Abstract
Surgical resection is a widely used method for the treatment of solid tumor cancers. However, the inhibition of tumor recurrence and metastasis are the main challenges of postoperative tumor therapy. Traditional intravenous or oral administration have poor chemotherapeutics bioavailability and undesirable systemic toxicity. Polymeric hydrogels with a three-dimensional network structure enable on-site delivery and controlled release of therapeutic drugs with reduced systemic toxicity and have been widely developed for postoperative adjuvant tumor therapy. Among them, because of the simple synthesis, good biocompatibility, biodegradability, injectability, and multifunctionality, iron-based hydrogels have received extensive attention. This review has summarized the general synthesis methods and construction principles of iron-based hydrogels, highlighted the latest progress of iron-based hydrogels in postoperative tumor therapy, including chemotherapy, photothermal therapy, photodynamic therapy, chemo-dynamic therapy, and magnetothermal-chemical combined therapy, etc. In addition, the challenges towards clinical application of iron-based hydrogels have also been discussed. This review is expected to show researchers broad perspectives of novel postoperative tumor therapy strategy and provide new ideas in the design and application of novel iron-based hydrogels to advance this sub field in cancer nanomedicine.
Similar content being viewed by others
Introduction
Cancer is one of the diseases with the highest mortality rate to severely obstruct life expectancy expansion and life quality improvement [1, 2]. According to the statistics of the World Health Organization in 2019, the number of patients who died from cancers have exceeded cardiovascular and cerebrovascular accidents, in more than 60% countries, to become the first or second leading cause of death of the group younger than 70 [3]. Surgery is the commonly used method to treat solid tumors [4]. However, the surgical resection cannot eradicate carcinoma tissue completely which poses a challenge to the clinical treatment. Some cancer focuses may inevitably remain on the wound tissue because of the infiltrative and invasive properties may significantly increase the risk of local recurrence and metastasis [5,6,7,8]. Some researchers considered that surgery enhances successful implantation of spilled tumor cells [9,10,11]. Besides, in clinical practice, eliminating cancerous tissue inevitably leads to large tissue defects, which not only impairs normal function and appearance, but also causes impact to the mental health of patients [12,13,14,15,16]. Meanwhile, the surgery will cause potential bacterial infection to impede wound healing and skin regeneration in the resection site [17,18,19,20,21]. Therefore, it is urgently needed to develop new therapeutic methods to meet the requirements of comprehensive treatment after radical surgery of tumor.
To eradicate residual tumor cells and reduce recurrence rates, radiotherapy and chemotherapy are generally used as adjuvant treatments alongside with surgery [22]. Systemic application of chemotherapy drugs is preferred with relatively higher effectiveness, especially in the treatment of hardly approachable or unknown tumor, because the drugs can affect cancer cells even outside their primary region [23]. However, conventional systematic chemotherapy still faces some challenges, mainly including low efficacy to reach the tumor sites and the ensuing adverse reactions [24,25,26]. Consequently, local chemotherapy would be beneficial, particularly in the case of approachable tumor tissue. In the drug-loaded system, drugs can be directly located at the cancerous region to avoid excessive drug circulation compared to systemic drug administration with suppressed serious side effects on normal tissues [27,28,29,30,31,32]. In local drug delivery systems, hydrogel-based cancer therapy platforms have attracted much attention. Hydrogel is a network polymer formed by crosslinking gel monomers with soft texture, excellent biocompatibility, adjustable biochemical and biophysical properties [33,34,35,36]. There are many kinds of hydrogels with different synthesis methods to meet a variety of application requirements [37]. Here, the advantages of hydrogel in postoperative treatment of tumors are listed. Firstly, high drug loading capacity [38,39,40] and good compatibility in drug co-encapsulation [41,42,43,44,45,46,47] can endow the system different functions and therapeutic effects. Drug-loaded hydrogels can be filled into the tumor site or cavity after the surgical resection of solid tumor to maintain the local drug concentration for a long time and greatly alleviate the possibility of recurrence of residual cancer cells [48]. Secondly, hydrogel can realize controllable drug releasing [49,50,51]. Hydrogels can respond to various stimuli [52], for example, light [53], near infrared light (NIR) [54,55,56], temperature [57, 58], pH [59], magnetic field [60], enzymes [61], ionic strength and electric field. The controlled release of drugs can be realized by virtue of the reversible expansion and contraction properties of hydrogels under specific stimulation conditions to fix the size of gel grids. This customizable antineoplastic protocol makes it possible to achieve more personalized cancer therapy. Thirdly, hydrogels also have great clinical practice potential in tissue reconstruction. As a powerful biomaterial, hydrogel has been widely used in tissue engineering, including spinal cord repairment [62], wound healing [63], tissue reconstruction [64, 65], and bone regeneration [66]. For example, hydrogel has unique advantages in the process of tissue reconstruction and plasticity after breast-conserving surgery for breast cancer [67]. After the visible lesions are surgically removed, hydrogels can be used to fill and repair the breast defects to inhibit tumor recurrence while restoring its original appearance. On the other hand, the hydrogel has good water absorption, moisture retention characteristics and air permeability to provide biomechanically supportive 3D microenvironments for cell proliferation and migration, showing potential application prospect in the field of serving as external auxiliary materials [68]. These dominant positions boost the development of hydrogel for comprehensive tumor therapy after surgery.
Polymer hydrogels can be prepared in different methods that can be classified into chemical or physical hydrogels depending on the cross-linking mechanism. The chemical or permanent hydrogels are formed by covalent crosslinking of polymer chains. While physical crosslinking hydrogel are made via hydrogen bonding, crystallization, hydrophobic interaction, entangled chains, ionic interaction, and coordination bond, giving them a reversible character [69]. In recent years, reasonably designed metal coordination cross-linked hydrogels by introducing metal ions have aroused wide interest among researchers. The doped inorganic metal ions, such as Cu [24, 70], Ag [71], Au [66], Zn [72], Fe and other metal elements, can endow hydrogels abundant biological functions [73]. Among them, Fe is an essential nutrient element for human body [74] and possesses good biocompatibility, playing a vital role in a multitude of biochemical reactions [75,76,77,78,79,80,81,82]. The addition of Fe element in hydrogel has been shown diversified curative effects, enabling it to meet various demands. In the experiment of synthesizing artificial muscle with hydrogel, Fe ions and other forming structures can enhance the strength of hydrogel, give it a certain contractility [83] and achieve self-recovery function [84]. Besides, Fe can be reduced to Fe2+ or Fe3+ in tumor or bacterial microenvironment to trigger Fenton reaction, and the produced hydroxyl radical (•OH) can effectively kill cancer cells and avoid infection in postoperative adjuvant therapy [85, 86]. Fe also has photothermal property. By in-situ heating, the hydrogel shows a significant promoting effect on angiogenesis and chronic wound healing in vivo [87, 88]. Meanwhile, Fe2+ itself shows the function in enhancing the proliferation of HUVEC and up-regulating the expression of endothelial cell-related genes such as vascular endothelial growth factor (VEGF) and Endothelial Nitric Oxide Synthases (eNOS) [89]. Fe3+ can contribute to the enhancement of the cohesion and interface adhesion that can be used in myocardial infarction recovery [90]. In addition, Fe makes great progress in tissue engineering, like assisting the stem cells proliferation and differentiation [91], promoting liver [92] and bone [93] regeneration, constructing artificial livers [92] and so on. Iron-based hydrogel can also be used for topical treatment, such as wound disinfection [94] and tendon tissue injury recovery [95]. What’s more, Fe has a great potential in adjuvant treatment after surgery for malignant tumors [96,97,98,99,100,101,102]. For example, with the help of the para-magnetism of Fe ions, under the external magnetic field, heat can be released to achieve the directional killing of tumor cells [103].
In general, hydrogel is a new type of biomaterial with great clinical application and development potential which can provide individualized synthesis in the fields of residual lesion removal, anti-infection, promotion of tissue healing, and reconstruction of defective tissue after tumor surgery. However, hydrogels still have many technical and conceptual challenges are waiting to be solved before successful clinical applications. For example, its biosafety, biodegradability, metabolic rate, and cytotoxicity require further study. At this stage, the short-term biocompatibility of hydrogels is only evaluated in animal models without human clinical trials to verify its effectiveness and long-term safety. Secondly, the common use of hydrogels is topical application. Compared with the mature treatment methods such as intravenous injection and oral administration, hydrogels require operators with higher technical operant level which may cause a negative impact on the promotion of hydrogels. Furthermore, hydrogels are composed of multiple component that can be applied on multiple therapeutic strategies, but the complicated system design obstacle its scale-up and quality control.
Overall, in this review, the latest progress of iron-based polymeric hydrogels with a three-dimensional (3D) network structure in postoperative tumor therapy have been highlighted, including chemotherapy, photothermal therapy (PTT), magnetic hyperthermia therapy (MHT), photodynamic therapy (PDT), chemo-dynamic therapy (CDT), and multimodal combination therapy. In addition, the advantages and limitations of these iron-based hydrogels in the categorized examples have been discussed and emphasized. Finally, the future directions of novel strategies to fabricate iron-based hydrogels will be reconsidered in the aspect of biosafety which are the key concerns related to medical translation. This review could hopefully offer researchers a broader perspective of iron-based hydrogels for the enhanced postoperative tumor therapy and provide new ideas in the design and application of novel iron-based hydrogels.
Application of Iron-based hydrogels for postoperative cancer therapy
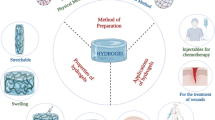
Chemotherapy have been widely used in postoperative tumor therapy, while the introduction of hydrogel platform greatly improves the efficacy of chemotherapy and potentially reduces systemic toxicity. In the last decades, with the rapid development of nanomedicine, new cancer treatments such as PTT, MHT, PDT, and CDT have attracted extensive attention due to their unique advantages including high specificity, low aggressiveness, and precise spatiotemporal selectivity. Simultaneously, the current trend in clinical studies has gradually shifted from a single treatment modality to the combined or multiple therapies since the synergistically enhanced interactions between two or more treatments results in significant super additive (namely “1 + 1 > 2”) therapeutic effects. Notably, due to the inherent advantages of Fe ions, many iron-based hydrogel platforms with integrated multiple treatments and functions have been established (Fig. 1) which has shown great application prospects in postoperative tumor therapy. Here in Table 1, some research publications in the recent years of iron-based hydrogel applied in postoperative treatment are listed.
Iron-based hydrogel for chemotherapy
Fe can promote the hydrogel aggregation in different ways to enhance the cohesion of the hydrogel and induce chelating to achieve the controlled release of drugs at the specific target. Recently, iron-based hydrogel as a novel drug delivery system have been developed to deliver a wide range of chemotherapeutic drugs. Yavvari et al. synthesized chitosan-catechol based hydrogel (CAT-CHIT) that was assembled by catechol-Fe (III) coordination interaction to achieve the effective delivery of chemo-therapy drugs (Fig. 2). CAT-CHIT contains two kinds of catechol which form different complexes with Fe (III) in different structures respectively. When the polymer solution is at an optimal Fe(III)-catechol molar ratio of 1:3, the cohesive interaction is necessary for hydrogel to be detected. At the same time, Fe (III) has a strong chelating ability with doxorubicin (DOX), and the formation of CAT-CHIT polymer become faster with the addition of Fe (III). In the drug release experiments, DOX was firstly released to maintain a high concentration of drug around the tumor, however the release was very slow due to the strong interaction of docetaxel (DTX) with hydrogel network which achieved the sequential release of drug [96]. The hydrogel has an ideal function of realizing comprehensive post operation treatment through chemotherapy, the competitive coordination ability of Fe(III) ions can target the goal of sequential, continuous and local delivery of two different chemotherapy drugs in the gel implanted near the tumor, and the self-healing property of the hydrogel can prolong the half-life of drug metabolism and remarkably improve the median survival rate of affected mice.
Schematic illustrations of the synthesis of Fe (III)-catechol hydrogels and their potential application in inhibiting tumor recurrence by sequential release of chemotherapeutic drugs. Reproduced with permission from Ref. [96]
In addition, as a magnetic species, under the premise of giving an external magnetic field, Fe can generate heat and enhance the drug release. Wu et al. designed a new type of magnetic supramolecular hydrogel (MSH) that was self-assembled by PEGylated Fe3O4 nanoparticles and α-CD through the inclusion complexation. It was capable for inhibiting the locoregional recurrence of cancer following the primary tumor resection in a breast mouse model (Fig. 3) [97]. In the layered structure, with Fe3O4 as the core, this structure ensures that drugs of different properties can be continuously delivered with different release profiles, so that hydrophobic molecule paclitaxel (PTX) and hydrophilic molecule DOX can be loaded simultaneously. MSH is gel-like under 37 ℃, after being injected into the body, the heat released from Fe3O4 by alternating current magnetic field (ACMF) can transform MSH from gel to liquid to match the irregular cavity after tumor resection and exert the therapeutic effect on tumor cells to the maximum extent by covering the whole residual tumor. Thermal generated under the ACMF by Fe3O4 promotes the release of DOX and PTX, significantly increases the cumulative release and maintains the effective therapeutic concentration ultimately. Besides, induction thermal mediated by magnetic iron nanoparticles provides the effect of thermal-induced cell damage. MSH has many advantages, for example, MSH with the characteristic of shear thinning can be easily injected into a surgical site through a needle. After being exposed to ACMF, hydrogel can perfectly fill the tissue cavity after tumor surgery through magnetothermal gel-sol transformation. The magnetic induced thermal effect promotes the controlled release of chemotherapy drugs at different rates, so that recurrence and metastasis of tumors are prevented in various methods.
Schematic illustrations of the synthesis process of MSH and their application of the MSH in inhibiting tumor recurrence and wounding healing under the ACMF. Reproduced with permission from Ref. [97]
Iron-based hydrogel for PTT
PTT has been considered as an effective method for antitumor and anti-infection due to its high efficiency, high selectivity, and low side effects [105,106,107,108,109]. Since the rapid proliferated tumor cells are more sensitive to temperature raise than normal cells, increasing temperature within a certain range could kill tumor cells without affecting others. In the experiment of Ma et al., an iron manganese silicate (FeMn (SiO4), FMS)-incorporated bioactive hydrogel was synthesized [110]. The synthesized FMS owns a hollow spherical structure with a rough surface and exhibits a good photothermal effect which is presumably attributed to its own olivine structure (Fig. 4A). Under the irradiation of NIR, FMS can absorb the photon energy and interact with the lattice vibration to enhance the lattice thermal vibration, and finally generate thermal through phonon scattering. Half of the iron atoms in Fe2SiO4 are occupied by Mn2+ which will lead to lattice distortion to a certain extent, thus enhancing the thermal vibration of the lattice under laser irradiation and improving the photothermal performance. In addition, the Fe released by FMS can promote the expression of VEGF, accelerate cell proliferation and migration (Fig. 4B), and promote wound healing. Therefore, FMS/SA composite hydrogel has good biological activity and can be used as a biological material for skin regeneration. Ma et al. believed that no one had studied FMS in the photothermal therapy field before their experiment. After FMS was mixed into sodium alginate hydrogel, a hydrogel with potential application value in the treatment of tumor-induced skin wound defect was obtained. FMS/SA composite hydrogel can play an ideal role in the comprehensive treatment of melanoma after resection with a good development prospect. However, there are still some limitations in this experiment. For example, the photothermal effect of FMS/SA composite hydrogel and its biological activity of promoting wound healing can only be verified by in vitro experiments without animal experiment to show the simulation of this hydrogel in animal or human body.
Schematic diagram of action mechanism and curative effect of FeMn(SiO4)-incorporated bioactive hydrogels. A Schematic illustrations of the preparation of FeMn(SiO4)-incorporated bioactive hydrogels and their application in postoperative cancer therapy. B The migration performance of human dermal fibroblasts (HDFs) on various time (0 h, 12 h) and the corresponding migration rate analysis (*p < 0.05). Reproduced with permission from Ref. [110]
Luo et al. synthesized a multifunctional bioactive therapeutics-repair-enabled citrate-iron hydrogel scaffold (GPDF) used for comprehensive post operation treatment of skin cancer (Fig. 5) [98]. In the presence of poly (citric acid-ethylene glycol) (PCG)-dopamine (PCD) and Fe3+, gelatin/methacrylic anhydride (GelMA) was photo-crosslinked to prepare a double-network GPDF hydrogel. Attributed to the double-network physical cross-linking of catchol-Fe3+ coordination and hydrogen bond between GelMA and PCD polymers, GPDF hydrogel obtained the ideal injectable and self-healing capability. In addition, the hydrogel has good antioxidant effect which eliminates cell oxidative stress and protects cells from ROS (reactive oxygen species) damage, thereby being beneficial to accelerate wound healing after tumor surgery. The hydrogel also has strong ultraviolet absorption capacity to protect tissues from ultraviolet radiation. In addition, Fe3+ can induce angiogenesis, inhibit the expression of TNF-α and up-regulate the expression of CD31 to promote wound healing and tissue repairment and plays an important role in the comprehensive postoperative treatment. Besides, after the Fe3+ is added into hydrogel, its photothermal performance can be significantly increased, the photothermal conversion efficiency is further improved, and the ideal killing effect of residual lesions after tumor surgery is achieved through the photothermal effect. Luo et al. developed an ideal multifunctional hydrogel with abundant functions such as injectability, ultraviolet shielding, wound healing promotion, and tumor recurrence inhibition. This work has confirmed the feasibility of a multifunctional platform for comprehensive post operation treatment which may obtain good application potential in future clinical practices. Although PTT could not substitute surgery, chemotherapy, or radiotherapy to become an independent tumor treatment method, it will be a supplement for higher treatment efficiency. Therefore, the treatment of tumors by increasing the local temperature of the lesion may have great potential in treating tumor in the future.
Schematic illustration for the fabrication process of GPDF hydrogel and the potential application of inhibiting tumor recurrence and promoting wound healing. Reproduced with permission from Ref. [98]
Iron-based hydrogel for MHT
MHT generally refers to the heat released by a specific inductive medium during exposure to an alternating magnetic field (AMF) with appropriate frequency and amplitude [111]. The magnetic field is highly selective to the human body, so it causes little harm to health and can effectively perform high-specific hyperthermia therapy on deep and inaccessible tumors [112, 113]. In addition, hyperthermia enhances the effect of chemotherapy through surgery [114], achieving reliable tumor killing effect. Therefore, iron-based hydrogel with magnetic induction is an ideal platform for tumor postoperative treatment.
Yan et al. synthesized an in situ formed magnetic hydrogel in response to body temperature, which is composed of iron oxide nanoparticles (Fe3O4@rGO, denoted as FG), used for hepatocellular carcinoma (HCC) postoperative treatment (Fig. 6) [115]. The addition of FG improved the mechanical properties of the hydrogel. Otherwise, under the action of AMF, FG nanosheets contributes to the effective MHT after liver cancer surgery while the effect on normal cells is negligible. The hydrogel also has advantages in hemostasis and vascular embolization. When blood interacted with the FG nanosheets in the hydrogel, the negative charge on the FG enhanced the interface stimulation to red blood cells and improved the coagulation ability. Thus, in the obtained hybrid hydrogel, the dopamine group functionalized polymer matrix can seal the wound and FG contributes to improve coagulation [116, 117]. At the same time, the hydrogel is stable in injectability, so it can accurately embolize the blood vessels around the focus. The purpose of treatment is achieved by embolizing the blood vessels around the lesions, reducing blood supply and promoting the atrophy of liver cancer lesions. These combined effects effectively enhance hemostasis after liver cancer and improve the final survival rate.
Schematic diagram shows the role of hydrogel in hemostasis, vascular embolization and MHT in postoperative treatment for HCC. Reproduced with permission from Ref. [115]
In the experiment from Gao et al., a new adapting magnetic hydrogel using ferromagnetic vortex-domain iron oxide (FVIOs) incorporated onto chitosan-based dynamic hydrogels and FVIO-functionalized hydrogel (FMH) was fabricated by the grafting-onto method (Fig. 7) [100]. The doping of FVIO in the hydrogel optimized the physical properties of the magnetic hydrogel, with rapid gelling, self-healing, and self-conforming abilities, endowing the hydrogel with good injectability. FMH has good adaptability for the easy infiltration into the small gap between the residual lesions after surgery, so it can perfectly cover the tissue defects after tumor resection surgery to ensure the selective release of the drugs carried in the hydrogel and achieve good therapeutic effect. Fe3O4’s primitive cubic inverse spinel structure is the magnetic source of FIVO. Attributing to their unique magnetic reversal process from a vortex-state to onion-state, FVIO perform superior heat induction capability [118]. With AMF stimulation, FMH showed good magnetocaloric property, increasing the temperature of the surrounding environment and killing the residual tumor cells in the wound rapidly. In addition, magnetic hyperthermia generated by hydrogel could promote the delivery of carried DOX to the nuclei of cancer cells, significantly enhancing the efficacy of chemotherapy. In summary, FMH can promote effective chemo-magnetocaloric synergistic treatment and effectively prevent tumor recurrence after surgery [100]. Compared with superparamagnetic iron oxide nanoparticles (SPIOs)-functionalized magnetic hydrogel, the possible side effects of the traditional SPIO-based magnetic hydrogel has been overcome with better self-adaptability. Moreover, under the stimulation of external AMF, the hydrogel containing FIVO shows superior stability to provide a long treatment period, while showing high induction heating and significant rheological properties. In addition, only a lower concentration of FVIO is required to show the same thermal therapeutic effect as SPIO.
Schematic diagram illustrates FMH with optimal adaptive functions for breast cancer postoperative treatment. Reproduced with permission from Ref. [100]
In summary, the doping of the Fe in the hydrogel can be used as a bridge to connect the magnetocaloric effect and the controlled release of drugs, therefore the heat can be released under an external magnetic field through the para-magnetism of the Fe element to kill the tumor. Furthermore, the controlled release of drugs in chemotherapy can be promoted through the raise of temperature, so that the comprehensive treatment after the tumor surgery can be achieved.
Iron-based hydrogel for CDT
CDT is a new ROS-mediated cancer treatment method based on in situ Fenton reaction in tumor site [102, 119, 120]. In the CDT process, metal ions such as Cu [121], Fe [122], Mn [123], catalyzed excessive endogenous H2O2 in tumor microenvironment (TME) by Fenton or Fenton-like reaction to generate highly toxic •OH [124], which can specifically cause the death of tumor cells.
In the experiment from Zhang et al., a multifunctional sodium alginate (SA) hydrogel immobilizing hemoglobin (Hb) and pH-sensitive fluorescent changing carbon quantum dots (CQDs) was designed [125] (Fig. 8). This is a new multifunctional implant with the effects of preventing tumor recurrence and infection after tumor resection, detecting pH of tumor microenvironment (TME) and stopping bleeding. With the tumor endogenous H2O2, the Fe2+ in Hb was able to generate toxic •OH by Fenton reaction, effectively killing the residual recurrent cancer cells and the infected bacteria which makes the hydrogel a good method for comprehensive postoperative treatment. First, the hydrogel has good water absorption ability to absorb exuded blood, thereby improving the local concentration of hemostatic factors, promoting thrombosis, reducing blood loss, and playing a role in effective hemostasis after surgery. Secondly, hydrogel can quickly capture the disseminated tumor cells into micropores, reduce the circulating tumor cell (CTC) level and the risk of tumor recurrence after operation. Thirdly, immobilized hemoglobin is a natural protein in human body, which is composed of four heme groups. Due to Fe2+ in the center of heme groups, Hb can undergo Fenton reaction in TME where H2O2 is over-expressed, producing •OH with high cancer cells toxicity and broad-spectrum antibacterial activity to reduce the risk of recurrence, metastasis, and wound infection in the short term after radical tumor resection.
Schematics illustrate the potential application of the inhibiting tumor recurrence and infection. Reproduced with permission from Ref. [125]
Iron-based hydrogel for synergistic CDT and PTT
CDT and PTT have a broad prospect in enhancing the anticancer effect. However, monotherapy equipped with a single model of anticancer efficacy is generally insufficient to induce an adequate therapeutic response. When PTT is combined with CDT, a synergistic therapeutic effect will be generated by PTT which can not only kill cancer cells, but also accelerate the generation of •OH in CDT [126]. The toxicity and side effects of drugs can be reduced at the same time, thereby achieving the ideal clinical effect [127].
Ma et al., fabricated a sprayable FS/SA composite hydrogel which was composited by β-FeSi2 (FS) and CaCl2. In this study, FS was utilized as a bioactive material for tumor postoperative recurrence inhabitation and skin wound healing for the first time (Fig. 9). Due to the local surface plasmon resonance phenomenon of particles, FS has a good photothermal effect with about 28.9% photothermal conversion efficiency that proportional to the content of Fe in the hydrogel, resulting in strong visible and near-infrared light absorption. FS can absorb the photon energy at 808 nm, triggering an electronic transition. The excited electrons can combine with the holes in a nonradiative manner to generate phonons to release the thermal radiation to the surrounding environment. In addition, the release of Fe ions in FS could trigger the Fenton reaction to produce •OH in the weak acidic environment, killing residual tumor cells and antibacterial. Therefore, due to the presence of FS, the prepared FS/SA hydrogel has excellent photothermal and chemical kinetic properties which can be flexibly controlled by adjusting the content of FS and the laser power density. In addition, the release of Fe ion in the hydrogel was also involved in the promotion of angiogenesis and wound healing. After being released from the hydrogel, Fe2+ is easily oxidized to Fe3+, the increased proportion of Fe3+ inhibits the expression activity of prolyl hydroxylase. The degradation of hypoxia inducible factor-1 (HIF-1α) proteasome increases the level of HIF-1α. The stable expression of HIF-1α can significantly up-regulate the expression of VEGF, thereby inducing angiogenesis. Fe ions can promote the migration and differentiation of endothelial cells by promoting the high expression of eNOS. In conclusion, FS has excellent biological activity in promoting endothelial cells migration, differentiation, as well as angiogenesis in vitro [89].
The schematic diagram of the preparation and function of sprayable FS/SA hydrogel. Reproduced with permission from Ref. [89]
Huang et al. synthesized a bioactive nanocomposite hydrogel by incorporating Ag2S nanodots conjugated Fe-doped bioactive glass nanoparticles (BGN-Fe-Ag2S) into biodegradable PEGDA and AIPH solution (Fig. 10) [99]. Under the irradiation of laser, Ag2S can release a certain amount of hyperthermia, induce the decomposition of AIPH, release alkyl free radicals, and then initiate the polymerization of PEGDA which leads to the gelation of hydrogel in the lesion and fixes BGN-Fe-Ag2S in the lesion. Based on the over-expressed H2O2 in the inflammatory microenvironment, •OH are generated by that Fenton reaction, and BGN-Fe was used as a growth promoter of wound tissues together with CDT agent to inhibit tumor growth and bacterial proliferation. Besides, nanocomposite hydrogel can be hydrolyzed under laser irradiation to produce growth factors and facilitate wound healing by promoting granulation tissue growth and collagen deposition. As an ideal growth promoter and chemokinetic therapeutic agent, iron-based hydrogel can realize the functional controllability of anti-infection, residual lesions eradication after tumor resection, enhanced wound healing and scar repairment. In general, injectable iron-based hydrogels are one of the promising candidates for mitigating postoperative side effects and improving patients’ quality of life.
Schematic illustration of the preparation of BGN-Fe-Ag2S hydrogels and their application in anticancer, antibacterial, and skin repairment. Reproduced with permission from Ref. [99]
Application of Iron-based hydrogels in other biomedical aspects
Iron-based hydrogels have great development potential in the field of tumor treatment which occupy an important position in the chemotherapy, MHT, PTT, CDT, chemokinetic therapy, and other fields. Moreover, because of the good water absorption, tissue compatibility, low cytotoxicity, together with the effects in promoting angiogenesis and inhibiting bacteria, iron-based hydrogel also plays an important role in tissue regeneration [128, 129], chronic wound healing promoting [130,131,132], anti-infection [133, 134], controlled drug release [135,136,137], image diagnosis [138]. Table 2 have listed the recent studies on the various clinical biological applications of iron-based hydrogels.
Fe (III)@TA microsphere, formed by coordination of ferric ions and tannic acid complexes, is one of the simplest metal-polyphenol networks [139]. Fe (III)@TA coating is a good platform to trigger the endosomal escape of nanoparticles, thus enhancing the treatment efficiency of bioactive factor [140]. Meanwhile, the drug loading efficiency and the durability of the peptide can be improved without damaging the peptide of normal structure, [141], making it commonly be used for drug delivery. In the experiment of Chen et al., a gelatin methacrylate (GelMA)/oxidized hyaluronic acid (OHA)/galactosylated chitosan (Gal-CS)/Fe (III)@TA@IGF-2 200 (TA200) hydrogel loaded with insulin-like growth factor 2 (IGF-2) was manufactured for liver regeneration [92]. Fe (III)@TA microspheres with uniform particle size are used as IGF-2 absorber to realize a long-term stable release, GelMA/OHA/Gal-CS@TA200 can meet the demands of liver tissue damage repairment through good biocompatibility and sustained release characteristics. In a conclusion, iron-based hydrogel may have the development potential in the fields of promoting liver regeneration and artificial liver in the future.
In the field of cartilage tissue repairment, iron-based hydrogels also have unique advantages. The hydrogels have 3D structure with high hydrophilicity and good biological which is similar to cartilage tissue, making it easier for stem cells to embed and recruit enough endogenous cells (especially stem cells) to induce cartilage regeneration. The bioactive substances can be added into the hydrogel base to simulate extracellular matrix (ECM) which promotes cell adhesion, proliferation, and differentiation. Wang et al. manufactured poly (γ-glutamic acid) (γ-PGA) hydrogel and Fe3+ ligand coordination to repair cartilage defect [129]. The secondary crosslinking of Fe3+ with the carboxyl of the γ-PGA further improves the crosslinking density of the hydrogel and increases the mechanical strength of the hydrogel [142], promoting proliferation and directed differentiation of mesenchymal stem cells (BMSCs) [143,144,145], inducing cartilage specific gene expression to enhance damage repair and cartilage tissues regeneration. Besides, The Fe3+ is involved in the induction of BMSCs differentiation into cartilage tissue which provides a novel idea for BMSCs cartilage formation and clinical cartilage regeneration.
Iron-based hydrogel is also an ideal biological dressing for preventing infection, promoting wound closure and tissue repairment due to its excellent biocompatibility and adjustable structure [146]. For example, in the EDTA-Fe3+ complexes crosslinked hyaluronic acid designed by Tian et al. [147], hyaluronic acid was degraded by hyaluronidase secreted from bacteria to release Fe3+ complex around the bacteria which was quickly absorbed by the surrounding bacteria and reduced to Fe2+. Then Fe2+ reacted with H2O2 to generate •OH, destroying proteins and nucleus to realize effective anti-infection [148, 149]. In addition, Fe3+ was used as a part of the physical cross-linking agent to fill the whole hydrogel, thereby greatly increasing the loading of the antibacterial agent and prolonging the effective period, enabling the hydrogel to continuously release the antibacterial agents until the hydrogel are completely decomposed or the bacteria surrounded are completely killed without inducing the drug resistance of the bacteria [150, 151].
Compared with the metal-free hydrogel (Table 3), the addition of Fe element improves and the clinical application potential of the hydrogel. For example, the intrinsic physical properties of the hydrogel substrate, such as the strength, injectability, self-healing property, can be greatly improved. In addition, the sensitivity to external stimulation, the drug carrying capacity, and the controlled release ability of the hydrogel are strengthened. To enrich the functions of the hydrogel, such as the photothermal effect, the magnetocaloric effect, and the integration of multiple functions, Fe can be added into the hydrogel in different forms. Compared with other metals (Table 4), Fe is an important trace element that can be digested and absorbed by the human body after the treatment. Meanwhile, the threshold of Fe poisoning is much higher than other metals, thus, obvious side effects will not be introduced and the metabolic burden of Fe to the human body is much smaller as well. In addition, Fe can induce Fenton reaction and produce amount of ROS to improve hydrogel’s antibacterial property and tumor killing function with the reduced risk of carcinogenicity than other metals. Fe is involved in the synthesis of a variety of active ingredients in the human body, and these bioactive ingredients can also be directly combined with the hydrogel to significantly improve the overall biocompatibility of the hydrogel. Meanwhile, Fe’ s magnetic properties can play an important role in the fields of Magnetic Resonance Imaging (MRI). At the same time, Fe has the unique characteristics of promoting angiogenesis and blade healing, especially in the fields of anti-infection of major surgical wounds and promoting healing. Therefore, the development of iron-based hydrogels in the clinical application should be a hot topic in future medical research.
Conclusion, discussion, and future perspective
Postoperative cancer therapy still faces great challenges due to the difficulties in tumor recurrence and metastasis inhibition. As personalized therapy have become an important goal of tumor research, hydrogels have shown great inhibition ability in cancer therapy. Due to Fe element’s unique functions and good biocompatibility, iron-based hydrogels displayed huge potential in postoperative cancer therapy. This review has summarized various synthesis methods and construction principles of iron-based hydrogels and highlighted the latest progress of iron-based hydrogels in postoperative tumor therapy, including chemotherapy, PTT, PDT, CDT, MHT, and multimodal combination therapy. Although some progresses have been achieved so far, there is still a long way to go until wide iron-based hydrogel applications are available in clinic and some challenges is still waiting to be fully addressed.
As it is known that iron-based hydrogels have advantageous properties in clinical transformation. For example, due to the simple preparation and readily available raw materials, the production cost of iron-based hydrogel is relatively low to reduce the economic burden on patients. As a good drug carrier, hydrogel can be loaded by a variety of bioactive drugs, such as growth factor, anti-inflammatory factor, or chemotherapy drugs, and integrate various of functions to achieve comprehensive treatment after radical surgical treatment of tumors. By virtue of the morphological changes of the hydrogel under certain stimulations, the tiny defects after tumor surgical resection can be fully covered to reduce the risks of tumor recurrence and metastasis. With the excellent water absorption capacity, hydrogel can be used to absorb tissue exudate around the wound when it is covered on the operation site to inhibit or relieve pain, control inflammation, and reduce scar formation. Hydrogel can accurately cover defects of surgical and isolate the tissue from the external environment which can be used to effectively reduce infection. With the good plasticity, hydrogel will not generate obvious stress and side effects to the surrounding normal tissues when it is applied to local areas.
Iron-based hydrogels have shown great advantages in the field of cancer nanomedicine, however, further clarifications should be made before clinical application. For example, at present, in the preparation of bioactive hydrogel, bioactive components have strict transportation and storage requirements which may be inactivated at certain ambient temperature and will affect the synthetizing process and character of hydrogel. However, there are few studies about the preservation conditions and shelf life of bioactive hydrogels. In addition, it is difficult to keep the hydrogels at suitable storage conditions when that are used by patients themselves, affecting the curative effect of hydrogel and reducing the patients’ treatment compliance. Intravenously injected nanodrugs may have relatively easy access to the intact tumor microenvironment and be sufficiently stimulated by the tumor microenvironment. While the postoperative inflammatory environment may be detrimental to the stimuli-responsive drug release of iron-based hydrogels which previously only occur in the tumor microenvironment. From this perspective, it is necessary to re-examine the hydrogel construction strategy.
As the post-surgery residual may disperse at multiple sites, the single beam’s NIR light of PTT/PDT of some hydrogels cannot reach tumor location. Therefore, the ideal photo-responsive therapeutic effect is hardly achieved. Due to the short application window of hydrogel and easy absorption by non-tumor tissues, it may cause certain immunogenicity. Furthermore, some components of hydrogel have the similar functions as the initiators of partial positive and negative feedback reactions in human body fluids, potentially causing lesions such as inflammatory reactions [165] and abnormal coagulation, even become life-threatening in severe cases. Its biosafety, biodegradability, metabolic rate, and cytotoxicity should be further studied. At the current stage, the short-term biocompatibility of hydrogels has only been evaluated in animal models without the long-term safety of hydrogels. The pharmacokinetics are easily elucidated in vitro, whereas the release rate and metabolism in vivo remain unclear, and the drug release per unit time is difficult to be precisely controlled. At the same time, there may be liver-kidney metabolism and excretion pathways of hydrogel, and kidney excretion pathways mostly depend on the diameter of the drug. Hydrogels are mostly macromolecular cross-linked structured with relatively large molecular weight and molecular size which may affect their metabolism and excretion efficiency [166]. In addition, hydrogels are composed by multiple components, the further combination with multiple therapeutic strategies makes the system be complicated and hard for scale-up and quality control. Some injectable hydrogels have converted into gel prematurely in the needle, thus, controlling the activation condition of hydrogels at more precise temperatures to reduce the risk of premature gelation.
Although the incorporation of Fe into the hydrogel can enrich the function of the hydrogel, its effect is mostly proportional to the content. When Fe exceeds a certain dose, iron poisoning might be caused. Furthermore, the design to involve the target of iron-based hydrogels is also relatively difficult because postoperative treatment is generally injected locally. Unlike the ordinary nanomedicines that can be targeted and accumulated inside the tumor after intravenous injection, the hydrogels are applied after surgery. After the site gels, some of the drug components are absorbed by the tumor rather than the hydrogel. In the future, the development of iron-based hydrogels with high stimuli responsiveness, high biocompatibility, high biodegradability, and high targeting should be focused. Although iron-based hydrogels have made great progress in the postoperative treatment of tumors, from the perspective of clinical transformation of tumor treatment drugs, hydrogels cannot completely replace the existing postoperative adjuvant therapy. Therefore, many factors restrict the clinical application of hydrogels which are also the challenges faced by many new polymer materials in the application.
In practical clinical application, the comprehensive treatment plan for postoperative patients should be determined according to their pathological type, but pathological results cost a long time. Meanwhile, the usage of some chemotherapeutic drugs such as anti-angiogenic drugs need several months interval from surgery which will lead to the inability to achieve the timely application of hydrogel to the surgical site during the operation. Second, along with the peristalsis of the gastrointestinal tract, when the hydrogel is applied to the pelvic and abdominal cavities, the existing site of the hydrogel may be changed, resulting in the decrease of the drug concentration at the surgical site of the tumor and affecting the therapeutic effect. Hydrogel has a certain viscosity, and as a foreign body, it will aggravate the adhesion after pelvic and abdominal surgery and increase the risk and difficulty of reoperation. In the recovery period of surgery, the presence of hydrogel may affect the recovery of gastrointestinal peristalsis, causing gastrointestinal flatulence, abdominal pain, and other discomforts, and even lead to serious postoperative complications such as intestinal obstruction.
Most of the experiments focused on the characterization and efficacy of hydrogels, but the studies in practical clinical application are still rare. And most of them are external dosage forms for anti-infection and promoting tissue healing. Hydrogels that are applied in vivo or used for tumor treatment are rare. As mentioned above, there are still many practical difficulties in clinical application that restrict the potential application of hydrogels and further limit the development of clinical human experiments. At present, the research on hydrogels mainly focuses on the level of cell and animal experiments, and the number of animal experiments is relatively small with a lack of safety evaluation and it does not meet the standards for human experiments. In addition, most of the hydrogel drugs that have entered clinical practice or clinical trials are external reagents, and very few hydrogels are given in vivo with short observation period. Therefore, there are inadequate clinical trial-based medical evidence on the long-term adverse reactions of its application. As a new type of biomaterial, most doctors and patients still have skepticism and wait-and-see attitude for the use of hydrogel in vivo which will cause certain difficulties in the recruitment of observation subjects for clinical trials. Finally, to ensure the reliability of hydrogel clinical experiments, it will cause practical problems such as long observation period, harsh entry conditions, and difficulty in tracking.
The hydrogels still have great potential in the future clinical practice. Firstly, the selected drugs for chemotherapy after tumor surgery are related to the pathological type of tumor. The existing research mostly focuses on the combination of a hydrogel substrate and one or more fixed chemotherapy drugs. To solve the arbitrary collocation of the hydrogel substrate and the chemotherapy drugs as well as the artificial and accurate formulation of the drug content may be the trend and focus of hydrogel development in the future. Secondly, most of the existing experiments focus on solid tumors such as breast cancer, lung cancer and skin cancer, while few studies are about non-solid tumors such as leukemia which means that the hydrogel has great development potential and prospects in the field of non-solid tumor treatment. With the unique biocompatibility of hydrogel, it may be a new idea of treatment. Hydrogels are same as human tissue matrix which have natural advantages in carrying bioactive substances, enzymes, DNA and RNA, and have great potential in the fields of developing tumor vaccines, immunotherapy, and gene therapy. In addition, tumors grow at sites protective barrier structures are difficult to be treated by conventional administration routes, the utilization of hydrogel may be a novel method. Future research can also focus on achieving high aggregation in the tumor microenvironment by virtue of the pH responsiveness, photothermal effect and other characteristics, reducing the hepatorenal toxicity and irritation of the existing contrast agents, and realizing the application in accurate cancer detection. The injectable multidisciplinary hydrogel-based delivery systems for the co-delivery and sequential release of different therapeutic agents are expected to maximize the overall therapeutic efficiency of cancer therapies and accelerate their clinical translation.
In the future, in terms of the composition of hydrogels, it is necessary to explore a simpler method with simpler components but multiple functions, for example, the development of iron-based hydrogels with high stimuli responsiveness, high biocompatibility, high biodegradability, and high targeting should be focused. In treatment methods aspect, besides treating the tumors directly with iron-based hydrogels, alleviating the inflammatory state in the surgical site to improve the tumor microenvironment should also be a promising postoperative cancer therapy method. Because of the improvement of inflammatory microenvironment, Epithelial-Mesenchymal Transition of tumor cells can be well inhibited which can also reduce the risk of CTC to colonize in distant organs. In addition, there is a substantial requirement to design appropriate hydrogels with the combined therapy strategies, including chemotherapy, immunotherapy, PTT, PDT, gene therapy, radiation therapy, and so on, improving postoperative tumor therapy in various synergistic modalities.
In summary, although iron-based hydrogels have made great progress in the postoperative treatment of tumors, from the perspective of clinical transformation of tumor treatment drugs, iron-based hydrogels still exist many limitations. It should not be ignored that these limitations prevent their further practical application. Fortunately, things are looking up, hydrogel is developing very rapidly under the current situation with more and more research force. If the shortcomings found in the existing research can be optimized, the functions can be centralized, and the advantages can be reasonably exerted, iron-based hydrogel will play an important role in future cancer therapy.
Availability of data and materials
Not applicable.
Abbreviations
- NIR:
-
Near infrared light
- ·OH:
-
Hydroxyl radical
- PTT:
-
Photothermal therapy
- MHT:
-
Magnetic hyperthermia therapy
- PDT:
-
Photodynamic therapy
- CDT:
-
Chemo-dynamic therapy
- CAT-CHIT:
-
Chitosan-catechol based hydrogel
- DOX:
-
Doxorubicin
- DTX:
-
Docetaxel
- MSH :
-
Magnetic supramolecular hydrogel
- PTX:
-
Molecule taxus chinensis
- ACMF:
-
Alternating current magnetic field
- VEGF:
-
Vascular endothelial growth factor
- GPDF :
-
Citrate-iron hydrogel scaffold
- PCD:
-
Poly (citric acid-ethylene glycol) (PCG)-dopamine
- GelMA:
-
Gelatin/methacrylic anhydride
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor necrosis factor-α
- AMF:
-
Alternating magnetic field
- HCC:
-
Hepatocellular carcinoma
- FG:
-
Iron oxide nanoparticles
- FVIOs:
-
Ferromagnetic vortex-domain iron oxide
- FMH:
-
FVIO-functionalized hydrogel
- SPIOs:
-
Superparamagnetic iron oxide nanoparticles
- TME :
-
Tumor microenvironment
- SA:
-
Sodium alginate
- Hb :
-
Hemoglobin
- CODs:
-
Carbon quantum dots
- CTC:
-
Circulating tumor cell
- FS :
-
β-FeSi2
- HIF-1:
-
Hypoxia inducible factor-1
- eNOS:
-
Endothelial Nitric Oxide Synthases
- BGN-Fe-Ag2S:
-
Ag2S nanodots conjugated Fe-doped bioactive glass nanoparticles
- PEGDA:
-
Polyethylene glycol diacrylate
- OHA:
-
Oxidized hyaluronic acid
- Gal-CS:
-
Galactosylated chitosan
- TA200:
-
Fe(III)@TA@IGF-2 200
- IGF-2:
-
Insulin-like growth factor 2
- ECM:
-
Simulate extracellular matrix
- γ-PGA:
-
Poly (γ-glutamic acid)
- BMSCs:
-
Mesenchymal stem cells
- MRI:
-
Magnetic Resonance Imaging
References
Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–30.
Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–90.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Simões MCF, Sousa JJS, Pais AACC. Skin cancer and new treatment perspectives: a review. Cancer Lett. 2015;357(1):8–42.
Orecchia R. Breast cancer. post-mastectomy radiotherapy reduces recurrence and mortality. Nat Rev Clin Oncol. 2014;11(7):382–4.
Zhuang B, Chen T, Xiao Z, Jin Y. Drug-loaded implantable surgical cavity-adaptive hydrogels for prevention of local tumor recurrence. Int J Pharm. 2020;577:119048.
Wang X, Xue J, Ma B, Wu J, Chang J, Gelinsky M, et al. Black bioceramics: combining regeneration with therapy. Adv Mater. 2020;32(48):e2005140.
Liang Y, Li M, Huang Y, Guo B. An integrated strategy for rapid hemostasis during tumor resection and prevention of postoperative tumor recurrence of hepatocellular carcinoma by antibacterial shape memory cryogel. Small. 2021;17(38):e2101356.
Raa ST, Oosterling SJ, van der Kaaij NP, van den Tol MP, Beelen RH, Meijer S, et al. Surgery promotes implantation of disseminated tumor cells, but does not increase growth of tumor cell clusters. J Surg Oncol. 2005;92(2):124–9.
Damen MPF, van Rheenen J, Scheele CLGJ. Targeting dormant tumor cells to prevent cancer recurrence. FEBS J. 2021;288(21):6286–303.
Ogawa T, Ogawa K, Shiga K, Furukawa T, Nagase H, Hashimoto S, et al. Upregulation of IGF2 is associated with an acquired resistance for cis-diamminedichloroplatinum in human head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2010;267(10):1599–606.
Aquil A, El Kherchi O, El Azmaoui N, Mouallif M, Guerroumi M, Benider A, et al. Predictors of mental health disorders in women with breast and gynecological cancer after radical surgery: A cross-sectional study. Ann Med Surg (Lond). 2021;65:102278.
Maass SW, Roorda C, Berendsen AJ, Verhaak PF, de Bock GH. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas. 2015;82(1):100–8.
Tsaras K, Papathanasiou IV, Mitsi D, Veneti A, Kelesi M, Zyga S, et al. Assessment of Depression and anxiety in breast cancer patients: prevalence and associated factors. Asian Pac J Cancer Prev. 2018;19(6):1661–9.
Jacob L, Bleicher L, Kostev K, Kalder M. Prevalence of depression, anxiety and their risk factors in German women with breast cancer in general and gynecological practices. J Cancer Res Clin Oncol. 2016;142(2):447–52.
Yang YL, Liu L, Wang XX, Wang Y, Wang L. Prevalence and associated positive psychological variables of depression and anxiety among Chinese cervical cancer patients: a cross-sectional study. PLoS ONE. 2014;9(4):e94804.
Etzkorn JR, Sharkey JM, Grunyk JW, Shin TM, Sobanko JF, Miller CJ, et al. Frequency of and risk factors for tumor upstaging after wide local excision of primary cutaneous melanoma. J Am Acad Dermatol. 2017;77(2):341–8.
Costa Svedman F, Spanopoulos D, Taylor A, Amelio J, Hansson J. Surgical outcomes in patients with cutaneous malignant melanoma in Europe - a systematic literature review. J Eur Acad Dermatol Venereol. 2017;31(4):603–15.
Yang B, Yin J, Chen Y, Pan S, Yao H, Gao Y, et al. 2D-black-phosphorus-reinforced 3D-printed scaffolds: a stepwise countermeasure for osteosarcoma. Adv Mater. 2018;30(10):1705611.
Xi Y, Ge J, Wang M, Chen M, Niu W, Cheng W, et al. Bioactive anti-inflammatory, antibacterial, antioxidative silicon-based nanofibrous dressing enables cutaneous tumor photothermo-chemo therapy and infection-induced wound healing. ACS Nano. 2020;14(3):2904–16.
Xi Y, Ge J, Guo Y, Lei B, Ma PX. Biomimetic elastomeric polypeptide-based nanofibrous matrix for overcoming multidrug-resistant bacteria and enhancing full-thickness wound healing/skin regeneration. ACS Nano. 2018;12(11):10772–84.
Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J. Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother. 2022;71(3):507–26.
Poláková L, Širc J, Hobzová R, Cocârță AI, Heřmánková E. Electrospun nanofibers for local anticancer therapy: Review of in vivo activity. Int J Pharm. 2019;558:268–83.
Zhang C, Sun W, Wang Y, Xu F, Qu J, Xia J, et al. Gd-/CuS-loaded functional nanogels for MR/PA imaging-guided tumor-targeted photothermal therapy. ACS Appl Mater Interfaces. 2020;12(8):9107–17.
Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, et al. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23(1):137–48.
Lukianova-Hleb EY, Kim YS, Belatsarkouski I, Gillenwater AM, O’Neill BE, Lapotko DO. Intraoperative diagnostics and elimination of residual microtumours with plasmonic nanobubbles. Nat Nanotechnol. 2016;11(6):525–32.
Hou X, Zhong D, Li Y, Mao H, Yang J, Zhang H, et al. Facile fabrication of multi-pocket nanoparticles with stepwise size transition for promoting deep penetration and tumor targeting. J Nanobiotechnol. 2021;19:111.
Lu L, Zhang Q, Wang Z, Gao L, Shen J. Peptide-modified nanoparticles for tumor targeting and molecular imaging. Curr Med Chem. 2021;28(31):6411–36.
Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact Mater. 2020;6(7):1973–87.
Widjaya AS, Liu Y, Yang Y, Yin W, Liang J, Jiang Y, et al. Tumor-permeable smart liposomes by modulating the tumor microenvironment to improve the chemotherapy. J Control Release. 2022;344:62–79.
Kim Y, Youn YS, Oh KT, Kim D, Lee ES. Tumor-targeting liposomes with transient holes allowing intact rituximab internally. Biomacromolecules. 2021;22(2):723–31.
Franco MS, Gomes ER, Roque MC, Oliveira MC. Triggered drug release from Liposomes: exploiting the outer and inner tumor environment. Front Oncol. 2021;11:623760.
Su J, Li J, Liang J, Zhang K, Li J. Hydrogel preparation methods and biomaterials for wound dressing. Life. 2021;11(10):1016.
Teixeira MO, Antunes JC, Felgueiras HP. Recent advances in fiber-hydrogel composites for wound healing and drug delivery systems. Antibiotics. 2021;10(3):248.
Mele E. Electrospinning of natural polymers for advanced wound care: towards responsive and adaptive dressings. J Mater Chem B. 2016;4(28):4801–12.
Kopecek J. Hydrogel biomaterials: A smart future? Biomaterials. 2007;28(34):5185–92.
Miranda CS, Ribeiro ARM, Homem NC, Felgueiras HP. Spun biotextiles in tissue engineering and biomolecules delivery systems. Antibiotics. 2020;9(4):174.
Xiao Y, Gu Y, Qin L, Chen L, Chen X, Cui W, et al. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf B Biointerfaces. 2021;200:111581.
Wang W, Wat E, Hui PC, Chan B, Ng FS, Kan CW, et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci Rep. 2016;6:24112.
Chen T, Yang Y, Peng H, Whittaker AK, Li Y, Zhao Q, et al. Cellulose nanocrystals reinforced highly stretchable thermal-sensitive hydrogel with ultra-high drug loading. Carbohydr Polym. 2021;266:118122.
Huang TH, Hsu SH, Chang SW. Molecular interaction mechanisms of glycol chitosan self-healing hydrogel as a drug delivery system for gemcitabine and doxorubicin. Comput Struct Biotechnol J. 2022;20:700–9.
Wang J, Youngblood R, Cassinotti L, Skoumal M, Corfas G, Shea L. An injectable PEG hydrogel controlling neurotrophin-3 release by affinity peptides. J Control Release. 2021;330:575–86.
Chen X, Tan B, Wang S, Tang R, Bao Z, Chen G, et al. Rationally designed protein cross-linked hydrogel for bone regeneration via synergistic release of magnesium and zinc ions. Biomaterials. 2021;274:120895.
Deller RC, Richardson T, Richardson R, Bevan L, Zampetakis I, Scarpa F, et al. Artificial cell membrane binding thrombin constructs drive in situ fibrin hydrogel formation. Nat Commun. 2019;10(1):1887.
Fu X, Chen T, Song Y, Feng C, Chen H, Zhang Q, et al. mRNA Delivery by a pH-Responsive DNA Nano-Hydrogel. Small. 2021;17(29):e2101224.
Zhang J, Guo Y, Pan G, Wang P, Li Y, Zhu X, et al. Injectable drug-conjugated DNA hydrogel for local chemotherapy to prevent tumor recurrence. ACS Appl Mater Interfaces. 2020;12(19):21441–9.
Yuan T, Shao Y, Zhou X, Liu Q, Zhu Z, Zhou B, et al. Highly permeable DNA supramolecular hydrogel promotes neurogenesis and functional recovery after completely transected spinal cord injury. Adv Mater. 2021;33(35):e2102428.
Wang M, Chen J, Li W, Zang F, Liu X, Qin S. Paclitaxel-nanoparticles-loaded double network hydrogel for local treatment of breast cancer after surgical resection. Mater Sci Eng C Mater Biol Appl. 2020;114:111046.
Dong Y, Li S, Li X, Wang X. Smart MXene/agarose hydrogel with photothermal property for controlled drug release. Int J Biol Macromol. 2021;190:693–9.
Han Y, Yang J, Zhao W, Wang H, Sun Y, Chen Y, et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact Mater. 2021;6(10):3596–607.
Zong S, Wen H, Lv H, Li T, Tang R, Liu L, et al. Intelligent hydrogel with both redox and thermo-response based on cellulose nanofiber for controlled drug delivery. Carbohydr Polym. 2022;278:118943.
Duong HTT, Thambi T, Yin Y, Kim SH, Nguyen TL, Phan VHG, et al. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials. 2020;230:119599.
Shao J, Ruan C, Xie H, Li Z, Wang H, Chu PK, et al. Black-phosphorus-incorporated hydrogel as a sprayable and biodegradable photothermal platform for postsurgical treatment of cancer. Adv Sci. 2018;5(5):1700848.
Zhao S, Zhang L, Deng L, Ouyang J, Xu Q, Gao X, et al. NIR-II responsive hydrogel as an angiogenesis inhibition agent for tumor microenvironment reprogramming. Small. 2021;17(47):e2103003.
Liang Y, Hao Y, Wu Y, Zhou Z, Li J, Sun X, et al. Integrated hydrogel platform for programmed antitumor therapy based on near infrared-triggered hyperthermia and vascular disruption. ACS Appl Mater Interfaces. 2019;11(24):21381–90.
Wang S, Zhang Z, Wei S, He F, Li Z, Wang HH, et al. Near-infrared light-controllable MXene hydrogel for tunable on-demand release of therapeutic proteins. Acta Biomater. 2021;130:138–48.
Zheng Y, Wang W, Zhao J, Wu C, Ye C, Huang M, et al. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr Polym. 2019;222:115039.
He Y, Yuan T, Wang X, Shen M, Ding L, Huang L, et al. Temperature sensitive hydrogel for preoperative treatment of renal carcinoma. Mater Sci Eng C Mater Biol Appl. 2020;111:110798.
Lin CW, Tseng SJ, Kempson IM, Yang SC, Hong TM, Yang PC. Extracellular delivery of modified oligonucleotide and superparamagnetic iron oxide nanoparticles from a degradable hydrogel triggered by tumor acidosis. Biomaterials. 2013;34(17):4387–93.
Wang H, Yi J, Mukherjee S, Banerjee P, Zhou S. Magnetic/NIR-thermally responsive hybrid nanogels for optical temperature sensing, tumor cell imaging and triggered drug release. Nanoscale. 2014;6(21):13001–11.
Zhao Z, Shen J, Zhang L, Wang L, Xu H, Han Y, et al. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater Sci. 2020;8(19):5306–16.
Wang C, Wang M, Xia K, Wang J, Cheng F, Shi K, et al. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact Mater. 2021;6(8):2523–34.
Yang Y, Zhao X, Yu J, Chen X, Wang R, Zhang M, et al. Bioactive skin-mimicking hydrogel band-aids for diabetic wound healing and infectious skin incision treatment. Bioact Mater. 2021;6(11):3962–75.
Chen J, Ning E, Wang Z, Jing Z, Wei G, Wang X, et al. Docetaxel loaded mPEG-PLA nanoparticles for sarcoma therapy: preparation, characterization, pharmacokinetics, and anti-tumor efficacy. Drug Deliv. 2021;28(1):1389–96.
Li Q, Xu S, Feng Q, Dai Q, Yao L, Zhang Y, et al. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact Mater. 2021;6(10):3396–410.
Liao J, Shi K, Jia Y, Wu Y, Qian Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact Mater. 2021;6(8):2221–30.
Yang X, Gao L, Wei Y, Tan B, Wu Y, Yi C, et al. Photothermal hydrogel platform for prevention of post-surgical tumor recurrence and improving breast reconstruction. J Nanobiotechnol. 2021;19(1):307.
Zhang M, Qiao X, Han W, Jiang T, Liu F, Zhao X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr Polym. 2021;266:118100.
Mondal S, Das S, Nandi AK. A review on recent advances in polymer and peptide hydrogels. Soft Matter. 2020;16(6):1404–54.
Xu Q, Chang M, Zhang Y, Wang E, Xing M, Gao L, et al. PDA/Cu bioactive hydrogel with “Hot Ions Effect” for inhibition of drug-resistant bacteria and enhancement of infectious skin wound healing. ACS Appl Mater Interfaces. 2020;12(28):31255–69.
Basha SI, Ghosh S, Vinothkumar K, Ramesh B, Kumari PHP, Mohan KVM, et al. Fumaric acid incorporated Ag/agar-agar hybrid hydrogel: A multifunctional avenue to tackle wound healing. Mater Sci Eng C Mater Biol Appl. 2020;111:110743.
Mao C, Xiang Y, Liu X, Cui Z, Yang X, Yeung KWK, et al. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano. 2017;11(9):9010–21.
Wu Y, Liang Y, Liu Y, Hao Y, Tao N, Li J, et al. A Bi2S3-embedded gellan gum hydrogel for localized tumor photothermal/antiangiogenic therapy. J Mater Chem B. 2021;9(14):3224–34.
Roemhild K, von Maltzahn F, Weiskirchen R, Knüchel R, von Stillfried S, Lammers T. Iron metabolism: pathophysiology and pharmacology. Trends Pharmacol Sci. 2021;42(8):640–56.
Khan MM, Deen KM, Shabib I, Asselin E, Haider W. Controlling the dissolution of iron through the development of nanostructured Fe-Mg for biomedical applications. Acta Biomater. 2020;113:660–76.
Schwartz AJ, Converso-Baran K, Michele DE, Shah YM. A genetic mouse model of severe iron deficiency anemia reveals tissue-specific transcriptional stress responses and cardiac remodeling. J Biol Chem. 2019;294(41):14991–5002.
Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344–61.
Cronin SJF, Woolf CJ, Weiss G, Penninger JM. The role of iron regulation in immunometabolism and immune-related disease. Front Mol Biosci. 2019;6:116.
Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–19.
Vogt AS, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF. On iron metabolism and its regulation. Int J Mol Sci. 2021;22(9):4591.
Sukhbaatar N, Weichhart T. Iron regulation: macrophages in control. Pharmaceuticals. 2018;11(4):137.
Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21:6–20.
Cui Y, Li D, Gong C, Chang C. Bioinspired shape memory hydrogel artificial muscles driven by solvents. ACS Nano. 2021;15(8):13712–20.
Yuan Y, Shen S, Fan D. A physicochemical double cross-linked multifunctional hydrogel for dynamic burn wound healing: shape adaptability, injectable self-healing property and enhanced adhesion. Biomaterials. 2021;276:120838.
Lin X, Fang Y, Hao Z, Wu H, Zhao M, Wang S, et al. Bacteria-triggered multifunctional hydrogel for localized chemodynamic and low-temperature photothermal sterilization. Small. 2021;17(51):e2103303.
Hao Y, Dong Z, Chen M, Chao Y, Liu Z, Feng L, et al. Near-infrared light and glucose dual-responsive cascading hydroxyl radical generation for in situ gelation and effective breast cancer treatment. Biomaterials. 2020;228:119568.
Sheng L, Zhang Z, Zhang Y, Wang E, Ma B, Xu Q, et al. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials. 2021;264:120414.
Han N, Xu Z, Cui C, Li Y, Zhang D, Xiao M, et al. A Fe(3+)-crosslinked pyrogallol-tethered gelatin adhesive hydrogel with antibacterial activity for wound healing. Biomater Sci. 2020;8(11):3164–72.
Ma W, Ma H, Qiu P, Zhang H, Yang Z, Ma B, et al. Sprayable beta-FeSi2 composite hydrogel for portable skin tumor treatment and wound healing. Biomaterials. 2021;279:121225.
Wu T, Cui C, Huang Y, Liu Y, Fan C, Han X, et al. Coadministration of an adhesive conductive hydrogel patch and an injectable hydrogel to treat myocardial infarction. ACS Appl Mater Interfaces. 2020;12(2):2039–48.
Huang J, Liang Y, Jia Z, Chen J, Duan L, Liu W, et al. Development of magnetic nanocomposite hydrogel with potential cartilage tissue engineering. ACS Omega. 2018;3(6):6182–9.
Chen J, Wang X, Ye H, Yu Z, Feng L, Zhou J, et al. Fe (III)@TA@IGF-2 microspheres loaded hydrogel for liver injury treatment. Int J Biol Macromol. 2020;159:183–93.
Cao Z, Wang D, Li Y, Xie W, Wang X, Tao L, et al. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci China Life Sci. 2018;61(4):448–56.
Deng H, Sun J, Yu Z, Guo Z, Xu C. Low-intensity near-infrared light-triggered spatiotemporal antibiotics release and hyperthermia by natural polysaccharide-based hybrid hydrogel for synergistic wound disinfection. Mater Sci Eng C Mater Biol Appl. 2021;118:111530.
Wang J, Wang L, Gao Y, Zhang Z, Huang X, Han T, et al. Synergistic therapy of celecoxib-loaded magnetism-responsive hydrogel for tendon tissue injuries. Front Bioeng Biotechnol. 2020;8:592068.
Yavvari PS, Pal S, Kumar S, Kar A, Awasthi AK, Naaz A, et al. Injectable, self-healing chimeric catechol-Fe(III) hydrogel for localized combination cancer therapy. ACS Biomater Sci Eng. 2017;3(12):3404–13.
Wu H, Song L, Chen L, Zhang W, Chen Y, Zang F, et al. Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents post-operative recurrence in a breast cancer model. Acta Biomater. 2018;74:302–11.
Luo M, Dorothy WD, Niu W, Wang Y, Zhao H, Qu X, et al. Bioactive therapeutics-repair-enabled citrate-iron hydrogel scaffolds for efficient post-surgical skin cancer treatment. Chem Eng J. 2022;431:133596.
Huang H, Wang X, Wang W, Qu X, Song X, Zhang Y, et al. Injectable hydrogel for postoperative synergistic photothermal-chemodynamic tumor and anti-infection therapy. Biomaterials. 2022;280:121289.
Gao F, Xie W, Miao Y, Wang D, Guo Z, Ghosal A, et al. Magnetic hydrogel with optimally adaptive functions for breast cancer recurrence prevention. Adv Healthc Mater. 2019;8(14):e1900203.
Xue W, Liu XL, Ma H, Xie W, Huang S, Wen H, et al. AMF responsive DOX-loaded magnetic microspheres: transmembrane drug release mechanism and multimodality postsurgical treatment of breast cancer. J Mater Chem B. 2018;6(15):2289–303.
Wang ZQ, Jia RR, Chen YH, Xie XY, Wang S, Huang JX. Supramolecular semiquinone radicals confined with DNAzymes for dissipative ROS generation and therapy. Nano Today. 2022;43:101402.
Zhang ZQ, Song SC. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials. 2016;106:13–23.
Ko S, Park JY, Oh YK. A Microbial Siderophore-inspired self-Gelling hydrogel for noninvasive anticancer phototherapy. Cancer Res. 2019;79(24):6178–89.
Yu C, Weili S, Qianyun T, Pingping L, Chengwu Z, Peng C, et al. Small-molecule diketopyrrolopyrrole-based therapeutic nanoparticles for photoacoustic imaging-guided photothermal therapy. Nano Res. 2017;10(3):794–801.
Lv X, Zhang J, Yang D, Shao J, Wang W, Zhang Q, et al. Recent advances in pH-responsive nanomaterials for anti-infective therapy. J Mater Chem B. 2020;8(47):10700–11.
Chen D, Zhong Z, Ma Q, Shao J, Huang W, Dong X. Aza-BODIPY-based nanomedicines in cancer phototheranostics. ACS Appl Mater Interfaces. 2020;12(24):26914–25.
Qing Y, Xuan H, Tian Z, Weili W, Dongliang Y, Jinjun S, et al. Near-infrared Aza-BODIPY dyes through molecular surgery for enhanced photothermal and photodynamic antibacterial therapy. Chem Res Chin Univ. 2021;37:951–9.
Chen D, Tang Q, Zou J, Yang X, Huang W, Zhang Q, et al. pH-responsive PEG-Doxorubicin-encapsulated Aza-BODIPY nanotheranostic agent for imaging-guided synergistic cancer therapy. Adv Healthc Mater. 2018;7(7):e1701272.
Ma W, Zhang H, Ma H, Wu C. Iron manganese silicate incorporated bioactive hydrogels for therapy of skin tumor. Prog Nat Sci Mater Int. 2022;33(1):1–8.
Spirou SV, Basini M, Lascialfari A, Sangregorio C, Innocenti C. Magnetic hyperthermia and radiation therapy: radiobiological principles and current practice. Nanomaterials. 2018;8(6):401.
Verma J, Lal S, Van Noorden CJ. Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma. Int J Nanomedicine. 2014;9:2863–77.
Gupta R, Sharma D. Evolution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem Neurosci. 2019;10(3):1157–72.
Singh A, Jain S, Sahoo SK. Magnetic nanoparticles for amalgamation of magnetic hyperthermia and chemotherapy: An approach towards enhanced attenuation of tumor. Mater Sci Eng C Mater Biol Appl. 2020;110:110695.
Yan X, Sun T, Song Y, Peng W, Xu Y, Luo G, et al. In situ thermal-responsive magnetic hydrogel for multidisciplinary therapy of hepatocellular carcinoma. Nano Lett. 2022;22(6):2251–60.
Quan K, Li G, Tao L, Xie Q, Yuan Q, Wang X. Diaminopropionic acid reinforced graphene sponge and its use for hemostasis. ACS Appl Mater Interfaces. 2016;8(12):7666–73.
Singh SK, Singh MK, Nayak MK, Kumari S, Shrivastava S, Grácio JJ, et al. Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano. 2011;5(6):4987–96.
Liu XL, Yang Y, Ng CT, Zhao LY, Zhang Y, Bay BH, et al. Magnetic vortex nanorings: a new class of hyperthermia agent for highly efficient in vivo regression of tumors. Adv Mater. 2015;27(11):1939–44.
Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z, et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew Chem Int Ed Engl. 2016;55(6):2101–6.
Hao Y, Gao Y, Fan Y, Zhang C, Zhan M, Cao X, et al. A tumor microenvironment-responsive poly(amidoamine) dendrimer nanoplatform for hypoxia-responsive chemo/chemodynamic therapy. J Nanobiotechnol. 2022;20(1):43.
Ma B, Wang S, Liu F, Zhang S, Duan J, Li Z, et al. Self-assembled copper-amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J Am Chem Soc. 2019;141(2):849–57.
Peiran Z, Zhongmin T, Xiaoyan C, Ziyu H, Xinhong H, Meng Z, et al. Ferrous-cysteine–phosphotungstate nanoagent with neutral pH fenton reaction activity for enhanced cancer chemodynamic therapy. Mater Horiz. 2019;6:369–74.
Cao S, Fan J, Sun W, Li F, Li K, Tai X, et al. A novel Mn-Cu bimetallic complex for enhanced chemodynamic therapy with simultaneous glutathione depletion. Chem Commun. 2019;55(86):12956–9.
Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: tumour Microenvironment-mediated Fenton and Fenton-like reactions. Angew Chem Int Ed Engl. 2019;58(4):946–56.
Zhang Q, Li Z, Zhang M, Wang W, Shen J, Ye Z, et al. Injectable In situ self-cross-linking hydrogels based on hemoglobin, Carbon Quantum Dots, and Sodium Alginate for Real-Time Detection of wound bacterial infection and efficient postoperative prevention of tumor recurrence. Langmuir. 2020;36(44):13263–73.
Yao J, Zheng F, Yao C, Xu X, Akakuru OU, Chen T, et al. Rational design of nanomedicine for photothermal-chemodynamic bimodal cancer therapy. Wiley interdisciplinary reviews. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(3):e1682.
Shi Y, Zhang J, Huang H, Cao C, Yin J, Xu W, et al. Fe-Doped Polyoxometalate as Acid-Aggregated Nanoplatform for NIR-II photothermal-enhanced chemodynamic therapy. Adv Healthc Mater. 2020;9(9):e2000005.
Karimi S, Bagher Z, Najmoddin N, Simorgh S, Pezeshki-Modaress M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int J Biol Macromol. 2021;167:796–806.
Wang P, Zhang W, Yang R, Liu S, Ren Y, Liu X, et al. Biomimetic poly(gamma-glutamic acid) hydrogels based on iron (III) ligand coordination for cartilage tissue engineering. Int J Biol Macromol. 2021;167:1508–16.
Ma M, Zhong Y, Jiang X. An injectable photothermally active antibacterial composite hydroxypropyl chitin hydrogel for promoting the wound healing process through photobiomodulation. J Mater Chem B. 2021;9(22):4567–76.
Huang T, Yuan B, Jiang W, Ding Y, Jiang L, Ren H, et al. Glucose oxidase and Fe3O4/TiO2/Ag3PO4 co-embedded biomimetic mineralization hydrogels as controllable ROS generators for accelerating diabetic wound healing. J Mater Chem B. 2021;9(31):6190–200.
Zhang D, Wang B, Sun Y, Wang C, Mukherjee S, Yang C, et al. Injectable enzyme-based hydrogel matrix with precisely oxidative stress defense for promoting dermal repair of burn wound. Macromol Biosci. 2020;20(6):e2000036.
Liang Y, Li Z, Huang Y, Yu R, Guo B. Dual-dynamic-bond cross-Linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano. 2021;15(4):7078–93.
Yang Y, Shi K, Yu K, Xing F, Lai H, Zhou Y, et al. Degradable hydrogel adhesives with enhanced tissue adhesion, superior self-healing, cytocompatibility, and antibacterial property. Adv Healthc Mater. 2022;11(4):e2101504.
Scheja S, Domanskyi S, Gamella M, Wormwood KL, Darie CC, Poghossian A, et al. glucose-triggered insulin release from Fe(3+) -cross-linked alginate hydrogel: experimental study and theoretical modeling. ChemPhysChem. 2017;18(12):1541–51.
Ziv-Polat O, Skaat H, Shahar A, Margel S. Novel magnetic fibrin hydrogel scaffolds containing thrombin and growth factors conjugated iron oxide nanoparticles for tissue engineering. Int J Nanomed. 2012;7:1259–74.
Hu Y, Jeong D, Kim Y, Kim S, Jung S. Preparation of succinoglycan hydrogel coordinated with Fe(3+) ions for controlled drug delivery. Polymers. 2020;12(4):977.
Su H, Han X, He L, Deng L, Yu K, Jiang H, et al. Synthesis and characterization of magnetic dextran nanogel doped with iron oxide nanoparticles as magnetic resonance imaging probe. Int J Biol Macromol. 2019;128:768–74.
Song B, Yang L, Han L, Jia L. Metal ion-chelated tannic acid coating for hemostatic dressing. Materials. 2019;12(11):1803.
Chen J, Li J, Zhou J, Lin Z, Cavalieri F, Czuba-Wojnilowicz E, et al. Metal-phenolic coatings as a platform to trigger endosomal escape of nanoparticles. ACS Nano. 2019;13(10):11653–64.
He Z, Hu Y, Gui Z, Zhou Y, Nie T, Zhu J, et al. Sustained release of exendin-4 from tannic acid/Fe (III) nanoparticles prolongs blood glycemic control in a mouse model of type II diabetes. J Controlled Release. 2019;301:119–28.
Lin P, Ma S, Wang X, Zhou F. Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv Mater. 2015;27(12):2054–9.
Giammanco GE, Carrion B, Coleman RM, Ostrowski AD. Photoresponsive polysaccharide-based hydrogels with tunable mechanical properties for cartilage tissue engineering. ACS Appl Mater Interfaces. 2016;8(23):14423–9.
Zhenhui L, Sijia L, Yiguan L, Zainen Q, Mingwei H, Fuben X, et al. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials. 2019;218:119190.
Ledo AM, Vining KH, Alonso MJ, Garcia-Fuentes M, Mooney DJ. Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta Biomater. 2020;110:153–63.
Li S, Chen N, Li X, Li Y, Xie Z, Ma Z, et al. Bioinspired double-dynamic-bond crosslinked bioadhesive enables post-wound closure care. Adv Funct Mater. 2020;30(17):2000130.
Tian R, Qiu X, Yuan P, Lei K, Wang L, Bai Y, et al. Fabrication of self-healing hydrogels with on-demand antimicrobial activity and sustained biomolecule release for infected skin regeneration. ACS Appl Mater Interfaces. 2018;10(20):17018–27.
Ji H, Dong K, Yan Z, Ding C, Chen Z, Ren J, et al. Bacterial hyaluronidase self-triggered prodrug release for chemo-photothermal synergistic treatment of bacterial infection. Small. 2016;12(45):6200–6.
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810.
Dwyer DJ, Kohanski MA, Collins JJ. Role of reactive oxygen species in antibiotic action and resistance. Curr Opin Microbiol. 2009;12(5):482–9.
Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, et al. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev. 2013;37(6):955–89.
Liu H, Shi X, Wu D, Kahsay KF, Deng L, Dong A, et al. Injectable, biodegradable, thermosensitive nanoparticles-aggregated hydrogel with tumor-specific targeting, penetration, and release for efficient postsurgical prevention of tumor recurrence. ACS Appl Mater Interfaces. 2019;11(22):19700–11.
Qian Q, Wang D, Shi L, Zhang Z, Qian J, Shen J, et al. A pure molecular drug hydrogel for post-surgical cancer treatment. Biomaterials. 2021;265:120403.
Leng Q, Li Y, Zhou P, Xiong K, Lu Y, Cui Y, et al. Injectable hydrogel loaded with paclitaxel and epirubicin to prevent postoperative recurrence and metastasis of breast cancer. Mater Sci Eng C. 2021;129:112390.
Lei N, Gong C, Qian Z, Luo F, Wang C, Wang H, et al. Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale. 2012;4(18):5686–93.
Yu Z, Xiao Z, Shuai X, Tian J. Local delivery of sunitinib and Ce6 via redox-responsive zwitterionic hydrogels effectively prevents osteosarcoma recurrence. J Mater Chem B. 2020;8(30):6418–28.
Li Q, Wen J, Liu C, Jia Y, Wu Y, Shan Y, et al. Graphene-nanoparticle-based self-healing hydrogel in preventing postoperative recurrence of breast cancer. ACS Biomater Sci Eng. 2019;5(2):768–79.
Lu Y, Wu C, Yang Y, Chen X, Ge F, Wang J, et al. Inhibition of tumor recurrence and metastasis via a surgical tumor-derived personalized hydrogel vaccine. Biomater Sci. 2022;10(5):1352–63.
Wang W, Zhang Q, Zhang M, Lv X, Li Z, Mohammadniaei M, et al. A novel biodegradable injectable chitosan hydrogel for overcoming postoperative trauma and combating multiple tumors. Carbohydr Polym. 2021;265:118065.
Shenqiang W, Hua Z, Li Z, Fang C, Zhao L, Hepeng Z, et al. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials. 2020;260:120314.
Ma H, Yu Q, Qu Y, Zhu Y, Wu C. Manganese silicate nanospheres-incorporated hydrogelsstarvation therapy and tissue regeneration. Bioact Mater. 2021;6(12):4558–67.
Chan F, Jiang O, Zhongmin T, Na K, Yuan L, LiYi F, et al. Germanene-based theranostic materials for surgical adjuvant treatment: inhibiting tumor recurrence and wound infection. Matter. 2020;3(1):127–44.
Talebian S, Shim IK, Foroughi J, Orive G, Vine KL, Kim SC, et al. 3D-Printed coaxial hydrogel patches with mussel-inspired elements for prolonged release of gemcitabine. Polymers. 2021;13(24):4367.
Wang X, Ma B, Xue J, Wu J, Chang J, Wu C. Defective black Nano-Titania thermogels for cutaneous tumor-induced therapy and healing. Nano Lett. 2019;19(3):2138–47.
Ni NY, Zhang XY, Ma YL, Yuan J, Wang DQ, Ma GQ, et al. Biodegradable two-dimensional nanomaterials for cancer theranostics. Coord Chem Rev. 2022;458:214415.
Ni NY, Su YQ, Wei YC, Ma YL, Zhao LZ, Sun X. Tuning nanosiliceous framework for nnhanced cancer theranostic applications. Adv Therap. 2021;4:2000218.
Acknowledgements
Not applicable.
Funding
We acknowledge the funding provided by the National Natural Science Foundation of China (22104073), Natural Science Foundation of Shandong (ZR2021QB119, 2022HWYQ-079, ZR2020MH066).
Author information
Authors and Affiliations
Contributions
HZ and MZ: writing-original draft. XZ, YG, JW: resources and figure processing. YM: writing-editing. HC and LC: conceptualization, resources, and supervision. XS and FW: writing-editing, supervision, and funding acquisition. All authors contributed to the article and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Zhang, M., Zhang, X. et al. Enhanced postoperative cancer therapy by iron-based hydrogels. Biomater Res 26, 19 (2022). https://doi.org/10.1186/s40824-022-00268-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40824-022-00268-4