Abstract
Background
Arachnoid cysts account for about 1% of all intracranial mass lesions. Intraparenchymal arachnoid cysts are an uncommon entity. Only a few reports are available in adults with the intraparenchymal arachnoid cyst.
Case presentation
We present a 40-year-old lady with right-side progressive hemiparesis, and radiology revealed a cystic mass located in the left posterior frontal area. She underwent craniotomy and excision of the cyst. In the postoperative period, she recovered well. Immunohistochemistry stains confirm arachnoid cyst.
Conclusion
This was a case of a pure frontal arachnoid cyst with delayed clinical presentation without any communication with sub-arachnoid space or ventricle.
Similar content being viewed by others
Background
Arachnoid cysts are seen in 4% of the population [1, 2]. Intracranial arachnoid cysts usually occur adjacent to the arachnoidal cistern or cerebral fissures [3]. Primary intraparenchymal location is rare for an arachnoid cyst. We reported a primary intraparenchymal arachnoid cyst located posterior part of frontal part devoid of communication with subarachnoid space or cerebral fissures in an adult patient. Park et al. [4] described a 65-year-old lady with intraparenchymal arachnoid cyst.
Case presentation
A 40-year-old female presented with dull aching, insidious onset, and progressively increasing headache for 2 years which was accompanied by progressive weakness of right upper limb and lower limb with a facial deviation toward the right side for 1 year. She also developed slurring of speech for 3 months without any difficulty in comprehension. Gradually, she became bedbound. Upon clinical examination, she had spastic hemiparesis of the right side. She had normal hemogram and ESR. Computerized tomogram (CT) scan (head) showed hypodense lesion in the left posterior frontal area with minimal mass effect without contrast enhancement (Fig. 2a). Contrast magnetic resonance imaging (MRI) (Fig. 1a–f) revealed a well-defined lesion in left posterior frontal area, hypointense on T1W, hyper on T2W, not enhancing on contrast. There was a thin rim of cortical tissue between subarachnoid space and cyst wall. Size of the lesion was 6 cm × 4 cm × 5 cm. She underwent left frontoparietal craniotomy and total excision of the cystic lesion. The cyst was entirely intraparenchymal without communication with ventricle or cerebral subarachnoid space. Wall of the cyst was yellowish, flimsy, and glistening. Histopathology of the mass revealed a cyst with delicate fibro collagenous cyst wall lined by flat to low cuboidal arachnoidal cells of single cell thickness [Fig. 2c] which are positively stained with vimentin and epithelial membrane antigen (EMA) and are negative for glial fibrillary acidic protein (GFAP) [Fig. 2d–f]. These cells are dispersed over a collagenous cyst wall and not a glial tissue. These features confirm that the cyst is an arachnoid cyst by nature and ruled out glioependymal cyst. She was discharged with residual right-side hemiparesis. After a 1-year follow-up, her condition improved, she was able to do a daily activity, and headache subsided.
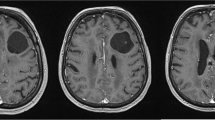
a MRI (axial) T1W shows a hypointense cystic lesion at the left posterior frontal area. b MRI (axial) T2W shows lesion was hypertense. c MRI (contrast) revealed no enhancement of the wall. d MRI (coronal) T2W shows no communication with lateral ventricle. e MRI T1W (coronal) contrast shows solitary cystic lesion without communication with subarachnoid space. f MRI T1W (sagittal) contrast shows thin rim of cerebral cortex between subarachnoid space and cyst wall
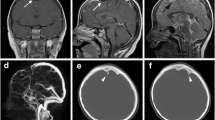
a Preoperative CT head (axial) shows hypodense lesion at the left posterior frontal area. b Post-op CT (axial) head shows excision of the cyst. c Histopathology revealed fibro collagenous cyst wall lined by flat to low cuboidal arachnoidal cells of single cell thickness suggestive of an arachnoid cyst. d Positive for vimentin. e Positive with epithelial membrane antigen (EMA) stain. f Slide was negative for glial fibrillary acidic protein (GFAP)
Discussion
Arachnoid cysts are benign congenital collections of cerebrospinal fluid. Arachnoid cysts are two types: (1) congenital cysts (true arachnoid cyst); (2) secondary arachnoid cysts [5–7]. Secondary (acquired) arachnoid cysts result from hemorrhage, trauma, and infection and usually communicate with the subarachnoid space [5]. The common locations of arachnoid cysts are the surface of the brain at the level of main brain fissures, such as sylvian (49%), interhemispheric fissures (5%), cerebral convexity (4%), vermis (9%), cerebellopontine angle (11%), suprasellar and sella turcica (9%), and supracollicular area (10%)1. Intraparenchymal location in an adult is rare; only one case was reported in English literature till now [4].
The genesis of arachnoid cyst is controversial, and it is sporadic or familial. Different hypothesis are discussed like (a) it arise due to agenesis of part of the brain, or (b) due to minor aberration in the development of arachnoid that leads to splitting or duplication of the membrane (endomeninx) followed by collection of CSF (1), or (c) defect in condensation of the mesenchyme or from abnormalities of CSF flow [3, 8, 9]. Sylvian fissure arachnoid cyst and suprasellar arachnoid cyst [10] developed due to the splitting of membrane followed by one-way CSF flow (valve mechanism) due to vascular pulsation from surrounding vessels (sylvian: MCA, suprasellar: basilar) [5].
The cell of origin in primary intraparenchymal is still unknown. It was extrapolated that it originates from cellular remnants because of embryonic malformation as it was seen in intramedullary arachnoid cysts [11]. Bayrakli et al. [12] reported genetic linkage of the intracranial arachnoid cyst and located chromosome 6q22.31-23.2.
Regarding cyst progression, three prevailing theories exist: (1) active secretion of fluid by cells in the cyst wall, (2) fluid influx due to an oncotic pressure gradient, and (3) trapping of fluid by a valve mechanism [6, 9]. Controversy exists about the content of cyst either CSF or different from CSF. Berle et al. [13] reported in the arachnoid cyst that Na-K-Cl co-transporter NKCC1 is upregulated. Hence, fluid content is slightly differing from normal CSF. Protein content is also high in cyst fluid compared to CSF [14]. Helland et al. [8] also mentioned the increased expression of NKCC1 gene and suggested NKCC1 inhibitor for prevention of cyst expansion.
However, in our case, there was no communication seen with CSF space radiological image or during surgery. So unlikely ball valve mechanism is responsible for cyst expansion. Probably, the fluid influx is the mechanism for cyst expansion as we have not done the NKCC1 expression.
The clinical manifestations of arachnoid cysts are variable and depend on location [2]. They cause neurological deficit through expansion that can compress normal neural tissue and obstruct cerebrospinal fluid flow. The most common presenting symptoms and signs are those of raised intracranial pressure, craniomegaly, and developmental delay in children [9]. Hydrocephalus has been estimated to be present in 30–60% of patients with arachnoid cysts. In our case, the patient presented with features of the intrinsic mass lesion with compression of surrounding brain parenchyma at the age of 40 years with 2 years symptoms. Park et al. [4] reported a 65-year-old lady presented at 65 years of age with a headache. Computerized tomography (CT) with contrast studies, magnetic resonance imaging (MRI), radioisotope scintigraphy, and cine phase-contrast MRI imaging are the different tools of investigation for arachnoid cyst [15] to diagnose the cyst and differentiate from other primary intraparenchymal cyst and look for communication with CSF space. Radiologically, we considered ependymal cyst, neuroenteric cyst, and dermoid/epidermoid as a differential diagnosis in our case.
Management options of arachnoid cysts are craniotomy with resection of the cyst walls, a shunting procedure, stereotactic aspiration or fenestration of the cyst cavity, and neuroendoscopic fenestration [1, 3, 9]. Park et al. [4] reported craniotomy and excision of cyst wall, and 6 months follow-up showed no recurrence. El-Ghandour et al. [16] reported a series of endoscopic management of intraparenchymal arachnoid cyst in children. In our case, we managed with craniotomy and complete excision of the cyst. No communication was seen with ventricle or subarachnoid CSF space. However, for another parenchymal cyst like an ependymal/neuroenteric cyst, the chance of recurrence is high if not removed completely.
Conclusion
The primary intraparenchymal arachnoid cyst is to be considered as a differential of the intraparenchymal cystic lesion in adult population. The prognosis is favorable after complete surgical excision.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- MCA:
-
Middle cerebral artery
References
Boutarbouch M, El Ouahabi A, Rifi L, Arkha Y, Derraz S, El Khamlichi A. Management of intracranial arachnoid cysts: institutional experience with initial 32 cases and review of the literature. Clin Neurol Neurosurg. 2008;110(1):1–7.
Pradilla G, Jallo G. Arachnoid cysts: case series and review of the literature. Neurosurg Focus. 2007;22(2):E7.
Ariai S, Koerbel A, Bornemann A, Morgala M, Tatagiba M. Cerebellopontine angle arachnoid cyst harbouring ectopic neuroglia. Pediatr Neurosurg. 2005;41(4):220–3.
Park KJ, Kang SH, Chae YS, Chung YG. Supratentorial arachnoid cyst located in the brain parenchyma: case report. Neurosurgery. 2011;68(1):E258–62.
Choi JU, Kim DS. Pathogenesis of arachnoid cyst: congenital or traumatic? Pediatr Neurosurg. 1998;29(5):260–6.
Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981;40(1):61–83.
Garcia-Conde M, Martin-Viota L. Arachnoid cysts: Embriology and pathology. Neurocirugia. 2015;26(3):137–42.
Helland CA, Aarhus M, Knappskog P, Olsson LK, Lund-Johansen M, Amiry-Moghaddam M, et al. Increased NKCC1 expression in arachnoid cysts supports secretory basis for cyst formation. Exp Neurol. 2010;224(2):424–8.
Cincu R, Agrawal A, Eiras J. Intracranial arachnoid cysts: current concepts and treatment alternatives. Clin Neurol Neurosurg. 2007;109(10):837–43.
Halani SH, Safain MG, Heilman CB. Arachnoid cyst slit valves: the mechanism for arachnoid cyst enlargement. J Neurosurg Pediatr. 2013;12(1):62–6.
Hyndman OR, Gerber WF. Spinal extradural cysts, congenital and acquired; report of cases. J Neurosurg. 1946;3(6):474–86.
Bayrakli F, Okten AI, Kartal U, Menekse G, Guzel A, Oztoprak I, et al. Intracranial arachnoid cyst family with autosomal recessive trait mapped to chromosome 6q22.31-23.2. Acta Neurochir. 2012;154(7):1287–92.
Berle M, Wester KG, Ulvik RJ, Kroksveen AC, Haaland OA, Amiry-Moghaddam M, et al. Arachnoid cysts do not contain cerebrospinal fluid: a comparative chemical analysis of arachnoid cyst fluid and cerebrospinal fluid in adults. Cerebrospinal Fluid Res. 2010;7:8.
Sandberg DI, McComb JG, Krieger MD. Chemical analysis of fluid obtained from intracranial arachnoid cysts in pediatric patients. J Neurosurg. 2005;103(5 Suppl):427–32.
Wester K. Peculiarities of intracranial arachnoid cysts: location, sidedness, and sex distribution in 126 consecutive patients. Neurosurgery. 1999;45(4):775–9.
El-Ghandour NM. Endoscopic treatment of intraparenchymal arachnoid cysts in children. J Neurosurg Pediatr. 2014;14(5):501–7.
Acknowledgements
Nil.
Funding
No funding utilized from any organization.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Contributions
SKK and KVLN helped in the conception and design. SKK, NBN, and KVLN drafted the manuscript. SKK, KVLN, NBN, and SS critically revised the manuscript. SKK, KVLN, NBN, and SS reviewed the final version of the manuscript and approved for submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethical approval was not required from the institute as it is a case report. In our institute, for case report no need to take ethical approval. An informed consent was taken from the patient to participate in the report.
Consent for publication
The inform consent was taken from the patient for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Konar, S., Rao, K.V.L.N., Nandeesh, B.N. et al. Delayed presentation of primary parenchymal arachnoid cyst in adult population: a rare location of a common cyst—case report and review of the literature. Egypt J Neurosurg 34, 16 (2019). https://doi.org/10.1186/s41984-019-0039-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-019-0039-6