Abstract
Background
Poplar fungal infections are difficult to control and result in severe economic loss. As a viable alternative to chemical pesticides, biocontrol is an effective safe method for disease control.
Results
Inhibitory activity of Bacillus velezensis 33RB and Aspergillus niger 46SF was evaluated against numerous phytopathogens. The bacterial strain displayed the highest inhibitory activity toward Colletotrichum gloeosporioides BJ02 and Fusarium oxysporum 20RF (61.2 and 49.4%, respectively). Also, the maximum inhibitory activity of A. niger 46SF was exhibited (75.51 and 70.83%) against C. gloeosporioides BJ02 and F. oxysporum 20RF, respectively. The minimum volume (6.25 ml) of sterilized cultural filtrate of bacterial and fungal strains significantly inhibited the growth of C. gloeosporioides BJ02 by 73.3 and 83.3%, respectively, and F. oxysporum 20RF reached 40.4 and 78.8%, respectively. B. velezensis 33RB and A. niger 46SF displayed the highest inhibition toward C. gloeosporioides BJ02 and F. oxysporum 20RF at neutral pH and pH 5, respectively. Moreover, the highest inhibitory activity of B. velezensis 33RB and A. niger 46SF was achieved at 37 °C and 28 °C, respectively. Pathogenicity tests on sterilized detached leaves indicated that these isolates could potentially affect anthracnose and fusarium wilt diseases. Several secondary bioactive metabolites that assured the biocontrol efficacy of tested microbes were detected by liquid chromatography–mass spectrometry (LC–MS). The most detectable compounds included organic acids such as fumaric, DL-malic, citric, isobutyric, and glutamic acids. Also, numerous fatty acids such as lauric, linoleic, oleic, stearic, and myristic acids with diverse biological functions, including antimicrobial properties, were determined.
Conclusions
Bacillus velezensis 33RB and A. niger 46SF were potential alternatives to chemical pesticides as biological control agents for the phytopathogens C. gloeosporioides BJ02 and F. oxysporum with environmentally friendly and sustainable properties.
Similar content being viewed by others
Background
The fast-growing Chinese white poplar trees (Populus tomentosa Carr.) offer significant ecological and economic benefits and high-quality wood for bioenergy production (Du et al. 2012). These trees are affected by severe diseases. Poplar anthracnose is caused by the fungus, Colletotrichum gloeosporioides, which infects the leaves and frequently causes premature defoliation (Huang et al. 2020). Fusarium spp. have also been linked to reddish-purple staining, necrosis, swelling, and the development of distinct cankers in poplar (Kwaśna et al. 2017). Chemical agents are used to treat these diseases; however, they pollute the ground and soil water reservoirs and cause an undesirable accumulation of chemical residues in the food chain (Barák 2017). Studies have focused on circumventing these consequences by reducing or replacing the use of chemical pesticides.
For these issues, beneficial microbes have been increasingly used as biological control agents. Endophytic bacteria can substitute chemical fertilizers and pesticides and increase plant output and resistance to biotic and abiotic stress (Shah et al. 2021). Their advantages include their ability to colonize internal plant tissues (Fadiji and Babalola 2020), control plant disease (Zhang et al. 2015), enhance crop growth (Faria et al. 2013) and resistance (Pavlo et al. 2011), and promote organic pollutant degradation (Zhang et al. 2014).
Antagonistic strains can control phytopathogenic fungi via mycoparasitism, competition, generation of antibiotics, and stimulation of defense responses (Jinal and Amaresan 2020). Endophytes produce many bioactive metabolites such as alkaloids, phenolic acids, steroids, quinones, saponins, terpenoids, and tannins, making them promising anticancer, antituberculosis, antimalarial, antiviral, anti-inflammatory, and antidiabetic agents. These bioactive substances also make the host plants resistant to biotic and abiotic stress (Fadiji and Babalola 2020).
Pageni et al. (2014) indicated that biocontrol bacterial strains such as Bacillus, Aureobacterium, Paenibacillus, Pseudomonas, Phyllobacterium, and Burkholderia produce various metabolic compounds such as lytic enzymes and antibiotics, which inhibit the growth of fungal phytopathogens, including Fusarium oxysporum, Sclerotium rolfsii, Rhizoctonia solani, and Verticillium dahliae. Bacillus species were frequently instead of chemical pesticides (Kamali et al. 2022). Additionally, fungal endophytes can protect the host plant from disease by reducing the harmful effect of phytopathogens (Ganley et al. 2008). It was reported that Aspergillus species can synthesize volatile and/or nonvolatile metabolites against numerous pathogenic fungi, including Pythium spp, Sclerotinia sclerotiorum, and F. oxysporum (Bhattacharyya and Jha 2012).
Recently developed techniques facilitated the investigation of plant growth-promoting rhizobacteria (PGPR) and endophytes for biocontrol applications (Sellami et al. 2021). The genomes of PGPR or endophytes were thoroughly studied using metagenomics, while their secretomes were extensively analyzed using LC–MS technology. These studies clarified the role of PGPR and endophytes as biocontrol agents against plant infections (Bailly and Weisskopf 2017).
The present study aimed to delineate the biocontrol activities of the endophytes against various plant pathogens, including F. oxysporum20FR and C. gloeosporioides BJ02, and identify several secondary antifungal metabolites using LC–MS spectroscopy.
Methods
An overview of the work done in this study is depicted in Fig. 1.
Endophytic microbes
Five bacterial strains, namely Enterobacter tabaci 24RB (MN540932.1), Bacillus velezensis 33RB (MN559965.1), Bacillus megaterium 59SB (MN540915.1), Pantoea eucrina 85LB (MN541091.1), and Bacillus aryabhattai 88LB (MN540958.1), and six fungal strains, Lasiosphaeriaceae sp. 17 RF (MN541090.1), Chaetomium globosum 37 RF (MN541117.1), Aspergillus niger 46SF (MN540962.1), Peyronellaea sp. 48SF (MN540968.1), Talaromyces amestolkiae 52SF (MN540956.1), and Alternaria sp. 63LF (MN541096.1), were isolated from different parts (roots, stems, and leaves) of Populus tomentosa (Sehim and Dawwam 2022).
Phytopathogens
Fusarium oxysporum 20RF, F. solani 15RF, C. gloeosporioides BJ02, and C. fructicola were used as the representative phytopathogens. The Colletotrichum isolates were kindly provided by the Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants at Beijing Forestry University, Beijing, China, while F. oxysporum 20RF and F. solani15RF were isolated from the roots of infected Populus tomentosa.
Isolation of Fusarium isolates
The infected root tissues of P. tomentosa were obtained from the greenhouse of Beijing Forestry University, Beijing, China (40° 0′ N, 116° 20′ E). Root parts were collected and rinsed under running tap water to remove the soil. Then, they were cut into 5–10 mm segments, surface sterilized using 1% sodium hypochlorite solution for 1–2 min, rinsed thrice using sterile distilled water, and dried on a sterile filter paper. The sterilized tissue segments were placed on potato dextrose agar (PDA) plates supplemented with 50 mg/l streptomycin sulfate. The plates were incubated at 28 °C for 5 days for fungal mycelial growth. Mycelial disks were excised from the growing point, subcultured on PDA slants, incubated at 28 °C for 5 days, and stored at 4 °C until further use (Yamauchi et al. 2004).
Molecular identification
The fungal DNA was extracted using the EZgene™ Fungal DNA Miniprep kit, according to the manufacturer’s instructions. The fungal ITS region was amplified using forward ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and reverse ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers (White et al. 1990). The PCR cycle included preheating for 5 min at 94 °C, followed by 35 cycles of 1 min at 94 °C, annealing at 55 °C for 40 s, extension at 72 °C for 1 min, and a final extension of 72 °C for 10 min. The size of the PCR products was checked using 1% agarose gel and sequenced on the ABI3730XL DNA Analyzer at the Beijing Ruibio Biotech, Co., Ltd.
In vitro assessment of endophytic microbes against several fungal phytopathogens
An antagonistic test to study the effect of endophytic bacterial and fungal isolates against several growth-inhibiting fungal pathogens was performed. The biocontrol strains were tested preliminarily using the dual culture method (Huang et al. 2015). The endophytic bacteria and fungi were placed at one end of the PDA plate (d = 90 mm), while the pathogenic fungi were placed on the other end, approximately 3 cm away. The plates were cultured at 28 °C for seven days. The control plates were inoculated only with the pathogens. Each treatment was performed in triplicate. The pathogens and control colony diameters were measured after incubation (Boukaew and Prasertsan 2014). The inhibition rate was determined using the formula below:
B. velezensis 33RB and A. niger 46SF against C. gloeosporioides and F. oxysporum were selected for further experiments as they displayed the highest growth inhibitory activity.
Optimizing cultural filtrate
Cultural filtrate method according to the method described by Dennis and Webster (1971) was performed. The bacterial and fungal isolates were inoculated separately for 48 h and 14 days, respectively, in liquid PD medium at 28 °C and 120 rpm. After incubation, the fermentation broth was centrifuged at 10,000 rpm at room temperature for 2 min to remove the remaining bacterial cells and mycelial fragments from the supernatant. The supernatant was transferred into fresh tubes and then filtered twice using sterilized filter paper, followed by 0.45 µm pore membrane filters. Four volumes (50, 25, 12.5, and 6.25 ml) of bacterial and fungal supernatants were mixed thoroughly with molten PDA medium to reach a final concentration of 100% (v/v). The filtrates were poured into Petri plates, and after solidification, a 5-mm disk of test pathogens was placed at the center of the plates. All the treatments were conducted in triplicates and incubated at 28 °C for seven days. The growth inhibition percentage was measured using the equation mentioned above.
Effect of cultural filtrate pH on the growth inhibition
The selected endophytic bacterial and fungal isolates were separately grown on PDA and distributed into media adjusted to five different pH values (3, 5, 7, 9, and 11). Sterilized cultural filtrate (12.5 ml) was added to an appropriate amount of molten PDA till a final concentration of 100% (v/v). After pouring into Petri dishes, it was inoculated with 5-mm mycelial disks containing a 5-day-old colony of test pathogens. After incubation at 28 °C for seven days, the colony diameter of the test pathogens was measured to assess the growth inhibition rate compared to control. This test was done in triplicate.
Effect of cultural filtrate temperature on the growth inhibition
To investigate the effect of temperature on fungal growth, the sterilized cultural filtrate was exposed to 4, 28, 37, 60, 80, and 100 °C for 30 min. After cooling the samples at room temperature, they were tested using the same procedure as the pH test. The formula for relative inhibition rate was described above.
Evaluation of biocontrol efficiency using detached leaves
To evaluate the biocontrol efficacy of bacterial and fungal isolates, sterilized leaves of P. tomentosa without any apparent disease symptoms were added into Petri dishes containing a wet filter paper. The petioles of the leaves were wrapped with sterilized pledges and soaked in sterile distilled water for 5 s. These leaves were inoculated with 10 μl of spore suspension with the different test pathogens (1 × 106 spore/ml). After 24 h, all the punctured locations on the leaves were injected with the same volume of biocontrol bacterial and fungal isolates. The positive control was treated with the pathogen only. The leaves were kept at 25 °C in the dark. After 14 days, the lesion diameters were observed and compared.
Identification of antimicrobial secondary metabolites using liquid chromatography–mass spectroscopy (LC–MS spectroscopy)
Metabolites preparation and extraction
The metabolites were extracted with partitioning with ethyl acetate according to the instructions by Novogene, Beijing, China. Then, the bacterial and fungal supernatants (100 μl) were combined with 400 μl of 80% methanol and 0.1% formic acid and vortexed. After centrifuging at 15,000 g, the samples were incubated at 4 °C for 5 min on ice. Then, some of the supernatants were diluted to a final concentration with 53% methanol using LC–MS grade water. The samples were subsequently transferred to fresh Eppendorf tubes and centrifuged at 15,000 g at 4 °C for 10 min. Finally, the supernatant was injected into the LC–MS/MS system for analysis.
UHPLC–MS/MS analysis
LC–MS/MS analyses were conducted using a Vanquish UHPLC system (Thermo Fisher), Beijing, China, coupled with an Orbitrap Q Exactive series mass spectrometer (Thermo Fisher). The samples were injected onto a Hyperil Gold column (100 × 2.1 mm, 1.9 μm) using a 16-min linear gradient at a flow rate of 0.2 ml/min. The eluents for the positive polarity mode were eluent A (0.1% formic acid in water) and eluent B (methanol). The eluents for the negative polarity mode were eluent A (5 mM ammonium acetate, pH 9) and eluent B (methanol). The solvent gradient was set as follows: 2% B, 1.5 min; 2–100% B, 12 min; 100% B, 14 min; 100–2% B, 14.1 min; 2% B, 17 min. The Q Exactive series mass spectrometer was operated in positive/negative polarity mode with a spray voltage of 3.2 kV, capillary temperature of 320 °C, sheath gas flow rate of 35 arb, and aux gas flow rate of 10 arb. KEGG database (http://www.genome.jp/kegg/) and LIPID MAPS database (http://www.lipidmaps.org/) were used to annotate the metabolites.
Data analysis
The data were statistically analyzed using R software (version 3.6.1) (Team 2016).
Results
Isolation and molecular identification of Fusarium isolates
Fusarium oxysporum 20RF and F. solani 15RF were isolated from the infected P. tomentosa root tissues and confirmed using molecular identification. They were submitted in the GenBank database under accession numbers (ON557730 and ON557719), respectively.
Antifungal activities against phytopathogens
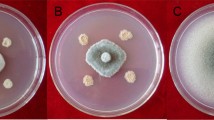
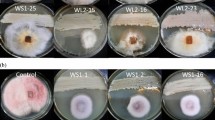
Antifungal activity of several plant growth-promoting microbes against phytopathogens such as F. oxysporum 20RF, C. gloeosporioidesBJ02, F. solani15RF, and C. fructicola using the dual culture method was assessed. The growth inhibitory effect of the endophytes against the pathogenic fungi is shown in Table 1 and Figs. 2, 3. B. velezensis33RB displayed the highest inhibitory activity toward C. gloeosporioidesBJ02 and F. oxysporum20RF (61.2 and 49.4%, respectively). However, A. niger46SF showed the strongest antagonistic effect against all the tested pathogenic fungi compared to the other selected isolates. The maximum inhibitory activities (75.51 and 70.83%) were exhibited against C. gloeosporioidesBJ02 and F. oxysporum 20RF, respectively.
Parameters affecting growth inhibition
Cultural filtrate
The sterilized B. velezensis33RB and A. niger46SF cultural filtrate significantly inhibited the growth of C. gloeosporioidesBJ02 and F. oxysporum20RF (Fig. 4A, D). With the increase in the volume of the sterilized cultural filtrate, the inhibitory activity increased. At minimum volume (6.25 ml), the relative inhibitory rates exhibited by B. velezensis33RB and A. niger46SF against C. gloeosporioides BJ02 and F. oxysporum 20RF were (73.3 and 40.4%) and (83.3 and 78.8%), respectively. Clearly, B. velezensis33RB and A. niger46SF strongly antagonized these phytopathogens.
Different key parameters affect the growth of phytopathogens. A Different cultural filtrate volumes, B pH values, and C different temperatures of Bacillus velezensis33RB suspension affect the growth of phytopathogens. D–F Different cultural filtrate concentrations, pH values, and different temperatures of Aspergillus niger 46SF suspension affect the growth of phytopathogens
Effect of pH
The sterilized cultural filtrate was tested under different pH values. Under strongly acidic, neutral, and weakly alkaline conditions, the effect of the sterilized B. velezensis 33RB and A. niger 46 SF cultural filtrates against C. gloeosporioidesBJ02 and F. oxysporum20RF were significantly different. At neutral pH, B. velezensis 33RB showed the highest inhibitions against C. gloeosporioides BJ02 and F. oxysporum20RF (86.6 and 59.4%, respectively). The maximum growth inhibitions were exhibited by A. niger46 SF against C. gloeosporioides BJ02 and F. oxysporum 20RF (85.7 and 81.2%, respectively) at pH 5. The inhibitory capacity decreased in strongly acidic and weakly alkaline conditions (Fig. 4B, E).
Effect of temperature
The B. velezensis33RB filtrate was thermally stable, as shown by the increase in the inhibition rate to over 65% when the filtrate temperature was increased from 4 to 80 °C. The inhibition rate declined at temperatures above 60 °C (Fig. 4C). B. velezensis33RB showed the highest inhibitions at 37 °C (72.2 and 87.2%) against F. oxysporum 20RF and C. gloeosporioides BJ02, respectively. While A. niger 46SF displayed the highest inhibition (85.18 and 80.78%) against C. gloeosporioides BJ02 and F. oxysporum 20RF, respectively, at 28 °C, its inhibitory activity decreased at temperatures above 28 °C (Fig. 4F).
Biocontrol efficiency on isolated leaves
As shown in Fig. 5, the disease control efficacy of B. velezensis 33RB and A. niger46 SF against anthracnose and fusarium wilt diseases. The lesions on the leaves treated with B. velezensis 33RB and A. niger46SF spore suspensions were smaller than that in control leaves, indicating that B. velezensis 33RB and A. niger46SF displayed significant preventive effects.
Photo of biocontrol efficacy of Bacillus velezensis 33RB and Aspergillus niger 46 SF against Colletotrichum gloeosporioides BJ02 and Fusarium oxysporum20RF. A Spore suspension of C. gloeosporioides BJ02 was applied firstly then sterilized cultural filtrate of B. velezensis 33RB, A. niger 46SF was applied to the same place. B Spore suspension of F. oxysporium 20RF was applied firstly then sterilized cultural filtrate of B. velezensis 33RB, A. niger 46SF was applied to the same place
LC–MS spectroscopy analysis of secondary metabolites in the culture extract of B. velezensis 33RB and A. niger 46SF
The presence of different antimicrobial metabolites using LC–MS spectroscopy was investigated. Thirty-three bioactive metabolites were found in the B. velezensis33RB culture extract using the negative ion mode (Additional file 1: Table S1). In the A. niger46SF culture extract, 36 metabolites were detected in the negative mode (Additional file 1: Table S2). Data showed that number of organic acids was predominant in the cultural extract for both antagonistic microbes. The most predominant acids were citric, valeric, fumaric, phosphoric, succinic, malic, valeric and tartaric acids. Also, numerous fatty acids such as linoleic, caprylic, nonanoic, decanoic, pentadecanoic, stearic, and myristic acids were detected in both microbial extracts.
The features of the B. velezensis 33RB and A. niger 46SF metabolites were also evaluated using KEGG pathway analysis (Additional file 1: Fig. S1). This revealed that the metabolites belonged to the amino acid, carbohydrate, energy, lipid, cofactors, vitamins, terpenoids and polyketides, xenobiotics biodegradation, membrane transport, and signal transduction pathways. The lipid map indicated that most metabolites were fatty acids and conjugates [FA01]. Eicosanoids [FA03], bile acids and derivatives [ST04], steroid conjugates [ST05], fatty alcohols [FA05], fatty amides [FA08], and octadecanoids [FA02] are also present in Additional file 1: Fig. S2. Thus, several bioactive secondary metabolites were found in B. velezensis 33RB and A. niger 46 SF culture filtrates, which might enable their antifungal activity.
Discussion
Plant fungal infections are hard to control and, hence, cause significant economic loss. Therefore, management approaches against plant fungal infection, particularly using biological control, are required (Sellem et al. 2017). A dual culture test as a representative test for screening biological control agents was performed. Endophytes with plant growth-promoting and antibacterial properties are potentially beneficial. Both endophytic microbes (B. velezensis 33RB and A. niger 46 SF) demonstrated potent biocontrol efficacy suggesting that these isolates can suppress the proliferation of phytopathogens, as they significantly inhibited at least three investigated microbial pathogens. Jinfeng et al. (2017) explored the antibacterial activity of endophytes and observed that their crude filtrate inhibited microbial growth. Our findings implied that bacterial and fungal endophytes could act as biocontrol agents by inhibiting harmful pathogens. Consistently, Huang et al. (2020) observed that out of the 15 bacterial strains, B. subtilis ZSH-1 displayed the highest antifungal activity against C. gloeosporioides (at a distance of 10 mm from the disk). Additionally, Tiwari et al. (2011) demonstrated that A. niger suppressed the growth of Trigonospila cingulata and Stereum hirsutum by 66.29 and 59.26%, respectively.
The cultural filtrate technique was used to assess the in vitro inhibitory effect of the secondary metabolites against mycopathogenic growth. Previous studies have shown that the Bacillus and Aspergillus cultural filtrates have several properties. Rong et al. (2019) found that the B. safensis B21 cultural filtrate displayed antifungal activity against Magnaporthe oryzae, which causes rice blast disease. Also, Li et al. (2018) confirmed that the sterilized B. tequilensis GYLH001 cultural filtrate significantly inhibited the growth of M. oryzae. Furthermore, Huang et al. (2020) showed that the sterilized B. subtilis ZSH-1 cultural filtrate displayed high antifungal activities against seven fungal phytopathogens (C. gloeosporioides, A. tenuissima, F. oxysporum, C. chrysosperma, Mucor sp., B. dothidea, and Absidia sp.) with inhibition rates ranging between 44 and 89.1%. Obtained results showed that the sterilized cultural filtrate could effectively inhibit the investigated pathogens at the lowest volume (6.25 ml). Similarly, Idan et al. (2017) found that a low concentration of secondary metabolites (12.5%) of a 14-day-old A. niger culture optimally inhibited P. oryza growth.
Effect of different temperature and pH ranges was evaluated to understand the physicochemical parameters for microbial growth. pH variations affected the production of secondary metabolites and antimicrobials, probably because the concentration of hydrogen ions might directly impact the cell behavior or might act indirectly due to the different dissociation levels of various components in the growth medium (Li et al. 2018). Resultantly, pH changes significantly affected the enzyme activities of microorganisms, and the generation, dissociation, and solubility of intermediate compounds (Kumar et al. 2003). Additionally, the incubation temperature is a physical component that might variably affect the microorganisms’ growth and generation of secondary metabolites (Jain et al. 2011).
The present results revealed that B. velezensis 33RB exhibited the highest inhibitions against C. gloeosporioidesBJ02 and F. oxysporum 20RF at neutral pH and was also thermally stable. Consistently, Li et al. (2018) found that the sterilized cultural filtrate of B. tequilensis GYLH001 showed the strongest inhibition against M. oryzae at pH 7 and was thermally stable. The inhibition rate reached 60%, when the filtrate’s temperature ranged between 4 and 80 °C. Beyond 60 °C, the inhibition rate decreased with an increase in temperature. The results revealed that A. niger46SF exhibited the maximum growth inhibitions against C. gloeosporioides BJ02 and F. oxysporum20RF at pH 5 and 28 °C. Similarly, Idan et al. (2017) observed that pH 5 was ideal for the production of antimicrobials by A. niger.
Poplar anthracnose is mainly treated using fungicidal treatments (Song et al. 2016). Numerous Bacillus species have been employed as biocontrol agents against C. gloeosporioides (Guerrero-Barajas et al. 2020). Bacterial secondary metabolites are organic, low-molecular-weight chemicals that suppress other microbes (Thomashow 2002). LC–MS analysis showed that the secondary metabolites produced by B. velezensis 33RB and A. niger 46SF isolates possessed several antimicrobial, herbicidal and insecticidal activities. Numerous bacterial genera, including Bacillus, Agrobacterium, Pantoea, Serratia, Pseudomonas, Streptomyces, and Stenotrophomonas, have been shown to produce antimicrobial metabolites with broad-spectrum activities (Ongena and Jacques 2008). Moreover, A. niger is a significant source of secondary metabolites (Al-Shaibani et al. 2013).
The metabolites detected in the present study were mostly organic acids such as fumaric, DL-malic, citric, isobutyric, 2-keto-glutamic, levulinic, succinic, malonic, and aspartic acids. All these acids reduced the pH and suppressed microbial growth (Eklund 1989). The hydrophobic and undissociated forms of the acid caused cell membrane diffusion and detachment from the cell, resulting in the release of hydrogen carbon ions and acidification of the cytoplasm (Piard and Desmazeaud 1991). Russell (1991) found that many types of fatty acids have antifungal and antibacterial characteristics. Several fatty acids, including lauric, linoleic, stearic, oleic, and myristic acids, which have been previously reported to have antibacterial and antifungal properties, were detected (Seidel and Taylor 2004). Hence, the presence of fatty acids might have contributed to the reduction of F. oxysporum20RF and C. gloeosporioidesBJ02 mycelial growth.
Conclusion
The results revealed the antifungal potential of the endophytic isolates B. velezensis33RB and A. niger 46SF, which showed antagonistic activity against taxonomically diverse fungal pathogens, especially Colletotrichum and Fusarium genera that infect Populus tomentosa. Different antimicrobial metabolites with diverse biological functions were determined using LC–MS spectroscopy. The most detectable compounds included organic acids such as fumaric, DL-malic, isobutyric, and glutamic acids. Also, numerous fatty acids such as lauric, linoleic, oleic, stearic, and myristic acids were determined. In summary, B. velezensis33RB and A. niger 46SF were promising biological control agents for anthracnose and fusarium wilt diseases management affecting P. tomentosa.
Availability of data and materials
The sequencing data generated and analyzed in this study are available in the NCBI Sequence Read Archive database (https://www.ncbi.nlm.nih.gov/), Accession Number: ON557719 and ON557730.
References
Al-Shaibani A, Shakarchi F, Ameen R (2013) Extraction and characterization of antibacterial compound from Aspergillus niger. J Al-Nahrain Univ Sci 16:167–174. https://doi.org/10.22401/JNUS.16.4.20
Bailly A, Weisskopf L (2017) Mining the volatilomes of plant-associated microbiota for new biocontrol solutions. Front Microbiol 8:1638
Barák I (2017) Spores and spore formers. Front Microbiol 8:1046
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Boukaew S, Prasertsan P (2014) Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot 61:1–10
Dennis C, Webster J (1971) Antagonistic properties of species-groups of Trichoderma: III. Hyphal interaction. Trans Br Mycol Soc 57:363-IN2
Du Q-Z, Zhang D-Q, Li B-L (2012) Development of 15 novel microsatellite markers from cellulose synthase genes in Populus tomentosa (Salicaceae). Am J Bot 99:e46–e48
Eklund T (1989) Organic acids and esters. Mech Action Food Preserv Proced 1:161–200
Fadiji AE, Babalola OO (2020) Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol 8:467
Faria DC, Dias ACF, Melo IS, de Carvalho Costa FE (2013) Endophytic bacteria isolated from orchid and their potential to promote plant growth. World J Microbiol Biotechnol 29:217–221
Ganley RJ, Sniezko RA, Newcombe G (2008) Endophyte-mediated resistance against white pine blister rust in Pinus monticola. For Ecol Manag 255:2751–2760
Guerrero-Barajas C, Constantino-Salinas EA, Amora-Lazcano E et al (2020) Bacillus mycoides A1 and Bacillus tequilensis A3 inhibit the growth of a member of the phytopathogen Colletotrichum gloeosporioides species complex in avocado. J Sci Food Agric 100:4049–4056
Huang H, Wu Z, Tian C et al (2015) Identification and characterization of the endophytic bacterium Bacillus atrophaeus XW2, antagonistic towards Colletotrichum gloeosporioides. Ann Microbiol 65:1361–1371
Huang H, Tian C, Huang Y, Huang H (2020) Biological control of poplar anthracnose caused by Colletotrichum gloeosporioides (Penz.) Penz. \& Sacc. Egypt J Biol Pest Control 30:1–9
Idan AA, Sijam K, Kadir J et al (2017) Biological control of Pyricularia oryzae using antifungal compounds produced by Aspergillus niger. Am J Plant Sci 8:2445
Jain P, Pundir RK et al (2011) Effect of fermentation medium, pH and temperature variations on antibacterial soil fungal metabolite production. J Agric Technol 7:247–269
Jinal NH, Amaresan N (2020) Evaluation of biocontrol Bacillus species on plant growth promotion and systemic-induced resistant potential against bacterial and fungal wilt-causing pathogens. Arch Microbiol 202:1785–1794
Jinfeng EC, Mohamad Rafi MI, Chai Hoon K et al (2017) Analysis of chemical constituents, antimicrobial and anticancer activities of dichloromethane extracts of Sordariomycetes sp. endophytic fungi isolated from Strobilanthes crispus. World J Microbiol Biotechnol 33:1–19
Kamali M, Guo D, Naeimi S, Ahmadi J (2022) Perception of biocontrol potential of Bacillus inaquosorum KR2-7 against tomato Fusarium wilt through merging genome mining with chemical analysis. Biology (basel) 11:137
Kumar DJ, Jain VK, Shanker G, Srivastava AK (2003) Citric acid production by solid state fermentation using sugarcane bagasse. Proc Biochem 38:1731–1738
Kwaśna H et al (2017) Health of poplars in plantations in the sanitary protection zones of Legnica and Głogów copper mills. Sylwan 161:639–647
Li H, Guan Y, Dong Y et al (2018) Isolation and evaluation of endophytic Bacillus tequilensis GYLH001 with potential application for biological control of Magnaporthe oryzae. PLoS ONE 13:e0203505
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Pageni BB, Lupwayi NZ, Akter Z et al (2014) Plant growth-promoting and phytopathogen-antagonistic properties of bacterial endophytes from potato (Solanum tuberosum L.) cropping systems. Can J Plant Sci 94:835–844
Pavlo A, Leonid O, Iryna Z et al (2011) Endophytic bacteria enhancing growth and disease resistance of potato (Solanum tuberosum L.). Bio Control 56:43–49
Piard JC, Desmazeaud M (1991) Inhibiting factors produced by lactic acid bacteria. 1. Oxygen metabolites and catabolism end-products. Lait 71:525–541
Rong S, Xu H, Li L et al (2019) Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic Biochem Physiol. https://doi.org/10.1016/j.pestbp.2019.09.003
Russell AD (1991) Mechanisms of bacterial resistance to non-antibiotics: food additives and food and pharmaceutical preservatives. J Appl Bacteriol 71:191–201
Sehim AE, Dawwam GE (2022) Molecular phylogenetics of microbial endophytes endowed with plant growth-promoting traits from Populus tomentosa. Egypt J Bot 62:797–810
Seidel V, Taylor PW (2004) In vitro activity of extracts and constituents of Pelagonium against rapidly growing mycobacteria. Int J Antimicrob Agents 23:613–619
Sellami M, Khlifi A, Frikha F et al (2021) Agro-industrial waste based growth media optimization for biosurfactant production by Aneurinibacillus migulanus. J Microbiol, Biotechnol Food Sci 2021:578–583
Sellem I, Triki M, Elleuch L, Cheffi M, Chakchouk-Mtibaa A, Slim S, Mellouli L (2017) The use of newly isolated Streptomyces strain TN258 as potential biocontrol agent of potato tubers leak caused by Pythium ultimum. J Basic Microbiol 57(5):393–401
Shah D, Khan MS, Aziz S et al (2021) Molecular and biochemical characterization, antimicrobial activity, stress tolerance, and plant growth-promoting effect of endophytic bacteria isolated from wheat varieties. Microorganisms 10:21
Song D, Zhang Y, Zhang L et al (2016) Sensitivities of poplar anthracnose fungi isolates to carbendazim and three C-14$α$-demethylation inhibitors. Chin J Pestic Sci 18:567–574
Team RC (2016) R: a language and environment for statistical computing. Vienna, Austria. Retrieved from https://www.R-project.org/
Thomashow LS (2002) Antibiotic production by soil and rhizosphere microbes in situ. Manual Environ Microbiol 636–647
Tiwari CK, Parihar J, Verma RK (2011) Potential of Aspergillus niger and Trichoderma viride as biocontrol agents of wood decay fungi. J Ind Acad Wood Sc 8:169–172
White TJ, Bruns T, Lee S et al (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18:315–322
Yamauchi N, Shimazu J, Satou M et al (2004) Physiological races and vegetative compatibility groups of butterhead lettuce isolates of Fusarium oxysporum f. sp. lactucae in Japan. J Gen Plant Pathol 70:308–313
Zhang X, Liu X, Wang Q et al (2014) Diesel degradation potential of endophytic bacteria isolated from Scirpus triqueter. Int Biodeter Biodegrad 87:99–105
Zhang J, Gu Y, Chi F et al (2015) Bacillus amyloliquefaciens GB1 can effectively control apple valsa canker. Biol Control 88:1–7
Acknowledgements
We gratefully acknowledge the Beijing Forestry University, Beijing, China, for supporting us and facilitating this work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
GED, AES, conceptualization, and supervision; GED, AES conducted the experiment; GED, AES wrote original draft preparation, GED, AES wrote review and editing. Both authors contributed equally to the work. Both authors have read, reviewed, and agreed to publish the version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
All the authors have given their consent to publish the submitted manuscript as an “Original paper” in EJBPC.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1. Bioactive secondary metabolites obtained from Bacillus velezensis 33RB using LC-MS/MS analysis. Table S2. Bioactive secondary metabolites extracted from A. niger 46SF using LC-MS/MS analysis. Fig. S1. KEGG Pathway of different metabolites produced by B. velenzensis and A. niger. (A&B) KEGG Meta negative and positive Annotation of B. velenzensis. (C&D) KEGG Meta negative and positive Annotation of A. niger. Fig. S2. Lipid maps analysis of bioactive metabolites of B. velenzensis and A. niger by negative and positive annotation. (A&B) Lipid maps of Meta negative and positive Annotation of B. velenzensis. (C&D) Lipid maps of Meta negative and positive Annotation of A. niger.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dawwam, G.E., Sehim, A.E. Promising biological agents represented in Bacillus velezensis 33RB and Aspergillus niger 46SF endophytic isolates for controlling Populus tomentosa wilt and anthracnose diseases. Egypt J Biol Pest Control 32, 144 (2022). https://doi.org/10.1186/s41938-022-00644-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00644-1