alamitos, CA, USA, pp 770–778
Huang G, Liu Z, Van Der Maaten L, et al (2017) Densely connected convolutional networks. In: 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), pp 2261–2269
Razavian AS, Azizpour H, Sullivan J, et al (2014) CNN Features Off-the-Shelf: An Astounding Baseline for Recognition. In: 2014 IEEE Conference on Computer Vision and Pattern Recognition Workshops, Ohio, United States, pp 512–519
Deng J, Dong W, Socher R, et al (2009) Imagenet: A large-scale hierarchical image database. In: 2009 IEEE Conference on Computer Vision and Pattern Recognition, pp 248–255
Shorten C, Khoshgoftaar TM (2019) A survey on image data augmentation for deep learning. Journal of Big Data 6(1):60
Bharti P, Mittal D, Ananthasivan R (2016) Computer-aided characterization and diagnosis of diffuse liver diseases based on ultrasound imaging. Ultrasonic Imaging 39(1):33–61
Aubé C, Bazeries P, Lebigot J, et al (2017) Liver fibrosis, cirrhosis, and cirrhosis-related nodules: Imaging diagnosis and surveillance. Diagnostic and Interventional Imaging 98(6):455–468
Jabarulla MY, Lee HN (2017) Computer aided diagnostic system for ultrasound liver images: A systematic review. Optik 140:1114–1126
Huang Q, Zhang F, Li X (2018) Machine learning in ultrasound computer-aided diagnostic systems: A survey. BioMed Research International 2018:10
Akkus Z, Cai J, Boonrod A, et al (2019) A survey of deep-learning applications in ultrasound: Artificial intelligence–powered ultrasound for improving clinical workflow. JACR Journal of the American College of Radiology 16(9):1318–1328
Decharatanachart P, Chaiteerakij R, Tiyarattanachai T, et al (2021) Application of artificial intelligence in chronic liver diseases: a systematic review and meta-analysis. BMC Gastroenterology 21(1):10
Page MJ, McKenzie JE, Bossuyt PM, et al (2021) The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372
Gao L, Zhou R, Dong C, et al (2021) Multi-modal active learning for automatic liver fibrosis diagnosis based on ultrasound shear wave elastography. In: 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), pp 410–414
Xue LY, Jiang ZY, Fu TT, et al (2020) Transfer learning radiomics based on multimodal ultrasound imaging for staging liver fibrosis. European Radiology 30(5):2973–2983
Ribeiro R, Marinho R, Velosa J, et al (2011) Chronic liver disease staging classification based on ultrasound, clinical and laboratorial data. In: 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, pp 707–710
Wang J, Guo L, Shi X, et al (2012) Real-time elastography with a novel quantitative technology for assessment of liver fibrosis in chronic hepatitis b. European Journal of Radiology 81(1):e31–e36
Meng D, Zhang L, Cao G, et al (2017) Liver fibrosis classification based on transfer learning and fcnet for ultrasound images. IEEE Access 5:5804–5810
Chen Y, Luo Y, Huang W, et al (2017) Machine-learning-based classification of real-time tissue elastography for hepatic fibrosis in patients with chronic hepatitis b. Computers in biology and medicine 89:18–23
Li W, Huang Y, Zhuang BW, et al (2019) Multiparametric ultrasomics of significant liver fibrosis: A machine learning-based analysis. European Radiology 29(3):1496–1506
Liu J, Wang W, Guan T, et al (2019) Ultrasound liver fibrosis diagnosis using multi-indicator guided deep neural networks. In: Suk HI, Liu M, Yan P, et al (eds) Machine Learning in Medical Imaging. Springer International Publishing, Cham, pp 230–237
Lee JH, Joo I, Kang TW, et al (2020) Deep learning with ultrasonography: automated classification of liver fibrosis using a deep convolutional neural network. European Radiology 30(2):1264–1273
Trombini M, Borro P, Ziola S, et al (2020) A digital image processing approach for hepatic diseases staging based on the glisson–capsule. In: 2020 2nd International Conference on Electrical, Control and Instrumentation Engineering (ICECIE), pp 1–6
Zamanian H, Mostaar A, Azadeh P, et al (2021) Implementation of combinational deep learning algorithm for non-alcoholic fatty liver classification in ultrasound images. Journal of biomedical physics & engineering 11(1):73–84
Feng X, Chen X, Dong C, et al (2022) Multi-scale information with attention integration for classification of liver fibrosis in b-mode us image. Computer Methods and Programs in Biomedicine 215:106,598
Z. S. Jabbar AAKA. Q. Al-Neami, Salih SM (2023) Liver fibrosis processing, multiclassification, and diagnosis based on hybrid machine learning approaches. Indonesian Journal of Electrical Engineering and Computer Science 29(3)
Xie Y, Chen S, Jia D, et al (2022) Artificial intelligence-based feature analysis of ultrasound images of liver fibrosis. Computational Intelligence and Neuroscience 2022:2859,987
Lei Ym, Zhao Xm, Guo Wd (2015) Cirrhosis recognition of liver ultrasound images based on svm and uniform lbp feature. In: 2015 IEEE Advanced Information Technology, Electronic and Automation Control Conference (IAEAC), pp 382–387
Liu X, Song JL, Wang SH, et al (2017) Learning to diagnose cirrhosis with liver capsule guided ultrasound image classification. Sensors (Basel, Switzerland) 17(1):149
Rabie R, Eltoukhy MM, al Shatouri M, et al (2018) Computer aided diagnosis system for liver cirrhosis based on ultrasound images. In: Proceedings of the 7th International Conference on Software and Information Engineering. Association for Computing Machinery, New York, NY, USA, ICSIE ’18, p 68–71
Aggarwal K, Bhamrah MS, Ryait HS (2019) Detection of cirrhosis through ultrasound imaging by intensity difference technique. EURASIP Journal on Image and Video Processing 2019(1):80
Mitani Y, Fisher RB, Fujita Y, et al (2020) Cirrhosis liver classification on b-mode ultrasound images by convolution neural networks with augmented images. International Journal of Machine Learning and Computing 10(6):723–728
Yang H, Sun X, Sun Y, et al (2020) Ultrasound image-based diagnosis of cirrhosis with an end-to-end deep learning model. In: 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), pp 1193–1196
Drazinos P, Tsantis S, Zoumpoulis P, et al (2020) A deep learning approach on cirrhosis diagnosis utilizing ultrasound b-mode images of segmented liver left lobes using liver biopsy as the gold standard. In: 2020 American Association of Physicists in Medicine (AAPM
Ossama A, Ahmed O, Hashem M, et al (2022) Automatic classification of diffuse liver diseases: Cirrhosis and hepatosteatosis using ultrasound images. In: 2022 2nd International Mobile, Intelligent, and Ubiquitous Computing Conference (MIUCC), pp 246–250
Ribeiro R, Sanches J (2009) Fatty liver characterization and classification by ultrasound. In: Araujo H, Mendonça AM, Pinho AJ, et al (eds) Pattern Recognition and Image Analysis. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 354–361
Acharya UR, Sree SV, Ribeiro R, et al (2012) Data mining framework for fatty liver disease classification in ultrasound: A hybrid feature extraction paradigm. Medical Physics 39(7Part1):4255–4264
Minhas FuAA, Sabih D, Hussain M (2012) Automated classification of liver disorders using ultrasound images. Journal of Medical Systems 36(5):3163–3172
Singh M, Singh S, Gupta S (2014) An information fusion based method for liver classification using texture analysis of ultrasound images. Information Fusion 19:91–96. Special Issue on Information Fusion in Medical Image Computing and Systems
Subramanya MB, Kumar V, Mukherjee S, et al (2015) A cad system for b-mode fatty liver ultrasound images using texture features. Journal of Medical Engineering & Technology 39(2):123–130
Liao YY, Yang KC, Lee MJ, et al (2016) Multifeature analysis of an ultrasound quantitative diagnostic index for classifying nonalcoholic fatty liver disease. Scientific Reports 6(1):35,083
Acharya UR, Fujita H, Bhat S, et al (2016a) Decision support system for fatty liver disease using gist descriptors extracted from ultrasound images. Information Fusion 29:32–39
Acharya UR, Fujita H, Sudarshan VK, et al (2016b) An integrated index for identification of fatty liver disease using radon transform and discrete cosine transform features in ultrasound images. Information Fusion 31:43–53
Kuppili V, Biswas M, Sreekumar A, et al (2017) Extreme learning machine framework for risk stratification of fatty liver disease using ultrasound tissue characterization. J Med Syst 41(10):1–20
Bharath R, Mishra PK, Rajalakshmi P (2018) Automated quantification of ultrasonic fatty liver texture based on curvelet transform and svd. Biocybernetics and Biomedical Engineering 38(1):145–157
Sharma V, Juglan K (2018) Automated classification of fatty and normal liver ultrasound images based on mutual information feature selection. IRBM 39(5):313–323
Biswas M, Kuppili V, Edla DR, et al (2018) Symtosis: A liver ultrasound tissue characterization and risk stratification in optimized deep learning paradigm. Computer Methods and Programs in Biomedicine 155:165–177
Byra M, Styczynski G, Szmigielski C, et al (2018) Transfer learning with deep convolutional neural network for liver steatosis assessment in ultrasound images. International Journal of Computer Assisted Radiology and Surgery 13(12):1895–1903
Amin MN, Rushdi MA, Marzaban RN, et al (2019) Wavelet-based computationally-efficient computer-aided characterization of liver steatosis using conventional b-mode ultrasound images. Biomedical signal processing and control 52:84–96
Bharath R, Rajalakshmi P (2019) Nonalcoholic fatty liver texture characterization based on transfer deep scattering convolution network and ensemble subspace knn classifier. In: 2019 URSI Asia-Pacific Radio Science Conference (AP-RASC), pp 1–4
Wu CC, Yeh WC, Hsu WD, et al (2019) Prediction of fatty liver disease using machine learning algorithms. Computer Methods and Programs in Biomedicine 170:23–29
Han A, Byra M, Heba E, et al (2020) Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat with radiofrequency ultrasound data using one-dimensional convolutional neural networks. Radiology 295(2):342–350
Cao W, An X, Cong L, et al (2020) Application of deep learning in quantitative analysis of 2-dimensional ultrasound imaging of nonalcoholic fatty liver disease. Journal of Ultrasound in Medicine 39(1):51–59
Che H, Brown LG, Foran DJ, et al (2021) Liver disease classification from ultrasound using multi-scale cnn. International Journal of Computer Assisted Radiology and Surgery 16(9):1537–1548
Chou TH, Yeh HJ, Chang CC, et al (2021) Deep learning for abdominal ultrasound: A computer-aided diagnostic system for the severity of fatty liver. Journal of the Chinese Medical Association 84(9)
Kim T, Lee DH, Park EK, et al (2021) Deep learning techniques for fatty liver using multi-view ultrasound images scanned by different scanners: Development and validation study. JMIR Med Inform 9(11):e30,066
Gaber A, Youness HA, Hamdy A, et al (2022) Automatic classification of fatty liver disease based on supervised learning and genetic algorithm. Applied Sciences 12(1)
Tahmasebi A, Wang S, Wessner CE, et al (2023) Ultrasound-based machine learning approach for detection of nonalcoholic fatty liver disease. Journal of Ultrasound in Medicine n/a(n/a)
Wu CH, Hung CL, Lee TY, et al (2022) Fatty liver diagnosis using deep learning in ultrasound image. In: 2022 IEEE International Conference on Digital Health (ICDH), pp 185–192
Sayed Abou Zaid AZ, Fakhr MW, Ali Mohamed AF (2006) Automatic diagnosis of liver diseases from ultrasound images. In: 2006 International Conference on Computer Engineering and Systems, pp 313–319
Acharya UR, Raghavendra U, Fujita H, et al (2016) Automated characterization of fatty liver disease and cirrhosis using curvelet transform and entropy features extracted from ultrasound images. Computers in Biology and Medicine 79:250–258
Owjimehr M, Danyali H, Helfroush MS, et al (2017) Staging of fatty liver diseases based on hierarchical classification and feature fusion for back-scan–converted ultrasound images. Ultrasonic Imaging 39(2):79–95
Xu SSD, Chang CC, Su CT, et al (2019) Classification of liver diseases based on ultrasound image texture features. Applied Sciences 9(2)
Mabrouk AG, Hamdy A, Abdelaal HM, et al (2021) Automatic classification algorithm for diffused liver diseases based on ultrasound images. IEEE Access 9:5760–5768
Pasyar P, Mahmoudi T, Kouzehkanan SZM, et al (2021) Hybrid classification of diffuse liver diseases in ultrasound images using deep convolutional neural networks. Informatics in Medicine Unlocked 22:100,496
Nastenko I, Maksymenko V, Galkin A, et al (2021) Liver pathological states identification with self-organization models based on ultrasound images texture features. In: Shakhovska N, Medykovskyy MO (eds) Advances in Intelligent Systems and Computing V. Springer International Publishing, Cham, pp 401–418
Xian Gm (2010) An identification method of malignant and benign liver tumors from ultrasonography based on glcm texture features and fuzzy svm. Expert Syst Appl 37(10):6737–6741
Mittal D, Kumar V, Saxena SC, et al (2011) Neural network based focal liver lesion diagnosis using ultrasound images. Computerized Medical Imaging and Graphics 35(4):315–323
Virmani J, Kumar V, Kalra N, et al (2013) Characterization of primary and secondary malignant liver lesions from b-mode ultrasound. Journal of Digital Imaging 26(6):1058–1070
Jeon JH, Choi JY, Lee S, et al (2013) Multiple roi selection based focal liver lesion classification in ultrasound images. Expert Systems with Applications 40(2):450–457
Virmani J, Kumar V, Kalra N, et al (2013) Svm-based characterization of liver ultrasound images using wavelet packet texture descriptors. Journal of Digital Imaging 26(3):530–543
Virmani J, Kumar V, Kalra N, et al (2014) Neural network ensemble based cad system for focal liver lesions from b-mode ultrasound. Journal of Digital Imaging 27(4):520–537
Kalyan K, Jakhia B, Dattatraya Lele R, et al (2014) Artificial neural network application in the diagnosis of disease conditions with liver ultrasound images. Advances in Bioinformatics 2014(6):14
Wu K, Chen X, Ding M (2014) Deep learning based classification of focal liver lesions with contrast-enhanced ultrasound. Optik 125(15):4057–4063
Sakr AA, Fares ME, Ramadan M (2014) Automated focal liver lesion staging classification based on haralick texture features and multi-svm. International Journal of Computer Applications 91(8):17–25
Gatos I, Tsantis S, Spiliopoulos S, et al (2015) A new automated quantification algorithm for the detection and evaluation of focal liver lesions with contrast-enhanced ultrasound. Medical Physics 42(7):3948–3959
Hwang YN, Lee JH, Kim GY, et al (2015) Classification of focal liver lesions on ultrasound images by extracting hybrid textural features and using an artificial neural network. Bio-Medical Materials and Engineering 26:S1599–S1611
Rani A, Mittal D (2016) Detection and classification of focal liver lesions using support vector machine classifiers. Journal of Biomedical Engineering and Medical Imaging 3:21
Hassan TM, Elmogy M, Sallam ES (2017) Diagnosis of focal liver diseases based on deep learning technique for ultrasound images. Arabian Journal for Science and Engineering 42(8):3127–3140
Krishnan KR, Radhakrishnan S (2017) Hybrid approach to classification of focal and diffused liver disorders using ultrasound images with wavelets and texture features. IET Image Processing 11:530–538(8)
Acharya UR, Koh JEW, Hagiwara Y, et al (2018) Automated diagnosis of focal liver lesions using bidirectional empirical mode decomposition features. Computers in Biology and Medicine 94:11–18
Yao Z, Dong Y, Wu G, et al (2018) Preoperative diagnosis and prediction of hepatocellular carcinoma: Radiomics analysis based on multi-modal ultrasound images. BMC Cancer 18(1):1089
Guo LH, Wang D, Qian YY, et al (2018) A two-stage multi-view learning framework based computer-aided diagnosis of liver tumors with contrast enhanced ultrasound images. Clinical Hemorheology and Microcirculation 69:343–354
Pan F, Huang Q, Li X (2019) Classification of liver tumors with ceus based on 3d-cnn. In: 2019 IEEE 4th International Conference on Advanced Robotics and Mechatronics (ICARM), pp 845–849
Schmauch B, Herent P, Jehanno P, et al (2019) Diagnosis of focal liver lesions from ultrasound using deep learning. Diagnostic and Interventional Imaging 100(4):227–233
Yang Q, Wei J, Hao X, et al (2020) Improving b-mode ultrasound diagnostic performance for focal liver lesions using deep learning: A multicentre study. EBioMedicine 56
Mostafiz R, Rahman MM, Islam AKMK, et al (2020) Focal liver lesion detection in ultrasound image using deep feature fusions and super resolution. Machine Learning and Knowledge Extraction 2(3):172–191
Tiyarattanachai T, Apiparakoon T, Marukatat S, et al (2021) Development and validation of artificial intelligence to detect and diagnose liver lesions from ultrasound images. PLOS ONE 16(6):1–14
Hu HT, Wang W, Chen LD, et al (2021) Artificial intelligence assists identifying malignant versus benign liver lesions using contrast-enhanced ultrasound. Journal of gastroenterology and hepatology 36(10):2875–2883
Căleanu CD, Sîrbu CL, Simion G (2021) Deep neural architectures for contrast enhanced ultrasound (ceus) focal liver lesions automated diagnosis. Sensors 21(12)
Ryu H, Shin SY, Lee JY, et al (2021) Joint segmentation and classification of hepatic lesions in ultrasound images using deep learning. European Radiology
Kim N, Lee WJ, Lee HJ (2023) Deep learning classification of focal liver lesions with contrast-enhanced ultrasound from arterial phase recordings. In: 2023 International Conference on Electronics, Information, and Communication (ICEIC), pp 1–3
Turco S, Tiyarattanachai T, Ebrahimkheil K, et al (2022) Interpretable machine learning for characterization of focal liver lesions by contrast-enhanced ultrasound. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 69(5):1670–1681
Singal AG, Mukherjee A, Elmunzer JB, et al (2013) Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Official journal of the American College of Gastroenterology 108(11)
Brehar R, Mitrea DA, Vancea F, et al (2020) Comparison of deep-learning and conventional machine-learning methods for the automatic recognition of the hepatocellular carcinoma areas from ultrasound images. Sensors 20(11)
Jin J, Yao Z, Zhang T, et al (2021) Deep learning radiomics model accurately predicts hepatocellular carcinoma occurrence in chronic hepatitis b patients: a five-year follow-up. American journal of cancer research 11(2):576–589
Ma QP, He Xl, Li K, et al (2021) Dynamic contrast-enhanced ultrasound radiomics for hepatocellular carcinoma recurrence prediction after thermal ablation. Molecular Imaging and Biology 23(4):572–585
Mitrea D, Badea R, Mitrea P, et al (2021) Hepatocellular carcinoma automatic diagnosis within ceus and b-mode ultrasound images using advanced machine learning methods. Sensors 21(6)
Zhou H, Jiang T, Li Q, et al (2021) Us-based deep learning model for differentiating hepatocellular carcinoma (hcc) from other malignancy in cirrhotic patients. Frontiers in Oncology 11
Feng X, Cai W, Zheng R, et al (2023) Diagnosis of hepatocellular carcinoma using deep network with multi-view enhanced patterns mined in contrast-enhanced ultrasound data. Engineering Applications of Artificial Intelligence 118:105,635
Mitrea DA, Brehar R, Nedevschi S, et al (2023) Hepatocellular carcinoma recognition from ultrasound images using combinations of conventional and deep learning techniques. Sensors 23(5)
Liang X, Lin L, Cao Q, et al (2016) Recognizing focal liver lesions in ceus with dynamically trained latent structured models. IEEE Transactions on Medical Imaging 35(3):713–727
Bilic P, Christ PF, Vorontsov E, et al (2019) The liver tumor segmentation benchmark (lits). CoRR abs/1901.04056
Ginneken BV, Heimann T, Styner M (2007) 3d segmentation in the clinic: A grand challenge. In: In: MICCAI Workshop on 3D Segmentation in the Clinic: A Grand Challenge. (2007
Kavur AE, Gezer NS, Barış M, et al (2021) Chaos challenge - combined (ct-mr) healthy abdominal organ segmentation. Medical Image Analysis 69:101,950
Gibson E, Giganti F, Hu Y, et al (2018) Automatic multi-organ segmentation on abdominal ct with dense v-networks. IEEE Transactions on Medical Imaging 37(8):1822–1834
Clark K, Vendt B, Smith K, et al (2013) The cancer imaging archive (tcia): Maintaining and operating a public information repository. Journal of Digital Imaging 26(6):1045–1057
Landman B, Xu Z, Igelsias JE, et al (2015) 2015 miccai multi-atlas labeling beyond the cranial vault - workshop and challenge. MICCAI
Yan K, Wang X, Lu L, et al (2018) DeepLesion: automated mining of large-scale lesion annotations and universal lesion detection with deep learning. Journal of Medical Imaging 5(3):1 – 11
De Luca V, Banerjee J, Hallack A, et al (2018) Evaluation of 2d and 3d ultrasound tracking algorithms and impact on ultrasound-guided liver radiotherapy margins. Medical Physics 45(11):4986–5003
Marrero JA, Kulik LM, Sirlin CB, et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology 68(2):723–750
Yopp AC, Mansour JC, Beg MS, et al (2014) Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Annals of Surgical Oncology 21(4):1287–1295
Acknowledgements
We would like to express our gratitude to anonymous reviewers for providing feedback in the early drafts of this manuscript to improve its quality. The research is supported by funding from the School of Computer Science and Engineering at the University of New South Wales (UNSW), Sydney.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research is supported by funding from the School of Computer Science and Engineering at the University of New South Wales (UNSW) Sydney, Australia.
Author information
Authors and Affiliations
Contributions
S.S. wrote the main manuscript. S.H. did changes especially to the Introduction section relevant to medical facts and terminology. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Human ethics
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, S., Hoque, S., Zekry, A. et al. Radiological Diagnosis of Chronic Liver Disease and Hepatocellular Carcinoma: A Review. J Med Syst 47, 73 (2023). https://doi.org/10.1007/s10916-023-01968-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-023-01968-7