Abstract
Ocean acidification (OA) and coastal eutrophication affect coastal marine organisms. We studied the physiological responses of Gracilariopsis lemaneiformis (Gracilariales, Rhodophyta) to increased concentrations of CO2 and NH4 +. Incubation treatments were applied at two different pH units (low, 7.5; high (control), 7.9) and three different NH4 + concentrations (low, 10; medium, 50; high, 100 μM). Growth, rates of photosynthetic oxygen evolution, and NH4 + uptake rates were affected by both elevated CO2 and NH4 + conditions. The changes in the pH of culture media were influenced by elevated CO2 or NH4 + treatments. However, chlorophyll fluorescence was affected only by the level of NH4 +. These results indicate that the physiological responses of G. lemaneiformis might be enhanced when the concentrations of CO2 and NH4 + rise. Therefore, cultures of this alga could provide a good mitigation solution against ongoing problems with OA and coastal eutrophication.
Similar content being viewed by others
Background
In coastal regions, marine organisms face serious environmental problems caused by anthropogenic eutrophication and, more recently, ocean acidification, or OA (Fei 2004; Doney et al. 2009; Boyd 2011). The combinations of ocean acidification and changing states of eutrophication affect the physiology of marine species (Reymond et al. 2013; Chen et al. 2016). A report by the Intergovernmental Panel on Climate Change (IPCC 2014) revealed that, since the Industrial Revolution, atmospheric CO2 concentrations have increased from 280 to 400 ppm while pH values have decreased 0.1 units. Orr et al. (2005) have predicted that pH values will decline by 0.3 to 0.4 units if atmospheric CO2 concentrations reach above 800 ppm. This OA phenomenon influences the physiology of marine biota such as calcifying species, phytoplankton, and seaweeds (Hinga 2002; Gao and Zheng 2010; Cornwall et al. 2012; Kram et al. 2016). Although OA can have a negative impact on the metabolism of calcifying organisms (Hofmann and Bischof 2014; Kram et al. 2016), many macroalgae respond positively under acidified conditions (Zou 2005; Sarker et al. 2013; Kram et al. 2016). For example, many macroalgal species utilize carbon-concentrating mechanisms (CCMs) that enable them to acquire CO2 from HCO3 −. When concentrations of dissolved inorganic carbon (DIC) are elevated, macroalgae take up this DIC and conserve energy by downregulating their CCMs (Beardall et al. 1998; Sarker et al. 2013). In this process, the growth of seaweeds might be enhanced under higher CO2 concentrations (Beardall et al. 1998; Sarker et al. 2013).
Coastal eutrophication is a severe challenge that has resulted from human activities such as aquaculture, industrialization, and urbanization. Higher concentrations of nutrients, e.g., nitrogen (N) and phosphorus (P), can improve photosynthetic rates in primary producers, including seaweeds (Chen et al. 2016; Xu et al. 2017b). However, excessive inputs of those nutrients can change the function and structure of a marine ecosystem (Valiela et al. 1997; Wu et al. 2015). In addition, eutrophication could also cause ocean acidification in the coastal zones because of the active respiration of bacteria (Cai et al. 2011).
Gracilariopsis lemaneiformis (Bory de Saint-Vincent) E.Y.Dawson, Acleto & Foldvik (Gracilariales, Rhodophyta, formerly Gracilaria lemaneiformis) is distributed in tropical and warm-temperate regions, and is cultivated worldwide, especially in Chile, China, and Taiwan (Armisen 1995; Tseng 2001; Yang et al. 2015). In China, this alga is cultivated on a large scale because it is an economically important source of agar (Fei 2004; Wang et al. 2010). Furthermore, this species has been studied as a valuable component of Integrated Multi-Trophic Aquaculture and as a bioremediation agent (Yang et al. 2006, 2015; Wu et al. 2015).
The physiological responses of G. lemaneiformis have previously been monitored in experiments that combined various CO2 levels with a range of nutrient concentrations or light intensities (Zou and Gao 2009; Xu et al. 2010; Xu and Gao 2012; Chen et al. 2017). The results from those studies indicated that plant growth, photosynthesis, rate of nutrient uptake, and levels of biochemical components and amino acids are increased under elevated CO2 conditions, light intensities, or nutrient concentrations (Zou and Gao 2009; Xu et al. 2010; Xu and Gao 2012; Chen et al. 2017). However, that earlier research did not focus on the physiological reactions of G. lemaneiformis under higher CO2 and ammonium (NH4 +) concentrations. Therefore, we looked at whether this species has the capacity to mitigate the effects of OA and coastal eutrophication by measuring growth rates, pH changes in the culture medium, oxygen evolution by photosynthesis, the rate of ammonium uptake (an indicator of plant capacity for nutrient removal), and chlorophyll fluorescence (for assessing photosynthetic efficiency). We then analyzed the interactions among different combinations of CO2 and NH4 + levels.
Methods
We collected Gracilariopsis lemaneiformis at culture farm near Nanao Island, Shantou, China (23° 20′ N, 116° 55′ E), in March of 2016. Samples were transported to the field station near the farm and washed several times to remove any epiphytes. They were acclimated for 24 h before the experiments under 20 °C, 100 μmol photons m−2 s−1 (12:12 light/dark; a LI-250 light meter, LI-COR, USA). For each treatment, fresh samples (2 g) were placed in culture vessels containing 250 mL of filtered seawater. All experiments utilized a factorial design that included two pH conditions (low 7.5; high 7.9, control) and three ammonium concentrations (low 10; medium 50; high 100 μM). The low NH4 + level was based on the ambient NH4 + (8.84 ± 1.42 μM) concentration at the sampling site. The medium and high NH4 + concentrations were assumed severe eutrophication conditions and were made by adding to ambient NH4 + level, respectively. The low-pH condition indicated elevated CO2 concentration in the future condition and high-pH condition has shown the ambient CO2 level. The six culture treatments (four replicates each) were identified as follows: LpHLA, low pH + low NH4 +; HpHLA, high pH + low NH4 +; LpHMA, low pH + medium NH4 +; HpHMA, high pH + medium NH4 +; LpHHA, low pH + high NH4 +; and HpHHA, high pH + high NH4 +. The low-pH medium was prepared by mixing filtered seawater with pure CO2-enriched seawater from a CO2 tank. Gattuso et al. (2010) indicated that mixing of high CO2 seawater is the reasonable method as similar as CO2 bubbling. The medium and high NH4 + concentrations were obtained by adding 50 or 100 μM NH4Cl, respectively, to the medium. All media were changed every day. Total alkalinity (TA) was measured by the electro-titration method (Gran 1952; precision ±4 μmol kg−1). The amounts of DIC and the pCO2 values were calculated by the CO2SYS program (Lewis and Wallace 1998) (Table 1). The dissociation constant and KSO4 values were defined by Millero et al. (2006) and Dickson (1990).
The growth of G. lemaneiformis was quantified by measuring the change in fresh weight (FW) after 3 days of incubation. The relative growth rate (RGR; % day−1) was calculated as follows:
where W 1 is the initial fresh weight, W 2 is the final fresh weight, and T is the incubation period (3 days).
Over a 12-h period, we monitored changes in pH in the culture media, the rate of oxygen evolution, and NH4 + uptake. An Orion-250A meter (Thermoscientific, USA) was used to measure pH at 0, 2, 4, 6, and 12 h. We also assessed the changes in pH in vessels containing media but no specimens (blanks), under low- and high-pH conditions.
Rates of photosynthetic oxygen evolution (μmol O2 g−1 FW h−1) were recorded with a Clark-type microelectrode oxygen sensor (Unisense, Denmark), which was calibrated with a mixture of C6H7NaO6 (sodium ascorbate) of 0.4 g and NaOH (sodium hydroxide) of 2 g in the 100-mL dilution water.
The rate of NH4 + uptake (μmol NH4 + g−1 FW h−1) was calculated as the amount lost from each culture medium over 12 h. The measurement method of NH4 + uptake rates were described by Parsons et al. (1984). The following equation was used in the calculation:
where S i is the initial concentration of NH4 +, S f is the final concentration after T hours of incubation, vol is the volume of the medium, and W is the fresh weight of each algal specimen.
Chlorophyll fluorescence was determined after 3 days with a Plant Efficiency Analyzer (PEA, Hansatech, UK). The maximum quantum yield (F v/F m) of Photosystem II was calculated as follows:
where F m is maximum fluorescence after dark adaptation and F o is minimum fluorescence after dark adaptation. Algal samples were placed in leaf-clip holders and F v/F m was measured by applying a saturating pulse after the samples were dark-adapted for 15 min.
One- and two-way ANOVA were conducted with all experimental data. Normality and homogeneity were investigated before the statistical analysis began. After that, Tukey’s tests were used to compare among treatments, and the threshold for statistically significant differences was set at p < 0.05. All analyses were performed with the SPSS software program (version 23.0).
Results
Results of physiological responses of Gracilariopsis lemaneiformis under elevated CO2 and NH4 + treatments were summarized in Table 2. During the incubation period, the relative growth rate of Gracilariopsis lemaneiformis increased under elevated CO2 and NH4 + treatments. The maximum RGR was 2.95 ± 0.20% day−1 under LpHHA conditions while the minimum RGR, 1.07 ± 0.21% day−1, was achieved under HpHLA conditions (Fig. 1). At the high NH4 + levels, RGR was greater at pH 7.5 than at pH 7.9 (HA: F = 6.04, p = 0.04).
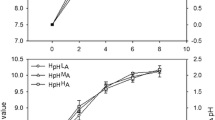
Alterations in pH in the culture media containing algal specimens were significantly affected by initial pH or NH4 + levels. The extent of variation in pH ranged from 0.44 ± 0.02 to 1.26 ± 0.18 units, and the maximum and minimum changes in pH were associated with LpHHA and HpHLA, respectively (Fig. 2; Table 3). At each pH level, the medium and high NH4 + concentrations were significantly different from the low NH4 + condition (LpH: F = 17.08, p = 0.01; HpH: F = 78.98, p < 0.01). At individual NH4 + levels, the change in pH was significantly greater in the low-pH treatment than in the high-pH treatment (LA: F = 6.65, p = 0.04; MA: F = 6.94, p = 0.04; HA: F = 138.86, p < 0.01). The pH values in blank culture media were remain constant over 12 h that pH values were affected only photosynthesis oxygen evolution of G. lemaneiformis.
Photosynthetic oxygen evolution was affected by elevated levels of CO2 and NH4 +. Rates were 62.28 ± 1.71 μmol O2 g−1 FW h−1 under HpHLA and 111.48 ± 0.95 μmol O2 g−1 FW h−1 under LpHHA (Fig. 3). When compared at the same initial pH, those rates increased significantly as the NH4 + level rose (LpH: F = 479.22, p < 0.01; HpH: F = 854.92, p < 0.01). Under medium and high NH4 + conditions, the rate was greater at low than at high pH but there was no significant difference at the low NH4 + level (LA: F = 0.26, p = 0.63; MA: F = 7.94, p = 0.03; HA: F = 7.67, p = 0.03).
The rate of NH4 + uptake was significantly affected by elevated concentrations of CO2 and NH4 +, with rates ranging from 0.84 ± 0.01 to 7.43 ± 0.03 μmol NH4 + g−1 FW h−1 (Fig. 4). Uptake was more rapid under LpHHA conditions and slowest under HpHLA treatment. When those rates were compared at the same pH, values increased under higher NH4 + concentrations (LpH: F = 3230.83, p < 0.01; HpH: F = 25,898.16, p < 0.01). Furthermore, at the same level of NH4 +, uptake was more rapid under LpHHA than under HpHHA treatment (HA: F = 6.50, p = 0.04).
When values of the chlorophyll fluorescence were used as a proxy to represent photosynthetic efficiency, they ranged from 0.55 ± 0.22 (HpHLA) to 0.64 ± 0.02 (LpHHA) (Fig. 5). Although values of F v/F m were significantly affected under high NH4 + levels and under both pH conditions (LpH: F = 44.64, p < 0.01; HpH: F = 15.91, p < 0.01), they were not significantly influenced by NH4 + conditions (LA: F = 0.60, p = 0.47; MA: F = 1.23, p = 0.31; HA: F = 0.92, p = 0.37).
Discussion
Growth of Gracilariopsis lemaneiformis was affected by elevated CO2 and NH4 + treatments and there were interactions of both factors. Previous studies have revealed similar results with G. lemaneiformis, Hypnea spinella, Chondrus crispus, Pyropia haitanensis , and Ulva pertusa (Yu and Yang 2008; Suárez-Álvarez et al. 2012; Sarker et al. 2013; Chen et al. 2016; Kang and Chung 2017). When DIC concentrations rise in the ocean, many macroalgal species conserve energy by regulating their CCMs, thereby improving their growth performance (Sarker et al. 2013). For example, red algae within the Gracilaria genus used external carbonic anhydrase to increase their capacity for HCO3 − utilization, which boosts their growth under elevated CO2 conditions (Israel and Beer 1992; García-Sánchez et al. 1994; Zou et al. 2004). Greater availability of nutrients can also enhance the growth of macroalgae (Yu and Yang 2008). Although greater accumulations of nitrogen can stimulate growth, Xu et al. (2010) reported that G. lemaneiformis development was influenced by either CO2 or the level of phosphorus, but those two factors did not show synergistic effects. Our results indicated that RGR were lower than those of other experiments. We speculated that short-term incubation period and high stocking density could be the reason of lower RGR. Kim et al. (2013) indicated that high stocking density might cause lower growth rates than those of light shading of the culture vessels. We assumed the high stocking density conditions of aquaculture farm to find realistic growth of G. lemaneiformis. In addition, Xu et al. (2017a) found that the RGRs of short-time cultivation period were higher than those of long-time cultivation time. They explained that decreased of RGRs resulted from the acclimation to culture conditions.
The pH values in the culture medium could be used as an indicator of physiological characteristics of the macroalgae (Maberly 1990; Murru and Sandgren 2004). The pH of our culture medium was altered in response to increases in CO2 or NH4 + concentrations. The addition of CO2 gas caused pH values to decline while the concentration of DIC, such as CO2(aq), and HCO3 − increased. This change in pH was more dramatic in the low-pH medium than under high-pH conditions. The DIC concentrations were elevated in the culture medium, which lead to increases in pH levels by photosynthesis (Zhang et al. 2012). In addition, we did not find any inhibition of photosynthesis. When pH values are >9.0, photosynthesis of some species is more likely to be inhibited (Maberly 1990; Björk et al. 2004). Zou et al. (2004) indicated that inorganic carbon (Ci) affinity and photosynthetic rates at pH 9.0 were decreased in G. lemaneiformis. In case of pH values over 9.0, this alga does not accumulate CO2 because of poor capacity of HCO3 − utilization (Zou et al. 2004).
Photosynthetic oxygen evolution in our Gracilariopsis lemaneiformis samples was also increased by elevated CO2 and NH4 + levels. The same has been reported for Hizikia fusiforme (currently, Sargassum fusiforme), Hypnea spinella, and Pyropia haitanensis (Zou 2005; Suárez-Álvarez et al. 2012; Chen et al. 2016). Zou et al. (2004) showed that the photosynthesis of G. lemaneiformis is already saturated under normal DIC concentrations found in natural seawater. However, we noted that this species was also influenced by the synergism between CO2 and NH4 +. Similarly, Xu et al. (2010) has shown that the maximum net photosynthetic rate for G. lemaneiformis is influenced by increases in both CO2 and phosphorus concentrations.
The uptake of NH4 + was influenced by treatment with exogenous CO2 and NH4 +. Research with Gracilaria sp., G. chilensis, Hizikia fusiforme, Gracilariopsis lemaneiformis, Pyropia yezoensis, P. haitanensis, and U. pertusa has demonstrated that all respond to elevated levels of CO2 or NH4 + (Gao et al. 1993; Zou 2005; Xu et al. 2010; Kang et al. 2014; Chen et al. 2016; Kang and Chung 2017). Increasing the concentration of CO2 can enhance nitrogen uptake and the activity of nitrate reductase (Gordillo et al. 2001; Xu et al. 2010; Hofmann et al. 2013; Liu and Zou 2015). In various seaweeds, the uptake of NH4 + is greater when the initial NH4 + concentration is high (Dy and Yap 2001). In addition, when more nutrients are available, they are more easily absorbed (Runcie et al. 2003; Pérez-Mayorga et al. 2011). Therefore, because NH4 + uptake by our G. lemaneiformis samples was more rapid under the elevated CO2 and NH4 + treatment, we speculate that this was due to the associated rise in photosynthesis.
Chlorophyll fluorescence, which can reflect photosynthetic efficiency, is enhanced by increased N concentrations (Dawes and Koch 1990). However, the role of CO2 is debatable with some studies showing that F v/F m is not affected by elevated CO2 levels (Hofmann et al. 2012; Olischläger et al. 2013; Kram et al. 2016) and others indicating that chlorophyll fluorescence increases under high CO2 concentrations (Chen et al. 2015). We found here that the photosynthetic efficiency of G. lemaneiformis was more strongly affected by greater NH4 + concentrations than by higher CO2 levels. Because of these contrasting reports, direct measurement of oxygen evolution is now considered a more appropriate method than chlorophyll fluorescence for monitoring changes in efficiency (Kram et al. 2016).
Our data provided evidence that the physiological activities of G. lemaneiformis are enhanced under elevated CO2 and NH4 + treatments. We predict that mass production of this species in China will increase in response to OA and eutrophication. Kim and Yarish (2014) indicated that productivity of Gracilaria sp. was enhanced under elevated CO2 levels with high stocking density and irradiance. The biomass production of G. chilensis was also increased at elevated CO2 levels or high nutrient concentrations (Buschmann et al. 1994). The increase of harvest can be used as agar materials, seafood, and feed for abalone (Tseng 2001; Fei 2004; Yang et al. 2015). Yang et al. (2015) have suggested that large-scale cultivation of Gracilaria can improve water quality by uptake of excessive nutrients and potential carbon sink absorber along the coast of China. Therefore, this alga could provide a good solution for mitigation against the problems associated with such marine challenges. If we are to achieve practical results, future investigations will require more large-scale, long-term experiments. Those projects must also focus on the synergistic effects of several environmental factors, e.g., temperature, light intensity, level of salinity, and nutrient concentrations.
Conclusions
The combined treatment of elevated CO2 and NH4 + heightened the physiological reactions of G. lemaneiformis, as demonstrated by changes in relative growth rates, rates of photosynthetic oxygen evolution, and the uptake of ammonium. The pH values were affected each elevated CO2 or NH4 + treatments. In contrast, we noted that chlorophyll fluorescence was affected only by altering the concentration of NH4 +.
Abbreviations
- OA:
-
Ocean acidification
- RGR:
-
Relative growth rates
References
Armisen R. World-wide use and importance of Gracilaria. J Appl Phycol. 1995;7:231–43.
Beardall J, Beer S, Raven JA. Biodiversity of marine plants in an era of climate change: some predictions based on physiological performance. Bot. Mar. 1998;41:113–24.
Björk M, Axelsson L, Beer S. Why is Ulva intestinalis the only macroalga inhabiting isolated rockpools along the Swedish Atlantic coast? Mar Ecol Prog Ser. 2004;284:109–16.
Boyd PW. Beyond ocean acidification. Nat Geosci. 2011;4:273–4.
Buschmann AH, Mora OA, Gómez P, Böttger M, Buitano S, Retamales C, Pedro A, Gutierrez A. Gracilaria chilensis outdoor tank cultivation in Chile: use of land-based salmon culture effluents. Aquac Eng. 1994;13:283–300.
Cai WJ, Hu X, Huang WJ, Murrell MC, Lehrter JC, Lohrenz SE, Chou WC, Zhai W, Hollibaugh JT, Wang Y, Zhao P. Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci. 2011;4:766–70.
Chen B, Zou D, Jiang H. Elevated CO2 exacerbates competition for growth and photosynthesis between Gracilaria lemaneiformis and Ulva lactuca. Aquaculture. 2015;443:49–55.
Chen B, Zou D, Ma J. Interactive effects of elevated CO2 and nitrogen-phosphorus supply on the physiological properties of Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol. 2016;28:1235–43.
Chen B, Zou D, Zhu M, Yang Y. Effects of CO2 levels and light intensities on growth and amino acid contents in red seaweed Gracilaria lemaneiformis. Aquac Res. 2017;48:2683–90.
Cornwall CE, Hepburn CD, Pritchard D, Currie KI, McGraw CM, Hunter KA, Hurd CL. Carbon-use strategies in macroalgae: differential responses to lowered pH and implications for ocean acidification. J Phycol. 2012;48:137–44.
Dawes CJ, Koch EW. Physiological responses of the red algae Gracllarla Verrucosa and G. Tikvahiae before and after nutrient enrichment. Bull Mar Sci. 1990;46:335–44.
Dickson AG. Standard potential of the reaction: AgCl (s)+ 12H2 (g)= Ag (s)+ HCl (aq), and and the standard acidity constant of the ion HSO4 − in synthetic sea water from 273.15 to 318.15 K. J Chem Thermodyn. 1990;22:113–27.
Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Annu Rev Mar Sci. 2009;1:169–92.
Dy DT, Yap HT. Surge ammonium uptake of the cultured seaweed, Kappaphycus alvarezii (Doty) Doty (Rhodophyta: Gigartinales). J Exp Mar Biol Ecol. 2001;265:89–100.
Fei X. Solving the coastal eutrophication problem by large scale seaweed cultivation. Hydrobiologia. 2004;512:145–51.
Gao K, Aruga Y, Asada K, Kiyohara M. Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J Appl Phycol. 1993;5:563–71.
Gao K, Zheng Y. Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Glob Chang Biol. 2010;16:2388–98.
García-Sánchez MJ, Fernández JA, Niell X. Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta. 1994;194:55–61.
Gattuso JP, Gao K, Lee K, Rost B, Schulz KG. Approaches and tools to manipulate the carbonate chemistry. In: Riebesell U, Fabry VJ, Hansson L, Gattuso JP, editors. Guide to best practices for ocean acidification research and data reporting. Luxembourg: Publications Office of the European Union; 2010. p. 41–52.
Gordillo FJ, Niell FX, Figueroa FL. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta. 2001;213:64–70.
Gran G. Determination of the equivalence point in potentiometric titrations of seawater with hydrochloric acid. Oceanol Acta. 1952;5:209–18.
Hinga KR. Effects of pH on coastal marine phytoplankton. Mar Ecol Prog Ser. 2002;238:281–300.
Hofmann LC, Bischof K. Ocean acidification effects on calcifying macroalgae. Aquat Biol. 2014;22:261–79.
Hofmann LC, Straub S, Bischof K. Competition between calcifying and noncalcifying temperate marine macroalgae under elevated CO2 levels. Mar Ecol Prog Ser. 2012;464:89–105.
Hofmann LC, Straub S, Bischof K. Elevated CO2 levels affect the activity of nitrate reductase and carbonic anhydrase in the calcifying rhodophyte Corallina officinalis. J Exp Bot. 2013;64:899–908.
IPCC. Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. New York: Cambridge University Press; 2014.
Israel A, Beer S. Photosynthetic carbon acquisition in the red alga Gracilaria conferta. Mar Bio. 1992;112:697–700.
Kang JW, Chung IK. The effects of eutrophication and acidification on the ecophysiology of Ulva pertusa Kjellman. J Appl Phycol. 2017; doi:10.1007/s10811-017-1087-5.
Kang YH, Kim S, Lee JB, Chung IK, Park SR. Nitrogen biofiltration capacities and photosynthetic activity of Pyropia yezoensis Ueda (Bangiales, Rhodophyta): groundwork to validate its potential in integrated multi-trophic aquaculture (IMTA). J Appl Phycol. 2014;26:947–55.
Kim JK, Duston J, Corey P, Garbary DJ. Marine finfish effluent bioremediation: effects of stocking density and temperature on nitrogen removal capacity of Chondrus crispus and Palmaria palmata (Rhodophyta). Aquaculture. 2013;414:210–6.
Kim JK, Yarish C. Development of a sustainable land-based Gracilaria cultivation system. Algae. 2014;29:217–25.
Kram SL, Price NN, Donham EM, Johnson MD, Kelly ELA, Hamilton SL, Smith JE. Variable responses of temperate calcified and fleshy macroalgae to elevated pCO2 and warming. ICES J Mar Sci. 2016;73:693–703.
Lewis E, Wallace DWR. Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon dioxide information analysis center. Oak Ridge: Oak Ridge National Laboratory, US Department of Energy; 1998.
Liu C, Zou D. Do increased temperature and CO2 levels affect the growth, photosynthesis, and respiration of the marine macroalga Pyropia haitanensis (Rhodophyta)? An experimental study. Hydrobiologia. 2015;745:285–96.
Maberly SC. Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J Phycol. 1990;26:439–49.
Millero FJ, Graham TB, Huang F, Bustos-Serrano H, Pierrot D. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar Chem. 2006;100:80–94.
Murru M, Sandgren CD. Habitat matters for inorganic carbon acquisition in 38 species of red macroalgae (Rhodophyta) from Puget sound, Washington. USA J Phycol. 2004;40:837–45.
Olischläger M, Bartsch I, Gutow L, Wiencke C. Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycol Res. 2013;61:180–90.
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner G, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig M, Yamanaka Y, Yool A. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–6.
Parsons TR, Maita Y, Lalli CM. A manual of chemical and biological methods for seawater analysis. New York: Pergamon Press; 1984.
Pérez-Mayorga DM, Ladah LB, Zertuche-González JA, Leichter JJ, Filonov AE, Lavín MF. Nitrogen uptake and growth by the opportunistic macroalga Ulva lactuca (Linnaeus) during the internal tide. J Exp Mar Biol Ecol. 2011;406:108–15.
Reymond CE, Lloyd A, Kline DI, Dove SG, Pandolfi JM. Decline in growth of foraminifer Marginopora rossi under eutrophication and ocean acidification scenarios. Glob Chang Biol. 2013;19:291–302.
Runcie JW, Ritchie RJ, Larkum AW. Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquat Bot. 2003;76:155–74.
Sarker MY, Bartsch I, Olischläger M, Gutow L, Wiencke C. Combined effects of CO2, temperature, irradiance and time on the physiological performance of Chondrus crispus (Rhodophyta). Bot. Mar. 2013;56:63–74.
Suárez-Álvarez S, Gómez-Pinchetti JL, García-Reina G. Effects of increased CO2 levels on growth, photosynthesis, ammonium uptake and cell composition in the macroalga Hypnea spinella (Gigartinales, Rhodophyta). J Appl Phycol. 2012;24:815–23.
Tseng CK. Algal biotechnology industries and research activities in China. J Appl Phycol. 2001;13:375–80.
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K. Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr. 1997;42:1105–18.
Wang Z, Wang G, Niu J, Wang W, Peng G. Optimization of conditions for tetraspore release and assessment of photosynthetic activities for different generation branches of Gracilaria lemaneiformis Bory. Chin J Oceanol Limnol. 2010;28:738–48.
Wu H, Huo Y, Hu M, Wei Z, He P. Eutrophication assessment and bioremediation strategy using seaweeds co-cultured with aquatic animals in an enclosed bay in China. Mar Pollut Bull. 2015;95:342–9.
Xu D, Schaum CE, Lin F, Sun K, Munroe JR, Zhang XW, Fan X, Teng LH, Wang YT, Zhuang ZM, Ye N. Acclimation of bloom-forming and perennial seaweeds to elevated pCO2 conserved across levels of environmental complexity. Glob Chang Biol. 2017a; doi:10.1111/gcb.13701.
Xu Z, Gao G, Xu J, Wu H. Physiological response of a golden tide alga (Sargassum muticum) to the interaction of ocean acidification and phosphorus enrichment. Biogeosciences. 2017b;14:671–81.
Xu Z, Gao K. NH4 + enrichment and UV radiation interact to affect the photosynthesis and nitrogen uptake of Gracilaria lemaneiformis (Rhodophyta). Mar Pollut Bull. 2012;64:99–105.
Xu Z, Zou D, Gao K. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Bot. Mar. 2010;53:123–9.
Yang Y, Chai Z, Wang Q, Chen W, He Z, Jiang S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015;9:236–44.
Yang YF, Fei XG, Song JM, Hu HY, Wang GC, Chung IK. Growth of Gracilaria lemaneiformis under different cultivation conditions and its effects on nutrient removal in Chinese coastal waters. Aquaculture. 2006;254:248–55.
Yu J, Yang YF. Physiological and biochemical response of seaweed Gracilaria lemaneiformis to concentration changes of N and P. J Exp Mar Biol Ecol. 2008;367:142–8.
Zhang N, Song J, Cao C, Ren R, Wu F, Zhang S, Sun X. The influence of macronitrogen (NO3 − and NH4 +) addition with Ulva pertusa on dissolved inorganic carbon system. Acta Oceanol Sin. 2012;31:73–82.
Zou D. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture. 2005;250:726–35.
Zou D, Gao K. Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia. 2009;48:510–7.
Zou D, Xia J, Yang Y. Photosynthetic use of exogenous inorganic carbon in the agarophyte Gracilaria lemaneiformis (Rhodophyta). Aquaculture. 2004;237:421–31.
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Korean Government (MSIP) (NRF-2016R1A2B1013637), to IKC.
Funding
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Korean Government (MSIP) (NRF-2016R1A2B1013637), to IKC.
Availability of data and materials
All datasets in this study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors conducted the field survey in the sampling stations. JW, Cicilia, and IK conducted experiments and wrote manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kang, J.W., Kambey, C., Shen, Z. et al. The short-term effects of elevated CO2 and ammonium concentrations on physiological responses in Gracilariopsis lemaneiformis (Rhodophyta). Fish Aquatic Sci 20, 18 (2017). https://doi.org/10.1186/s41240-017-0063-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41240-017-0063-y