Abstract
We present a rare finding of the arachnoid matter invaginating into the base of middle cranial fossa and creating an abnormal space. Presented entity was incidentally found in head CT scan of 12-year-old male. Based on the radiological characteristics in CT scans and MR images, the diagnosis of intradiploic arachnoid cyst (AC) was suggested. After surgical intervention and histopathological analysis of the specimen, the diagnosis was confirmed. We assume this is the first description of large intrasphenoid AC without any traumatic or iatrogenic cause. The literature provides many different terms for the phenomenon. We are proposing the term intradiploic arachnoid diverticulum as the more accurate for capturing the essence of the phenomenon. It provides clear differentiation of the entities from classical arachnoid cysts since they are of different anatomical localization (intradural vs. extradural) and etiopathogenesis. Management with arachnoid diverticulum is not yet established, but observation with serial imaging studies should be recommended as primary management in case of asymptomatic cyst. When cyst is symptomatic, surgical treatment may be required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachnoid cysts (ACs) are benign collections of fluid which develop inside the arachnoid mater of the cranium or vertebral column [1]. ACs represent approximately 1% of all intracranial lesions, and they are most commonly found in the middle cranial fossa in the area of Sylvian fissure [2]. When advanced radiologic tools such as computed tomography (CT) or magnetic resonance imaging (MRI) became widespread, the diagnosis of ACs started to increase [3]. There are a few possible causes of the development of ACs but no definite etiology has yet been found. While most of them are intradural, the intradiploic localization is related to the discontinuity of the dura and breaching of the arachnoid membrane [4]. Indications for surgical treatment seem to be obvious in the case of symptomatic patients, while the management of asymptomatic arachnoid cysts is still debatable. Intracystic shunt and surgical or endoscopic fenestration are most widely considered as proper interventional treatment, but intradiploic localization qualifies finding as a specific subtype and, in most cases, limits possible treatment to open surgery only.
Clinical presentation
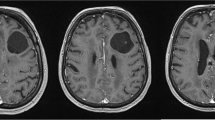
A 12-year-old male patient has been admitted to the emergency department due to a mild head injury without loss of consciousness. He has been complaining of headaches in the parietooccipital area caused by direct trauma. He did not present nausea, double vision, or any other sign or symptom. There was no medical history of previous traumatic accidents, chronic diseases, allergies, and undergone procedures, including dental. During a neurological examination, he has presented no focal deficits. Non-contrast CT has revealed no acute post-traumatic intracranial findings, such as bone fracture (neither base of the skull nor calvaria), intracranial bleeding, signs of cerebral edema, and midline shift. In the area of the floor of the middle cranial fossa on the left side, an accidental finding was made. Large, (approximate dimensions, 3.3 cm ap × 2.6 cm cor × 2.5 cm cc) hypodense lesion of cerebrospinal fluid (CSF) attenuation was visualized within a greater wing of sphenoid bone (Fig. 1).
The lesion was limited with generally dense, cortical bone wall, occasionally thin and spiculated. There was no component of associated thickening of soft tissue. The osseous wall of surrounding cranial spaces such as the sphenoid and maxillary sinus, orbit, and nasal cavity appeared intact. There was no radiologic evidence for the destruction of cranial base foramina and fissures (i.e., foramen rotundum, foramen ovale, foramen spinosum, inferior orbital fissure). MRI revealed irregular space with CSF intensity signal: low in T1W, FLAIR, high in T2W (Fig. 2).
No restriction of diffusion was described in diffusion-weighted (DW) sequence. In addition, the connection between the inside of the lesion and subarachnoid space was discovered and visualized in the region of the slight absence of the osseous floor of the middle cranial fossa. Based on the clinical status and radiological findings, the patient was qualified for surgical intervention.
Management
The pterional approach was performed—the periosteum and temporalis muscle have been cut in a typical fashion and the periosteal flap was preserved for further reconstruction. Rectangle-shaped craniotomy has been made, and the dura of the middle cranial fossa has been shown.
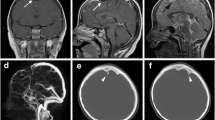
The dissection has been continued medially until visualization of the communicating channel between the dura on the floor of the cranial fossa and the intradiploic space of the greater wing of the sphenoid bone (Fig. 3).
Intraoperative view. Characteristic craniometrical points and lines such as pterion (circle), coronal suture (intermittent line), squamous suture (dotted line), and superior temporal line (continuous line) were marked on the skull (a). Pterional craniotomy was performed, and periosteal flap was preserved (b). After dissection and mobilization of the dura, intradiploic space was visualized. Cyst is marked with white arrows (c)
The superior osseous wall of the cyst has been drilled, and the arachnoid-like tissue covering the cyst inside has been removed and preserved for histopathology examination as well as the fluid content of the cyst. Subdural space has been revised and duroplasty has been performed with the use of artificial materials. Intradiploic space has been filled with periosteal flap.
The were no abnormal findings described in the post-op CT scan. During the post-operative period, no complications such as CSF leakage, wound infection, or excessive pain complaints were observed. The patient was dismissed in good general condition, with no neurological deficits.
Histopathology examination of the specimen revealed that the cyst wall consisted of the membrane of leptomeninx and dura mater cells. Based on radiological, surgical, and histopathological findings, the diagnosis of intradiploic arachnoid cyst has been made.
Discussion
Bright was the first who defined, in 1831, a group of specific intracranial findings as “serous cysts forming in connection with the arachnoid and apparently lying between its layers” based on cadaveric studies [5]. As modern radiologic tools have become more available, ACs have been widely described in the literature. Well-developed prenatal diagnostics and increased availability of CT/MR imaging are the cause of early detection of arachnoid cysts—most of them are recognized before an individual reaches 30 years of age [5]. ACs are most commonly discovered incidentally while running diagnostics of pathologies unrelated to the location and development of the arachnoid cyst and post-traumatic events [6, 7].
The characteristic symptoms associated with ACs were not revealed in a population-based study [8]; however, the most common include headache, weakness, seizures, and hydrocephalus [5]. On the other hand, headache is one of the most common complaints in the population; thus, its coexistence with arachnoid cyst may be simply accidental. This approach may also be supported by cases in which spontaneous improvement has been observed without any treatment [9]. In a group of pediatric patients with confirmed presence of ACs, the most common primary indications for radiological diagnostics were seizure, headache, developmental delay, and acute mental status change [10].
One of the most important factors that correlates with the symptomatic appearance of ACs is the localization of the lesion. A higher risk of symptomatic presentation has suprasellar region, cerebello-pontine angle, ambient cistern, or quadrigeminal cistern [9]. The infratentorial location has frequent association with other intra- or extracranial developmental abnormalities as investigated by Harrison [11], McCullough et al. [12], or Menezes et al. [13].
There are many classification systems of arachnoid cysts and currently the most widely used was proposed by Galassi et al. [14]. This system divides ACs into 3 types, with type I as the mildest and type III as the most severe [14]. One must bear in mind Galassi’s classification describes arachnoid cysts located in the middle cranial fossa only.
Considering the etiopathogenesis of these phenomena, ACs can be divided into two large groups:
-
1.
Primary—occur more often than secondary; these are caused by congenital malformation developing during gestation such as duplication or splitting of the arachnoid mater; further expansion of the cyst can occur because of 3 possible mechanisms [15]:
-
Fluid production by the cyst wall,
-
A ball-valve mechanism,
-
Osmotic gradient between the cyst and subarachnoid space.
-
-
2.
Secondary—developing as an effect of potentially harmful or infectious factors (i.e., meningitis, head injury, tumor, surgical intervention) [7].
Despite common histological features, the intraosseous or intradiploic arachnoid cyst should be considered a separate clinical issue. The key factor for such an entity to arise is the discontinuity of the inner cortical bone of the skull. It may involve any bone of the calvaria or the base of the skull [6]. Another essential feature is a rupture of the dura—it facilitates breaching out of the arachnoid membrane and expanding into the diploe [4]. It seems obligatory since we did not find any description in the literature of the intradiploic and simultaneously intradural cyst. Primary intraosseous expansion of the arachnoid matter is probably an effect of CSF entering the diploe through the preexisting dehiscences or natural foramina with the force of intracranial pressure [6]. However, the non-traumatic variety of intradiploic cyst is very rare—usually seen in the occipital region, the temporal and frontal skull base [16, 17].
The secondary ACs develop usually at the base of the trauma that causes bone fracture and tears the dura. When both the inner and outer table of the skull is fractured, invagination of the arachnoid mater into the osseous defect creates an obstacle on the way of healing the fracture. If that happens, the complication of head injury known as growing skull fracture may occur. It is usually seen in childhood and contributes to an incidence of 0.05–1.6% of pediatric skull fractures [18] (Table 1).
We assume this is the first description of large intrasphenoid AC without any traumatic or iatrogenic cause. The literature provides many different terms for the phenomenon. D’Almeida and King [19] named that intradiploic cerebrospinal fluid fistula, whereas Weinand et al. [4] were the first who used the term intradiploic arachnoid cyst in 1989. Among others, there are leptomeningeal cyst or post-traumatic arachnoid cyst [1]. As the intradiploic arachnoid membrane can be compared to a pouch opening from one space to another, we are proposing the term intradiploic arachnoid diverticulum as the more accurate for capturing the essence of the phenomenon. It provides clear differentiation of the entities from classical arachnoid cysts since they are of different anatomical localization (intradural vs. extradural) and etiopathogenesis. Our comparison of all those findings is presented in Table 2.
CT scans followed by MRI give quite obvious radiologic visualization of the arachnoid cysts and diverticula. However, during the management of AC-like lesions, one should consider dermoid or epidermoid cyst, mucocele, aneurysmal bone cyst, plasmacytoma, metastasis, or cystic fibrous dysplasia [1, 4]. Sharma et al. [20] in their report of a giant intradiploic cyst of occipital bone advised exploratory surgery, which was not performed because of patient’s refusal. In our case, the decision of surgical treatment was made based on the radiological appearance. The uncommon localization of the feature and bone destruction around it led the authors to decide about the surgery. The lack of appropriate recommendations and descriptions of the management of such entities in the literature is unsatisfactory. Our present experience gives us a new perspective on the treatment of asymptomatic arachnoid cysts, and we would like to recommend observation with serial imaging studies, for example, with non-enhanced MRI after 6–12 months.
Conclusions
-
ACs may be asymptomatic and usually they are discovered incidentally while running diagnostics after head trauma.
-
Non-contrast CT scans followed by basic sequences of MR imaging are often enough in terms of differential diagnosis.
-
Surgical treatment may be necessary in case of symptomatic ACs.
-
We propose a new term—intradiploic arachnoid diverticulum—for intraosseous localization of arachnoid entities.
-
Treatment of arachnoid diverticulum is not yet established, but observation with serial imaging studies should be recommended as primary management in case of asymptomatic cyst.
Availability of data and material
Please contact authors for data requests (Adrian Drożdż MD—email: adrian.drozdz@wum.edu.pl). The data belong to the Department of Pediatric Neurosurgery, Bogdanowicz Memorial Hospital for Children, and are not available to share unless in the form included in the manuscript.
References
Kumar Lahiri A, Chilvers G (2018) Nontraumatic intradiploic arachnoid cyst of the sphenoid bone. Radiol Case Rep 8;13(3):576–579. https://doi.org/10.1016/j.radcr.2018.02.011
Samii M, Carvalho GA, Schuhmann MU, Matthies C (1999) Arachnoid cysts of the posterior fossa. Surg Neurol 51(4):376–382. https://doi.org/10.1016/s0090-3019(98)00095-0
Kandenwein JA, Richter HP, Börm W (2004) Surgical therapy of symptomatic arachnoid cysts - an outcome analysis. Acta Neurochir (Wien) 146(12):1317–22; discussion 1322. https://doi.org/10.1007/s00701-004-0359-4
Weinand ME, Rengachary SS, McGregor DH, Watanabe I (1989) Intradiploic arachnoid cysts. Report of two cases J Neurosurg 70(6):954–958. https://doi.org/10.3171/jns.1989.70.6.0954
Pradilla G, Jallo G (2007) Arachnoid cysts: case series and review of the literature. Neurosurg Focus 22:E7. https://doi.org/10.3171/foc.2007.22.2.7
Adrien J, Verillaud B, Bresson D, Tran H, Kania R, Sauvaget E, Guichard JP, Herman P (2015) Petrous and sphenoid arachnoid cysts: diagnosis and management. Head Neck 37(6):823–828. https://doi.org/10.1002/hed.23677
Koutsimpelas D, Mueller-Forell W, Stoeter P, Hey O, Mann WJ (2010) Arachnoid cyst with extraordinary extracranial extension in the skull base as a result of an iatrogenic defect of the middle cranial fossa floor: case report and literature review. J Craniomaxillofac Surg 38(4):284–287. https://doi.org/10.1016/j.jcms.2009.03.005
Rabiei K, Jaraj D, Marlow T, Jensen C, Skoog I, Wikkelsø C (2016) Prevalence and symptoms of intracranial arachnoid cysts: a population-based study. J Neurol 263(4):689–694. https://doi.org/10.1007/s00415-016-8035-1
Al-Holou WN, Terman S, Kilburg C, Garton HJL, Muraszko KM, Maher CO (2013) Prevalence and natural history of arachnoid cysts in adults: clinical article. J Neurosurg 118(2):222–231. https://doi.org/10.3171/2012.10.JNS12548
Al-Holou WN, Yew AY, Boomsaad ZE, Garton HJL, Muraszko KM, Maher CO (2010) Prevalence and natural history of arachnoid cysts in children: clinical article. J Neurosurg Pediatr 5(6):578–585. https://doi.org/10.3171/2010.2.PEDS09464
Harrison MJ (1971) Cerebral arachnoid cysts in children. J Neurol Neurosurg Psychiatry 34(3):316–323. https://doi.org/10.1136/jnnp.34.3.316
McCullough DC, Harbert JC, Manz HJ (1980) Large arachnoid cysts at the cranial base. Neurosurgery 6(1):76–81. https://doi.org/10.1227/00006123-198001000-00011
Menezes AH, Bell WE, Perret GE (1980) Arachnoid cysts in children. Arch Neurol 37(3):168–172. https://doi.org/10.1001/archneur.1980.00500520066012
Galassi E, Tognetti F, Gaist G, Fagioli L, Frank F, Frank G (1982) CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol 17(5):363–369. https://doi.org/10.1016/0090-3019(82)90315-9
Isaacson B, Coker NJ, Vrabec JT, Yoshor D, Oghalai JS (2006) Invasive cerebrospinal fluid cysts and cephaloceles of the petrous apex. Otol Neurotol 27(8):1131–1141. https://doi.org/10.1097/01.mao.0000244353.26954.71
Açikgöz B, Tekkök IH (2002) Post-traumatic intradiploic leptomeningeal fistula and cyst. J Clin Neurosci 9(4):468–473. https://doi.org/10.1054/jocn.2002.1066
Granata F, Alafaci C, Passalacqua M, Cacciola F, Caffo M, Salpietro FM, Vinci SL, Longo M (2011) Non-traumatic sphenoidal intradiploic arachnoid cyst as a cause of trigeminal neuralgia. A case report. Neuroradiol J 30;24(6):867–71. https://doi.org/10.1177/197140091102400606
Jaiswal M, Gandhi A, Sharma A, Mittal RS (2015) Occipital post-traumatic intradiploic arachnoid cyst converted to pseudomeningocele after re-trauma: a rare complication of rare pathology. Pediatr Neurosurg 50(1):53–55. https://doi.org/10.1159/000369936
D’Almeida AC, King RB (1981) Intradiploic cerebrospinal fluid fistula. Report of two cases J Neurosurg 54(1):84–88. https://doi.org/10.3171/jns.1981.54.1.0084
Sharma R, Gupta P, Mahajan M, Sharma P, Gupta A, Khurana A (2016) Giant nontraumatic intradiploic arachnoid cyst in a young male. Radiol Bras 49(5):337–339. https://doi.org/10.1590/0100-3984.2013.0022
Author information
Authors and Affiliations
Contributions
A. Drożdż: conceptualization; methodology; formal analysis and investigation; writing (lead)—original draft preparation; review and editing. T. Wojciechowski: formal analysis and investigation; writing (support)—original draft preparation; review and editing. B. Ciszek: conceptualization; formal analysis and investigation; writing—review and editing. Z. Stopa: resources; writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study abides by the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The authors declare that this manuscript has not been published elsewhere and is not under consideration by another journal.
The authors declare that the study complies with the current law in Poland.
Consent to participate
Informed consent was obtained from legal guardian.
Consent for publication
Legal guardian signed informed consent regarding publishing clinical data and intraoperative photographs.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Drożdż, A., Wojciechowski, T., Ciszek, B. et al. Large intradiploic arachnoid cyst of the skull in child—a case report and new terminology proposition. Childs Nerv Syst 40, 1271–1276 (2024). https://doi.org/10.1007/s00381-023-06255-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06255-x