Abstract
A survey for naturally occurring entomopathogenic nematodes (EPNs) was conducted in various agricultural fields in central Turkey, Nevsehir, between 2015 and 2016 years. EPNs were recovered from 20 of 112 soil samples (17.9%). Seventeen isolates were identified as Steinernema feltiae (Rhabditida: Steinernematidae). One was unknown Steinernema sp. (Rhabditida: Steinernematidae), and two were Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae). The most common species was S. feltiae, which was recovered from 5 out of 8 sites. The pathogenicity of these isolates was evaluated on the last larval instar of Galleria mellonella L. (Lepidoptera: Pyralidae) at different concentrations (25, 50, 100 IJs) in the laboratory at 25 ± 1 °C. Maximum mortality rate (87%) was achieved from the H. bacteriophora AVB-15 isolate at the lowest concentration after 48 h post-inoculation, while the only isolate that caused maximum mortality of (100%) at the concentration of 50 IJs/ml, 24 h post-inoculation was S. feltiae DDKB-17 isolate. All isolates showed high pathogenicity on G. mellonella last instar ranging from 63 to 100% at the concentration of 100 IJs/ml, 48 h post-inoculation. The present survey revealed that these EPNs are commonly present at Nevsehir, and they might have a good potential in biological control of insect pests.
Similar content being viewed by others
Background
Long-standing improper use of pesticides has led to an increased risk of contamination of the environment and harmful effects on food security. Concerns about the destructive effects of chemical pesticides have prompted a growing number of researchers across the world to find more sustainable and environmentally friendly novel control methods that offer the production of safer foods to consumers in the management of pests (Canhilal et al. 2016). Entomopathogenic nematodes (EPNs) from the families Heterorhabditidae and Steinernematidae possess many features that distinguish them from other EPNs and enable them to provide a more successful control against insect pests that live in both soil and cryptic habitats (Hazir et al. 2004; Kaya et al. 2006 and Lacey and Georgis 2012). Recently, EPNs as biological control agents proved to be successful in the management of major insect pests in agriculture (Yuksel et al. 2018; Yuksel and Canhilal 2018 and Majić et al. 2019). Therefore, there is a great scientific interest in both obtaining EPNs and determining their efficacy against the most damaging species (Canhilal et al. 2016 and Kepenekci et al. 2018).
The occurrence and distribution of EPN have been studied in some parts of Turkey, but no study has been conducted at Nevsehir (Kepenekci and Susurluk 2000; Canhilal et al. 2016 and Canhilal et al. 2017).
The objective of the present study was to determine the distribution and species composition of EPNs at Nevsehir Province, and to evaluate their pathogenicity on Galleria mellonella L.
Materials and methods
A survey took place between 2015 and 2016 years, and soil samples were collected randomly with a hand shovel from different habitats such as forest, pasture, field crops, vegetable, and fruit orchards following rainy days in spring (March–June) and autumn (September–October) (Majić et al. 2018). A total of 112 soil samples were collected. Each one (approximately 1 kg) consisted of 6–8 subsamples taken at a depth of 10–15 cm over an area of 30–40 m2. Samples were placed in polyethylene bags to avoid dehydration, kept in a cool box (approximately at 15 °C) and transported to the laboratory. The hand shovel was sterilized with 70% ethanol after each sampling.
EPN were recovered from the soil samples using Galleria traps method described by Bedding and Akhurst (1975). Subsamples, taken from each soil sample, were placed into a clean plastic box with 8 individuals of G. mellonella last instar. The boxes were covered by lids allowing air flow, turned upside down every 24 h to allow up and down free movement of the wax moth larvae inside the plastic boxes and raise their chance to meet nematode infective juveniles (IJs). The boxes were kept in the dark at 25 °C for 1 week. The Galleria larvae were checked at 3-day intervals for the presence of the dead larvae, and insect cadavers were placed individually to modified White traps (White 1927) for the emergence of the infective-stage juveniles. Emerging IJs within the first week were cleaned by distilled water and each nematode isolate was tested against 10 G. mellonella larvae to confirm Koch’s postulates for pathogenicity (Kaya and Stock 1997). The juveniles emerging from newly inoculated larvae were maintained on G. mellonella larvae, with regular inoculation each month and stored at 11 °C after rinsed three times with sterile distilled water.
The morphometric assessments of IJs and adults were made using a drawing camera (camera lucida) (Axioplan Axiophoto) attached to a ZEISS (MC100 SPOT) light microscope based on the criteria suggested by Stock and Kaya (1996), Hominick et al. (1997), and Nguyen and Hunt (2007). For morphometric characterization of each isolate, 20 first-generation hermaphrodites of Heterorhabditis species and 20 IJs and 20-s-generation males of Steinernema species were selected at random by dissecting different G. mellonella cadavers after 2–4 and 5–7 days post-infection, respectively. Selected specimens of each isolate were killed by heat, fixed (Seinhorst 1959) in TAF solution (triethanolamine formalin), and transferred to glycerin to mount on slides (Kaya and Stock 1997).
The following taxonomic characters were measured: (L) total body length; (W) maximum body diameter, (ABD) anal body diameter, (EP) excretory pore position, (ES) distance from anterior end to base of pharynx, (NR) distance from anterior end to nerve ring position, (T) tail length, a (total body length divided by maximum body diameter); b (total body length divided by distance from anterior end to base of pharynx); c (body length divided by tail length); (SL) the length of the spicules, (GU) length of the gubernaculum, D% (excretory pore position divided by distance from anterior end to base of pharynx); E% (excretory pore position divided by tail length). After morphological characteristics were made, isolates were placed into related species-groups.
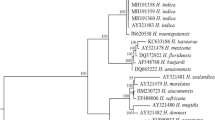
Molecular characterization of each isolate was performed by ITS rDNA (internal transcribed spacer) sequences. DNA extraction was performed, following the protocol of Waeyenberge et al. (2000). Newly harvested live 5–10 IJs of each population were used for DNA extraction. The ITS region of the isolates rDNA was amplified by a total of 50 μl PCR reaction mixture containing of 2 μl DNA, 32 μl ddH2O, 15 μl of Dream Taq PCR Master Mix (Fermentas Life Sciences, Germany), 0.5 μM of forward primer 5′-CGTAACAAGGTAGCTGTAG-3′ and 0.5 μl of the 5′-TCCTCCGCTAAATGATATG-3′ (Joyce et al. 1994). The PCR products were sequenced in a sequencing facility in both directions at Refgen, (Ankara, Turkey) and the amplified fragments of Heterorhabditis and Steinernema isolates (Rhabditida: Heterorhabditidae and Steinernematidae) were compared to the sequences of the Heterorhabditis and Steinernema species available in GenBank (NCBI).
A dose-response bioassay was performed by the isolates obtained against last instar larvae of G. mellonella to evaluate their virulence in a Petri dish arena. Ten G. mellonella larvae were placed in sterile 9-cm Petri dishes including 2 moist filter papers (100 × 15 mm) and exposed to IJs suspended in 1 ml of sterile water containing concentrations of 25, 50, and 100 IJs. The Petri dishes applied at different concentrations of IJs were put in a dark incubator at 25 ± 1 °C, R.H. 60%, and only sterile water was applied to the Petri dishes used for control treatments. The bioassay was replicated 3 times and mortality rates were recorded first and second-day post-treatment. Dead larvae were removed from the Petri dishes, incubated individually on modified White Traps at 25 ± 1 °C and dissected to confirm the presence of nematodes.
The data obtained were analyzed by factorial analysis of variance (ANOVA) using SPSS (Version 11.0) statistical software package and Tukey’s multiple range test (P < 0.05) was used to separate mean values.
Results and discussion
EPNs were isolated from 20 of the 112 soil samples collected from the districts of Hacibektas, Urgup, Avanos, Kozakli, Gulsehir, Acigol, Derinkuyu, and Nevsehir Center with a (17.9%) recovery rate. No nematodes were isolated from the soil samples collected from Gulsehir and Hacibektas districts. Urgup District had the highest proportion of positive soil samples (35%) among others. Vegetable habitats demonstrated a higher recovery frequency (35%) than other habitats and 12 soil samples collected from the forest habitats yielded no nematodes (Table 1).
Most of the nematode isolates belonged to the genus Steinernema spp., which were isolated from 18 (90%) out of 20 positive soil samples. The most common species was Steinernema feltiae, which was recovered from 17 soil samples (85%), followed by Heterorhabditis bacteriophora, found only in 2 soil samples (10%) and unidentified Steinernema sp. found only in one soil sample (5%) according to the morphometrical and molecular examination of the isolates (Tables 2 and 3).
The virulence of the obtained isolates was evaluated on G. mellonella in Petri dish experiments. The results indicated that all isolates were effective and caused significant mortality rates ranging between 13 and 100% on the first day and 30 and 100% on the second day post-treatment at 25 °C. Larval mortality increased as the concentration of the isolates tested gradually increased and no mortality occurred in control treatments. The maximum mortality rate (100%) on G. mellonella larvae was achieved by S. feltiae DDKB-17 isolate at all concentrations and exposure times, except on the first day at the concentration of 25 IJs. Four of the isolates (UTP-5, UMK-7, AVB-15, and DDKB-17) generally caused higher mortality rates than others at all concentrations and exposure times (Figs. 1 and 2).
The mortality rate (%) of entomopathogenic nematode isolates on Galleria mellonella larvae on the first day after treatment at 25 °C (25, 50, and 100 IJs). UKK-1, MKB-2, UCK-3, KBC-4, UTP-5, DTK-6, MCB-8, UCY-9, DTF-10, DDKY-11, MAY-12, ATB-13, UCB-16, DDKB-17, DME-18, UIP-19, and DMB-20: Steinernema feltiae isolate, UMK-7 and AVB-15: Heterorhabditis bacteriophora isolate, MBB-14: Steinernema sp
The mortality rate (%) of entomopathogenic nematode isolates on Galleria mellonella larvae on the second day after treatment at 25 °C (25, 50, and 100 IJs). UKK-1, MKB-2, UCK-3, KBC-4, UTP-5, DTK-6, MCB-8, UCY-9, DTF-10, DDKY-11, MAY-12, ATB-13, UCB-16, DDKB-17, DME-18, UIP-19, and DMB-20: Steinernema feltiae isolate, UMK-7 and AVB-15: Heterorhabditis bacteriophora isolate, MBB-14: Steinernema sp
The present study is the first comprehensive survey that indicates the common presence and wide distribution of EPNs in the Cappadocia Region, Nevsehir. During the survey, 20 EPN isolates were obtained by a recovery rate of (17.9%) from 112 soil samples collected from 8 districts throughout the province. The recovery rate of EPN isolates (17.9%) was similar to that reported at Karaman province (19.2%) (Yavuzaslanoglu et al. 2016) but remarkably higher than other surveys having recovery rates of (4.71%) in Rize (Ozer et al., 1995), 9% at Adana and Kahramanmaras provinces (Canhilal et al. 2016), 2.03% throughout Turkey (Hazır et al. 2003a). Recovery rates recorded for other European countries in sub-tropical regions were; 13.8% in Southern Italy (Tarasco and Triggiani 2016), 9.5% in Egypt (Shamseldean and Abd-Elgawad 1994) and 4.6% in Spain (Del Pino and Palomo 1996). The reason behind a high recovery rate in the present study might be due to the fact that the soil samples were collected from a narrow area of land at the most appropriate time, especially after rainfall as soil moisture and temperature are among the most crucial factors in the survival of EPNs in the soil environment (Wright 1992; Gaugler and Kaya 1990 and Ehlers and Peters 1996). Of the isolates obtained, the most prevalent ones were S. feltiae (Filipjev 1934) with the ratio of (85%), while the occurrence of H. bacteriophora (Poinar, 1985) (10%), and Steinernema sp. (5%) were rare. This is in agreement with the earlier studies. S. feltiae has been found as the most common EPNs species isolated in the other surveys conducted in Turkey, followed by H. bacteriophora (Laznik et al. 2009; Canhilal et al. 2016 and Canhilal et al. 2017). Both of these EPNs are widely distributed throughout the world. Even though S. feltiae has been found in different climatic zones, it is one of the species that is well-adapted to cold and continental climate conditions (Hazir et al. 2001). When considering the climate of Nevsehir, as continental and its distance from coastal areas, it is an expected result for S. feltiae to be the most isolated EPN species. Although H. bacteriophora is known to be a species that is more adapted to tropical and subtropical areas (Grewal et al. 1994), it has been isolated from many areas with different climatic conditions (Griffin et al. 1999). This suggests that EPNs are distributed in varying frequencies on earth (Griffin et al. 1999).
All the isolates tested had the ability to kill Galleria larvae, but their pathogenicity differed remarkably among different species and/or isolates. In the present study, mortality rates varied between 63 and 100% among the isolates at the concentration of 100 IJs/ml after 48-h exposure time. Similar mortality rates ranging between 78 and 100% were found at the concentration of 100 IJ/ml after 72 h in another study (El Khoury et al. 2018). Tarasco and Triggiani (2016) reported that the mortality rates of G. mellonella larvae varying between 78 and 100%, when exposed to different isolates of S. feltiae and H. bacteriophora, following 72 h of exposure to 100 IJs/ml at 25 °C. In this study, the most virulent steinernematid isolate (S. feltiae, DDKB-17) caused (100%) mortality at the lowest concentration (25 IJ/ml) after 48 h of nematode exposure. Considering the application concentration and exposure time, S. feltiae DDKB-17 isolate appeared to be more virulent than other isolates in other studies. Differences in pathogenicity among the EPNs isolates belonging to the same species have been previously well documented (Canhilal 2013 and Tarasco and Triggiani 2016). These differences in pathogenicity may be due to the application techniques used in these studies or the soil habitats of these nematode species, which is one of the most important factors affecting the performance of EPNs isolates.
Conclusions
Obtained results indicate that the EPNs isolates were highly effective against the G. mellonella larvae where the isolates virulent behavior of S. feltiae UTP-5, DDKB-17 S. feltiae, UMK-7 H. bacteriophora, and AVB-15 H. bacteriophora performed better than the other isolates at all concentrations and exposure times. Therefore, they might have a great potential in the biological control of insect pests. The present study provides insight into the distributions of EPNs in Turkey and further studies are needed to determine the pathogenicity of these isolates against major agricultural insect pests for the selection of the appropriate EPNs isolates.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21(1):109–110
Canhilal R (2013) Comparison of efficacy of nine new heterorhabditid isolates (Rhabditida: Heterorhabditidae) in Tenebrio molitor (Coleoptera.Tenebrionidae). Afr J Microbiol Res 6(7): 1597–1602.
Canhilal R, Waeyenberge L, Toktay H, Bozbuga R, Çetintas R, Imren M (2016) Distribution of Steinernematids and Heterorhabditids (Rhabditida: Steinernematidae and Heterorhabditidae) in the southern Anatolia region of Turkey. Egypt J Biol Pest Co 26(4):1-6.
Canhilal R, Waeyenberge L, Yuksel E, Koca AS, Deniz Y, Imren M (2017) Assessment of the natural presence of entomopathogenic nematodes in Kayseri soils, Turkey. Egypt J Biol Pest Cont 27(2):237-44.
Del Pino FG, Palomo A (1996) Natural occurrence of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Spanish soils. J Invertebr Pathol 68(1):84–90
Ehlers RU, Peters A (1996) Entomopathogenic nematodes in biological control: feasibility, perspectives and possible risks. In: Hokkanen HMT, Lynch JM (eds) Biological control: benefits and risks. Cambridge University Press, Cambridge
El Khoury YARA, Oreste M, Noujeim E, Nemer N, Tarasco E (2018) Effect of temperature on the pathogenicity of Mediterranean native entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from natural ecosystems. REDIA 101:123–127
Filipjev IN (1934) Eine newe art der gattung Neoaplectana steiner nebst bemerkungen uber die systematishe sellung der letzteren. Magasin de parasitologie de l'Institut zoologique des Sciences del 4: 229–240.
Gaugler R, Kaya HK (1990) Entomopathogenic nematodes in biological control. Reissued 2018 by CRC press, Boca Raton
Grewal PS, Lewis EE, Gaugler R, Campbell JF (1994) Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology 108(2):207–215
Griffin CT, Dix I, Joyce SA, Burnell AM, Downes MJ (1999) Isolation and characterisation of Heterorhabditis spp. (Nematoda: Heterorhabditidae) from Hungary, Estonia and Denmark. Nematology 1:321–332
Hazir S, Kaya HK, Stock SP, Keskin N (2004) Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turkish J Biol 27(4):181–202
Hazir S, Stock SP, Kaya HK, Koppenhöfer AM, Keskin N (2001) Developmental temperature effects on five geographic isolates of the entomopathogenic nematode Steinernema feltiae (Nematoda: Steinernematidae). J Invertebr Pathol 77(4):243–250
Hazır S, Stock SP, Keskin N (2003a) Diversity and distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Turkey. Biodivers Conserv 12: 375–386.
Hominick WM, Briscoe BR, del Pino FG, Heng J, Hunt DJ, Kozodoy E, Mracek Z, Nguyen KB, Reid AP, Spiridonov S, Stock P, Sturhan D, Waturu C, Yoshida M (1997) Biosystematics of entomopathogenic nematodes: current status, protocols and definitions. J Helminthol 71(4):271–298
Joyce SA, Reid A, Driver F, Curran J (1994) Application of polymerase chain reaction (PCR) methods to the identification of entomopathogenic nematodes. In: Burnell AM, Ehlers RU, Masson JP, (eds). COST 812 Biotechnology: Genetics of Entomopathogenic Nematode-Bacterium Complexes, Proceedings of Symposium and Workshop. St Patrick’s College, Maynooth, Co. Kildare, Ireland.DG XII, Luxembourg: European Commission, pp. 178–187.
Kaya HK, Aguillera MM, Alumai A, Choo HY, De la Torre M, Fodor A, Ganguly S, Hazir S, Lakatos S, Pye A, Wilson M, Yamanaka S, Yang H, Ehlers RU (2006) Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biol Control 38(1):134–155
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lawrence AL (ed) Manual of 12 techniques in insect pathology. Academic Press, Wapato
Kepenekci I, Hazir S, Oksal E, Lewis EE (2018) Application methods of Steinernema feltiae, Xenorhabdus bovienii and Purpureocillium lilacinum to control root-knot nematodes in greenhouse tomato systems. Crop Prot 108:31–38
Kepenekci I, Susurluk LA (2000) Turkiye icin yeni bir Entomopatojen nematod turu; Heterorhabditis marelatus Lui and Berry, 1996 (Rhabditida: Heterorhabditidae) Tarım Bilim Derg 6: 59–64.
Lacey LA, Georgis R (2012) Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematol 44:218–225
Laznik Ž, Tóth T, Lakatos T, Vidrih M, Trdan S (2009) First record of Steinernema feltiae (Filipjev) (Rhabditida: Steinernematidae) in Slovenia. Helminthologia 46:135–138
Majić I, Sarajlić A, Lakatos T, Tóth T, Raspudić E, Puškadija Z, Kanižai ŠG, Laznik Ž (2019) Virulence of new strain of Heterorhabditis bacteriophora from Croatia against Lasioptera rubi. Plant Protect Sci 55:134–141
Majić I, Sarajlić A, Lakatos T, Tóth T, Raspudić E, Zebec V, Šarić GK, Kovačić M, Laznik Ž (2018) First report of entomopathogenic nematode Steinernema feltiae (Rhabditida: Steinernematidae) from Croatia. Helminthologia 55:256–260
Nguyen K, Hunt D (2007) Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. Brill, Boston
Ozer N, Keskin N, Kirbas Z (1995) Occurrence of entomopathogenic nematodes (Steinernematidae: Heterorhabditidae) in Turkey. Nematologica 41:639–640.
Poinar GO (1985) Neoaplectana intermedia n. sp. (Steinernematidae: Nematoda) from South Carolina. Revue de Nematologie 8: 321–327.
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4(1):67–69
Shamseldean MM, Abd-Elgawad MM (1994) Natural occurrence of insect pathogenic nematodes (Rhabditida: Heterorhabditidae) in Egyptian soils. Afro-Asian J Nematol 4(2):151–154
Tarasco E, Triggiani O (2016) Survey of Steinernema and Heterorhabditis (Rhabditida: Nematoda) in Southern Italian soils. Entomologica 31:117-123.
Tarasco E, Oreste M, Li X, Liu Q (2016) Infectivity of Mediterranean native Entomopathogenic nematodes (Steinerne¬ matidae and Heterorhabditidae) from natural habitats in relation to temperature. Redia 98(1):109–114
Waeyenberge L, Ryss A, Moens M, Pinochet J, Vrain TC (2000) Molecular characterisation of 18 Pratylenchus species using rDNA restriction fragment length polymorphism. Nematology 2(2):135–142
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303
Wright PJ (1992) Cool temperature reproduction of steinernematid and heterorhabditid nematodes. J Invertebr Pathol 60(2):148–151
Yavuzaslanoglu E, Gozel U, Gozel C, Aydogdu M (2016) Distribution of the entomopathogenic nematodes in apple growing areas of Karaman, Turkey. Pakistan J Nematol 34(1):53–62
Yuksel E, Canhilal R (2018) Evaluation of local isolates of entomopathogenic nematodes for the management of black cutworm, Agrotis ipsilon Hufnagel (Lepidoptera: Noctuidae). Egypt J Biol Pest Cont 28(1):82
Yuksel E, Taskesen YE, Erarslan D, Canhilal R (2018) Effectiveness of different entomopathogenic nematode species against the variegated cutworm, Peridroma saucia (Hubner) (Lepidoptera: Noctuidae). Egypt J Biol Pest Cont 28(1):8
Acknowledgements
We thank Prof. Dr. Halil Elekcioğlu (Cukurova University), Assoc. Prof. Dr. Mustafa İMREN (Abant Izzat Baysal University), Abdurrahman Sami KOCA (Abant Izzat Baysal University), and Ece Bortecine KASAPOGLU (Cukurova University) for their guidance in morphometric and molecular analysis.
Funding
This study was funded by Project No. FYL-2017-7664 by the office of scientific research projects of Erciyes University. The funders had no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
EY and RC conceived and designed the research. EY conducted the experiments, analyzed the data, and wrote the manuscript. Both authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All applicable institutional and national guidelines for the care and use of animals were followed.
Consent for publication
Both authors of this manuscript accepted that the paper is submitted for publication in the Journal of Pest Science, and reported that this paper has not been published or accepted for publication in another journal, and it is not under consideration at another journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yuksel, E., Canhilal, R. Isolation, identification, and pathogenicity of entomopathogenic nematodes occurring in Cappadocia Region, Central Turkey. Egypt J Biol Pest Control 29, 40 (2019). https://doi.org/10.1186/s41938-019-0141-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-019-0141-9