Abstract
Recently, a considerable amount of literature has emerged around the theme of neuroinflammation linked to neurodegeneration. Glaucoma is a neurodegenerative disease characterized by visual impairment. Understanding the complex neuroinflammatory processes underlying retinal ganglion cell loss has the potential to improve conventional therapeutic approaches in glaucoma. Due to the presence of multiple barriers that a systemically administered drug has to cross to reach the intraocular space, ocular drug delivery has always been a challenge. Nowadays, studies are focused on improving the current therapies for glaucoma by utilizing nanoparticles as the modes of drug transport across the ocular anatomical and physiological barriers. This review offers some important insights on the therapeutic advancements made in this direction, focusing on the use of nanoparticles loaded with anti-inflammatory and neuroprotective agents in the treatment of glaucoma. The prospect of these novel therapies is discussed in relation to the current therapies to alleviate inflammation in glaucoma, which are being reviewed as well, along with the detailed molecular and cellular mechanisms governing the onset and the progression of the disease.
Similar content being viewed by others
Background
Glaucoma, a prime cause of irreversible blindness, refers to a group of ocular disorders with multifactorial etiology. As of now, it is considered a neurodegenerative disease in both the eye and brain [1]. In 2020, approximately 76 million people suffered from glaucoma and this number is expected to reach 112 million by 2040 [2]. These complex neurodegenerative disorders are characterized by optic neuropathy which is potentially progressive and visible changes can be seen at the optic nerve head (ONH) [3]. Glaucomatous optic neuropathy indicates a structural damage to the optic nerve with the corresponding loss of function. The structural damage is observed through the neurodegeneration of retinal ganglion cell (RGC) axons and deformation of lamina cribrosa with a concomitant diffuse and localized nerve fiber bundle pattern [4]. Undetected glaucoma in the early stages increases the risk of visual field loss [5]. The visual acuity may be spared at the early stage of the disorder, but progression of the neurodegenerative changes may result in the complete loss of vision [6].
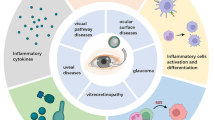
Mechanisms underlying the development and progression of glaucoma at the level of RGC axons at the ONH remain unclear [7]. However, studies have indicated that early neuroinflammatory response is potentially a contributing factor to glaucomatous optic neuropathy (Fig. 1) [8]. Immunological surveillance in retina mediated by astrocytes, microglia, and other blood-derived immune cells is hypothesized to be associated with pro-inflammatory events leading to RGC damage [9, 10]. Several studies also found substantial evidence on the detrimental impact to axons, cell bodies, and dendrites of the ganglion cells during the early stage of experimental glaucoma in animal models [11, 12]. Interestingly, dampening certain pro-inflammatory pathways appears to have a neuroprotective effect on RGCs, particularly on events at the ONH during the early stages of glaucoma, further demonstrate the role of neuroinflammation in its pathogenesis [13]. Regardless of the initiation of insults, the neurodegeneration of ganglion cell axons is associated with the loss of ganglion cell bodies via apoptosis [14]. Since RGCs are unable to regenerate their axons, their loss is irreparable, which can disable the eye from generating connections to the brain and result in lifelong visual loss [14]. However, it calls for a greater concern because its prevalence is on the rise, and unlike in the case of cataracts, there is no effective therapy available [15].
Relationship between microglia and astrocytes in glaucomatous neurodegeneration. Upon injury, they release immunological signals, which include pro-inflammatory cytokines, subsequently triggering secondary mechanisms exacerbating the neuronal injury and eventually cell death. Created with BioRender.com (2022)
The goal of any glaucoma treatment is to prevent vision loss. Most recent therapies have been focused on lowering intraocular pressure (IOP), as it is the only proven treatment for glaucoma; elevated IOP is considered the primary risk factor for the initiation and progression of the disease [3]. However, a significant number of glaucoma patients show worsening visual fields even when the IOP is controlled [16]. Although higher baseline IOP and older age are regarded as consistent and predisposing factors for glaucoma progression, several other factors should not be overlooked [17, 18]. In particular, individuals with family history of glaucoma, genetic predisposition, medications for pre-existing conditions such as systemic hypertension and diabetes, high myopia with great disc torsion of the optic disc and thinner lamina cribrosa at the ONH, and central corneal thickness, are amongst the factors reported that can influence the development of the disease [19, 20]. Nevertheless, to this day, IOP remains as the cardinal modifiable parameter in the management and treatment of glaucoma. In spite of that, most treatments for controlling IOP are associated with adverse effects and none of the current anti-glaucoma medications provide retinal neuroprotection by preventing RGC loss [21]. Although more than a few emerging therapeutic agents seem to have the potential to provide neuroprotection in human glaucoma, none of them have been clinically approved so far. Thus, there remains a need for therapeutic interventions that can provide maximal retinal neuroprotective effects in glaucoma with minimal adverse effects.
Glaucoma drug therapy typically employs topical instillations of eye drops. Although other glaucoma treatments such as surgical and laser therapy are increasingly utilized in the clinical setting, conventional eye drop remains the primary treatment for the majority of glaucoma cases [21]. Owing to various anatomical and physiological barriers in the eye, it is highly challenging for the drug to reach the target site [22]. Topical application of the glaucoma drug is predicted to reach the target tissue at the amount not higher than 5% of the applied amount due to the rapid clearance mechanisms at the corneal surface [23]. In addition, poor instillation by patients, especially the elderly, and the drug overspill are other contributing factors to the low bioavailability of ocular drugs in glaucoma [24]. To circumvent these obstacles, a new paradigm for glaucoma medical therapy is needed to fulfil the gold standard of the treatment criteria, including the efficient reduction of the IOP in such a way that the visual field is not compromised, and the optic nerve protected without causing tachyphylaxis and without generating other local and systemic adverse effects. It is also important to consider a treatment that can promote patient compliance and applicability in diverse patient populations [25].
Among many new therapeutic innovations for the treatment of glaucoma, nanoparticles (NPs) occupy a prominent place [26]. In this review, we have examined the potential of NPs in the treatment of glaucoma by emphasizing the mitigation of neuroinflammation, all to circumvent the drawbacks in the current glaucoma therapies.
Main text
Modulation of neuroinflammation in glaucoma
Neuroinflammation in glaucoma can take place at different physiological locations, but it is most prominent at the posterior segment of the eye (i.e., retina and optic nerve; Fig. 2) and the brain (i.e., superior colliculus and lateral geniculate). It can also occur peripherally in blood vessels. Nonetheless, the primary focus has been on the RGCs. In the ONH, most research has demonstrated the critical concern of glaucoma in which the RGC soma, synapses and dendrites show the effects of neuroinflammation and peripheral immune responses. Recent studies have demonstrated leukocytic recruitment into the ONH and the retina, which may contribute to the development of the glaucoma [13, 27].

(Adapted from “Structure of The Retina”, by BioRender.com (2022). Retrieved and edited from https://app.biorender.com/biorender-templates)
The overview of human retinal cells and layers
In the pathophysiology of glaucoma, RGC axons are the first to be affected. Mechanical alterations to lamina cribrosa, neurotrophic signaling, direct RGC pressure, and neuroglial activation, such as that of microglia or astrocytes, are among the initial stimuli for this event. Müller glia, astrocytes, and microglia are the ‘resident cells’ that stimulate innate immune responses in the retina and optic nerve. The astrocytes play a crucial role in controlling homeostatic conditions for neurons by maintaining neurovascular coupling (neuronal activity/local blood flow) [28, 29]. When activated, astrocytes undergo morphological alteration and proliferation to the area of injury. Severe astrocytosis, an abnormal increase in the number of astrocytes that leads to inflammatory responses, has been reported in glaucoma and is known to be involved in the onset of the disease [30]. Some studies have reported that ONH astrocytes have a phagocytotic effect which are able to engulf synaptic materials and cellular debris [31, 32]. Nevertheless, the extent of effects of astrocytosis is still the subject of ongoing research.
In glaucomatous pathophysiology, microglia and macroglia are the key players responsible for immunoregulation in the retina [33]. These cells are responsible in several key functions including providing nutritional and structural support, regulating metabolic activity and homeostasis, phagocytosis as well as levels of cytokines and neurotrophic factors [34].
Major neuroinflammatory cells in glaucoma
Microglia is the key cell type controlling neuronal function and homeostasis. They are phagocytes that play a vital role in the innate immune response. As resident macrophages, its presence is undoubtedly ubiquitous in the central nervous system (CNS) [35]. This cell is the first to react toward the site of injury by stimulating inflammatory cascades and recruiting other inflammatory cells, such as astrocytes. In an in vivo study of glaucoma, activated microglia were found to increase in number in glaucoma; however, it is not certain whether these reactions are beneficial [36]. Initially, microglia were assumed to have ascended from the yolk sac of macrophages that had entered the brain during the development of fetus. However, more recently, it is believed to have come from circulated monocytes, which later differentiated into microglia [37]. Microglia are responsible for homeostasis of the neural circuits and angiogenesis in the retinal development. In mature retina, microglia help in neuronal signaling and integrity of synaptic transmission [38,39,40]. Some studies have suggested that inactivation of retinal and ONH microglia using drugs known as minocycline lowers the neurodegenerative actions [41, 42]. Astrocytes, microglia, and macrophages are believed to be involved in neuronal inflammation, with aging among the causative factors [43].
In glaucomatous eyes, activation of microglia has been detected at an earlier stage, whereas the aggregation, activation and redistribution of microglia is seen even before RGC injury has taken place in a DBA/2J mouse model of chronic hereditary glaucoma [44]. The early phase of the glaucomatous model in mice also involved the monocytic recruitment and other pathologies with neuronal damage [45]. Microglial activation and proliferation can lead to detrimental effects on RGCs through the secretion of pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and reactive oxygen species (ROS) [46]. In another study utilizing the same animal model, the transcriptome of ONH microglia was seen to change drastically in the metabolic, phagocytotic, inflammatory and sensome pathways [47]. This was confirmed by another study, which showed an increased activity of microglia and their density in the retina, including the optic nerve when IOP was elevated [36]. This finding supports the hypothesis that the chronic ocular hypertension inhibits the homeostasis-regulating function of microglia [47]. Despite all these findings, the role of microglia is still debatable. Few studies have suggested that RGC injury can be worsened by microglia when the inflammatory mediators such as TNF-α, interleukin 1β (IL-1β), IL-6, matrix metalloproteinases, Fas ligands (FasL), and ROS are released [46, 48].
Macroglia are predominantly Müller cells and astrocytes that share similar transcriptomic profiles and functions [49]. Out of these two cell types, the prime macroglial cells are Müller cells, which can be found across the retina. Their cell bodies lie in the inner nuclear layer, which elongates into two trunks that extend their ends into the inner limiting membrane. The inner limiting membrane separates the retina from the vitreous body and is one of the most significant barriers for ocular drug delivery [50]. For a detailed overview of the inner limiting membrane, the reader is referred to the work by Peynshaert et al. [50,51,52]. Müller cells are essential in maintaining the structural integrity of the retina. They are also important regulators for cell metabolism in the retina [53]. Müller cells are anatomical conduits among the retinal neurons, including the cellular environment. Hence, they help in maintaining retinal homeostasis. Astrocytes are the foremost glial cells found at the ONH. As the dominant component of glial cells in the CNS, astrocytes engage in a variety of critical functions, such as ionic balance regulation, metabolic supply and its structural maintenance, neurotransmitter transmission, and synaptic plasticity [54]. Collectively and together with microglia, astrocytes and Müller cells ensure a smooth process for the synaptic activity to occur by maintaining ion and neurotransmitter levels.
When an injury occurs to the retina, macroglia can be stimulated to release glial fibrillary acidic protein (GFAP) and other extracellular matrix proteins [55, 56]. In the glaucomatous retina, there is an increased amount of GFAP immunostaining and macroglia display a hypertrophic morphology, suggesting the existence of retinal gliosis in glaucoma [57]. Several models of glaucoma have showed that the number of astrocytes increases with increased GFAP immunoreactivity, and this was also seen on the extracellular matrix remodeling on the ONH. GFAP is an intermediate filament of the glial cell cytoskeleton that upsurges when astrocytes transform into their reactive state [55]. Furthermore, some studies have shown that ONH astrocytes play a significant role in the engulfment of optic axons, including stimulation of axon degradation, which is one of the proposed mechanisms for the sectorial nature of RGC loss in glaucoma [32]. The inflammatory response in glaucoma is activated and mediated early on through a process called astrogliosis. Several inflammatory pathways were activated by astrocytes when rats were injected with hypertonic saline into their episcleral veins, leading to a high IOP [58]. The activation of inflammatory pathways includes tumor necrosis factor α (TNF-α) signaling, nuclear factor kappa B (NF-κB) activation, autophagy, and inflammasome-associated regulators.
Inflammatory pathways
TNF-α and toll-like receptors (TLRs) pathways are among the crucial complement cascades in glaucomatous neuroinflammation. Furthermore, there are various associated inflammatory mediators involved such as cytokines and prostaglandins [59], and pathways such as β2-microglobulin and cluster of differentiation 3 (CD3) [60]. Studies are currently focused on the classical pathway of the complement cascade whereby RGCs detect stimuli of injuries and activates the complement component 1 (C1) complex, a giant proteolytic enzyme [61,62,63]. This is followed by activation of the C3 convertase, which can attract leukocytes and further activates C5 convertase. C5 convertase would recruit more leukocytes and stimulate cell lysis through the membrane attack complex. In the complement cascade in a glaucomatous eye, glial cells such as astrocytes would amplify the RGC signal to boost microglia response and even attract monocytes, especially in the ONH and inner plexiform layer. Rather than killing the RGCs, the complement system is believed to protect them from further damage and maintain their function [64]. Still, based on the previous glaucomatous animal model, the nature of the neuroinflammatory mechanism is thought to possess an evidently damaging role on the disease [65, 66].
Meanwhile, TLRs pathway induce glaucomatous neuroinflammation in two ways, either through polymorphism of TLR4 alleles [67] or increase in TLR4 protein expression in the retina of glaucoma animal models [68]. Different TLRs recognize different stimuli. For instance, TLR3 detects double-stranded RNA of foreign substances, whereas TLR4 focused on detecting an endogenous ligand, for example, tenascin-C, which is elevated in the glaucomatous ONH [69, 70]. It has also been found that monocytes and microglia have more detecting TLRs as compared to astrocytes. Even though some studies have examined TLRs in RGC injury, more investigation is needed to further delineate the role of TLRs in human glaucoma.
Another critical activator of neuroinflammation in glaucoma is TNF-α, which is produced by astrocytes and especially microglia [71, 72]. Studies have reported that polymorphism [73, 74] and increment of TNF-α in the vitreous body, retina and optic nerve are associated with glaucoma [75, 76]. Parallel to FasL’s downstream actions, TNF-α also triggers RGC cell death [77, 78]. In a vitreous glaucoma mouse model, oligodendrocyte and RGC damages are induced by soluble murine TNF-α while TNF-α suppression prevented these damages [43, 79]. This is similar to the extent of blocking FasL activity by pharmacotherapy [80]. Although TNF-α inhibitors seem to have a neuroprotective property in clinical settings, further research is needed to clarify this, especially in glaucomatous diseases [74].
Several pathways of neuroinflammation have been proposed, although more extensive studies are required for both in vivo and human glaucoma. The continued elucidation of these pathways is essential for defining therapeutic targets that have clinical benefit.
Current investigational therapies to alleviate inflammation in glaucoma
Immunomodulatory drugs
With respect to the neurodegenerative potential of neuroinflammation, several molecules have showed the potential to act as ‘neuroprotectors’. Citicoline (cytidine 5′-diphosphocholine) exemplifies a naturally endogenous compound that has been evaluated for its protective role on RGC in glaucoma [81]. A number of in vitro and in vivo studies have demonstrated the neuroprotective role of citicoline via increased dopamine retinal levels, enhanced anti-apoptotic effect, restrained thinning of the retinal nerve fiber layer (RNFL), regeneration of neurites, defense against glutamate excitotoxicity, and minimized RGC impairment, thereby enhancing a better visual field [82]. A significant reduction in the apoptotic nuclei pathway of cell death with contrasting synaptic loss achieved with citicoline treatment showed its efficacy in protecting against the excitotoxic neuronal damage and thus delayed the progression of glaucoma [83, 84]. Citicoline also plays a crucial role in the regeneration of the axon through sphingomyelin synthesis, which stabilizes the plasma membrane of RCG axons, thereby suppressing free fatty acids and protecting against the redox imbalance [85]. Experimental studies in adult male Albino rabbits treated with citicoline had demonstrated a higher dopaminergic neurotransmission in the brain compared to the untreated group, and highlighted the influence of citicoline on retinal catecholamine levels [86]. The usage of citicoline against retinal damage eventually proved to have a neuroprotective effect in kainic acid-induced neurotoxicity in vivo [87]. Shuettauf et al. investigated the anti-apoptotic effect of mitochondria-dependent cell death mechanism by delivering citicoline with lithium, with the outcome being a rise in the RGC density [88]. In a randomized clinical trial, Parisi et al. observed, enhanced retinal and visual functions in a glaucoma patient who received citicoline [89]. A follow-up electrophysiological analysis of glaucomatous visual dysfunction, which was carried out in conjunction with hypotensive therapy, further confirmed citicoline to be a fitting medical treatment for glaucoma within an extended period of time [90]. The effects of citicoline administered through oral and intramuscular approaches were subsequently tested on glaucoma patients with moderate visual defects, showing improvement in retinal function [91]. In a similar study conducted by Ottobelli et al. patients with progressing glaucoma were supplemented with an oral citicoline solution and the follow-up visual examinations showed reduced rate of mean progression by the end of the study after the treatment [92]. Lanza et al. demonstrated neuroprotective effect of oral citicoline, which slowed down the progression of primary open-angle glaucoma (POAG). The citicoline therapy assessment by standard automated white-on-white perimetry showed a stable and highly significant mean deviation (MD) of progression over time in treated patients compared with untreated patients [93]. Another method carried out in a different study acknowledged that intravenous therapy can be a means for citicoline to reduce the progression of glaucoma in conjunction with citicoline eye drops as the IOP lowering treatment. Visual field and RNFL loss detected were much lower on average [94]. Studies involving the encapsulation of citicoline eye drops in a liposomal formulation conducted by Parisi et al. also suggested improved retinal bioelectrical responses with enhanced visual cortex bioelectricity [95]. Overall, these findings showed the crucial role of citicoline as a neuroprotective compound for managing glaucoma, yet further clinical trials with larger sample sizes are highly needed to gather more understanding in relation to the dose-response and clinical effects.
The renin-angiotensin aldosterone system (RAAS) is a complex endocrine system that has a major function in the regulation of hemodynamic stability and fluid balancing. Upon the occurrence of hypotension in the body, granular cells of renal juxtaglomerular apparatus release the renin enzyme, which cleaves angiotensinogen to angiotensin II (Ang II) via Ang II type 1 receptor (AT1-R) [96]. Recent evidence suggests the prospect of utilization of AT1-R antagonists as a treatment for several conditions such as hypertension, blood pressure and cardiovascular diseases, mainly due to the pro-inflammatory effects of Ang II and aldosterone [97]. Studies have proven that administration of AT1-R blockers is not only able to transverse the blood-brain barrier and communicate with AT1-R to minimize the infarct volume, but also extenuate inflammatory and oxidative stress in the retina and brain [98]. Yang et al. showed that the AT1-R signaling blockade of candesartan succeeded in averting the retinal neuronal death in a rat model of chronic glaucoma [99]. Similarly, the orally active AT1-R antagonist candesartan inhibited toll-like receptor 4 (TLR4-apoptosis signal-regulating kinase 1 pathway), which supported the activation of RAAS in the innate immune response, expediting neural cell death [96]. The conclusion is a significant neuroprotective effect of Ang II against RGC loss.
Natural products
Aside from the standard therapy used currently, which involves IOP reduction through medical drugs, laser and surgical therapy, herbal medicine is one of the primary alternatives chosen in the management of glaucoma [100]. In the nineteenth century, active compounds were directly isolated from plants [101]. Plants such as ginkgo biloba, saffron, and phytochemicals such as epigallocatechin-3-gallate and resveratrol are known as traditional remedies used in glaucoma pathology [102].
Among various antioxidative compounds present, Ginkgo (Ginkgo biloba), which originated from China 250 million years ago, has been recognized for its therapeutic effects in several pathologies, including neurodegenerative diseases [103]. The beneficial component of this living fossil tree is found in the ginkgo extract, which contains polyphenolic flavonoids that stabilize the mitochondria at organelle level, and also exerts multiple therapeutic properties, including the antioxidant, antimicrobial, neuroprotective and antiapoptotic effects [104, 105]. Extract 761 (EGb761), obtained from leaves of the ginkgo plant, has been effective in treating Alzheimer’s dementia and cognitive impairment. Therefore, researchers attempted to use EGb761 in the treatment of glaucoma due to the analogous biological and mechanistic features between these two chronic disorders [106]. Namely, both Alzheimer’s dementia and glaucoma are age-related pathologies, experiencing the RGC degeneration and deposition of extracellular fibrils in the exfoliation syndrome, indicating that both are likely derived from similar misfolding mechanisms [107]. In previous studies, both short- and long-term effects of the ginkgo biloba extract (GBE) were tested and the extract was used to treat pre-existing patients with normal tension glaucoma (NTG), often resulting in a significant improvement of visual acuity [108, 109]. However, Guo et al. who performed a randomized, crossover clinical trial, failed to demonstrate the effect of GBE to improve progressing visual defects within normal NTG patients, likely due to the smaller sample size and shorter time periods applied during the study [110]. The administration of GBE also showed an increasing end diastolic velocity in the ophthalmic artery and NTG throughout clinical cross-over trials, highlighting the desirable effect of the drug on the retinal blood flow in glaucoma disorders [111, 112]. Shim et al. supported these findings by showing the escalating MD upon GBE and bilberry anthocyanin treatment [113]. In a similar study, the standardized EGb761 extract demonstrated a progressing pharmacological effect on the oxidative stress with improved vascular circulation in both in vitro and in vivo experiments, highlighting the neuroprotective effect of the drug against the hypoxic injury of RGCs [114]. These findings have emphasized the prospect of this natural medicine in treating glaucoma. However, its usage has yet to become widely recognized in public.
Saffron, the dried stigmas originating from the Crocus sativus flower of the Iridaceae family in Greece, has been commonly used in cooking as an aromatizing and coloring seasoning [115]. The major constituents in saffron are natural carotenoid compounds, namely crocin and crocetin [116]. Its usage in the medical field has been recognized in the treatment of various diseases due to the wide therapeutic spectrum, including neuroprotective, anti-inflammatory, anti-oxidant and anti-genotoxic activity [117]. Both saffron compound extracts, crocin and crocetin, showed an enhanced neuroprotective effect through repression of activated microglia neurotoxicity. The development of intracellular ROS and nitric oxide is inhibited with a slower release of TNF-α and IL-1β [118]. These beneficial aspects can be observed in animal models of neurodegenerative ocular diseases and patients suffering from diabetic retinopathy and age-related macular degeneration (AMD). Studies in animal models with retinal damage emphasized the role of crocin in saffron as an inhibitor of the ischemic damage and a stabilizer of the ocular blood flow, alongside the neuroprotective effect provided by crocetin [119]. In a pilot study, Bonyadi et al. investigated the influence of an aqueous saffron extract on the IOP in the eyes of POAG patients and showed that the treatment significantly decreased the mean baseline IOP compared to the control group by the end of the therapy [120]. Despite the limited studies on saffron in glaucoma disease, the saffron extract emerges as an important therapeutic agent for potential clinical use.
Epigallocatechin-gallate (EGCG) is a type of catechin mainly found in green tea. It is well known as a robust antioxidant with multifunctional properties and has been investigated for its contribution to neuroprotection in human corneal epithelial cell culture models and animal models of glaucoma [121, 122]. Earlier findings not only demonstrated its therapeutic effect on the axon and the bodies of RGCs in optic nerve crush and N-methyl-d-aspartate (NMDA) toxicity studies, but also showed an elevation in the survival rates of RGCs via oral administration [123, 124]. In a similar study, which also used oral EGCG, the drug was shown to be a potent penetrator into the retina, where it reduced both the injury caused by ischemia and in vitro white light-induced apoptosis in RGC-5 cells [125]. Falsini et al. claimed a higher amplitude detection in the OAG group compared to the ocular hypertension (OHT) group in a pattern electroretinogram analysis, thus supporting the prospect of short-term supplementation of EGCG [121]. ECGC does not only provide protection against the oxidative stress, but also has the capability to weaken the glutamate-induced cytotoxicity by decreasing the ionotropic calcium influx [126]. These outcomes showed that EGCG is a suitable neuroprotective agent for the glaucoma treatment. However, there is a need to perform further studies to determine the long-term benefits, the component activity, and the precise dosage requirements for EGCG in the glaucoma treatment.
Resveratrol (RSV), also known as 3,5,4′-trihydrocystilbene, a nonflavonoid polyphenol compound derived from plant sources such as grapes, blueberries and apples, has been developed into an effective phytoalexin [127]. It has diverse roles in relation to the well-being of humans, by virtue of biological attributes including antioxidant, anti-inflammatory and neuroprotective functions [128]. In POAG patients, RSV was shown to interrupt intracellular ROS, inhibit the release of inflammatory cytokines and slow down the accretion of carbonylated proteins, hence supporting the neuroprotective action of the drug against the RGC apoptosis and the ability to slow down the progression of glaucoma [129]. Other studies also demonstrated this neuroprotective effect of RSV, including the delay in the RGC loss upon dosing with RSV and riluzole. Although both single and combined administrations were effective, an improved and better RGC protection was provided through the combined therapy [130]. Luo et al. showed that sirtuin 1 (SIRT1) activation by RSV confers neuroprotection in mice with ischemia–reperfusion injury (IR) via Akt activation and mitochondrial apoptotic suppression with a verified concentration of intravitreal injection, and thus contributed to the understanding of the mechanism of action important for the clinical usage of RSV [131]. Inhibition of endothelin-1, a vasoactive peptide in glaucoma, highlighted a pivotal effect of RSV [132]. Moreover, researchers have suggested the induction of mitochondrial biogenesis by RSV to alleviate glaucomatous retinopathy. This is due to the efficiency of RSV in reducing derivative-serum in the RGC-5 cell line by subcellular translocation of SIRT1 dependent proliferator-activated receptor-gamma coactivator 1 alpha [133]. In addition, Shamsher et al. studied the in vitro and in vivo neuroprotective effects of RSV and curcumin nanoparticle formulations with ~ 70% encapsulation efficiency [134].
One of the examples of long-standing, well-conducted research and development of an anti-inflammatory agent comes from curcumin, a major active compound of turmeric, Curcuma longa [135]. Curcumin has shown exceptional promise for the beneficial modulation of numerous signaling molecules (e.g., pro-inflammatory cytokines, NF-κB, apoptotic proteins, and C-reactive protein) in multiple diseases, including cancers and inflammatory and neurodegenerative disorders. Apart from anti-inflammatory properties, curcumin exerts antioxidant, anti-microbial and anti-tumorigenic activity. Owing to these properties, curcumin has been extensively studied in vitro and in vivo in the context of many inflammatory, autoimmune, and degenerative diseases of both anterior and posterior segment, and has been suggested as an adjuvant therapy [136]. In a retinal ischemic injury animal model, curcumin was reported to prevent ischemic damage to the RGC and microvasculature via suppression of NF-κB signal transducer as well as activation of transcription 3, and monocyte chemotactic protein 1 expression [137]. The chemical properties in curcumin with anti-inflammatory and antioxidant functions have been suggested to be associated to its hydroxyl and methoxy group, which deregulates TNF-α and pro-inflammatory interleukins which lead to the downregulation of STAT pathways. In both in vitro and in vivo experimental glaucoma studies, curcumin has shown antioxidant effects, as demonstrated by the improved cell viability of microglial cells, reduced intracellular ROS and apoptosis of RGCs [138]. These findings should make an important contribution to the therapeutic potential of curcumin in clinical ophthalmology, notwithstanding that this potential is restricted by a few adverse factors, including extremely poor bioavailability and water solubility [139]. The active fraction of curcumin detected in the blood is often suboptimal, for which reason increased doses are needed to achieve the proper therapeutic effect [140]. To overcome these limitations, several approaches such as the use of enhancers, analogues and nanocarriers to provide a hydrophobic environment for poorly water-soluble curcumin have been reported and extensively reviewed by other [141,142,143]. An in vivo study by Davis et al. demonstrated < 95% encapsulation efficiency with good stability upon the formulation of topical curcumin-loaded, Pluronic-F127 stabilized d-α-tocopherol polyethene glycol 1000 succinate NPs (< 20 nm) [143]. Similarly, Cheng et al. developed a formulation consisting curcumin-latanoprost NPs (~ 161 nm), which resulted in a sustained-release profile with low oxidative stress-mediated damage via ROS production and apoptosis in vitro and in vivo [144]. These studies highlight the potential of curcumin to provide a neuroprotective therapy in glaucoma. Of note, the use of nanocarriers is one of the most prospective approaches in improving curcumin delivery. With the development of a nanocarrier suitable for utilization as a topical formulation, the bioavailability of curcumin could be drastically improved [143]. One of the promising drug carriers for the delivery of curcumin has been the amphiphilic polymer polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer, Soluplus [145]. Although this and other types of carriers must be further investigated, they provide important opportunities for advancing the understanding of curcumin as an anti-inflammatory agent and, potentially, as a neuroprotective therapy in glaucoma.
The above mentioned substances were extensively studied in both IOP-dependent and -independent types of glaucoma. Outside traditional clinical settings, the progression of glaucoma can be controlled, yet it still cannot substitute the conventional therapeutic management of glaucoma; hence, further studies are required. It is noteworthy that an increasing number of experimental studies currently consider molecular targets in the modulation of inflammatory responses activated by microglial cells, RGCs and other retinal cells that elicit downstream actions of inflammatory pathways responsible for glaucomatous neurodegeneration (Table 1). Although the therapeutic anti-inflammatory potential of the various agents seems to be encouraging, their neuroprotective effects could be attributed to other factors, including the route of delivery to the target tissues, which may have an impact on patient safety and compliance in clinical practice [3]. Most of the therapeutic agents also require a proper formulation to provide optimal neuroprotection, particularly in promoting RGC survival under glaucomatous conditions. To fully explore the potential of these therapies and their biocompatibility for the treatment of glaucoma in humans, further investigations are necessary to develop formulations that can be administered non-invasively.
Potential of nanoparticles in drug delivery
Current perspective on glaucoma therapies targeting neuroprotective agents
Neuroprotection in glaucoma refers to any IOP-independent intervention that preserves the optic nerve by preventing or delaying RGC and axonal degeneration [146]. Regardless of the various definitions, neuroprotection is a therapeutic approach directed at keeping RGCs alive and functional in progressive glaucomatous optic neuropathy [147]. Data from randomized controlled clinical trials show that even with excellent IOP control the disease is still exacerbated in some patients [148]. Therefore, the idea of IOP-independent treatment strategies in glaucoma should be extensively investigated.
There is a vast amount of literature on identifying neuroprotective agents targeting the mechanisms proposed that underlie RGC damage in glaucoma [149]. Glutamate excitotoxicity antagonists, neurotrophic factors, and oxidative stress suppression are some of the studied neuroprotective agents with favorable neuroprotective activities. For further details regarding these, the reader is referred to the comprehensive reviews by Sharif and others [3, 150,151,152,153]. However, results from human clinical trials have been inconclusive and non-consequential [154]. Over time, literature reports have shifted towards inflammatory and immune responses, supporting the notion that neuroinflammation could be the key player in the mechanism underlying retinal damage in glaucoma, potentially having a reciprocal causative role in the pathology [69]. Since the mediators of neuroinflammation activate the immune system within the CNS, they may have either harmful or beneficial effects on RGC survival. This justifies a dire need for better therapeutic strategies. As such, the development of new therapies aimed at modulating rather than suppressing neuroinflammation might also produce the highly sought-after neuroprotective effects.
Challenges in ocular drug delivery
The outcomes of clinical trials testing for the safety and efficacy of neuroprotective agents demonstrate clear challenges in the aspect of drug delivery [146]. Poor drug delivery could be a factor largely contributing to the failure of the drug in clinical studies. The most common administration routes for glaucoma drug delivery are intravenous, subcutaneous, topical, and oral [155]. However, through these routes, ocular drugs are prone to absorption into the systemic circulation, which may result in low dose delivery to the target tissue and increase systemic risks. The low dose delivery could be also due to the poor solubility of the drug entailing high degradation rate and the failure to pass through the cornea and across the blood-retina barrier [156]. For topical administration, the corneal and the non-corneal routes control and influence the absorption [22]. The course that the drug molecules take towards the target cell in the intraocular environment starts with their passive diffusion via barriers formed by tight interconnected junctions. The components of these barriers include the precorneal pocket, corneal epithelium, the blood aqueous barrier, the retinal pigment epithelium and the blood capillary endothelial cells (choroidal barrier), all of which inevitably restrict the permeation of drug molecules into the intraocular chamber where they are to carry out their pharmacological action, resulting in an inefficient therapy [157]. Even if it were perfectly efficient, this trajectory would hardly allow for the access of the drug to the posterior area of the eye, where RGCs and the optic nerve reside. The most common route of administration to treat this posterior segment of the eye in experimental studies has been through intravitreal injection [158]; this is an immediate and direct route, which can increase the therapeutic drug delivery to the vitreous cavity and bypass the aforementioned barriers [159]. The intravitreal route is safe and effective, but due the invasiveness of the procedure, it is accompanied by side effects such as elevated IOP, cataract formation, bleeding and the risk of ocular infections [159]. This route is particularly problematic because drugs can be prevented from reaching the target tissue by the posterior vitreoretinal interface, including the inner limiting membrane of the retina and the vitreous cortex [160]. Figure 3 shows a basic schematic of the eye with anatomical barriers and common routes of drug delivery.

(Adapted from “Anatomy of the Human Eye”, by BioRender.com (2022). Retrieved and edited from https://app.biorender.com/biorender-templates)
Schematic diagram of the human eye with barriers [tear film (precorneal), corneal, conjunctival, blood aqueous, vitreoretinal interface to blood-retinal barrier] and the common route of administration. Primary methods of drug delivery to the eye are topical (1), local ocular [e.g., intravitreal (2) and subconjunctival (3)] and systemic [i.e., oral (4) and intravenous (5)]. Topical instillation is the most widely preferred non-invasive route of drug administration to treat diseases affecting the anterior segment and, potentially, the posterior segment. The ocular barriers block the entry of the most active molecules; hence, effective drug delivery systems are required to facilitate the passage of the drug across these barriers and transport the given pharmaceutical compound to its target site to achieve an optimal therapeutic effect [151]
The eyes are easily accessible in terms of delivering the drug into the body, yet the drug distribution is one of the most challenging endeavors. Therefore, the development of safer and more efficient drug delivery systems is vital for therapeutic purposes. Research conducted to date address these challenges and there is a growing consensus that the characteristics of ideal drug carriers are as follows [161, 162]:
-
Particle size reduction and direct interaction with target cells or tissues (adhesive properties);
-
Improved drug retention time in the precorneal area and promoted drug tissue permeation as well as optimal tissue absorption;
-
Improved solubility of poorly soluble drugs (e.g., oral delivery of lipophilic drugs into the systemic circulation) and prolonged drug shelf-life;
-
Biodegradability and biocompatibility;
-
Absence of irritant features to reduce the drug dosing regimens and improve patient adherence to medication;
-
Protection of sensitive therapeutic molecules (e.g., small molecule drugs and bioactive agents) against degradation agents such as enzymes;
-
Targeted and controlled drug release characteristics that provide dose accuracy, reducing or preventing side effects and being ideal for long-term treatments.
The above criteria could be achieved by implementing NPs as drug carriers in ophthalmic drug formulations. This platform transports the drug molecules across biological barriers by physically or chemically attaching the molecules to the NPs [163]. The large surface-to-volume ratio of NPs, along with other chemical characteristics, enables mucoadhesive properties that aid in drug adhesion to the mucosa of the corneal tissue, hence potentially increasing the drug contact time with the ocular tissue [164]. Apart from the abovementioned, NPs have been shown to improve patient self-care and compliance in terms of reducing the frequency of topical eye drop instillations, ultimately reducing the required doses and the risk of adverse effects [161]. NPs for ocular drug delivery can not only improve the solubility of drugs so as to reach the posterior segment of the eye, but also enhance the cellular uptake and protect the drug from degradation [165]. By formulating NPs with currently available ophthalmic solutions or investigational drugs, a greater potential for an effective glaucoma therapy can be ascertained in the future. Tabular overview of current investigated polymeric and lipid based-NPs with incorporated ophthalmic substances as compared to pure substances in ocular tissues is presented in Table 2.
Nanoparticles as ocular drug delivery systems
NPs are ultrafine solid structures that vary in morphology and have at least one spatial dimension in the range between 1 and 100 nm, and sometimes up to 500 nm, for larger particles. In the field of drug delivery, NPs are formulated to enhance the penetration and drug targeting of the active compound, while promoting a sustained release [166]. Due to their miniscule nature, NPs can often easily infiltrate the anatomical barriers in the CNS [164], such as the blood-brain and blood-retinal barriers, and thus directly provide a maximal drug bioavailability to the target cells [157]. In NPs, the drug-loading capacity is dependent on a few factors, including chemistry and microstructure, but also size, especially for NPs carrying their drug payload on the surface. Smaller NPs in these cases provide a higher loading capacity than the larger ones due to their higher specific surface area. NPs can exhibit a wide variety of morphologies, which help to serve the specific purposes to provide an effective therapy.
Multiple studies have attempted to develop drug-encapsulated NPs for the delivery to anterior and posterior segments of the eye. Conjugating ocular drugs onto NPs has been shown to boost eye permeation, particularly pass through the precorneal barrier [167]. In neurodegenerative diseases associated with inflammation, extensive studies have exploited drug-encapsulating NPs [168]. Some of the NPs that have been employed in neurodegenerative experimental studies, including polymeric and lipid based ones, have emerged as the key players in the domain of anti-inflammatory drug carriers [169].
Generally, in the drug delivery field for neurodegenerative and ocular diseases, NPs are most commonly made of soft carbonaceous materials, such as polymers and/or lipids [170]. Both lipid and polymeric NPs have successfully delivered drugs for several therapeutic purposes, while protecting the encapsulated drugs from enzymatic degradation and controlling their release. NPs made of natural or synthetic polymers and proteins [e.g., chitosan, poly(ethylene glycol) (PEG), polycaprolactone, sodium alginate, and albumin] usually take the form of finely dispersed latexes [171]. Compared to other nanomaterials such as the inorganic ones (e.g., zinc oxide or aluminum oxide), the former have caused a minimal eye irritation and prolonged retention of drugs, and thus allow for the circumvention of multiple medications and dose reduction [162]. However, compared to nanomicelles, a type of nanocarrier, polymeric-based NPs have been unable to escape the rapid loss of the instilled solution from the precorneal integument and the nasolacrimal drainage system. To overcome this limitation, NPs with mucoadhesive properties (i.e., chitosan and hyaluronic acid) were developed [172]. Of note, both polymeric- and lipid-based NPs have successfully delivered drugs for a number of therapeutic purposes, while protecting the encapsulated drugs from enzymatic degradation and controlling their release [166]. Figure 4 shows the benefits of drug loaded NPs administered through the corneal and blood-retinal barriers.

(Adapted from “Anatomy of the Human Eye”, “Structure of the Retina” and “Tear Film Structure”, by BioRender.com (2022). Retrieved and edited from https://app.biorender.com/biorender-templates)
Overview of nanoparticle-mediated ocular drug delivery through the corneal barrier (a) and blood-retinal barrier (b). The potential advantages of the use of nanoparticles as an approach for improving current glaucoma medication (c)
In the beginning of the application of NPs in ocular drug delivery, different carbon-based NPs were developed with the aim of producing a sustained drug release in the precorneal pocket. This was due to the majority of ophthalmic formulations being administered as eye drops, which in their conventional forms are poorly bioavailable on the corneal surface and intraocular tissues. Among the earliest NPs were those made of acrylic polymers such as poly-alkylcyanoacrylate (PACA), which extended the time of drug contact with the eye surface. There was increased drug action duration, however, this resulted in ocular toxicity. Later, polyacrylamide NPs began to replace PACA for the same purpose [173].
Polyester NPs (e.g., polycaprolactone) have emerged in the recent times as a key biodegradable material for ocular drug delivery, largely thanks to the acute tolerance of the ocular surface to them. At the same time, polyester NPs can increase drug efficacy. For example, an ophthalmic betaxolol, a beta-adrenergic blocking agent, displayed its optimal pharmacological effect when encapsulated by polycaprolactone (hydrophobic NPs) owing to its gradual release. NPs made of biodegradable materials such as hyaluronic acid or composed of hydrophilic polysaccharides (components of the vitreous body) have also been proven safe for incorporation into ophthalmic solutions. Other NPs composed of polymeric-based materials have also improved the drug delivery interaction with the cornea, and thus allowed for the controlled drug release and the treatment of the ocular disease of the outer segment. It was postulated that nanocarriers coated with bioadhesive polymers (e.g., PACA and cyclosporine-A) can enhance the penetration of the embedded drug and improve the stability in the lacrimal fluid, which has been shown to prevent the enzymatic degradation of the delivered drug [173].
Recently, several studies have been performed to upgrade these standard NP formulations by reforming NP surface properties such as coating with functional groups [155]. One example is lectin, a glycoprotein that exhibits extremely high binding affinities for specific carbohydrate groups present on the surface of corneal epithelial cells [174]. Accordingly, better tissue penetration was demonstrated for positively charged NPs, unlike in the case of negatively charged NPs, which get electrostatically repelled from the cell membrane. To improve the adhesion on the mucosal surface e.g., at the periocular and oral mucosa, for a sustained drug release and efficient absorption, NPs were formulated with different bioadhesive polymers [173].
Due to their optimal size for the penetration of ocular barriers, NPs usually do not impose eye irritation, thereby limiting the frequency of drug administration as well as maintaining sustained drug release [161]. Lately, there has been an increased emergence of reports on NPs (e.g., polymeric [175, 176] and lipid-based [177, 178]) for drug-eluting contact lenses and corneal implants. Such commercialized medical devices provide a sustained and burst drug release with high bioavailability to the anterior and posterior segments of the eye, which may improve patient adherence as compared to eye drop medications [179]. Furthermore, contact lenses are typically used to correct refractive errors (e.g., myopia and hyperopia), which may positively impact patients' adherence towards their treatment regiment, particularly for those with both errors and glaucoma. Despite potential benefits, this approach is associated with potential safety risks and other limiting factors pertaining to production and storage [179]. As a result, topical eye drops continue to be the preferred first-line treatment option for glaucoma. Nonetheless, a substantial increase of studies is now being conducted to address the drawbacks of drug-eluting contact lens, making it possible for delivering medications to the eye and commercialization in the future. Among many others, Chauhan et al. have been working extensively on developing novel loaded contact lenses employing diverse NPs formulations. While this is an intriguing topic, it is beyond the focus of this review. For further information on this subject, refer to the cited reviews by Chauhan and others [180,181,182,183] as well as their recent published works on the fabrication of ophthalmic drug-eluting contact lenses using various nanomaterials [180,181,182, 184,185,186,187,188].
In the posterior eye segment, prolonged drug delivery could be achieved with the application of NPs, depending on their size and characteristics of the surface. In addition, prolonged and effective transscleral drug delivery through intravenous administration (blood and lymph circulation) could be achieved by conjugating the right moieties onto NPs. Intravitreal administration, for example, enables macromolecular drugs to reach the retina and reduces systemic toxicities. Through this technique, the encapsulated drug molecules can be accumulated at the retinal pigment epithelium layer, and thus maximize the therapeutic effects [158]. Dexamethasone-loaded poly lactic-co-glycolic acid (PLGA) NPs administered intravitreally in rabbits, for example, elevated the cellular uptake with stable bioavailability in the vitreous fluid, chorioretina, and plasma compared to the unconjugated dexamethasone [189]. The same features were observed for human serum albumin NPs, where the conjugated drug molecules successfully infiltrated the retina layers through specific pathway in the Müller cells, relying on endocytosis and exocytosis [190]. Of note, more investigations are needed to ascertain the ocular tissue penetration of NPs loaded with high molecular weight drugs, especially through the vitreoretinal interface, which is one of the major obstacles to reach the inner retina upon intravitreal administration. Indeed, not all NPs allow the drug molecules to be efficiently dispersed through the vitreous fluid or pass through the inner limiting membrane. The efficiency of the therapeutics to cross this membrane are highly dependent on their ability to migrate from the injection site towards the retina, which is often determined by the size of the NPs along with their other physicochemical properties. For further reading on this subject, the reader is referred to the recent and ongoing work by Peynshaert et al. [50,51,52]. Nevertheless, in view of all that has been mentioned so far, one may suppose that NPs have the potential to serve as an effective intravitreal drug delivery system.
A few studies have been dedicated to the attempts to limit the drug clearance, given that most administered NPs pool in the liver and spleen and are removed by the reticuloendothelial system after the administration [191]. Interestingly, NPs are flexible and can escape opsonization by the macrophages if coated with extra surface layers, such as PEG [192]. By controlling properties of NPs, it is possible to achieve maximal therapeutic effects, minimal side effects as well as highest solubility for targeted drugs [192]. In order to prolong the drug retention and enhance its corneal permeability and bioavailability, it is important to select appropriate NPs in terms of their chemistry, size, shape, surface charge and other physicochemical properties [193]. Herein, the suitable biocompatible ocular drug delivery system depends on the target tissue, the route of administration, and the characteristics of the drug to be incorporated into the NPs. Despite all of the advantages, however, the high permeability of NPs pose a high risk, as shown in several brain studies [194]. For instance, zinc oxide NPs and the anatase phase of titanium dioxide can easily bypass the blood-brain barrier via multiple routes and induce neuroinflammation with the potential to be neurotoxic [195]. Hence, the careful selection of carriers is of prime importance when designing the drug delivery system utilizing NPs.
Application of different nanoparticles as anti-inflammatory drug carriers
Polymer-based NPs
Polymeric NPs have played a major role in the advancement of NP-mediated drug delivery, given that they have proven successful for alleviating numerous diseases [155]. Polymeric NPs can form nanocapsules (surface-vesicular systems) or nanospheres (matrix systems) depending on their internal structure and preparation method (Fig. 5). While the former systems contain a drug encapsulated within a liquid core cavity, the latter ones contain a structural polymeric matrix where the drug is physically and uniformly dispersed [196]. In addition to being incorporated inside a polymeric matrix, the drugs can also be adsorbed on the NP surface. These biodegradable NPs ranging from 10 to 100 nm are the most commonly studied in the ocular drug delivery field [173]. Polymeric NPs have been proven superior compared to other types of NPs for ocular application primarily due to their properties including biodegradability, lesser toxicity, similarity in stiffness compared to the soft tissues, good encapsulation capacity as well as controlled release manner, alongside biocompatibility and mucoadhesiveness [170]. Different types of polymeric NPs can be produced by directly processing different monomers or by using derived polymers obtained through polymerization [173]. They are also applicable in producing many different NPs, which can improve drawbacks of conventional drug delivery systems.
Polymeric NPs have been shown to improve the stability of easily volatile substances and may act as preservatives in ophthalmic solutions [197]. This results in a greater efficiency and effectiveness in transporting maximal concentrations of active pharmaceutical ingredients to the targeted site, making them an ideal choice for modifying drugs in cancer therapy. Apart from drugs, polymeric NPs have been used as gene delivery carriers [173]. Furthermore, high bioavailability of polymeric NPs is related to their ability to concentrate at a targeted spot via passive or ligand-mediated mechanisms. This method offers the possibility for reduction of required dose(s) and the side effects associated with them. Despite all these advantages, these types of NPs may still potentially cause toxicity due to the organic solvents incorporated in the final formulation and deterioration of the polymer, which may produce systemic pernicious aftermath effects [171]. Nevertheless, most polymeric NPs provide beneficial properties required for the use in drug delivery in ophthalmology, fundamentally relying on the capability to retain drugs in ocular tissues. By interacting with mucin, mucoadhesive polymers help to minimize the elimination of drugs from the surface of the eye and therefore can increase the drug bioavailability at the precorneal area. Several examples of these types of polymers with modified surface characteristics are PLGA, PEG, poloxamers, poloxamines, hyaluronic acid, chitosan, sodium alginate and polyacrylic acid [170].
One of the widely utilized materials for polymeric NPs is PLGA [198]. PLGA has been approved by the United States Food and Drug Administration (US FDA), owing to its high biodegradability and biocompatibility demonstrated by long-term clinical trials [199]. In in vitro studies, dexamethasone-encapsulated PLGA has been shown to successfully bypass the human placenta with high bioavailability [200]. An empirical study conducted using prednisolone on C6 cells, a type of cancer cell resembling astrocytes, has shown that prednisolone-encapsulated PLGA NPs attenuated pro-inflammatory cytokines including TNF-α and nitric oxide, surpassing the effects of naked prednisolone [201]. On the other hand, in a glaucoma study using the rabbit’s cornea, a combination of dexamethasone and melatonin loaded PLGA NPs has been observed to significantly reduce the level of IOP [189]. This study associated the neuroprotective effect with the enhanced corneal penetration and sustained release of dexamethasone and melatonin by NPs. Interestingly, PLGA NPs are capable of encapsulating several active pharmacologic drugs simultaneously such as dexamethasone and melatonin for further improvement in delivering the drug [202]. For example, in an ex vivo rat brain tissue study, PLGA NPs coated with PEG exhibited rapid infiltration compared with uncoated NPs, indeed suggesting that coated NPs improved drug permeation [202].
In addition, prolonged delivery of therapeutic drugs is one of the most significant factors for successful neuroprotective therapy in glaucoma. To reach its target, NPs should be able to avoid the uptake by the mononuclear phagocytic system (MPS) of the host [203], which is responsible for opsonization and phagocytosis. Upon delivering the drugs, NPs are often specially designed to avoid or harness the MPS to reduce inflammatory effects, subsequently improving the payload delivery and the drug therapeutic efficacy [203]. It is established that PEG-coated PLGA NPs improve drug uptake and clearance. For instance, hydrophobic PLGA NPs coated with hydrophilic PEG exhibited an antagonism against opsonization and phagocytosis along with prolonged circulation time in the blood compared with NPs prepared without PEG [204]. Moreover, in a study done using anthocyanin, a phenolic compound with a high antioxidant activity, PLGA-PEG NPs encapsulating this compound were shown to effectively abolish the expression levels of inflammatory markers including NF-kB, TNF-α, and inducible nitric oxide synthase (iNOS) as well as apoptotic markers such as bcl-2 associated x (Bax), b-cell lymphoma-2 (Bcl-2), and caspase-3 protein against amyloid beta peptide 1-42 (Aβ1-42)-induced neurodegenerative effects in SH-SY5Y cell lines [205]. Taken together, these PEG-coated PLGA NPs can improve therapeutic drug delivery by inhibiting both neuroinflammatory and neuroapoptotic pathways. Although the delivery of anti-inflammatory drugs with PLGA has yet to be studied in glaucoma, this highlights its strong potential as a nanocarrier, particularly for the treatment of neuroinflammation.
Lipid-based NPs
Lipid-based nanocarriers are at the forefront of the rapidly developing drug delivery systems for various diseases. Here, we hypothesize that the lipid-based NP system is also one of the most promising drug delivery systems for treating glaucoma, improving drawbacks associated with the conventional treatment. Topical liposomal nanocarrier is one widely used lipid-based nanocarrier in preclinical and early clinical studies, efficiently delivering ophthalmic solutions such as timolol maleate into the vitreous and retina [206]. In many respects, lipid NPs are superior carriers compared to liposomes and polymeric NPs. The main benefits of these NPs are that they do not require organic solvents, which generate toxic degradation products, to be formulated unlike the polymeric NPs. As a result, they exhibit low in vivo toxicity as well as protect and stabilize the loaded drug molecules from degradation, while offer the controlled drug release capacity.
Lipid NPs are composed of o/w (oil-in-water) emulsions, which is a combination of a lipid nucleus with an amphiphilic surfactant acting as the stabilizer. As such, they are able to transmit both hydrophilic or hydrophobic drugs [207]. Lipids in a liquid state can transform to a solid state of various structures (e.g., steroids, monoglycerides, diglycerides, and triglycerides) and be dispersed in an aqueous solution at room and body temperature. In this context, lipid NPs are the aqueous dispersion of spherical vesicles made of ionized lipids with a positive charge at the neutral pH. They range in size from 40 to 1000 nm and can be classified into two categories: solid lipid NPs (SLN) and nanostructured lipid carriers (NLC) (Fig. 5) [208].
Initially, SLNs were formulated to improve the available drug delivery systems such as polymeric NPs and liposomes. SLNs are made of solid lipids derived from water, co-emulsifiers, and emulsifiers. As the more improvised version of drug carriers than the aforementioned ones, SLNs deliver several advantages, including the improved drug loading capacity, prolonged duration of drug release, higher drug bioavailability with a better stability for unstable molecules against chemical degradation, improved safety as well as good cost-effectiveness ratio, particularly for high-scale production [207]. SLNs also enhance the corneal absorption and conjunctival uptake, as shown in studies done on anterior and posterior eye tissues, and thus extend the drug retention period [209,210,211]. These studies have shown the potential of implementing SLNs formulation in clinical practice.
Nanostructured lipid carriers (NLCs), on the other hand, were designed as the alternatives to compensate for the prominent drawbacks of SLNs, such as their very limited drug-loading capacity [212]. NLCs are formed from a mixture of solid and liquid lipids that adopts an amorphous solid matrix state at room and body temperature. NLCs exhibit high drug tolerance due to the physiological and biodegradable lipids constituting them. Moreover, NLCs offer a higher drug loading capacity and extended drug release time compared to the SLNs, which includes both hydrophilic and lipophilic drugs [207]. In general, there are three types of NLCs: the imperfect, non-shaped (amorphous) and the multiple structures. The imperfect type refers to a mixture of fatty acids blended to create several lipid formations in a crystal structure (disorganized matrix) with gaps which provide the space for lipophilic drugs to enter the particles. On the contrary, the amorphous type does not have a crystalline matrix, hence it prevents premature drug ejection. Lastly, the multiple structures type consist of several compartments of a liquid lipid in a matrix of a solid lipid. This NLC type is utilized to avoid drug decomposition caused by the solid lipid. The development of NLC formulations has been demonstrated in ocular drug delivery to both the posterior and anterior parts of the eye [213]. For example, Luo and co-authors reported NLC chitosan-coated genistein formulation delivered via a topical administration, which enhanced the transcorneal penetration with an increased bioavailability of the drug molecules in the aqueous humor compared to the conventional solution [214]. Furthermore, triamcinolone acetonide encapsulated NLC showed enhanced therapeutic efficacy in mice. The developed formulation was able to reach the posterior segment of the eye via the corneal and non-corneal pathways upon topical administration [215]. These studies prove that the NLC formulations are good candidates for ocular drug delivery for treating glaucoma.
Overall, in comparison with other particulate systems, lipid NPs provide many advantages. From the commercial perspective, lipid NPs are feasible and easy to engage in a large-scale production [216]. Since lipids are biocompatible, lipid NPs are highly tolerated by the body. Furthermore, drug formulations with emulsifiers could have a better stability profile for both hydrophilic and lipophilic drugs, meaning they would be able to control and extend their retention time in the body. Besides, the important characteristics of lipid NPs including the optimal particle size, surface charge, drug entrapment efficiency, drug encapsulation and elimination, which enable them to protect the incorporated drugs from enzymatic degradation in the eye. This eventually provides a good adhesion onto the cornea/periocular tissues. Since they are composed of lipids, these NPs help to reach the lipid layer of the tear film, which directly improves the drug delivery and drug bioavailability for topical instillation. The natural affinity of lipid NPs for the lipid layers can be further augmented by endowing the given fatty acid chains with a positive surface charge, given that cationic lipids and surfactants extend the retention time of emulsion drops on the epithelial layer of the cornea [207]. Interestingly, studies done on the cytotoxicity of both SLNs and NLCs demonstrate that they are well tolerated and do not cause irritation to the ocular tissue. Still, non-ionic surfactants are occasionally required to minimize the toxic effects of the drug conjugated to lipid NPs [212]. Yet, these NPs can facilitate the passage of non-ionized drugs across various barriers, such as the precorneal film, while maintaining the neutral form of the encapsulated drug in its active form [212].
Conclusion
Several biological and nanoparticle-assisted agents have been evaluated in the experimental models of glaucoma, but none of them have passed the clinical trials. This possibly has to do with the complex molecular processes governing neuroprotection that are yet to be elucidated. Turbulence in the immune response surveillance is regarded as the prime source of the disease progression, including that in autoimmune and other neurodegenerative diseases. We, therefore, have discussed the prospect of tackling the inflammatory response at the early stages of glaucoma. Traditionally, the absence of the symptoms rarely prompts a clinical evaluation, let alone a treatment for the disease, notwithstanding the fact that most forms of glaucoma are asymptomatic in the early stages. It is also conceivable that IOP-lowering treatments may not be effective in circumventing the progression of the disease, as they lead to the progressive damage to the RGCs. The ineffectiveness of the available treatments has contributed to the improvement and development of several drug delivery systems. In ocular drug delivery systems, NPs have a vast applicability and potential to improve the efficacy of the current available treatments for glaucoma. NPs may enhance the current therapies by modulating drug solubility and subsequently enhancing bioavailability. They may also assist the drugs to permeate the critical barriers en route to their ocular target, but also extend the drug delivery timescale. The adverse effects at large may be minimized too as targeted delivery and improved bioavailability reduce the need for higher doses. Therefore, combining NPs with biological or small-molecule agents with the ability to counteract the inflammatory response in glaucomatous neurodegeneration can potentially move the field of glaucoma therapy forward.
Availability of data and materials
Not applicable.
Abbreviations
- Aβ142:
-
Amyloid beta peptide 1-42
- AMD:
-
Age-related macular degeneration
- Ang II:
-
Ang II angiotensin 1, 2
- AP-1:
-
Activator protein 1
- AT1-R:
-
Angiotensin type 1 receptor
- Bax:
-
Bcl-2 associated x
- Bcl-2:
-
B-cell lymphoma-2
- BDNF:
-
Brain-derived neurotrophic factor
- C1, C3, C5, C1Q:
-
Complement component 1, 3, 5, 1Q
- CD3:
-
Cluster of differentiation 3
- CNS:
-
Central nervous system
- COX-2:
-
Cyclooxygenase-2
- EGCG:
-
Epigallocatechin-gallate
- ET-1:
-
Endothelin-1
- FasL:
-
Fas ligand
- GBE:
-
Ginkgo biloba extract
- GFAP:
-
Glial fibrillary acidic protein
- IL-1β, IL-6, IL-8, IL-18:
-
Interleukin 1β, 6, 8, 18
- iNOS:
-
Inducible nitric oxide synthase
- IOP:
-
Intraocular pressure
- IP10:
-
Interferon gamma-induced protein 10
- Jak:
-
Janus kinase
- MD:
-
Mean deviation
- MgAT:
-
Magnesium acetyltaurate
- MIP-1α, MIP-β, MIP-2:
-
Macrophage inflammatory protein 1α, β, 2
- MPS:
-
Mononuclear phagocytic system
- NF-κB:
-
Nuclear factor kappa B
- NLCs:
-
Nanostructured lipid carriers
- NLRP1, NLRP3:
-
NOD-, LRR-family pyrin domain containing 1, 3
- NMDA:
-
N-Methyl-d-aspartate
- NTG:
-
Normal tension glaucoma
- OHT:
-
Ocular hypertension
- ONH:
-
Optic nerve head
- PACA:
-
Poly-alkylcyanoacrylate
- PAMAM:
-
Poly(amidoamine)
- PEG:
-
Poly(ethylene glycol)
- PLGA:
-
Poly lactic-co-glycolic acid
- POAG:
-
Primary open-angle glaucoma
- NPs:
-
Nanoparticles
- RAAS:
-
Renin-angiotensin aldosterone system
- RGC:
-
Retinal ganglion cell
- ROS:
-
Reactive oxygen species
- RNFL:
-
Retinal nerve fiber layer
- RSV:
-
Resveratrol
- SIRT1:
-
Sirtuin 1
- SLNs:
-
Solid lipid nanoparticles
- SOD-2:
-
Super oxide dismutase 2
- TLR1, TLR4:
-
Toll-like receptor 1, 4
- TNF-α:
-
Tumor necrosis factor-alpha
References
Marchesi N, Fahmideh F, Boschi F, Pascale A, Barbieri A. Ocular neurodegenerative diseases: interconnection between retina and cortical areas. Cells. 2021;10(9):2394.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90.
Sharif NA. Glaucomatous optic neuropathy treatment options: the promise of novel therapeutics, techniques and tools to help preserve vision. Neural Regen Res. 2018;13(7):1145–50.
Casson RJ, Chidlow G, Wood JP, Crowston JG, Goldberg I. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol. 2012;40(4):341–9.
Heijl A, Bengtsson B, Oskarsdottir SE. Prevalence and severity of undetected manifest glaucoma: results from the early manifest glaucoma trial screening. Ophthalmology. 2013;120(8):1541–5.
Jammal AA, Thompson AC, Mariottoni EB, Urata CN, Estrela T, Berchuck SI, et al. Rates of glaucomatous structural and functional change from a large clinical population: the Duke Glaucoma Registry Study. Am J Ophthalmol. 2021;222:238–47.
Tamm ER, Ethier CR, Dowling JE, Downs C, Ellisman MH, Fisher S, et al. Biological aspects of axonal damage in glaucoma: a brief review. Exp Eye Res. 2017;157:5–12.
Tezel G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol. 2013;13(1):23–31.
Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–79.
Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest. 2012;122(4):1246–61.
Distante P, Lombardo S, Verticchio Vercellin AC, Raimondi M, Rolando M, Tinelli C, et al. Structure/function relationship and retinal ganglion cells counts to discriminate glaucomatous damages. BMC Ophthalmol. 2015;15:185.
Vidal-Sanz M, Salinas-Navarro M, Nadal-Nicolás FM, Alarcón-Martínez L, Valiente-Soriano FJ, de Imperial JM, et al. Understanding glaucomatous damage: anatomical and functional data from ocular hypertensive rodent retinas. Prog Retin Eye Res. 2012;31(1):1–27.
Soto I, Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med. 2014;4(8):a017269.
Qu J, Wang D, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010;91(1):48–53.
Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11): e11686.
Bengtsson B, Heijl A. Lack of visual field improvement after initiation of intraocular pressure reducing treatment in the Early Manifest Glaucoma Trial. Invest Opthalmol Vis Sci. 2016;57(13):5611–5.
Ahmed AYA, Althomali IHM, Althobaiti OMA, Assery MSF, Alsini AAN. Risk factors associated with glaucoma disease progression. Egypt J Hosp Med. 2018;73(3):6331–6.
Zhang N, Wang J, Chen B, Li Y, Jiang B. Prevalence of primary angle closure glaucoma in the last 20 years: a meta-analysis and systematic review. Front Med (Lausanne). 2021;7:624179.
Park HYL, Shin DY, Jeon SJ, Kim YC, Jung Y, Kim EK, et al. Predicting the development of normal tension glaucoma and related risk factors in normal tension glaucoma suspects. Sci Rep. 2021;11(1):16697.
McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10(2):71–8.
Dikopf MS, Vajaranant TS, Edward DP. Topical treatment of glaucoma: established and emerging pharmacology. Expert Opin Pharmacother. 2017;18(9):885–98.
Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008;5(5):567–81.
Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26(5):1197–216.
Hennessy AL, Katz J, Covert D, Protzko C, Robin AL. Videotaped evaluation of eyedrop instillation in glaucoma patients with visual impairment or moderate to severe visual field loss. Ophthalmology. 2010;117(12):2345–52.
Thygesen J, Burk R, Carassa R, Crichton A, Goñi FJ, Menage M, et al. Criteria for choosing clinically effective glaucoma treatment: a discussion panel consensus. Curr Ther Res Clin Exp. 2007;68(3):127–36.
Kwon S, Kim SH, Khang D, Lee JY. Potential therapeutic usage of nanomedicine for glaucoma treatment. Int J Nanomed. 2020;15:5745–65.
Duarte JN. Neuroinflammatory mechanisms of mitochondrial dysfunction and neurodegeneration in glaucoma. J Ophthalmol. 2021;2021:4581909.
Patabendige A, Singh A, Jenkins S, Sen J, Chen R. Astrocyte activation in neurovascular damage and repair following ischaemic stroke. Int J Mol Sci. 2021;22(8):4280.
Thurgur H, Pinteaux E. Microglia in the neurovascular unit: blood-brain barrier-microglia interactions after central nervous system disorders. Neuroscience. 2019;405:55–67.
Ghosh AK, Rao VR, Wisniewski VJ, Zigrossi AD, Floss J, Koulen P, et al. Differential activation of glioprotective intracellular signaling pathways in primary optic nerve head astrocytes after treatment with different classes of antioxidants. Antioxidants (Basel). 2020;9(4):324.
Liu Y, Wu J, Yan LJ, Clark AF. High glucose impairs optic nerve head astrocyte phagocytosis prior to retinal ganglion cell degeneration. Invest Opthalmol Vis Sci. 2018;59(9):3549.
Nguyen JV, Soto I, Kim KY, Bushong EA, Oglesby E, Valiente-Soriano FJ, et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci. 2011;108(3):1176–81.
Reichenbach A, Bringmann A. Glia of the human retina. Glia. 2020;68(4):768–96.
Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia–neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1–40.
Chong RS, Martin KR. Glial cell interactions and glaucoma. Curr Opin Ophthalmol. 2015;26(2):73–7.
Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest Opthalmol Vis Sci. 2010;51(12):6448–60.
Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol. 2015;7(8):a020537.
Chen M, Luo C, Zhao J, Devarajan G, Xu H. Immune regulation in the aging retina. Prog Retin Eye Res. 2019;69:159–72.
Silverman SM, Wong WT. Microglia in the retina: roles in development, maturity, and disease. Annu Rev Vis Sci. 2018;4:45–77.
Rathnasamy G, Foulds WS, Ling EA, Kaur C. Retinal microglia—a key player in healthy and diseased retina. Prog Neurobiol. 2019;173:18–40.
Abcouwer SF, Lin CM, Shanmugam S, Muthusamy A, Barber AJ, Antonetti DA. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflamm. 2013;10:149.
Mélik Parsadaniantz S, Réaux-le Goazigo A, Sapienza A, Habas C, Baudouin C. Glaucoma: a degenerative optic neuropathy related to neuroinflammation? Cells. 2020;9(3):535.
Adornetto A, Russo R, Parisi V. Neuroinflammation as a target for glaucoma therapy. Neural Regen Res. 2019;14(3):391–4.
Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519(4):599–620.
Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, et al. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis Model Mech. 2015;8(5):443–55.
Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94.
Tribble JR, Harder JM, Williams PA, John SW. Ocular hypertension suppresses homeostatic gene expression in optic nerve head microglia of DBA/2 J mice. Mol Brain. 2020;13(1):81.
Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81(6):1345–51.
Xue W, Cojocaru RI, Dudley VJ, Brooks M, Swaroop A, Sarthy VP. Ciliary neurotrophic factor induces genes associated with inflammation and gliosis in the retina: a gene profiling study of flow-sorted, Müller cells. PLoS One. 2011;6(5):e20326.
Peynshaert K, Devoldere J, Minnaert AK, De Smedt SC, Remaut K. Morphology and composition of the inner limiting membrane: species-specific variations and relevance toward drug delivery research. Curr Eye Res. 2019;44(5):465–75.
Peynshaert K, Devoldere J, Forster V, Picaud S, Vanhove C, De Smedt SC, et al. Toward smart design of retinal drug carriers: a novel bovine retinal explant model to study the barrier role of the vitreoretinal interface. Drug Deliv. 2017;24(1):1384–94.
Peynshaert K, Devoldere J, De Smedt SC, Remaut K. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv Drug Deliv Rev. 2018;126:44–57.
Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, et al. Müller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci. 2007;104(20):8287–92.
Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27(24):6473–7.
Varela HJ, Hernandez MR. Astrocyte responses in human optic nerve head with primary open-angle glaucoma. J Glaucoma. 1997;6(5):303–13.
Lukowski SW, Lo CY, Sharov AA, Nguyen Q, Fang L, Hung SS, et al. A single-cell transcriptome atlas of the adult human retina. EMBO J. 2019;38(18):e100811.
Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Opthalmol Vis Sci. 2003;44(7):3025–33.
Tezel G, Yang X, Luo C, Cai J, Powell DW. An astrocyte-specific proteomic approach toInflammatory responses in experimental rat glaucoma. Invest Opthalmol Vis Sci. 2012;53(7):4220–33.
Samelska K, Zaleska-Żmijewska A, Bałan B, Grąbczewski A, Szaflik JP, Kubiak AJ, et al. Immunological and molecular basics of the primary open angle glaucoma pathomechanism. Cent Eur J Immunol. 2021;46(1):111–7.
Gramlich OW, Godwin CR, Heuss ND, Gregerson DS, Kuehn MH. T and B lymphocyte deficiency in Rag1−/− mice reduces retinal ganglion cell loss in experimental glaucoma. Invest Opthalmol Vis Sci. 2020;61(14):18.
Bosco A, Anderson SR, Breen KT, Romero CO, Steele MR, Chiodo VA, et al. Complement C3-targeted gene therapy restricts onset and progression of neurodegeneration in chronic mouse glaucoma. Mol Ther. 2018;26(10):2379–96.
Gassel CJ, Reinehr S, Gomes SC, Dick HB, Joachim SC. Preservation of optic nerve structure by complement inhibition in experimental glaucoma. Cell Tissue Res. 2020;382(2):293–306.
Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, et al. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener. 2016;11:26.
Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation—neuro-protection and -degeneration. J Neurochem. 2008;107(5):1169–87.
Madeira MH, Ortin-Martinez A, Nadal-Nícolas F, Ambrósio AF, Vidal-Sanz M, Agudo-Barriuso M, et al. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci Rep. 2016;6:27532.
Bouhenni R, Dunmire J, Sewell A, Edward DP. Animal models of glaucoma. J Biomed Biotechnol. 2012;2012:692609.
Lin Z, Huang S, Sun J, Xie B, Zhong Y. Associations between TLR4 polymorphisms and open angle glaucoma: a meta-analysis. Biomed Res Int. 2019;2019:6707650.
Wang H, Song X, Li M, Wang X, Tao Y, Xiya X, et al. The role of TLR4/NF-κB signaling pathway in activated microglia of rats with chronic high intraocular pressure and vitro scratch injury-induced microglia. Int Immunopharmacol. 2020;83:106395.
Wei X, Cho KS, Thee EF, Jager MJ, Chen DF. Neuroinflammation and microglia in glaucoma: time for a paradigm shift. J Neurosci Res. 2019;97(1):70–6.
Wiemann S, Reinhard J, Faissner A. Immunomodulatory role of the extracellular matrix protein tenascin-C in neuroinflammation. Biochem Soc Trans. 2019;47(6):1651–60.
Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989;491(2):394–7.
Wang B, Chen T, Wang J, Jia Y, Ren H, Wu F, et al. Methamphetamine modulates the production of interleukin-6 and tumor necrosis factor-alpha via the cAMP/PKA/CREB signaling pathway in lipopolysaccharide-activated microglia. Int Immunopharmacol. 2018;56:168–78.
Xin X, Gao L, Wu T, Sun F. Roles of tumor necrosis factor alpha gene polymorphisms, tumor necrosis factor alpha level in aqueous humor, and the risks of open angle glaucoma: a meta-analysis. Mol Vis. 2013;19:526–35.
Tekeli O, Turacli ME, Egin Y, Akar N, Elhan AH. Tumor necrosis factor alpha-308 gene polymorphism and pseudoexfoliation glaucoma. Mol Vis. 2008;14:1815–8.
Kondkar AA, Sultan T, Almobarak FA, Kalantan H, Al-Obeidan SA, Abu-Amero KK. Association of increased levels of plasma tumor necrosis factor alpha with primary open-angle glaucoma. Clin Ophthalmol. 2018;12:701–6.
Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, et al. Neurodegenerative and inflammatory pathway components linked to TNF-α/TNFR1 signaling in the glaucomatous human retina. Invest Opthalmol Vis Sci. 2011;52(11):8442–54.
Borkenstein A, Faschinger C, Maier R, Weger M, Theisl A, Demel U, et al. Measurement of tumor necrosis factor-alpha, interleukin-6, Fas ligand, interleukin-1α, and interleukin-1β in the aqueous humor of patients with open angle glaucoma using multiplex bead analysis. Mol Vis. 2013;19:2306–11.
Gregory MS, Hackett CG, Abernathy EF, Lee KS, Saff RR, Hohlbaum AM, et al. Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One. 2011;6(3):e17659.
Zanon-Moreno V, Raga-Cervera J, García-Medina JJ, Benitez-Del-Castillo J, Vinuesa-Silva I, Torregrosa S, et al. New horizons for the treatment of glaucoma. I: neuroinflammation and inflammasomes. Arch Soc Esp Oftalmol (Engl Ed). 2018;93:e7–9.
Malarkannan S. Molecular mechanisms of FasL-mediated ‘reverse-signaling.’ Mol Immunol. 2020;127:31–7.
Iulia C, Ruxandra T, Costin LB, Liliana-Mary V. Citicoline—a neuroprotector with proven effects on glaucomatous disease. Rom J Ophthalmol. 2017;61(3):152–8.
Parisi V, Oddone F, Ziccardi L, Roberti G, Coppola G, Manni G. Citicoline and retinal ganglion cells: effects on morphology and function. Curr Neuropharmacol. 2018;16(7):919–32.
Han YS, Chung IY, Park JM, Yu JM. Neuroprotective effect of citicoline on retinal cell damage induced by kainic acid in rats. Korean J Ophthalmol. 2005;19(3):219–26.
Matteucci A, Varano M, Gaddini L, Mallozzi C, Villa M, Pricci F, et al. Neuroprotective effects of citicoline in in vitro models of retinal neurodegeneration. Int J Mol Sci. 2014;15(4):6286–97.
Oddone F, Rossetti L, Parravano M, Sbardella D, Coletta M, Ziccardi L, et al. Citicoline in ophthalmological neurodegenerative disease: a comprehensive review. Pharmaceuticals. 2021;14(3):281.
Rejdak R, Toczołowski J, Solski J, Duma D, Grieb P. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res. 2002;34(3):146–9.
Park CH, Kim YS, Noh HS, Cheon EW, Yang YA, Yoo JM, et al. Neuroprotective effect of citicoline against KA-induced neurotoxicity in the rat retina. Exp Eye Res. 2005;81(3):350–8.
Schuettauf F, Rejdak R, Thaler S, Bolz S, Lehaci C, Mankowska A, et al. Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat. Exp Eye Res. 2006;83(5):1128–34.
Parisi V, Manni G, Colacino G, Bucci MG. Cytidine-5′-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology. 1999;106(6):1126–34.
Parisi V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5′-diphosphocholine (citicoline): a study of 8 years of follow-up. Doc Ophthalmol. 2005;110(1):91–102.
Parisi V, Coppola G, Centofanti M, Oddone F, Angrisani AM, Ziccardi L, et al. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog Brain Res. 2008;173:541–54.
Ottobelli L, Manni G, Centofanti M, Iester M, Allevena F, Rossetti L. Citicoline oral solution in glaucoma: is there a role in slowing disease progression? Ophthalmologica. 2013;229(4):219–26.
Lanza M, Gironi Carnevale UA, Mele L, Bifani Sconocchia M, Bartollino S, Costagliola C. Morphological and functional evaluation of oral citicoline therapy in chronic open-angle glaucoma patients: a pilot study with a 2-year follow-up. Front Pharmacol. 2019;10:1117.
Rossetti L, Iester M, Tranchina L, Ottobelli L, Coco G, Calcatelli E, et al. Can treatment with citicoline eyedrops reduce progression in glaucoma? The results of a randomized placebo-controlled clinical trial. J Glaucoma. 2020;29(7):513–20.
Parisi V, Oddone F, Roberti G, Tanga L, Carnevale C, Ziccardi L, et al. Enhancement of retinal function and of neural conduction along the visual pathway induced by treatment with citicoline eye drops in liposomal formulation in open angle glaucoma: a pilot electrofunctional study. Adv Ther. 2019;36(4):987–96.
Semba K, Namekata K, Guo X, Harada C, Harada T, Mitamura Y. Renin-angiotensin system regulates neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014;5(7):e1333.
Di Raimondo D, Tuttolomondo A, Buttà C, Miceli S, Licata G, Pinto A. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des. 2012;18(28):4385–413.
Fujita T, Hirooka K, Nakamura T, Itano T, Nishiyama A, Nagai Y, et al. Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Invest Opthalmol Vis Sci. 2012;53(7):4099–110.
Yang H, Hirooka K, Fukuda K, Shiraga F. Neuroprotective effects of angiotensin II type 1 receptor blocker in a rat model of chronic glaucoma. Invest Opthalmol Vis Sci. 2009;50(12):5800–4.
Suvarna V, Sarkar M, Chaubey P, Murahari M, Sangave PC. Role of natural products in glaucoma management. In: Preedy VR, Watson RR, editors. Handbook of nutrition, diet, and the eye. Cambridge: Academic Press; 2019. p. 221–30.
Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431–41.
Al Owaifeer AM, Al Taisan AA. The role of diet in glaucoma: a review of the current evidence. Ophthalmol Ther. 2018;7(1):19–31.
Achete De Souza G, de Marqui SV, Matias JN, Guiguer EL, Barbalho SM. Effects of Ginkgo biloba on diseases related to oxidative stress. Planta Med. 2020;86(6):376–86.
Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis. 2012;18:390–402.
Ige M, Liu J. Focus: plant-based medicine and pharmacology: herbal medicines in glaucoma treatment. Yale J Biol Med. 2020;93(2):347.
Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;1:CD003120.
Ghiso JA, Doudevski I, Ritch R, Rostagno AA. Alzheimer’s disease and glaucoma: mechanistic similarities and differences. J Glaucoma. 2013;22(Suppl 5):S36–8.
Lee J, Sohn SW, Kee C. Effect of Ginkgo biloba extract on visual field progression in normal tension glaucoma. J Glaucoma. 2013;22(9):780–4.
Quaranta L, Bettelli S, Uva MG, Semeraro F, Turano R, Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110(2):359–62.
Guo X, Kong X, Huang R, Jin L, Ding X, He M, et al. Effect of Ginkgo biloba on visual field and contrast sensitivity in Chinese patients with normal tension glaucoma: a randomized, crossover clinical trial. Invest Opthalmol Vis Sci. 2014;55(1):110–6.
Chung HS, Harris A, Kristinsson JK, Ciulla TA, Kagemann C, Ritch R. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999;15(3):233–40.
Park JW, Kwon HJ, Chung WS, Kim CY, Seong GJ. Short-term effects of Ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J Ophthalmol. 2011;25(5):323–8.
Shim SH, Kim JM, Choi CY, Kim CY, Park KH. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J Med Food. 2012;15(9):818–23.
Cho HK, Kim S, Lee EJ, Kee C. Neuroprotective effect of Ginkgo biloba extract against hypoxic retinal ganglion cell degeneration in vitro and in vivo. J Med Food. 2019;22(8):771–8.
José Bagur M, Alonso Salinas GL, Jiménez-Monreal AM, Chaouqi S, Llorens S, Martínez-Tomé M, et al. Saffron: an old medicinal plant and a potential novel functional food. Molecules. 2018;23(1):30.
Bathaie SZ, Farajzade A, Hoshyar R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech Histochem. 2014;89(6):401–11.
Koşar M, Başer KH. Chapter 13—Beneficial effects of saffron (Crocus sativus L.) in ocular diseases. In: Sarwat M, Sumaiya S, editors. Safron. Pittsburgh: Academic Press; 2020. p. 155–63.
Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648(1–3):110–6.
Ishizuka F, Shimazawa M, Umigai N, Ogishima H, Nakamura S, Tsuruma K, et al. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol. 2013;703(1–3):1–10.
Jabbarpoor Bonyadi MH, Yazdani S, Saadat S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: a pilot study. BMC Complement Altern Med. 2014;14:399.
Falsini B, Marangoni D, Salgarello T, Stifano G, Montrone L, Di Landro S, et al. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefe’s Arch Clin Exp Ophthalmol. 2009;247(9):1223–33.
Luo LJ, Lai JY. Epigallocatechin gallate-loaded gelatin-g-poly (N-isopropylacrylamide) as a new ophthalmic pharmaceutical formulation for topical use in the treatment of dry eye syndrome. Sci Rep. 2017;7(1):9380.
Chen F, Jiang L, Shen C, Wan H, Xu L, Wang N, et al. Neuroprotective effect of epigallocatechin-3-gallate against N-methyl-d-aspartate-induced excitotoxicity in the adult rat retina. Acta Ophthalmol. 2012;90(8):e609–15.
Shen C, Chen L, Jiang L, Lai TY. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci Lett. 2015;600:132–6.
Zhang B, Rusciano D, Osborne NN. Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res. 2008;1198:141–52.
Lee JH, Song DK, Jung CH, Shin DH, Park J, Kwon TK, et al. (–)-Epigallocatechin gallate attenuates glutamate-induced cytotoxicity via intracellular Ca2+ modulation in PC12 cells. Clin Exp Pharmacol Physiol. 2004;31(8):530–6.
Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–35.
de La Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007;35(5):1156–60.
Luna C, Li G, Liton PB, Qiu J, Epstein DL, Challa P, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47(1):198–204.
Pirhan D, Yüksel N, Emre E, Cengiz A, Kürşat YD. Riluzole-and resveratrol-induced delay of retinal ganglion cell death in an experimental model of glaucoma. Curr Eye Res. 2016;41(1):59–69.
Luo J, He T, Yang J, Yang N, Li Z, Xing Y. SIRT1 is required for the neuroprotection of resveratrol on retinal ganglion cells after retinal ischemia-reperfusion injury in mice. Graefes Arch Clin Exp Ophthalmol. 2020;258(2):335–44.
Corder R, Douthwaite JA, Lees DM, Khan NQ, Viseu Dos Santos AC, Wood EG, et al. Endothelin-1 synthesis reduced by red wine. Nature. 2001;414(6866):863–4.
Liu GS, Zhang ZS, Yang B, He W. Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life Sci. 2012;91(17–18):872–7.
Shamsher E. Formulation of potential phytochemicals with neuroprotective action for the treatment of Alzheimer’s disease, glaucoma and multiple sclerosis [doctoral dissertation]. London, England: University College London; 2021.
Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, et al. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 2012;7(2):e31211.
Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10(3):511–45.
Wang L, Li C, Guo H, Kern TS, Huang K, Zheng L. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS One. 2011;6(8):e23194.
Yue YK, Mo B, Zhao J, Yu YJ, Liu L, Yue CL, et al. Neuroprotective effect of curcumin against oxidative damage in BV-2 microglia and high intraocular pressure animal model. J Ocul Pharmacol Ther. 2014;30(8):657–64.
Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS. 2013;15(1):195–218.
Franzone F, Nebbioso M, Pergolizzi T, Attanasio G, Musacchio A, Greco A, et al. Anti-inflammatory role of curcumin in retinal disorders. Exp Ther Med. 2021;22(1):790.
Noureddin SA, El-Shishtawy RM, Al-Footy KO. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur J Med Chem. 2019;182:111631.
Pandey A, Chaturvedi M, Mishra S, Kumar P, Somvanshi P, Chaturvedi R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon. 2020;6(11):e05469.
Davis BM, Pahlitzsch M, Guo L, Balendra S, Shah P, Ravindran N, et al. Topical curcumin nanocarriers are neuroprotective in eye disease. Sci Rep. 2018;8(1):11066.
Cheng YH, Ko YC, Chang YF, Huang SH, Liu CJL. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp Eye Res. 2019;179:179–87.
Chen Y, Lu Y, Lee RJ, Xiang G. Nano encapsulated curcumin: and its potential for biomedical applications. Int J Nanomed. 2020;15:3099–120.
Nucci C, Martucci A, Giannini C, Morrone L, Bagetta G, Mancino R. Neuroprotective agents in the management of glaucoma. Eye. 2018;32(5):938–45.
Almasieh M, Levin LA. Neuroprotection in glaucoma: animal models and clinical trials. Annu Rev Vis Sci. 2017;3:91–120.
Rusciano D, Pezzino S, Mutolo MG, Giannotti R, Librando A, Pescosolido N. Neuroprotection in glaucoma: old and new promising treatments. Adv Pharmacol Sci. 2017;2017:4320408.
Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019;210(4):180–7.
Khatib TZ, Martin KR. Neuroprotection in glaucoma: towards clinical trials and precision medicine. Curr Eye Res. 2020;45(3):327–38.
Tsai JC. Innovative IOP-independent neuroprotection and neuroregeneration strategies in the pipeline for glaucoma. J Ophthalmol. 2020;2020:9329310.
Skopiński P, Radomska-Leśniewska DM, Izdebska J, Kamińska A, Kupis M, Kubiak AJ, et al. New perspectives of immunomodulation and neuroprotection in glaucoma. Cent Eur J Immunol. 2021;46(1):105–10.
Sharif NA. iDrugs and iDevices Discovery Research: preclinical assays, techniques, and animal model studies for ocular hypotensives and neuroprotectants. J Ocul Pharmacol Ther. 2018;34(1–2):7–39.
Kolko M. Present and new treatment strategies in the management of glaucoma. Open Ophthalmol J. 2015;9(1):89–100.
Kim HM, Woo SJ. Ocular drug delivery to the retina: current innovations and future perspectives. Pharmaceutics. 2021;13(1):108.
Delplace V, Ortin-Martinez A, Tsai ELS, Amin AN, Wallace V, Shoichet MS. Controlled release strategy designed for intravitreal protein delivery to the retina. J Control Release. 2019;293:10–20.
Occhiutto ML, Maranhão RC, Costa VP, Konstas AG. Nanotechnology for medical and surgical glaucoma therapy—a review. Adv Ther. 2020;37(1):155–99.
Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64.
Meyer CH, Krohne TU, Issa PC, Liu Z, Holz FG. Routes for drug delivery to the eye and retina: intravitreal injections. Dev Ophthalmol. 2016;55:63–70.
Tavakoli S, Peynshaert K, Lajunen T, Devoldere J, del Amo EM, Ruponen M, et al. Ocular barriers to retinal delivery of intravitreal liposomes: impact of vitreoretinal interface. J Control Release. 2020;328:952–61.
Omerović N, Vranić E. Application of nanoparticles in ocular drug delivery systems. Health Technol. 2019;10:61–78.
Khiev D, Mohamed ZA, Vichare R, Paulson R, Bhatia S, Mohapatra S, et al. Emerging nano-formulations and nanomedicines applications for ocular drug delivery. Nanomaterials. 2021;11(1):173.
Rizvi SAA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26(1):64–70.
Nowak M, Brown TD, Graham A, Helgeson ME, Mitragotri S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng Transl Med. 2020;5(2):e10153.
Yadav KS, Rajpurohit R, Sharma S. Glaucoma: current treatment and impact of advanced drug delivery systems. Life Sci. 2019;221:362–76.
Nieto González N, Obinu A, Rassu G, Giunchedi P, Gavini E. Polymeric and lipid nanoparticles: which applications in pediatrics? Pharmaceutics. 2021;13(5):670.
Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13(3–4):144–51.
Singh A, Chokriwal A, Sharma MM, Jain D, Saxena J, Stephen BJ. Therapeutic role and drug delivery potential of neuroinflammation as a target in neurodegenerative disorders. ACS Chem Neurosci. 2017;8(8):1645–55.
Baby N, Patnala R, Ling EA, Dheen ST. Nanomedicine and its application in treatment of microglia-mediated neuroinflammation. Curr Med Chem. 2014;21(37):4215–26.
Almeida H, Amaral MH, Lobão P, Silva AC, Loboa JMS. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: present and future considerations. J Pharm Pharm Sci. 2014;17(3):278–93.
Souto EB, Doktorovova S, Gonzalez-Mira E, Egea MA, Garcia ML. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr Eye Res. 2010;35(7):537–52.
Silva MM, Calado R, Marto J, Bettencourt A, Almeida AJ, Gonçalves L. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar Drugs. 2017;15(12):370.
Swetledge S, Jung JP, Carter R, Sabliov C. Distribution of polymeric nanoparticles in the eye: implications in ocular disease therapy. J Nanobiotechnol. 2021;19(1):10.
Mishra A, Behura A, Mawatwal S, Kumar A, Naik L, Mohanty SS, et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem Toxicol. 2019;134:110827.
Song J, Zhang Z. Brinzolamide loaded core-shell nanoparticles for enhanced coronial penetration in the treatment of glaucoma. J Appl Biomater Funct Mater. 2020;18:2280800020942712.
Prakash M, Dhesingh RS. Nanoparticle modified drug loaded biodegradable polymeric contact lenses for sustainable ocular drug delivery. Curr Drug Deliv. 2017;14(4):555–65.
Zhang L, Zhang C, Dang H. Controlled brimonidine release from nanostructured lipid carriers-laden silicone contact lens to treat glaucoma. J Drug Deliv Sci Technol. 2021;66:102753.
Liu Z, Jiao Z, Luo R, Fu J. Travoprost-loaded PEGylated solid lipid nanoparticle-laden silicone contact lens for managing glaucoma. J Drug Deliv Sci Technol. 2021;66:102731.
Lanier OL, Christopher KG, Macoon RM, Yu Y, Sekar P, Chauhan A. Commercialization challenges for drug eluting contact lenses. Expert Opin Drug Deliv. 2020;17(8):1133–49.
Gulsen D, Chauhan A. Ophthalmic drug delivery through contact lenses. Invest Opthalmol Vis Sci. 2004;45(7):23427.
Dixon P, Shafor C, Gause S, Hsu KH, Powell KC, Chauhan A. Therapeutic contact lenses: a patent review. Expert Opin Ther Pat. 2015;25(10):1117–29.
Chauhan A, Radke CJ. Modeling the vertical motion of a soft contact lens. Curr Eye Res. 2001;22(2):102–8.
Yu Y, Guerriero T, Carpenter J, Chauhan A. Transport of polymers in contact lenses and impact on lubricity. Colloids Surf A Physicochem Eng Asp. 2020;603:125123.
Desai DT, Maulvi FA, Desai AR, Shukla MR, Desai BV, Khadela AD, et al. In vitro and in vivo evaluation of cyclosporine-graphene oxide laden hydrogel contact lenses. Int J Pharm. 2022;613:121414.
Dandamudi M, McLoughlin P, Behl G, Rani S, Coffey L, Chauhan A, et al. Chitosan-coated PLGA nanoparticles encapsulating triamcinolone acetonide as a potential candidate for sustained ocular drug delivery. Pharmaceutics. 2021;13(10):1590.
Ubani-Ukoma U, Gibson D, Schultz G, Silva BO, Chauhan A. Evaluating the potential of drug eluting contact lenses for treatment of bacterial keratitis using an ex vivo corneal model. Int J Pharm. 2019;565:499–508.
Dixon P, Fentzke RC, Bhattacharya A, Konar A, Hazra S, Chauhan A. In vitro drug release and in vivo safety of vitamin E and cysteamine loaded contact lenses. Int J Pharm. 2018;544(2):380–91.
Kapoor Y, Dixon P, Sekar P, Chauhan A. Incorporation of drug particles for extended release of Cyclosporine A from poly-hydroxyethyl methacrylate hydrogels. Eur J Pharm Biopharm. 2017;120:73–9.
Pan X, Liu X, Zhuang X, Liu Y, Li S. Co-delivery of dexamethasone and melatonin by drugs laden PLGA nanoparticles for the treatment of glaucoma. J Drug Deliv Sci Technol. 2020;60:102086.
Koo H, Moon H, Han H, Na JH, Huh MS, Park JH, et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials. 2012;33(12):3485–93.
Kaminskas LM, Boyd BJ. Nanosized drug delivery vectors and the reticuloendothelial system. In: Prokop A, editor. Intracellular delivery, vol. 5. Fundamental biomedical technologies. Dordrecht: Springer; 2011. p. 155–78.
Gref R, Domb A, Quellec P, Blunk T, Müller RH, Verbavatz J, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev. 1995;16(2–3):215–33.
Liu D, Lian Y, Fang Q, Liu L, Zhang J, Li J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int J Biol Macromol. 2018;116:1026–36.
Leite PE, Pereira MR, Granjeiro JM. Hazard effects of nanoparticles in central nervous system: searching for biocompatible nanomaterials for drug delivery. Toxicol In Vitro. 2015;29(7):1653–60.
Sharma AK, Singh V, Gera R, Purohit MP, Ghosh D. Zinc oxide nanoparticle induces microglial death by NADPH-oxidase-independent reactive oxygen species as well as energy depletion. Mol Neurobiol. 2017;54(8):6273–86.
Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640–59.
Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–34.
Shibata A, Yada S, Terakawa M. Biodegradability of poly (lactic-co-glycolic acid) after femtosecond laser irradiation. Sci Rep. 2016;6:27884.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3(3):1377–97.
Ali H, Kalashnikova I, White MA, Sherman M, Rytting E. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int J Pharm. 2013;454(1):149–57.
Acharya S, Guru BR. Prednisolone encapsulated PLGA nanoparticles: characterization, cytotoxicity, and anti-inflammatory activity on C6 glial cells. J Appl Pharm Sci. 2020;10(4):14–21.
Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, et al. A dense poly (ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4(149): 149ra119.
Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510.
Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2(1):108–19.
Amin FU, Shah SA, Badshah H, Khan M, Kim MO. Anthocyanins encapsulated by PLGA@ PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1–42-induced oxidative stress. J Nanobiotechnol. 2017;15(1):12.
Yu S, Wang QM, Wang X, Liu D, Zhang W, Ye T, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm. 2015;480(1–2):128–36.
Sánchez-López E, Espina M, Doktorovova S, Souto E, García M. Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye-Part II-Ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2017;110:58–69.
Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–13.
Kakkar S, Karuppayil SM, Raut JS, Giansanti F, Papucci L, Schiavone N, et al. Lipid-polyethylene glycol based nano-ocular formulation of ketoconazole. Int J Pharm. 2015;495(1):276–89.
Abrishami M, Abrishami M, Mahmoudi A, Mosallaei N, Vakili Ahrari Roodi M, Malaekeh-Nikouei B. Solid lipid nanoparticles improve the diclofenac availability in vitreous after intraocular injection. J Drug Deliv. 2016;2016:1368481.
del Pozo-Rodriguez A, Pujals S, Delgado D, Solinís M, Gascón A, Giralt E, et al. A proline-rich peptide improves cell transfection of solid lipid nanoparticle-based non-viral vectors. J Control Release. 2009;133(1):52–9.
Poovi G, Vijayakumar TM, Damodharan N. Solid lipid nanoparticles and nanostructured lipid carriers: a review of the effect of physicochemical formulation factors in the optimization process, different preparation technique, characterization, and toxicity. Curr Nanosci. 2019;15(5):436–53.
Garcês A, Amaral M, Sousa Lobo JM, Silva AC. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharm Sci. 2018;112:159–67.
Luo Q, Zhao J, Zhang X, Pan W. Nanostructured lipid carrier (NLC) coated with chitosan oligosaccharides and its potential use in ocular drug delivery system. Int J Pharm. 2011;403(1–2):185–91.
Araújo J, Nikolic S, Egea MA, Souto EB, Garcia ML. Nanostructured lipid carriers for triamcinolone acetonide delivery to the posterior segment of the eye. Colloids Surf B Biointerfaces. 2011;88(1):150–7.
Dingler A, Gohla S. Production of solid lipid nanoparticles (SLN): scaling up feasibilities. J Microencapsul. 2002;19(1):11–6.
Lambuk L, Iezhitsa I, Agarwal R, Agarwal P, Peresypkina A, Pobeda A, et al. Magnesium acetyltaurate prevents retinal damage and visual impairment in rats through suppression of NMDA-induced upregulation of NF-κB, p53 and AP-1 (c-Jun/c-Fos). Neural Regen Res. 2021;16(11):2330–44.
Nor Arfuzir NN, Agarwal R, Iezhitsa I, Agarwal P, Ismail NM. Magnesium acetyltaurate protects against endothelin-1 induced RGC loss by reducing neuroinflammation in Sprague dawley rats. Exp Eye Res. 2020;194:107996.
Cammalleri M, Dal Monte M, Amato R, Bagnoli P, Rusciano D. A dietary combination of forskolin with homotaurine, spearmint and B vitamins protects injured retinal ganglion cells in a rodent model of hypertensive glaucoma. Nutrients. 2020;12(4):1189.
Jiang N, Li Z, Li Z, Zhang Y, Yu Z, Wan P, et al. Laquinimod exerts anti-inflammatory and antiapoptotic effects in retinal ischemia/reperfusion injury. Int Immunopharmacol. 2020;88:106989.
Krishnan A, Kocab AJ, Zacks DN, Marshak-Rothstein A, Gregory-Ksander M. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J Neuroinflamm. 2019;16(1):184.
Kuai L, Peng J, Jiang Y, Zheng Z, Zhou X. Apolipoprotein E-mimetic peptide COG1410 enhances retinal ganglion cell survival by attenuating inflammation and apoptosis following TONI. Front Neurosci. 2019;13:980.
Jia Y, Jiang S, Chen C, Lu G, Xie Y, Sun X, et al. Caffeic acid phenethyl ester attenuates nuclear factor-κB-mediated inflammatory responses in Müller cells and protects against retinal ganglion cell death. Mol Med Rep. 2019;19(6):4863–71.
Yang Y, Xu C, Chen Y, Liang JJ, Xu Y, Chen SL, et al. Green tea extract ameliorates ischemia-induced retinal ganglion cell degeneration in rats. Oxid Med Cell Longev. 2019;2019:840720.
Lin C, Wu F, Zheng T, Wang X, Chen Y, Wu X. Kaempferol attenuates retinal ganglion cell death by suppressing NLRP1/NLRP3 inflammasomes and caspase-8 via JNK and NF-κB pathways in acute glaucoma. Eye. 2019;33(5):777–84.
Huang R, Liang S, Fang L, Wu M, Cheng H, Mi X, et al. Low-dose minocycline mediated neuroprotection on retinal ischemia–reperfusion injury of mice. Mol Vis. 2018;24:367–78.
Georgiou T, Wen YT, Chang CH, Kolovos P, Kalogerou M, Prokopiou E, et al. Neuroprotective effects of omega-3 polyunsaturated fatty acids in a rat model of anterior ischemic optic neuropathy. Invest Opthalmol Vis Sci. 2017;58(3):1603–11.
Lambert WS, Carlson BJ, Formichella CR, Sappington RM, Ahlem C, Calkins DJ. Oral delivery of a synthetic sterol reduces axonopathy and inflammation in a rodent model of glaucoma. Front Neurosci. 2017;11:45.
Chien JY, Sheu JH, Wen ZH, Tsai RK, Huang SP. Neuroprotective effect of 4-(phenylsulfanyl) butan-2-one on optic nerve crush model in rats. Exp Eye Res. 2016;143:148–57.
Huang SP, Tsai RK. Efficacy of granulocyte-colony stimulating factor treatment in a rat model of anterior ischemic optic neuropathy. Neural Regen Res. 2014;9(16):1502–5.
Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine (Chichester). 2010;6(2):324–33.
Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Nanoparticles laden in situ gel for sustained ocular drug delivery. J Pharm Bioallied Sci. 2013;5(2):162–5.
Giannavola C, Bucolo C, Maltese A, Paolino D, Vandelli MA, Puglisi G, et al. Nanoparticles laden in situ gel for sustained ocular drug delivery. Pharm Res. 2003;20(4):584–90.
Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012;6(9):7595–606.
Sharma PK, Chauhan MK. Optimization and characterization of brimonidine tartrate nanoparticles-loaded in situ gel for the treatment of glaucoma. Curr Eye Res. 2021;46(11):1703–16.
Singh K, Shinde U. Chitosan nanoparticles for controlled delivery of brimonidine tartrate to the ocular membrane. Pharmazi. 2011;66(8):594–9.
Kao HJ, Lo YL, Lin HR, Yu SP. Characterization of pilocarpine-loaded chitosan/carbopol nanoparticles. J Pharm Pharmacol. 2006;58(2):179–86.
Yuan XB, Yuan YB, Jiang W, Liu J, Tian EJ, Shun HM, et al. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. Int J Pharm. 2008;349(1–2):241–8.
Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008;68(3):513–25.
Mahmoud AA, El-Feky GS, Kamel R, Awad GE. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int J Pharm. 2011;413(1–2):229–36.
Nagarwal RC, Kumar R, Pandit J. Chitosan coated sodium alginate-chitosan nanoparticles loaded with 5-FU for ocular delivery: in vitro characterization and in vivo study in rabbit eye. Eur J Pharm Sci. 2012;47(4):678–85.
Bhatta RS, Chandasana H, Chhonker YS, Rathi C, Kumar D, Mitra K, et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int J Pharm. 2012;432(1–2):105–12.
Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Hyaluronic acid modified chitosan nanoparticles for effective management of glaucoma: development, characterization, and evaluation. J Drug Target. 2010;18(4):292–302.
Ryu M, Nakazawa T, Akagi T, Tanaka T, Watanabe R, Yasuda M, et al. Suppression of phagocytic cells in retinal disorders using amphiphilic poly (γ-glutamic acid) nanoparticles containing dexamethasone. J Control Release. 2011;151(1):65–73.
Bessone CDV, Martinez SM, Luna JD, Marquez MA, Ramírez ML, Allemandi DA, et al. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Exp Eye Res. 2020;200:108222.
Bhagav P, Upadhyay H, Chandran S. Brimonidine tartrate-eudragit long-acting nanoparticles: formulation, optimization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2011;12(4):1087–101.
Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238(1–2):241–5.
Liu Z, Zhang X, Wu H, Li J, Shu L, Liu R, et al. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev Ind Pharm. 2011;37(4):475–81.
Wang F, Chen L, Zhang D, Jiang S, Shi K, Huang Y, et al. Methazolamide-loaded solid lipid nanoparticles modified with low-molecular weight chitosan for the treatment of glaucoma: vitro and vivo study. J Drug Target. 2014;22(9):849–58.
Attama AA, Reichl S, Müller-Goymann C. Sustained release and permeation of timolol from surface-modified solid lipid nanoparticles through bioengineered human cornea. Curr Eye Res. 2009;34(8):698–705.
Dang H, Dong C, Zhang L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm Dev Technol. 2022;27(2):127-33.
Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box–Behnken design. Int J Nanomed. 2011;6:683–92.
Wadetwar RN, Agrawal AR, Kanojiya PS. In situ gel containing Bimatoprost solid lipid nanoparticles for ocular delivery: in-vitro and ex-vivo evaluation. J Drug Deliv Sci Technol. 2020;56:101575.
Hippalgaonkar K, Adelli GR, Hippalgaonkar K, Repka MA, Majumdar S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: development, characterization, and in vitro evaluation. J Ocul Pharmacol Ther. 2013;29(2):216–28.
Liu R, Liu Z, Zhang C, Zhang B. Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: improving in vivo ocular bioavailability. J Pharm Sci. 2012;101(10):3833–44.
Araújo J, Gonzalez-Mira E, Egea M, Garcia M, Souto E. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm. 2010;393(1–2):168–76.
Gonzalez-Mira E, Egea MA, Souto EB, Calpena AC, García ML. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology. 2010;22(4):045101.
Üstündağ-Okur N, Gökçe EH, Bozbıyık Dİ, Eğrilmez S, Ertan G, Özer Ö. Novel nanostructured lipid carrier-based inserts for controlled ocular drug delivery: evaluation of corneal bioavailability and treatment efficacy in bacterial keratitis. Expert Opin Drug Deliv. 2015;12(11):1791–807.
Attama AA, Reichl S, Müller-Goymann CC. Diclofenac sodium delivery to the eye: in vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int J Pharm. 2008;355(1–2):307–13.
Chen YS, Green CR, Teague R, Perrett J, Danesh-Meyer HV, Toth I, et al. Intravitreal injection of lipoamino acid-modified connexin43 mimetic peptide enhances neuroprotection after retinal ischemia. Drug Deliv Transl Res. 2015;5(5):480–8.
Acknowledgements
Not applicable.
Funding
This work was supported by grant from Ministry of Education, Malaysia (Grant Number: FRGS/1/2020/SKK06/USM/03/2).
Author information
Authors and Affiliations
Contributions
LL, NAAS, MZS, and AJAJ performed literature search and drafted the manuscript. SA, NAAN, VU, RK, and RM supervised and revised the manuscript. All authors contributed to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Author VU was employed by the company TardigradeNano LLC. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lambuk, L., Suhaimi, N.A.A., Sadikan, M.Z. et al. Nanoparticles for the treatment of glaucoma-associated neuroinflammation. Eye and Vis 9, 26 (2022). https://doi.org/10.1186/s40662-022-00298-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40662-022-00298-y