Abstract
Entomopathogenic nematodes (EPNs) are a group of biological control agents that are characterized by their ability to search for hosts, safety to non-target insects and environment, and their ability to be used combined with agricultural chemicals. The objectives of this study were to isolate EPNs from agricultural soil in Egypt and study their virulence against the great wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae), for further use in biological control program. Two out of 20 soil samples collected from orchards cultivated with olives and mango were positive for the presence of EPNs, using the Galleria baiting technique. The positive soil samples were sandy clay loam. Sequencing of the internal transcribed spacer (ITS) region indicated that the isolates obtained belong to Heterorhabditis indica. The ITS sequences were submitted to the National Center for Biotechnology Information (NCBI) and registered under the accession nos. MH553167 and MK300683. The efficacy of the isolates was tested on G. mellonella, using different nematodes’ concentrations. Using 50 IJs/larvae from H. indica Aborawash and ERSAG2 showed 100 and 86% mortality rate after 48 h, respectively. The penetration rate reported in dead G. mellonella was 40% at H. indica Aborawash, while it was 35% in case of ERSAG2.
Similar content being viewed by others
Background
Entomopathogenic nematodes (EPNs) of the families Heterorhabditidae and Steinernematidae are obligate deadly insect parasites that spend part of their life cycles in hosts (Adams et al. 2006). The life cycle of EPNs includes an egg stage, 4 juvenile stages, and an adult stage. The 3rd juvenile stage of EPNs is referred to be the “infective juveniles” (IJs) (Poinar 1990). Once the IJs penetrate the host, they release bacteria that live symbiotically within the EPNs’ gut. Once released into the host, the bacteria multiply quickly and under optimal conditions causing host mortality within 24 to 48 h (Shapiro-Ilan and Gaugler 2019). EPNs are distributed worldwide and include more than 16 Heterorhabditis species and at least 60 species of Steinernema (Nguyen and Hunt 2007).
Due to the extensive use of insecticides, insects have acquired resistance during recent decades (Andaló et al. 2018). EPNs represent an alternative control method to insecticides as it is an environmentally safer option, not harmful to humans, animals, plants, or earthworms (Le Vieux and Malan 2013).
Recently, sequencing of different regions of the genome of EPNs has become the most suitable approach, not only for determining phylogenetic relationships, but also for species delimitation as morphological identification is not an accurate method (Stock 2009).
DNA sequencing of the nuclear ribosomal DNA (rDNA), internal transcribed spacer regions (ITS1 and ITS2), mitochondrial DNA (mtDNA) gene, the 18S rRNA gene, and the 5.8S and the 28S rRNA genes, which contain variable and conserved regions (Stock 2009), has proven useful for studying speciation, phylogenetic relationships, and molecular evolution in the Heterorhabditidae (Adams et al. 1998; Stock 2009).
Detection and identification of indigenous EPN isolates, adapted to local environmental and climatic conditions, are necessary for future integrated pest management programs that aim to control insect pests.
The major objectives of this study were to isolate and identify EPNs from olive and mango orchards in Egypt based on ITS region of rDNA and to study their virulence against G. mellonella.
Material and methods
Sampling
Soil samples were collected from cultivated orchards of olives and mangos in Giza and Ismailia Governorates, Egypt, in 2016. Twenty soil samples were collected. Four subsamples at a depth of 20 cm were mixed thoroughly and used to create a single sample of 1 kg for each location. Two hundred fifty grams of the subsample was placed into a plastic container, identified by vegetation and date, and then transferred to the laboratory for further processing. The soil samples were processed by the insect-baiting G. mellonella (Galleria trap) method (Bedding and Akhurst 1975).
Five last instar larvae of G. mellonella were placed inside perforated 250 g plastic boxes, filled with soil, which were then embedded in the soil sample. Soil samples were stored at 25 °C, and every 5 days, dead G. mellonella showing symptoms of EPNs’ infection were removed and replaced by new ones. This procedure was carried out for 10 days. The dead G. mellonella larvae were transferred to White traps (White 1927), and infective juvenile (IJs) collected during the following days and stored at 16 °C in distilled water.
Nematode identification
Nematode DNA were extracted from IJs, using the method described by Kary et al. (2009) as follows: 2000 (IJs) from the nematodes were crushed in 15 μl 1× PCR buffer and then transferred to a pre-cooled sterilized 1-ml tube containing 10 μl of the same buffer. The tubes were incubated at − 70 °C for 15 min and thawed at 60 °C, then inoculated with 5 μl of 60 μg ml−1 proteinase K. Then, they were incubated for 2 h at 65 °C, and after that, they were heated at 95 °C for 15 min, followed by centrifugation at 15.000 rpm for 15 min; the supernatant containing DNA was collected and stored at − 70 °C until use. One percent agarose gel electrophoresis and spectrophotometer were used to determine the quality and quantity of DNA.
Internal transcribed spacer (ITS) regions of rDNA were amplified by using PCR. Primers specific to the ITS rDNA region were designed as follows: forward primer TW81 (5-GTTTCC GTA GGT GAA CCT GC-3) and reverse primer AB28 (5-ATA TGC TTA AGT TCA GCG GGT-3) (Joyce et al. 1994) were used.
PCR conditions included initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 55 °C for 1 min 30 s, and 72 °C for 2 min, followed by a final extension at 72 °C for 7 min. The PCR products were analyzed by 1.5% agarose gels with TAE buffer. The PCR products were purified using Wizard® SV Gel and PCR clean-Up system Kit (Promega), following the manufacturer’s instructions. PCR products were sequenced, using sequence-specific primers with a BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystem, USA) and carried out on ABI PRISM 310 Genetic Analyzer (PE Applied Biosystems, USA) in both directions by the Macrogen Inc. service, South Korea.
The identity of approximately 700 bp sequences was confirmed by a BLAST (Basic Local Alignment Search Tool) search at NCBI. The obtained sequences were submitted and located at the NCBI database with the accession numbers.
Phylogenetic analysis
Sequence alignments of ITS, region of the rDNA gene, were assembled, using the Phylogeny.fr (Robust Phylogenetic Analysis for the Non-Specialist). Phylogenetic relationships among isolates were reconstructed by maximum likelihood (ML) methods. Sequence was compared with those from reference organisms/strains available in the GenBank database.
Assessment of nematode isolates’ biological activities
Insecticidal activity of the two isolates
Experimental infections were carried out to determine the virulence of the 2 isolates against the larvae of G. mellonella. Hatched larvae were grown in the laboratory and incubated at 26 ± 2 °C and in the dark. Third instar G. mellonella larvae were used for evaluating the efficacy of the nematodes. In concentration response experiments, 10, 25, or 50 IJs/larvae in microcentrifuge tubes were used, while the control ones were prepared as they were only treated with distilled water. Each concentration of nematodes was tested against 20 larvae and repeated 3 times and 20 larvae as control. They were then incubated in a dark growth chamber at 26 ± 2 °C for 48 h. The larval mortality rate recognized by the change of their color usually red/purple for Heterorhabditis was calculated after 48 h.
Penetration ability of the two isolates
Penetration ability was tested against the larvae of G. mellonella, infected with 10 IJs/larvae in microcentrifuge tubes. The experiment was incubated at 26 ± 2 °C, and larval mortality rate was recorded daily. Dead larvae were rinsed by tap water to remove nematodes from the larvae surface and placed individually in Petri dishes with moist filter paper and incubated for a further 2 days. Insects were dissected under a stereoscopic microscope. The penetration rate was calculated as the percentage of the initial IJs that had invaded the insect host (Glazer and Lewis 2000).
Identification by scanning electron microscopy
Non-activated IJs were treated by 0.5–0.6% sodium hypochlorite for 1 min to dissolve L2 cuticle sheath and immediately washed 3 times by 0.8% NaCl solution. Nematodes that were fully activated in vitro for 18 h were washed 3 times by 0.8% NaCl solution. The washed non-activated IJs and activated nematodes were processed and observed by SEM (Ragsdale et al. 2011).
Statistical analysis
The mortalities in the control and in the treatments were determined 2 days post-beginning of the experiment. Data obtained were subjected to an analysis of variance (ANOVA) with significant difference values calculated as Tukey’s statistic at P ≤ 0.05 (SAS Institute 2002). A standard probit analysis was used to calculate LC50 of insects under test (SAS Institute 2002).
Results and discussion
Among the 20 soil samples collected from 4 collecting points, 2 soil samples (10%) were positive by recording the occurrence of Heterorhabditis species. The soil type and temperature are important factors affecting the distribution of EPNs in the soil. In the present survey, the 2 positive soil samples were classified as sandy clay loam and the temperatures at all the 4 collecting points were reported as 25 °C. This agrees with the data presented by Singh et al. (2015) and Laznik and Trdan (2016) who concluded that the application of Steinernema feltiae, S. carpocapsae, and H. bacteriophora at 20 and 25 °C resulted to mortality rates of over 57% for granary weevils.
Molecular characterizations
For identifying the 2 isolates of EPNs at the species level, DNA sequencing of the ITS PCR-amplified products revealed that its size is 900 bp (Fig. 1) containing the partial sequence of ITS1 and ITS2, and the whole 5.8S rRNA gene was obtained and sequenced (BLASTed) against the nucleotide collection (nr/nt) database. Approximately 700 bp DNA sequence was compared with sequences of related species and new isolates available in GenBank with accession no. Based on sequence homology, the isolates ERSAG2 and H. indica (Aborawash) indicated a 99% similarity with Heterorhabditis indica, which agree with Isaac et al. (2004) that to avoid taxonomic inflation, they consider the obtained isolates in their results as a member of the previously well-characterized species. Therefore, in the present study, based on the morphological data and phylogenetic analysis, the new isolates were grouped with H. Indica species in a phylogenetic clade.
Phylogenetic analysis
Nematodes isolated from the positive samples were identified based on ITS sequences compared with the EPN sequences from members of the “Indica” group derived from the National Center for Biotechnology Information (NCBI), using BLAST (Basic Local Alignment Tool) as references. It classified the new isolates in a clade with other isolates of H. indica. Phylogenetic relationships among isolates were reconstructed by maximum likelihood methods by using the Phylogeny.fr. program (Fig. 2); also, the alignment of ITS1 sequence from H. indica (Aborawash) and ERSAG2 was distinguishable as shown in Fig. 3.
Maximum likelihood ITS phylogenetic trees of H. indica species using ITS sequence. Based on nucleotide sequences of H. indica (Heterorhabditis indica Aborawash and ERSAG2) and reference strains (alignment length approximately 700 bp using phylo_tree analysis (Phylogeny.fr. program))
The phylogenetic tree shows that H. indica (Aborawash) and H. indica Pak. S. H. 400 belong to the same clade. Considering the great geographical distances among sites of occurrence for the “indica subgroup” nematodes (i.e., Pakistan, India, and Egypt) might be due to what Dolgin et al. (2008) called “latitudinal clades.” Thus, isolates of H. indica around the globe might also be “latitudinal clades,” but more extensive studies are needed to test this hypothesis. Shehata et al. (2019) reported that H. indica is considered to be a heat-tolerant nematode (infecting insects at 30 °C or higher). As well, indigenous EPNs must be favored since they are probably more adaptive without any risk to Egyptian fauna and flora. Also H. indica had high yields in vivo and in vitro (Shapiro-Ilan and Gaugler 2019). It can recoup its short shelf life via local production. Also, it is considered as a useful model system to study an insect immune mechanism.
Identification by scanning electron microscopy
Analysis by the use of SEM provides an understanding of the surface structure of the nematodes. H. indica IJs showed a sheath protecting the entire body of the nematode, tessellate on the anterior region and are characterized by longitudinal ridges throughout almost the entire body length as seen in Fig. 4a, b. Figure 4c is a hermaphrodite as it has huge lips with prominent labial papillae at the apex of the lips, while Fig. 4d is the infective juvenile.
Assessment of virulence against G. mellonella and penetration rate
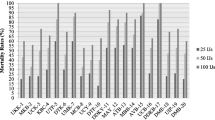
The insecticidal activities of EPNs to insect hosts can be highly changeable as it depends on factors associated with the nematode biology. The isolates ERSAG2 and H. indica (Aborawash) (Heterorhabditis indica) were used for further testing in order to determine their ability to be used as biological control agents. G. mellonella larvae were susceptible to the tested dose response assay for EPN isolates. Two days after inoculation, with the 2 isolates, both development and visual changes in the infected G. mellonella were documented, which were red/purple in color. The emergence of IJs from G. mellonella was ideal at 25 °C than other temperatures giving high mortality percentage. H. indica (Aborawash) had a great virulence causing 100% larval mortality, 48 h post-treatment (Fig. 5). The maximum mortality rate of the larvae was caused by H. indica (Aborawash) at a concentration of 50 IJs/larva (100%), while the minimum was recorded in the larvae treated with isolate ERSAG2 at 10 IJs/larva (50%) after 48 h. Both EPN species did not cause larval mortality 24 h after treatment at all used concentrations. The overall mortality of the larvae of G. mellonella 48 h after treatment with EPNs ranged between 86 and 100% (Fig. 5) for ERSAG2 and H. indica (Aborawash), respectively; the mortality rate reached 100% for ERSAG2, 72 h after treatment. An increase in larval mortality with the increase of exposure time was observed (Fig. 5). These findings agree with Leite et al. (2007), when H. indica was used for controlling Bradysia mabiusi larvae at concentration of 5.7 and 22.6 IJ/cm2 and the efficiency reaching 75 and 85% in green house, and also with Lalramliana and Yadav (2010) by using concentration of 50 IJ/host and the mortality was 100% after 96 h.
Lalitha et al. 2018 reported that following infection of the host, H. indica releases symbiotic bacteria, which produce and release several toxin complex resulting in activation of immune response such as antimicrobial peptides, phenoloxidase systems, antioxidant and detoxification enzyme, or causing insect mortality.
The calculated LC50 for ERSAG2 and H. indica Aborawash on the G. mellonella larvae after 48 h were 11.5 and 8.4 IJs/larva, respectively (Table 1), while the calculated LC50 for ERSAG2 and H. indica (Aborawash) on the larvae after 72 h were 8.9 and 7.4 IJs/larva, respectively (Table 1). The LC50 values implied that H. indica (Aborawash) was more virulent against the larvae of G. mellonella than ERSAG2.
Penetration rate
To be used as an insect pathogen, the EPN species have to penetrate their host and reproduce. The results of the penetration assay showed that both EPN species were able to penetrate and reproduce within the hemocoel of G. mellonella larvae. Caroli et al. (1996) reported that EPN species show differences in the penetration rates and are influenced by different hosts and concentrations of IJs. The highest penetration rate of nematode (40%) was found in dead G. mellonella larvae infected with H. indica (Aborawash), while it was 35% in dead G. mellonella larvae upon dissection in case of ERSAG2 and it mostly contained hermaphrodites, which nearly agrees with El-Lakwah and Azazy (2010) who reported that the penetration rate of H. indica was 32.66%, which is originally from far west, USA.
Conclusion
The present study indicated that the native EPN isolates were more virulent than non-native species. Furthermore, research on host ranges and characterization of these 2 isolates can be considered for using in biological control programs to minimize the use of chemical pesticides.
Availability of data and materials
All datasets are presented in the main manuscript.
Abbreviations
- EPNs:
-
Entomopathogenic nematodes
- G. mellonella :
-
Galleria mellonella L.
- ITS:
-
Internal transcribed spacer
- NCBI:
-
National Center for Biotechnology Information
- SEM:
-
Scanning electron microscopy
References
Adams BJ, Burnell AM, Powers TO (1998) A phylogenetic analysis of the genus Heterorhabditis (Nemata: Rhabditidae) based on internal transcribed spacer1 DNA sequence data. J Nematol 30:22–39
Adams BJ, Fodor A, Koppenhofer HS, Stackebrandt E, Stock SP, Klein MG (2006) Biodiversity and systematics of nematode bacterium entomopathogens. Biol Control 37:32–49
Andaló V, Kellin PR, Fábio JC, Mieko J, De Faria LS, De Assis GA, Barbosa LR (2018) Entomopathogenic nematodes for the control of Gryllus sp. (Orthoptera: Gryllidae) under laboratory and field conditions. Arq Inst Biol 85:1–7
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic nematodes in soil. Nematologica 21:109–110
Caroli L, Glazer I, Gaugle R (1996) Entomopathogenic nematode infectivity assay: comparison of penetration rate into different hosts. Biocontrol Sci Tech 6(2):227–234
Dolgin ES, Félix MA, Cutter AD (2008) Hakuna Nematoda: genetic and phenotypic diversity in African isolates of Caenorhabditis elegans and C. briggsae. Heredity 100:304–315
El-Lakwah SF, Azazy AM (2010) Physiological and biological studies of some entomopathogenic nematode species of families (steinernematidae and heterorabditidae). Egypt Acad J Biolog Sci 2(2):45–54
Glazer I and Lewis E E (2000) Bioassays for entomopathogenic nematodes. (Edited by Navon, A and Ascher K.R.S). Bioassays of entomopathogenic microbes and nematodes 229-249.
Isaac NJB, Mallet J, Mace GM (2004) Taxonomic inflation: its influence on acroecology and conservation. Trends Ecol Evol 19:464–469
Joyce SA, Burnell AM, Powers TO (1994) Characterization of Heterorhabditis isolates by PCR amplification of segments of mtDNA and rDNA genes. J Nematol 26:260–270
Kary NE, Niknam G, Griffin CT, Mohammadi SA, Moghaddam M (2009) A survey of entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in the north-West Iran. Nematology 11:107–116
Lalitha K, Karthi S, Vengateswari G, Karthikraja R, Peruma P, Shivakumar MS (2018) Effect of entomopathogenic nematode of Heterorhabditis indica infection on immune and antioxidant system in lepidopteran pest Spodoptera litura (Lepidoptera: Noctuidae). J Parasit Dis 42(2):204–211
Lalramliana Yadav AK (2010) Occurrence of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Meghalaya. NE India Sci Vis 10(3):89–100
Laznik Ž, Trdan S (2016) Attraction behaviors of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) to synthetic volatiles emitted by insect damaged carrot roots. J Pest Sci 4:977–984
Le Vieux PD, Malan AP (2013) The potential use of entomopathogenic nematodes to control Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). S Afr J Enol Vitic 34(2):296–306
Leite LG, Tavares FM, Bussóla RA, Amorim DS, Ambrós CM, Harakava R (2007) Virulence of entomopathogenic nematodes (Nemata: Rhabditida) against larva of the fungus gnat Bradysia mabiusi (lane, 1959) and persistence of Heterorhabditis indica Poinar et al. 1992 on organic substrates. Arquivos do Instituto Biológico 74:337–342
Nguyen K B and Hunt D (2007) Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. 816 pp. Leiden, E.J. Brill.
Poinar G O (1990) Biology and taxonomy of Steinernematidae and Heterorhabditidae. In: Entomopathogenic nematodes in biological control, pp. 23–61. Edited by R. Gaugler and H.K. Kaya. Boca Raton, FL: CRC Press.
Ragsdale EJ, Ngo PT, Crum J, Ellisman MH, Baldwin JG (2011) Reconstruction of the pharyngeal corpus of Aphelenchus avenae (Nematoda: Tylenchomorpha), with implications for phylogenetic congruence. Zool J Linnean Soc 161:1–30
Sas Institute (2002) SAS/stat User’s guide. SAS Institute Inc., Cary, NC, USA
Shapiro-Ilan D I and Gaugler R (2019) Nematodes: Rhabditida: Steinernematidae & Heterorhabditidae. In: Biological control: a guide to natural enemies in North America. Edited by A. Shelton, Cornell University.
Shehata IE, Hammam MM, El-Borai FE, Duncan LW, Abd-Elgawad MMM (2019) Comparison of virulence, reproductive potential, and persistence among local Heterorhabditis indica populations for the control of Temnorhynchus baal (Reiche & Saulcy) (Coleoptera: Scarabaeidae). Egypt J Biol Pest Control 29:32
Singh SP, Yadav AK, Shachi V, Tripathi CPM (2015) Diversity analysis of entomopathogenic nematodes against Helicoverpa armigera (Hübner) from Tarai region of IGP, India. Current Life Sciences 1(1):15–23
Stock S P (2009) Molecular approaches and the taxonomy of insect-parasitic and pathogenic nematodes. pp. 71–100 in Stock, S.P., Vandenburg, J., Glazer, I. & Boemare, N. (Eds) Insect pathogens: molecular approaches and techniques. Wallingford, Oxon, UK, CAB International Press.
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303
Acknowledgements
We would like to thank Prof. Dr. Ahmed M. El-Sharkawy (Genetic Engineering Research Center, Faculty of Agriculture, Cairo University, Egypt) for helping in the critical reading of the manuscript.
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
E.K. participated in the experimental design and practical work and coordinated in the manuscript writing. R.E. Moghieab coordinated in the manuscript writing and participated in the discussion. A.M.A. participated in the experimental design, practical work, and manuscript writing. I.S. participated in the discussion and provided the intellectual support to this research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khashaba, E.H.K., Moghaieb, R.E.A., Abd El Azim, A.M. et al. Isolation, identification of entomopathogenic nematodes, and preliminary study of their virulence against the great wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae). Egypt J Biol Pest Control 30, 55 (2020). https://doi.org/10.1186/s41938-020-00257-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00257-6