Abstract
Background
Botrytis cinerea can cause serious disease on lots of plant hosts during growth and postharvest storage. Biocontrol is known to be eco-friendly methods to control pathogens. Plant endophytic bacteria are generally considered as beneficial organisms, since they can promote plant growth and enhance plant immune system. Thus, screening biological control agents is very important for sustainable plant protection.
Results
Fifty-six endophytic bacteria were obtained from wild grape. Sixteen isolates and their extracts exhibited significant antifungal activity against B. cinerea. Particularly, strain JRX-YG39 with the strongest inhibition ability had a broad-spectrum antifungal activity. Combining 16S rDNA analysis and the phylogenetic results based on gyrA and gyrB genes, JRX-YG39 was assigned as Bacillus velezensis. JRX-YG39 could produce bioactive VOCs and obviously depressed mycelia growth of B. cinerea. It was confirmed that VOCs released by JRX-YG39 could significantly promote growth and induce defense of Arabidopsis thaliana. Thirty-one bioactive secondary metabolites were further identified from JRX-YG39 culture by gas chromatography–mass spectrometry analysis. Dibutyl phthalate, a potential antifungal substance, was the major compound accounting for 78.65%.
Conclusions
B. velezensis JRX-YG39 has wide broad-spectrum antagonistic activity and significant plant promotion activity. Hence, B. velezensis JRX-YG39 will provide a valuable constituent of modern agricultural practice as biofertilizers and biocontrol agents.
Similar content being viewed by others
Background
Botrytis cinerea can cause serious disease on more than 1400 plant hosts during growth and postharvest storage [1]. Chemical control is still a major measure to prevent grey mold on plant. However, B. cinerea has developed multiple fungicide resistance due to its high genetic variability and rapid reproduction [2, 3]. Additionally, the spraying fungicides in large quantities poses a great threat to the environment and human safety.
Biocontrol including the use of biological control agents and their active compounds has been considered as a sustainable approach to control plant diseases [4,5,6]. Plant endophytic bacteria are generally considered as beneficial organisms, since they can promote plant growth and enhance plant immune system [7, 8]. In recent years, lots of reports indicated that endophytic bacteria strains from genus Bacillus displayed significant potential in biological control of gray mold. The Bacillus genus possess genetic biodiversity and has been considered as biocontrol candidates against pathogens. For example, B. subtilis strains MBI 600 and QST 713 has been registered for the use against plant diseases, especially the latter was particularly effective against B. cinerea [9]. Meanwhile, B. amyloliquefaciens could prevent fruit diseases and plant pathogens such as B. cinerea, Monilinia fructicola, M. laxa, Penicillium digitatum, P. expansum and P. italicum [9,10,11]. B. halotolerans successfully inhibited B. cinerea, Fusarium oxysporum f. sp. radicis-lycopersici, Alternaria alternata, Rhizoctonia bataticola, and Phytophthora infestans [12, 13]. Recently, several studies have confirmed that B. velezensis is a valuable member of significant bioactivity on both pathogen suppression and plant growth promotion [14, 15].
Accumulated evidence has demonstrated that endophytes can produce active secondary metabolites including soluble substances and volatile organic compounds (VOCs). Bacillus are capable of produce a spectrum of bioactive secondary metabolites including nonribosomal peptide synthetases and polyketide synthases [15,16,17,18,19]. Indeed, VOCs produced by bacteria in Bacillus, Pseudomonas and Enterobacter genera demonstrated significantly antifungal activity. These VOCs included terpenes, esters, ketones, hydrocarbons, sulfur derivatives, acids, etc. [20, 21]. Several studies indicated that the plant growth promoting ability of endophytic bacteria had host specificity [22, 23]. The objectives of our study are to 1) isolation and identification of endophytic bacteria with bioactivity inhabiting wild grape in Zhong-tiao mountains; 2) determine the antagonistic activity and plant promotion activity of strain JRX-YG39; 3) identified the predominant volatile antifungal compounds from JRX-YG39, and elucidated the inhibitory mechanism.
Results
Isolation and identification of endophytic bacteria from wild grape
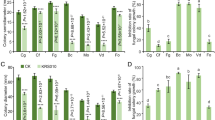
In total, 56 endophytic bacteria were isolated from wild grape tissue using NA medium. All the isolates were performed preliminary screening for antifungal activity. By antagonistic assay in plates, 16 isolates showed significant activities, accounting for 28.57% of the total isolates (Table1). According to the 16 S rRNA alignment results, most strains belonged to Bacillus velezensis. As shown in Table 1 and Fig. S1, 16 isolates could good control mycelia growth of B. cinerea, among which seven isolates owned more than 50% of inhibition activity. Five isolates showed more than 50% of inhibition rate against A. alternata. Strains JRX-YG39 and YB-K1 could obviously suppress F. pernambucanum growth with inhibition rate more than 50%. In addition, strains JRX-YG39, JRX-QT40, and YB-K1 could control C. gloeosporioides with inhabitation rate were 81.91%, 51.23%, and 66.74%, respectively (Fig. S1). Particularly, strain JRX-YG39 demonstrated excellent antifungal activity and was selected for the subsequent study.
Identification of strain JRX-YG39
Endophytic bacterium JRX-YG39 was identified using sequencing of 16S rRNA, DNA gyrase subunit A gene (gyrA), and DNA topoisomerase (ATP-hydrolyzing) subunit B gene (gyrB). The 16S rRNA, gyrA, and gyrB were deposited in Genbank with accession numbers ON413871, ON507281, and ON507282, respectively.
We performed 16S rRNA sequence alignment with type strains on NCBI. The result indicated that the strain JRX-YG39 demonstrated 99.65% identity to B. velezensis (NR_116240.1) (Table 1). To further determine the classification of JRX-YG39, we constructed neighbor-joining tree based on gyrA and gyrB genes. As shown in Fig. 1, both gyrA and gyrB phylogenetic trees demonstrated that JRX-YG39 was affiliated with B. velezensis. Thus, JRX-YG39 was identified as B. velezensis and named after B. velezensis JRX-YG39.
Antifungal activity assay of JRX-YG39 fermentation extract
Oxford cup experiment results demonstrated that strain B. velezensis JRX-YG39 fermentation broth owned antifungal substances (Fig. 2). Compared with control group, pathogen colonies in treatment were obviously smaller and sparser, which indicated that the bacterial strain could significantly suppress mycelium growth. The inhibition rates ranged from 50 to 85%. The maximal inhibition rates were observed against B. cinerea (85.48% ± 1.87).
Bioactivity of VOCs released by JRX-YG39
Double plate assay indicated that strain JRX-YG39 could produce antagonistic activity VOCs inhibiting growth of B. cinerea (Fig. 3). Compared with Escherichia coli DH-5α, JRX-YG39 could influence B. cinerea growth with inhibition rate up to 80%. While B. cinerea had grown all over the plate in the control group.
Growth promotion effect of JRX-YG39 VOCs on A. thaliana seedlings
Plant growth promotion effect of B. velezensis JRX-YG39 was measured in Figs. 4 and 5. By contrast, A. thaliana seedlings demonstrated increased growth with dark green leaves, longer main root, and developed lateral roots. The endophytic strain significantly (LSD’s multiple range test, p < 0.05) enhanced number of lateral roots, main root length, and the fresh weight after culture for three weeks (Fig. 5). Compared to control (about 42), the average number of lateral roots treated with JRX-YG39 was 100 (Fig. 5A). The length of main root in treatment group was 2.6 cm, while the control group was only 1.8 cm (Fig. 5B). In addition, and the fresh weight of A. thaliana plantlets treated with B. velezensis JRX-YG39 reached 0.48 g, which was much greater than that in control (Fig. 5C).
Resistance improvement effect of JRX-YG39
After incubation, A. thaliana plantlets were challenged with B. cinerea conidia suspension for resistance test. Compared with control group, A. thaliana plantlets treated with JRX-YG39 showed obviously lower disease incidence with 19.38% (Fig. 6 A; Fig. S2). Lesion diameter of treated group was 1.9 mm, and control group had bigger lesion about 3.2 mm in diameter (Fig. 6B). The results illustrated VOCs released by JRX-YG39 had induced plantlet resistance contributing to decrease of disease incidence and lesion diameter.
Identification of VOCs of JRX-YG39 culture by gas chromatography–mass spectrometry
Fermentation extract of B. velezensis JRX-YG39 was conducted GC–MS assay under optimized conditions. Assay results showed that the VOCs contained 31 substances including alcohols, aldehydes, olefins, organic acids, and lipids, etc. (Table S1). According to NIST library data, main chemical compounds with area percent more than 1% were identified as 3-methyl-butanoic acid, 2-methyl-butanoic acid, 3,5-dimethoxy- phenol, dibutyl phthalate, phenol, 2-Nonanone, l-leucine-N-cyclopropylcarbonyl-hexadecyl-ester, [1,2-a]pyrazine-1,4-dione-hexahydro-3-(2-methylpropyl)-Pyrrolo, (S-E)-2,3,7-trimethyl-4-Octene, and (Z)-9-octadecenamide. Particularly, results revealed a high content of dibutyl phthalate (78.65%) among all compounds.
Discussion
Although vast documentation on the pathogenic mechanisms and control have deposited, B. cinerea remains a remarkable challenge to overcome and promote its biocontrol. Therefore, isolation and evaluation microbe with broad-spectrum bioactive is essential for sustainable plant protection. Endophytes are considered as important high potential biological control agents, and have been found widely in various plants [24]. Several reports have indicated that plant living different environment could own diverse not only the soil and rhizosphere [25] but also phyllosphere [26] and endosphere communities [27]. Exploration of wild plant endophytes will provide new candidates for development of new and effective bioactive compounds in further plant disease management.
In this study, we isolated 16 endophytic bacteria with antifungal activity against four pathogens from wild grape in Zhong-tiao mountain. Most of these isolates belonged to Bacillus spp. based on 16S rRNA. Strain JRX-YG39 was identified as B. velezensis according to 16S rRNA, gyrA gene, and gyrB gene sequence analysis. JRX-YG39 demonstrated strong antifungal activity against B. cinerea by producing soluble and diffusible substances. Endophytic Bacillus spp. with antimicrobial activity have been documented in other studies. B. amyloliquefaciens possessed obvious inhibition effect on Fusarium oxysporum, Penicillium spp., Colletotrichum spp., B. cinerea, and Sclerotinia sclerotiorum [28, 29]. B. halotolerans could suppress mycelia growth of B. cinerea and F. oxysporum [30, 31]. In addition, B. velezensis is a valuable species as potential biocontrol agent against many phytopathogens. For example, endophytic B. velezensis B-36 isolated from lotus tissues showed strong antagonistic activities against lotus rot caused by F. oxysporum [14]. B. velezensis strain SDTB038 displayed good control of potato late blight (Phytophthora infestans) in greenhouses and field [32]. Strain JRX-YG39 could greatly inhibit the growth of four important phytopathogens, particularly inhibitory rate reached to 87.41% when treatment against B. cinerea on plate. Bacillus species can produce a set of bioactive secondary metabolites that potentially control plant pathogens [15].
,Strain JRX-YG39 significantly enhanced A. thaliana growth and triggered plant defense response by producing bioactive VOCs. Accumulated documents indicated that many bacteria displayed prominent plant growth promotion effect [15, 33]. Similar reports showed that endophytic Bacillus strains from tomato could obviously promote growth of tomato seedlings [34]. Additionally, growth promotion effects on A. thaliana roots were much stronger than that in leaves when incubation with strain JRX-YG39 (Fig. 4). In another study, B. subtilis strain GB03 displayed much stronger enhancement on roots elongation of A. thaliana seedlings compared to leaves [35]. Furthermore, it has been known that Bacillus can stimulate induced plant systemic resistance by producing various substances [8, 15, 36, 37]. In this study, disease incidence of A. thaliana seedlings challenged with B. cinerea decreased significantly, compared to control group. Lesion diameter of diseased leaves was about 1.9 mm, which was much smaller than that in control treatment. These results indicated JRX-YG39 could successfully boost plant defenses and decrease disease incidence.
In total, 31 bioactive secondary metabolites were further identified from the B. velezensis JRX-YG39 fermentation extract by GC–MS. Particularly, dibutyl phthalate was the major compound accounting for 78.65%. Dibutyl phthalate was reported as strongly antifungal compound in several actinomycete strains [38, 39]. Another study reported that dibutyl phthalate from marine Pseudomonas could inactivate cathepsin B [40]. Phenol and hexadecane were critical for antifungal activity to B. cinerea and A. alternaria [41]. VOCs released by Bacillus sp. BCT9 contained 2-Nonanone that leading to increase of lateral root length on Lactuca sativa seedlings [42]. Function of other bioactive substances emitted by JRX-YG39 need to be demonstrated in further study. Thus, B. velezensis JRX-YG39 could be selected as potential biocontrol agents to manage B. cinerea.
Conclusion
Endophytic bacterium B. velezensis JRX-YG39 could produce active soluble and volatile compounds contributing to antifungal activity, plant growth promotion, and resistance enhance effect. It had a great potential to provide novel agents for gray mold management in future.
Materials and methods
Microbes and plants
Four pathogens Botrytis cinerea LK-7, Fusarium pernambucanum FQ-17, Alternaria alternata YJHB-6, and Colletotrichum gloeosporioides PG-10 were isolated from diseased plants and stored in refrigerator at 4℃. Wild grape (Vitis heyneana Roem. et Schult) samples were obtained from the primitive ecological area of Zhong-Tiao Mountains in Shanxi Province, China (latitude 35◦09′ N, longitude 111◦20′ E, and elevation 400 m). Arabidopsis thaliana seeds were gift from Chinese Academy of Agricultural Sciences.
Isolation, screening and identification of endophytic bacteria from wild grape
Endophytic bacteria were isolated from wild grape samples by using nutrient agar (NA) medium according to the method of described previously [43]. The isolated strain was maintained in glycerol solution (30%) at − 20◦C.
The genomic DNA of candidate strains were extracted using a bacterial genomic DNA isolation kit (Biotech Corporation, Beijing, China). The harvested DNA was detected by the agarose gel electrophoresis and quantified by Qubit® 2.0 Fluorometer (Thermo Scientific). 16S rRNA was amplified using the universal primers 27 F and 1492R according to previous studies [41]. Candidate strains were preliminarily identified by alignment of 16S rRNA sequences with BLAST online software (https://blast.ncbi.nlm.nih.gov/Blast).
Phylogenetic trees construction of JRX-YG39 based on gyrA and gyrB genes
To further distinguish strain JRX-YG39, DNA gyrase subunit A gene (gyrA) and DNA topoisomerase (ATP-hydrolyzing) subunit B gene (gyrB) were amplified according to previous researches [32, 41]. Amplification products were sequenced and deposited in NCBI. Then sequences alignment was performed with CLUSTAL W in Mega-X software [44]. Phylogenetic trees were constructed based on gyrA and gyrB according to the neighbour-joining approach with bootstrap values based on 1000 replications, respectively.
Antifungal activity assay of JRX-YG39 fermentation extract
After preliminary screening of antifungal strains, JRX-YG39 was selected for further study. Strain JRX-YG39 was cultured with liquid NA medium in a 150-mL flask at 30 ℃, 180 rpm, for 24 h. The fermentation broth was collected and was centrifuged for 10 min at 4000 × g to collect supernatant. Then Oxford cup experiment was performed as follows: a 5-mm hyphal disk of plant pathogen (Botrytis cinerea, Fusarium pernambucanum, Alternaria alternata, and Colletotrichum gloeosporioides) was placed in the middle of a PDA plate, then two Oxford cups were placed in each side of about 2.5-cm distance from the pathogen, 100 µL of JRX-YG39 supernatant was added in the Oxford cups. Plates were incubated at 24 ◦C in dark for 5–7 days. The inhibition percentage was evaluated according to the method of Gao et al. [41]. Control Petri dishes contained only two mycelia disks of the fungal strains. The experiment was repeated three times.
Double plate assay of VOCs released by JRX-YG39
Double plate assay was used to study antagonistic activity of volatile organic compounds (VOCs) against B. cinerea according to methods in previous study [41]. A 5-mm hyphal disk of B. cinerea culture was placed in the middle of a PDA plate. Then a NA plate spread with 20 µL of JRX-YG39 culture was inverted over the pathogen plate and incubated. Control treatment consisted of mycelia disc of pathogen and Escherichia coli DH-5α. Plates were sealed with cling wrap and incubated at 24 ◦C in dark 5–7 days. The inhibition percentage was calculated as described earlier.
Co-culture A. thaliana seedlings with JRX-YG39
According to previous study [42], 10 mL of Murashige and Skoog medium (MS) and NA medium were poured into a two-compartment petri dish, respectively. After medium solidification, sterilized A. thaliana seeds were planted on the MS medium. Then a disk of strain JRX-YG39 was put at the middle of NA medium. The plates without bacteria were used as control. The seedlings were cultivated in a growth chamber with a 16/8-h day/night schedule at 20 ◦C. After 21 days, the fresh weight, length of root, number of root hairs were measured. Each treatment consisted of three replicates.
Inoculation A. thaliana seedlings with B. cinerea
After treatment with bacterial strain JRX-YG39 as described above, A. thaliana seedlings were inoculated with B. cinerea conidia suspension (1 × 106/ mL) by a 1-mL syringe. Then disease incidence and lesion diameter on leaves were calculated. Each treatment consisted of twenty seedlings.
Analyses of VOCs by gas chromatography–mass spectrometry
The strain JRX-YG39 was streaked in NA medium at 30℃ for 10 h. Single colony was cultured in 100 mL LB liquid medium at 30℃, 200 RPM /min for 24 h. Then 20 µL JRX-YG39 suspension was transferred to 300 mL LB at 30℃, 200 RPM /min for 48 h. Next, the fermentation liquid was extracted with ethyl acetate (1:1) for 3 times, and the extraction liquid was concentrated by rotary evaporation at 35℃.
Subsequently, the strain extract was first dissolved in the chromatographic-grade methanol and filtered through a 0.2-µm filter. The solution was injected into a gas capillary column (DB-17MS, 30 m × 0.25 mm × 0.25 µm) of a gas chromatography (5975C Inert XL MSD, Agilent, United States). Helium was used as a carrier gas with a flow rate of 1 ml min−1. The mass spectrometer (EI with replaceable horn) was operated in the electron ionization (EI) mode at 70 eV with a continuous scan from 50 to 800 m/z. The peaks were identified by matching the mass spectra with the National Institute of Standards and Technology (NIST, United States) library.
Statistical analysis
All data were analyzed using analysis of variance (ANOVA) by the SPSS statistical package (version 22, SPSS Inc., Chicago, IL, United States). The t-test was applied to compare means between different subjects. The statistical differences between means were assessed at the level of p < 0.05.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. and supplementary material online. The 16S rRNA sequence of endophytic bacteria generated and analysed during the current study are available in the GenBank repository with accession number ON413862—ON413876, and MW642498. gyrA, and gyrB sequences of JRX-YG39 strain were also deposited in GenBank with accession numbers ON507281 and ON507282, respectively.
References
Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol. 2007;8:561–80. https://doi.org/10.1111/j.1364-3703.2007.00417.x.
Haidar R, Fermaud M, Calvo-Garrido C, Roudet J, Deschamps A, Ar RH, et al. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol Mediterr. 2017;55:301–22. https://doi.org/10.14601/Phytopathol_Mediterr-18079.
Leroch M, Plesken C, Weber RWS, Kauff F, Scalliet G, Hahn M. Gray mold populations in german strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl Environ Microbiol. 2013;79(1):159–67. https://doi.org/10.1128/AEM.02655-12.
Abbey JA, Percival D, Abbey Lord Asiedu SK, Prithiviraj B, Schilder A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci Technol. 2019;29:241–62. https://doi.org/10.1080/09583157.2018.1548574.
Roca-Couso R, Flores-Félix JD, Rivas R. Mechanisms of action of microbial biocontrol agents against Botrytis cinerea. J Fungi (Basel). 2021;7(12):1045. https://doi.org/10.3390/jof7121045.
Lugtenberg BJJ, Caradus JR, Johnson LJ. Fungal endophytes for sustainable crop production. FEMS Microbiol Ecol. 2016;92(12):1–17. https://doi.org/10.1093/femsec/fiw194.
Bolívar-Anillo, HJ, Garrido C, Collado IG. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem Rev. 2019; 5. https://doi.org/10.1007/s11101-019-09603-5.
Santoyo G, Moreno-Hagelsieb G, Orozco-MosquedaMdel C, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–9. https://doi.org/10.1016/j.micres.2015.11.008.
Calvo H, Marco P, Blanco D, Oria R, Venturini ME. Potential of a new strain of Bacillus amyloliquefaciens BUZ-14 as a biocontrol agent of postharvest fruit diseases. Food Microbiol. 2017;63:101–10. https://doi.org/10.1016/j.fm.2016.11.004.
Gotor-vila A, Teixid N, Casals C, Torres R, De Cal A, Guijarro B, et al. Biological control of brown rot in stone fruit using Bacillus amyloliquefaciens CPA-8 under field conditions. Crop Prot. 2017;102:72–80. https://doi.org/10.1016/j.cropro.2017.08.010.
WoldemariamYohannes K, Wan Z, Yu Q, Li H, Wei X, Liu Y, et al. Prebiotic, probiotic, antimicrobial, and functional food applications of Bacillus amyloliquefaciens. J Agric Food Chem. 2020;68(50):14709–27. https://doi.org/10.1021/acs.jafc.0c06396.
Slama Ben H, Cherif-silini H, Bouket AC, Qader M. Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against Fusarium. Front Microbiol. 2019;9:1–24. https://doi.org/10.3389/fmicb.2018.03236.
Wang F, Xiao J, Zhang Y, Li R, Liu L, Deng J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mould in postharvest strawberry fruit. 2021;174: 111456. https://doi.org/10.1016/j.postharvbio.2020.111456.
Wang GF, Meng JF, Tian T, Xiao XQ, Zhang B, Xiao YN. Endophytic Bacillus velezensis strain B-36 is a potential biocontrol agent against lotus rot caused by Fusarium oxysporum. J Appl Microbiol. 2020;128(4):1153–62. https://doi.org/10.1111/jam.14542.
Rabbee MF, Ali MS, Choi J, Hwang BS, Jeong SC, Baek KH. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24(6):1046. https://doi.org/10.3390/molecules24061046.
Wu L, Wu HJ, Qiao J, Gao X, Borriss R. Novel routes for improving biocontrol activity of Bacillus based bioinoculants. Front Microbiol. 2015;6:1–13. https://doi.org/10.3389/fmicb.2015.01395.
Chowdhury SP, Uhl J, Grosch R, Alquéres S, Pittroff S, Dietel K, et al. Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the Lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol Plant-Microbe Interact. 2015;28:984–95. https://doi.org/10.1094/MPMI-03-15-0066-R.
Cao Y, Pi H, Chandrangsu P, Li Y, Wang Y, Zhou H, et al. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci Rep. 2018;8:1–14. https://doi.org/10.1038/s41598-018-22782-z.
Li Y, Gu Y, Li J, Xu M, Wei Q, Wang Y. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front Microbiol. 2015;6:1–15. https://doi.org/10.3389/fmicb.2015.00883.
Dhouib H, Zouaria I, Ben Abdallaha D, Belbahri L, Taktaka W, Triki MA, et al. Potential of a novel endophytic Bacillus velezensis in tomato growth promotion and protection against Verticillium wilt disease. Biol Control. 2019;139: 104092. https://doi.org/10.1016/j.biocontrol.2019.104092.
Elkahoui S, Djébali N, Yaich N, Azaiez S, Hammami M, Essid R, et al. Antifungal activity of volatile compounds-producing Pseudomonas P2 strain against Rhizoctonia solani. World J Microbiol Biotechnol. 2015;31:175–85. https://doi.org/10.1007/s11274-014-1772-3.
Sanggu Kim S, Ng WK, Dong Y, Das S, Tan Reginald BH. Preparation and physicochemical characterization of trans-resveratrol nanoparticles by temperature-controlled antisolvent precipitation. J Food Engineering. 2012;108(1):37–42. https://doi.org/10.1016/j.jfoodeng.2011.07.034.
Afzala I, Shinwaria ZK, Sikandarb S, Shahzad S. Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microb Res. 2019;221:36–9. https://doi.org/10.1016/j.micres.2019.02.001.
Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, et al. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol. 2016;7:906. https://doi.org/10.3389/fmicb.2016.00906.
Calleja-Cervantes ME, Menéndez S, Fernández-González AJ, Irigoyen I, Cibriáin-Sabalza JF, Toro N, et al. Changes in soil nutrient content and bacterial community after 12 years of organic amendment application to a vineyard. Eur J Soil Sci. 2015;66:802–12. https://doi.org/10.1111/ejss.12261.
Campisano A, Antonielli L, Pancher M, Yousaf S, Pindo M, Pertot I. Bacterial endophytic communities in the grapevine depend on pest management. PLoS ONE. 2014;9:e112763. https://doi.org/10.1371/journal.pone.0112763.
Sébastien B, Mónica Z, Floriane L, Eva T, Abhishek A, Agnès D, et al. Endophytes and epiphytes from the grapevine leaf microbiome as potential biocontrol agents against phytopathogens. Front Microbiol. 2019;10:2726. https://doi.org/10.3389/fmicb.2019.02726.
Ye W, Sun Y, Tang Y, Zhou W. Biocontrol potential of a broad-spectrum antifungal strain Bacillus amyloliquefaciens B4 for postharvest loquat fruit storage. Postharvest Biol Tech. 2021;174: 111439. https://doi.org/10.1016/j.postharvbio.2020.111439.
Massawe VC, Hanif A, Farzand A, Mburu DK, Ochola DK, Wu L, et al. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology. 2018;108(12):1373–85. https://doi.org/10.1094/PHYTO-04-18-0118-R.
Wang F, Xiao J, Zhang Y, Li R, Liu L, Deng J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mould in postharvest strawberry fruit. Postharvest Biol Tech. 2021;174: 111456. https://doi.org/10.1016/j.postharvbio.2020.111456.
Slama HB, Cherif-Silini H, ChenariBouket A, Qader M, Silini A, Yahiaoui B, et al. Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against Fusarium. Front Microbiol. 2019;9:3236. https://doi.org/10.3389/fmicb.2018.03236.
Yan H, Qiu Y, Yang S, Wang Y, Wang K, Jiang L, et al. Antagonistic activity of Bacillus velezensis SDTB038 against Phytophthora infestans in potato. Plant Dis. 2021;105(6):1738–47. https://doi.org/10.1094/PDIS-08-20-1666-RE.
Beneduzi A, Ambrosini A, Passaglia LMP. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet Mol Biol. 2012;35:1044–51. https://doi.org/10.1590/S1415-47572012000600020.
Chaouachi M, Marzouk T, Jallouli S, Elkahoui S, Djébali N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of botrytis cinerea. Postharvest Biol Technol. 2012;172: 111389. https://doi.org/10.1016/j.postharvbio.2020.111389.
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta. 2007;226:839–51. https://doi.org/10.1007/s00425-007-0530-2.
Ongena M, Jacques P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16:115–25. https://doi.org/10.1016/j.tim.2007.12.009.
Blake C, Christensen MN, Kovács ÁT. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol Plant Microbe Interact. 2021;34(1):15–25. https://doi.org/10.1094/MPMI-08-20-0225-CR.
Ahsan T, Chen J, Zhao X, Irfan M, Wu Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express. 2017;7:54. https://doi.org/10.1186/s13568-017-0351-z.
Zhang L, Zhang H, Huang Y, Peng J, Xie J, Wang W. Isolation and evaluation of Rhizosphere Actinomycetes with potential application for biocontrolling Fusarium Wilt of banana caused by Fusarium oxysporum f. sp. cubense tropical race 4. Front Microbiol. 2021;12:763038. https://doi.org/10.3389/fmicb.2021.763038.
Hoang V, Yong L, Kim SK. Cathepsin b inhibitory activities of phthalates isolated from a marine pseudomonas strain. Bioorg Med Chem Lett. 2008;18(6):2083–8. https://doi.org/10.1016/j.bmcl.2008.01.097.
Gao Z, Zhang B, Liu H, Han J, Zhang Y. Identification of endophytic Bacillus velezensis zsy-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol Control. 2017;105:27–39. https://doi.org/10.1016/j.biocontrol.2016.11.007.
Fincheira P, Parra L, Mutis A, Parada M, Quiroz A. Volatiles emitted by bacillus sp. bct9 act as growth modulating agents on lactuca sativa seedlings. Microbiol Res. 2017;203:47. https://doi.org/10.1016/j.micres.2017.06.007.
Dobereiner J, Reis VM, Paula MA, Olivares FL. Endophytic diazotrophs in sugarcane, cereals and tuber plants. In: Palacios R, Mora J, Newton W. E., editors. New Horizons in Nitrogen Fixation. Dordrecht: Kluwer Academic; 1993. p. 671–6. https://doi.org/10.1007/978-94-017-2416-6_55.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9. https://doi.org/10.1093/molbev/msy096.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science Foundation of Shanxi Province (20210302123084 and 20210302123087), the National Natural Science Foundation of China (31501665), Science and Technology Innovation Project of Universities of Shanxi (2021l468), Science and Technology Program of Yuncheng (YCKJ-2021029 and YCKJ-2021030), and Scientific research project of characteristic agricultural product development group (SKX-202205).
Author information
Authors and Affiliations
Contributions
Experiments were designed by Baozhen Feng and Peiqian Li. Baozhen Feng, Dandan Chen, Ruixue Jin, performed the experiments. Data were analyzed by Ruixue Jin and Erqin. The manuscript was written by Baozhen Feng. Peiqian Li reviewed the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Antifungal activity of 16 endophytic bacteria against four phytopathogens. A to D represented growth inhibition of Botrytis cinerea, Alternaria alternata, Fusarium pernambucanum, and Colletotrichum gloeosporioides after antagonist with 16 endophytic bacteria, respectively. Fig. S2. Symptom difference on A. thaliana seedlings treatment with B. cinerea conidia suspension. A. A. thaliana seedlings co-cultured with B. velezensis JRX-39 for 21 days and then inoculated with B. cinerea; B. control, A. thaliana seedlings cultured for 21 days on MS medium and inoculated with B. cinerea conidia suspension, showed more severe disease. Arrows indicated diseased leaves. Table S1. Identification of VOCs of the Bacillus JRX-YG39 by GC-MS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Feng, B., Chen, D., Jin, R. et al. Bioactivities evaluation of an endophytic bacterial strain Bacillus velezensis JRX-YG39 inhabiting wild grape. BMC Microbiol 22, 170 (2022). https://doi.org/10.1186/s12866-022-02584-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02584-0