Abstract
Glaucoma is the second leading cause of blindness worldwide. Even though significant advances have been made in its management, currently available antiglaucoma therapies suffer from considerable drawbacks. Typically, the success and efficacy of glaucoma medications are undermined by their limited bioavailability to target tissues and the inadequate adherence demonstrated by patients with glaucoma. The latter is due to a gradual decrease in tolerability of lifelong topical therapies and the significant burden to patients of prescribed stepwise antiglaucoma regimens with frequent dosing which impact quality of life. On the other hand, glaucoma surgery is restricted by the inability of antifibrotic agents to efficiently control the wound healing process without causing severe collateral damage and long-term complications. Evolution of the treatment paradigm for patients with glaucoma will ideally include prevention of retinal ganglion cell degeneration by the successful delivery of neurotrophic factors, anti-inflammatory drugs, and gene therapies. Nanotechnology-based treatments may surpass the limitations of currently available glaucoma therapies through optimized targeted drug delivery, increased bioavailability, and controlled release. This review addresses the recent advances in glaucoma treatment strategies employing nanotechnology, including medical and surgical management, neuroregeneration, and neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This article offers a comprehensive review of nanotechnology-based treatments for patients with glaucoma. |
Nanotechnology-based drugs will probably be incorporated into the arsenal of glaucoma specialists in the near future, allowing benefits such as reduced side effects, and less frequent dosing, among others. |
Toxicity issues related to nanotechnology-based treatments have yet to be addressed to test their safety. |
Further human studies in nanomedicine for glaucoma treatment are needed until this promising pharmacological innovation becomes available in the ophthalmologist’s daily therapeutic practice. |
Introduction
Glaucoma is the second leading cause of blindness worldwide [1, 2] and the most frequent cause of irreversible blindness. Indeed, the World Health Organization estimates that 5.2 million cases of blindness are a result of glaucoma (15% of the total burden of world blindness) [3]. Glaucoma is often characterized by elevated intraocular pressure (IOP), caused predominantly by blockage of the outflow system leading to the progressive death of retinal ganglion cells (RGCs) and resulting in optic neuropathy [4].
The main goal of virtually all glaucoma therapies today is to reduce IOP by either suppressing aqueous synthesis or by enhancing trabecular meshwork (TM) and uveoscleral outflow of aqueous humor [5,6,7]. Other proposed modalities for the treatment of glaucoma include laser [8], surgery [9], and non-IOP-dependent therapies such as neuroprotection [10].
Recently, research has focused on nanoparticles and their unique properties, which can provide a platform to surpass the limitations of traditional glaucoma therapies. Although some reviews have been published [11–15], we aimed to expand available evidence and provide a comprehensive summary of recent advances in nanotechnology-based antiglaucoma therapies with a focus on the following topics: hypotensive drugs, wound healing modulation, neuroprotection, and neuroregeneration.
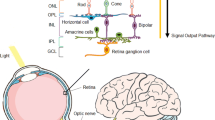
Figure 1 summarizes the main therapeutic targets in glaucoma treatment according to their anatomical location. Importantly, all of them could benefit from nanotechnology-based strategies.
Targeting Intraocular Pressure Reduction
Elevated IOP is considered the key risk factor for optic nerve damage in glaucoma, and most patients benefit from IOP-lowering therapies [16]. Despite the evidence that factors other than IOP are involved in the pathogenesis of glaucoma [17, 18], current treatment algorithms are based on decreasing IOP. First-line glaucoma therapy typically starts with eye drops that lower IOP by two mechanisms: suppression of aqueous humor production (beta-blockers, alpha-agonists, and carbonic anhydrase inhibitors) or increase of aqueous humor outflow though the trabecular or uveoscleral pathways (pilocarpine, epinephrine, or prostaglandin analogues) [19]. In patients where topical medications are not sufficient to controI IOP, laser or surgery can be indicated. We will discuss both treatment strategies in the scenario of recent advancements in nanomedicine. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Drug Delivery Routes, Features, and Limitations of Current Glaucoma Medical Therapy
Although medical therapy of glaucoma is effective, it relies on a delivery system that is generally inefficient and presents several drawbacks. First, eye drops have to be administered 1–3 times daily, which is frequently associated with suboptimal use and lack of adherence [20]. This is a more pronounced issue in the elderly population that is predominantly affected by glaucoma [21]. A previous report showed that 60% of patients expressed one or more problems with taking their glaucoma medications, which results in a decreased adherence to long-term therapy [22]. Furthermore, following instillation, the bioavailability of topical glaucoma medications in the anterior chamber is very low [23]. Typical aqueous humor bioavailability of a drug following the administration of eye drops ranges from 1% to 10% in animal [24] and human studies [25]. This is due to the various physiological barriers to drug penetration into the eye [26].
There are two routes that control and influence the absorption of topically administered medication: the corneal and the non-corneal routes. Small molecules with a hydroxyl group have high corneal permeability, favoring the corneal route [27]. The trajectory of the drug molecules starts with their passive diffusion via the corneal epithelium, through the stroma and endothelium into the anterior chamber, where the drug will carry out its pharmacological action [28] or will bind to melanin in the ciliary body and iris [29], or plasma proteins [30], which prolongs its half-life.
The remaining drug and drug metabolites will be removed via the trabecular meshwork through Schlemm’s canal into the systemic bloodstream (conventional route), or via the uveoscleral route (unconventional outflow). Both of these pathways lead to the systemic blood circulation [31]. The “unconventional” route that includes the ciliary muscle, supraciliary and suprachoroidal spaces, may drain aqueous humor and/or drugs and their metabolites through two possible pathways: a uveovortex pathway where they enter the choroid to drain through the vortex veins, and a uveoscleral pathway where they pass across the sclera to be resorbed by orbital vessels [32]. In addition to these two routes, there is a third route, the “uveolymphatic pathway”, defined by Yücel et al. to describe a novel pathway for aqueous humor drainage through lymphatic channels in the human ciliary body, which can become a novel target for glaucoma treatment [33–35]. Drug penetration in the iris and diffusion via the aqueous humor flow occurs with a small portion of the drug (depending on the molecular weight and lipophilicity). Drug concentrations in the vitreous will be 10–100 times lower than in the aqueous humor and cornea, respectively [27].
The so-called non-corneal absorption route is preferred by drugs with low corneal permeability, such as proteins and large molecules. These large compounds penetrate the eye via the conjunctiva and/or sclera [36, 37]. This route delivers drugs to the vitreous via passive diffusion and allows 20 times lower drug concentration in the anterior chamber compared to the corneal route [38]. However, the conjunctiva and underlying sclera are more permeable and have a higher surface of absorption for the bloodstream than the cornea [39]. Furthermore, some hypotensive topical drugs, such as timolol and betaxolol, have been shown to accumulate in Tenon’s capsule, periocular tissues, and sclera of normal cynomolgus monkeys, and of glaucomatous patients under long-term topical therapy [40, 41]. These findings indicate that periocular accumulation of certain medications could provide differentiated access to the posterior segment and proximal vasculature of the eye.
Systemic absorption of topical medications can lead to systemic side effects such as hypotension, bronchospasm, and bradycardia (beta-blockers), or dry mouth and fatigue (alpha-agonists) [19]. Prostaglandin analogues are prodrugs which have the advantage of being cleaved to their active forms inside the eye, thereby minimizing side effects in nontarget organs [42].
There are numerous ongoing studies aimed at offering neuroprotective or neuroregenerative treatments [16], but clinical trials have been unsuccessful in the past at demonstrating their effectiveness in preventing glaucoma progression [43–45]. One of the main challenges of this approach has been how to allow access of the drug to the back of the eye, where the optic nerve and RGCs reside. There are two pathways to allow drugs to reach these posterior structures: crossing the vitreous and the inner limiting membrane of the retina, or accessing the retina through the vascular system. Intravitreal injections allow direct access to the vitreous cavity and bypass this barrier, but increase the risk for serious ocular adverse events, such as endophthalmitis [46]. The intravenous route is accompanied by systemic side effects and is particularly problematic since drugs must cross the blood–retinal barrier to reach the target tissue [47].
To overcome the multiple limitations of current medical antiglaucoma treatments, the development of safer and more efficient drug delivery systems is imperative. These attributes may be reached through the use of nanomedicine, which may improve solubility, reduce tissue irritation, prolong shelf life, and provide dose accuracy, sustained release, targeted delivery, and bioavailability [12].
Even though nanoparticulate systems can potentially improve drug bioavailability of the active loaded ingredients, they can also be selectively removed more or less quickly from the target organ by conventional, uveoscleral, and uveolymphatic routes, depending on the direct uptake of the nanoparticle, and their physicochemical specificities. Therefore, further pharmacokinetic studies specific for each nanosystem employed for therapeutic purposes will be required.
Role and Value of Nanoparticles
Nanoparticles (NPs) are tiny structures most commonly spherical in shape with sizes in the nanometer range (1–100 nm). As a result of their minuscule size, nanoparticles can bypass biological barriers, providing drug access directly to the target cells [48]. Moreover, smaller nanoparticles have a higher drug-loading capacity than the larger ones, owing to their higher surface area [49].
NPs are utilized in a wide range of shapes such as nanoemulsion [50], liposomes [51], dendrimers [52], nanospheres [53], hydrogels [54], nanocrystals [55], cyclodextrins [56], nanodiamonds [57], microspheres [58], niosomes [59], nanofibers [60], and nanocapsules [61]. Figure 2 shows a schematic representation of various nanoparticles mentioned throughout this article.
NPs can be designed to enhance active compound penetration [62] and drug targeting [63] and to promote controlled release [64]. Currently, NPs are generally made from sundry materials including polymers, lipids, and metals. Lipid and polymer NPs may be employed successfully to carry drugs [65], isolate their contents from degradation, and regulate their release [66]. Metallic NPs have specific physicochemical properties that serve both diagnostic [67] and therapeutic purposes [68, 69]. Injected nanoparticles accumulate in the liver and spleen and are mostly eliminated by the reticuloendothelial system soon after administration. It is, however, possible to employ a coating that can prevent opsonization and recognition of NPs by the macrophages. Indeed, NPs may be covered by extra layers to achieve special therapeutic properties, to reduce side effects, or to increase solubility [70]. Engineered NPs may be created to help the molecule to find its target. For instance, docetaxel and ketoconazole loaded in solid lipid nanoparticles had their surface modified with folic acid to improve brain targeting [71]. Another example is hyaluronic acid-modified lipid–polymer hybrid NPs, which were designed to improve the ocular bioavailability of hydrophilic moxifloxacin hydrochloride, in order to prolong precorneal retention, and enhance its corneal permeability and ocular bioavailability [72].
The selection of the appropriate system depends on the target tissue, route of administration, and the characteristics of the drug to be incorporated into the NPs (size, stability, hydrophobicity, etc.). However, there are common physicochemical properties that all nanosystems must possess, such as biocompatibility, biodegradability, absence of toxicity, and stability, which can provide effective and safe pharmacological action, better than those obtained by traditional drug formulations.
Emergence of Nanomedicine Formulations in Glaucoma
Several drugs traditionally employed in glaucoma treatment, e.g., timolol, carteolol, brimonidine, pilocarpine, brinzolamide, bimatoprost, travoprost, and latanoprost [73], have been investigated in nanomedicine formulations [11, 12, 15]. We emphasize here that all of these formulations are still under investigation and are not currently approved for clinical use. In the following pages, we describe nanosystems loaded with the most utilized drugs for the treatment of glaucoma.
Pilocarpine
Pilocarpine has by and large been avoided by ophthalmologists because of several undesirable adverse effects, most of which are due to its non-selective ocular and systemic action as a muscarinic receptor agonist: intense miosis, ocular irritation, myopic shift, bronchial mucus secretion, bronchospasm, vasodilation, bradycardia, excessive salivation, sweating, and diarrhea. Pilocarpine has been used in a nitrate or hydrochloride pharmaceutical formulation, usually at a 0.5–4% concentration administered four times daily because of the short duration of action. When administered as a solution, it has a reasonable water solubility, which shortens the residence time of aqueous solutions in the eye. Hence, the real obstacle with pilocarpine solutions has been its very low ocular bioavailability (0.1–3%) [74, 75] arising from its poor lipophilicity.
Research efforts have been devoted to optimizing the activity and tolerability of pilocarpine [76]. Many nanosystems have been tried in order to improve pilocarpine’s bioavailability, solubility, and stability and to extend its pharmacological activity [77]. Table 1 shows examples of pilocarpine nanoparticulate drug delivery systems.
Timolol Maleate
Timolol maleate is a non-selective beta-adrenergic blocker that acts by inhibiting the beta-adrenergic receptors in the ciliary body epithelium, promoting the reduction of aqueous humor production and resulting in decreased IOP [87]. The hydrophilic nature of timolol maleate leads to slow corneal diffusion and low bioavailability [88]. Timolol maleate absorption from the eye into the systemic circulation, via nasolacrimal drainage, causes systemic adrenergic beta-blockage and high incidence of respiratory and cardiovascular side effects [89]. To improve the pharmacological features of timolol maleate, it is necessary to enhance its corneal penetration and reduce systemic absorption, which may be attained by the use of nanocarriers. Table 2 shows examples of timolol maleate-loaded nanoparticulate drug delivery systems.
Carbonic Anhydrase Inhibitors
Carbonic anhydrase (CA) is a generic denomination to designate several isoenzymes distributed in diverse proportions in the various tissues responsible for catalyzing the reversible hydration of carbon dioxide [104]. In the eye, CA is a key enzyme in aqueous humor production. Nonpigmented ciliary epithelial cells are the site of aqueous humor secretion, where two CA isoenzymes (CA-II and CA-IV) are the predominant forms. The inhibition of CA activity in the ciliary processes promotes decreased aqueous humor secretion and consequently leads to lowering of the IOP [105]. CA-II is considered the most relevant intracytoplasmic isoenzyme responsible for aqueous humor secretion [106]. Therefore, selective pharmacological inhibition of CA-II to achieve an ocular hypotensive effect is the objective in the treatment of glaucoma [106].
Three different CA inhibitors (CAI) molecules, all of them sulfonamide derivatives, are currently used in clinical practice to reduce elevated IOP in glaucoma. Acetazolamide is administered orally, and dorzolamide and brinzolamide are both administered topically [107]. Acetazolamide is a compound that indiscriminately inhibits most of the CA isozymes (CA-I, CA-II, CA-IV, CA-VA, CA-VB, CA-VII, CA-XIII, and CA-XIV) present in other organs than the eye [108–110], and although systemically administered acetazolamide is very effective as an intraocular hypotensive agent, the non-selective inhibition of the enzyme results in a myriad of undesirable side effects [108–110]. Dorzolamide and brinzolamide are potent nanomolar inhibitors of human CA-II isozyme [106, 111–113], with less activity against CA-IV, CA-XII, and CA-I [114].
Acetazolamide
Acetazolamide (ACZ) is a CAI used systemically to obtain a substantial and immediate reduction of IOP in glaucoma management. It is currently considered one of the most effective available IOP-lowering agents [115]. Limitations of orally administered ACZ include several systemic adverse events (e.g., metabolic acidosis, paresthesias, fatigue, diuresis, malaise, anorexia, loss of libido, renal function impediment, etc.) [116]. As a rule, large oral doses of ACZ are necessary to obtain the desired IOP reduction, probably because of its low bioavailability and relative instability [117]. Topical administration of ACZ would have been preferred over systemic administration, but as a result of the pharmacological limitations of topical ACZ (e.g., poor permeability via the corneal epithelium, short residence time, frequent instillations, loss of drug through nasolacrimal drainage, poor penetration coefficient (only 1–6% of the active compound actually reaches intraocular tissues) [118], poor biphasic solubility, and poor corneal bioavailability) [119], many researchers have tried to develop optimized ACZ topical drug delivery systems, some employing nanotechnology-based approaches [115, 117, 120–124]. Table 3 shows examples of ACZ-loaded nanoparticulate systems.
Dorzolamide
Dorzolamide (DRZ) is a CAI used extensively in the treatment of glaucoma. Inhibition of carbonic anhydrase results in decrease of aqueous humor production and IOP reduction. The first commercially available topical CAI was 2% DRZ hydrochloride solution (Trusopt®) [131], available since 1995. When topical 2% DRZ is used as monotherapy, it reduces IOP by up to 23% from baseline [132]. However, as a result of its low pH and high viscosity, 2% DRZ causes significant ocular irritation, stinging, or burning [133]. Moreover, topical 2% DRZ requires 2–3 daily doses, which hinders long-term adherence to treatment [134]. Therefore, there is a clear clinical demand to enhance the delivery efficiency of DRZ, and nano-based drug systems appear to have the potential to fulfil this goal. Table 4 demonstrates examples of dorzolamide-loaded nanoparticulate systems.
Brinzolamide
Brinzolamide is a water-insoluble CAI, which acts on the ciliary processes and decreases aqueous humor secretion. This medication is employed widely in stepwise glaucoma therapy most often in combination with prostaglandin analogues or in conjunction with brimonidine and beta-blockers [141]. Brinzolamide is formulated commercially as a 1% aqueous suspension, because lower concentrations resulted in insufficient therapeutic effect [114]. As a result of the solubilization demand of particles on the ocular surface and because of its high lipophilicity, brinzolamide aqueous suspension has a low bioavailability [142], which may elicit blurred vision after administration [143] and foreign body sensation, often leading to discomfort after instillation [144]. For these reasons, novel topical ocular delivery systems for this drug must surpass current problems. Table 5 shows examples of brinzolamide-loaded nanoparticulate systems.
Brimonidine
Brimonidine (BRD) is an α2-adrenergic agonist which acts through two mechanisms: it promotes an acute reduction in aqueous production, associated with an increase in uveoscleral outflow [151]. Nevertheless, BRD exhibits low bioavailability through the corneal stroma (1–7%) [152] and shows a short duration of action that leads to the necessity of three instillations during the day [153]. Consequently, BRD demonstrates a relatively reduced adherence, as evidenced by the rate of adherence, which ranges from 31% to 67% at 12 months [20]. The reluctance of patients to instill their eye drops many times a day on a regular basis justifies the need to develop new BRD formulations. Table 6 shows examples of BRD-loaded nanoparticulate systems.
Prostaglandin Analogues
Topically instilled prostaglandin F2α (PGF2α) analogues (latanoprost, travoprost, bimatoprost, and tafluprost) have been the fist-line choice in the medical management of glaucoma over the last decade, owing to their favorable safety profile, efficacy, and patient compliance [160, 161]. All PGF2α analogues lead to common side effects, such as eyelash growth, darkening of the iris and periocular pigmentation, conjunctival hyperemia, and rare adverse reactions (e.g., damage to the blood–aqueous barrier, cystoid macular edema, and prostaglandin-associated periorbitopathy) [162]. These side effects reduce patient compliance. To overcome the side effects, to reduce dosing, to optimize the efficacy and safety of this class of drugs, and to get a reliable dosing technology delivered continuously over a prolonged time (weeks or more), novel nanoformulations of PGF2α analogues may prove greatly advantageous. Table 7 shows examples of prostaglandin analogue-loaded nanoparticulate systems.
Glaucoma Drainage and Nanodevices
Glaucoma drainage implants are often employed in glaucoma care when medical therapy, laser procedures, and standard filtration surgery fail to sufficiently control IOP [172]. The main types of glaucoma drainage devices available commercially are the Molteno, the Baerveldt, and the Ahmed, all of them designed to create an alternate aqueous pathway by channeling aqueous humor from the anterior chamber to the collection plate positioned under the conjunctiva [173, 174].
The long-term success of glaucoma drainage implants, however, is reduced as a result of the fibrous reaction developing around the end-plate, which leads to encapsulation and reduction of aqueous humor absorption. Epstein [175] was the first author to consider that aqueous humor in the subconjunctival space, in itself, would be a stimulus for the fibrovascular proliferation in the episcleral tissue. The intensity of the fibrotic reaction was attributed to a multifactorial origin, although not all pathogenetic factors are well understood. Some elements, such as the type of biomaterial, design and/or size of the end-plate, and the patient’s immune response, certainly influence the formation of fibrous tissue around the valve plate [176]. Therefore, refinement of biomaterials, as well as the associated use of antifibrotic agents with more advanced drug delivery nanosystems may meaningfully improve the long-term success of glaucoma drainage devices.
A novel double-layered porous coating for Ahmed glaucoma valves based on biodegradable poly(lactic-co-glycolic acid) (PLGA) was developed by Ponnusamy et al. [177] to achieve continuous drug release of antifibrotic agents [mitomycin C (MMC) and/or 5-fluorouracil (5-FU)] to the subconjunctival space. This novel poriferous coating for the Ahmed glaucoma drainage implant may have the advantage of modifying the degradation pattern of the polymer, and the drug release features. The purpose of this double-layered film is to provide a burst of MMC release followed by a slow release of 5-FU, which would prevent fibroblast proliferation over the most active period of postoperative wound healing (0–28 days). Results showed a very rapid release of MMC within 1 day, followed by the initial release of 5-FU within the first 3–5 days, which lasted for 3 to 4 weeks. Cytotoxicity assays have demonstrated significant cytotoxic activity from day 1 which persisted until the PLGA film was almost totally degraded.
Pan et al. [178] were able to manufacture a nano-drainage device fabricated through microelectromechanical systems (MEMS; miniaturized devices containing submillimeter features). This nano-drainage implant is made of a nanoporous membrane (mimicking the drainage function of the trabecular meshwork) connected to an integrated polymeric shaft inserted through the sclera into the anterior chamber, thus enabling a bypass route for the aqueous humor outflow. The nano-filtration membrane provides the designed flow resistance to the aqueous humor flow, but clogging of proteins inside the nanopores from plasma perfusion significantly increased the resistance. Further efforts will be necessary to apply modifications to the nanoporous membrane to reach the ideal aqueous outflow.
Harake et al. [179] designed an anti-biofouling micro-device which provides an innovative platform, also using MEMS, for IOP reduction in patients with glaucoma. This novel device has an array of parallel micro-channels built inside to yield controlled aqueous outflow resistance. Polyethylene glycol (PEG), a synthetic hydrogel, was chosen as the primary device material owing to its favorable biocompatibility and its antifouling properties, aiming to reduce protein clogging of the micro-channels [180]. PEG 214 and PEG 4000 were jointly used to avoid channel swelling. In vitro, continuous testing under artificial flow conditions showed that the device design containing 23 channels provided a pressure drop of about 10 mmHg.
Ferrofluids are the colloidal mixtures of magnetic nanoparticles (ferri- or ferromagnetic particles) suspended in a non-magnetic, inert fluid, such as water or an organic solvent [181]. As a result of their nanosize (tipically 10–100 nm in diameter), the nanoparticles are subject to Brownian motion. Brownian motion or pedesis is the random movement of microscopic particles in suspension mixed in a fluid, and it results from the averaged impacts of fast-moving molecules of the surrounding medium [182]. Magnetite (Fe3O4) particles are composed of a single magnetic domain and show superparamagnetic properties, i.e., a drag force along field gradients. If a static magnetic field exists, the interfacial force causes the ferrofluid to conform to its boundaries. Accordingly, a ferrofluid in a capillary tube can operate as an on/off valve to flow through capillary blockade. A permanent magnet may be utilized to keep the ferrofluid in the capillary and a secondary magnet may be utilized to regulate the force necessary to form the capillary barrier. Whenever the pressure exerted on the ferrofluid by the liquid surpasses the magnetic force between the ferrofluid and the secondary magnet, flow can start after the barrier is broken. Paschalis et al. [183] designed a novel, replaceable, ferromagnetic valve tube able to afford pressure regulation without the requirement of subconjunctival encapsulation. Preliminary in vivo results in rabbits showed that during the 2 weeks of follow-up, there were no signs of inflammation or infection at the surgical site. The mean IOP value in the valve implanted eye (11.8 ± 2 mmHg) was significantly lower than the contralateral control eye (14 ± 3 mmHg), and a continuous flow at the outlet tip of the valve was observed until the last follow-up.
Biomaterial improvement provided by nanoparticle-coated glaucoma drainage devices, associated with design changes and innovative drug delivery systems may potentially reduce fibrous reaction, and improve their efficiency.
Nanoparticle-Based Antimetabolites and Wound Healing Modulation
Currently, trabeculectomy is still the gold standard surgical treatment for glaucoma. Postoperative wound healing produces a hypercellular response in the subconjunctival tissues, which may gradually close the filtration site, limiting its long-term success [184]. Subconjunctival antifibrotics, such as MMC and 5-FU, have been used to reduce the generation of scar tissue, by inhibiting fibroblast proliferation [184–187].
In order to improve the efficiency of these antifibrotics drugs, nanoformulations have been recently employed. Hou et al. [188] have developed a new method to prepare MMC-loaded polylactic acid (PLA) nanoparticles, employing soybean phosphatidylcholine to improve the liposolubility of MMC. Results showed refined formulation characteristics and longer sustained drug release. Gomes dos Santos et al. [189] designed nanosized complexes of antisense TGFβ2 phosphorothioate oligonucleotides (PS-ODN) with polyethylenimine (PEI), and naked PS-ODN encapsulated into poly(lactide-co-glycolide) microspheres, based on the hypothesis that the encapsulation of antisense TGFβ2 ODN-PEI nanosized complexes in different types of porous particles could be an efficacious system for oligonucleotide delivery in vivo. Results showed that the MS-AsPS-ODN-PEI nanosized complex significantly improved the bleb survival rate following trabeculectomy (100% at 42 days) in rabbits compared to controls (0% of survival at day 21). The improved efficiency of the complexes released from microspheres is attributed to a superior intracellular delivery in conjunctival cells, along with the inhibition of TGFβ2.
Mitomycin C is considered the most effective antifibrotic agent used in glaucoma filtering surgery [190]. It is used in more than 85% of the trabeculectomies, but severe adverse effects, such as conjunctival thinning, bleb leaks, hypotony, and infection, limit its safety [190]. Our group has been working on the development of a safer and effective alternative nanoparticle-based antifibrotic agent. Paclitaxel associated with lipid nanoemulsions (LDE-PTX) was tested on rabbits undergoing trabeculectomy [191]. Subconjunctival injection of LDE-PTX was as effective as MMC in preventing scarring after trabeculectomy, but with substantially less toxicity to the conjunctiva and ciliary body. After these promising results in the animal model, we have recently started the first clinical trial to investigate the effectiveness of LDE-PTX in patients with primary open-angle glaucoma (POAG) undergoing trabeculectomy.
Duan et al. [192] investigated the effects of IkappaB kinase subunit beta (IKKβ) inhibition using RNA interference (RNAi) technology on the proliferation of human Tenon’s capsule fibroblasts (HTFs), which have a fundamental role in the scarring process. Ye et al. [193] developed a small interfering RNA (siRNA) targeting IKKβ (IKKβ-siRNA) associated with a ternary cationic nano-copolymer called CS-g-(PEI-b-mPEG) as the vehicle delivered into HTFs. They found that the expression of IKKβ was downregulated, the activation of nuclear factor kappa B (NF-κB) in the HTFs was inhibited, and the proliferation of HTFs was suppressed through the blocking of the NF-κB pathway, effects that improved the surgical outcome in a non-human primate model of trabeculectomy.
There is increasing interest in the use of angiogenesis-inhibiting compounds, especially those that act against vascular endothelial growth factor (VEGF), aiming to modulate the effects of VEGF on the migration and proliferation of human fibroblasts in Tenon’s capsule [194]. VEGF is upregulated in the aqueous humor of the glaucoma rabbit model as well as in patients with glaucoma, and it stimulates fibroblast proliferation in vitro [195]. Bevacizumab is a full-length humanized non-selective monoclonal antibody directed against all isoforms of VEGFA, which has been reported to inhibit proliferation of fibroblasts in vitro and to reduce angiogenesis and collagen deposition in eyes undergoing trabeculectomy [194–197]. Han et al. [198] investigated the use of bevacizumab combined with PEG-PCL-PEG (PECE) hydrogel, by intracameral injection, after experimental glaucoma filtration surgery (GFS). Results demonstrated that the antifibrotic effect of bevacizumab-loaded PECE hydrogel was superior to bevacizumab alone in the prevention of postoperative scarring after GFS in the rabbits.
Neuroprotection and Neuroregeneration and Neurotrophic Factors
Although IOP is considered the main risk factor for the development and progression of glaucoma, RGC loss may continue in spite of IOP reduction in some patients with glaucoma [199, 200]. The physiopathology behind RGC death is complex and has been attributed to various factors, including oxidative stress, intermittent ischemia, defective axon transport, excitotoxicity, loss of electrical activity, and trophic factor withdrawal [16], which justifies the development of neuroprotection/neuroregeneration strategies.
Glial cell-derived neurotrophic factor (GDNF) is an important neurotrophic factor implicated in the growth, differentiation, maintenance, and regeneration of specific neuronal populations in the adult brain, as well as in peripheral neurons [201].
In vitro and in vivo studies have shown that GDNF may rescue photoreceptor and RGC functions during retinal degeneration [202]. Previous studies have demonstrated that exogenous GDNF is an interesting therapeutic approach for neurodegenerative diseases affecting the retina, including glaucoma [203]. Checa-Casalengua et al. [203] described a novel protein microencapsulation method to ensure protection of the biological factor during this procedure, allowing the controlled release of proteins to keep their bioactivity for prolonged periods of time. GDNF in combination with vitamin E released from the microspheres was active for at least 3 months after a single intravitreal injection in an animal model of glaucoma. There was a significant increase of RGC survival compared with GDNF alone, vitamin E alone, or blank microspheres, which indicates that this novel formulation may be clinically applicable as a neuroprotective tool in the treatment of glaucomatous optic neuropathy. Ward et al. [204] demonstrated long-term RGC survival in a spontaneous glaucoma animal model by injecting slow-release PLGA microspheres containing GDNF into the vitreous. Early treatment resulted in 3.5 times greater RGC density than untreated mice after 15 months.
In a rat glaucoma model, animals were treated with GDNF injected into their vitreous cavity. 10% GDNF-microsphere emulsion resulted in a significant reduction of optic nerve head excavation, and increased RGC survival, compared with 2% GDNF-microspheres, blank microspheres, and saline solution [205]. In order to evaluate the pharmacokinetics of GDNF, García-Caballero et al. [206] analyzed its levels after a single intravitreal injection of GDNF/vitamin E microspheres in rabbits. This method allowed a sustained controlled release of GDNF for up to 6 months.
Ciliary neurotrophic factor (CNTF) has also been shown to be neuroprotective on motor neurons following injury to the adult central nervous system [207], and also for neurons in other regions in degenerative diseases [208–210]. Effective neuroprotection could be reached if sustained and local delivery without adverse side effects were achievable, but sustained CNTF delivery has proven arduous to accomplish and control. Nkansah et al. [211] examined the effects of CNTF nanospheres on neural stem cells (NSC) and also on the factors that influence controlled CNTF delivery from microspheres and nanospheres. Protein bioactivity after encapsulation was studied treating neural stem cells with CNTF released from nanospheres and compared to those treated with unencapsulated CNTF. Results showed that encapsulated CNTF provided NSC differentiation with no loss of potency compared to the unencapsulated growth factor.
In addition, Pease et al. [212] demonstrated the neuroprotective effect of virally mediated overexpression of CTNF and brain-derived neurotrophic factor (BDNF) in experimental glaucoma. Injection of adeno-associated viral vectors carrying either BDNF, CTNF, or both, in laser-induced glaucoma eyes, was performed. Combined CNTF-BDNF and BDNF overexpression alone had no noticeable improvement in RGC axon survival, but CNTF alone showed a significant protective effect, with 15% less RGC death.
The induction of heat shock proteins (HSPs) has been investigated as a modality for the protection of optic nerve damage in glaucoma [213–215]. Caprioli et al. [214] conducted the first demonstration of the neuroprotective effects of 72-kDa HSPs in cultured RGCs and Müller cells after hyperthermia and sublethal hypoxia. Since then, many researchers have been attempting to apply the induction of HSPs as a neuroprotective strategy in patients with glaucoma.
The calcium (Ca2+)-binding protein oncomodulin is a potent macrophage-derived growth factor for RGCs and other neurons. Yin et al. [216] demonstrated that, in vivo, oncomodulin released from nanoparticles (microspheres) promotes regeneration in the mature rat optic nerve.
More recently, Jeun et al. [217] investigated a new approach to use localized HSPs—a promising treatment modality for the ocular neuroprotection—without the unwanted side effects typically occurring with the current clinical approaches to induce HSPs in RGCs. These authors proposed the induction of HSPs by local magnetic hyperthermia utilizing engineered superparamagnetic Mn0.5Zn0.5Fe2O4 nanoparticle agents (EMZF-SPNPAs). The results showed that the EMZF-SPNPAs possess all the ideal characteristics as an in vivo localized HSP induction agent. Moreover, the authors also used an innovative infusion technique, which delivers the silica-coated EMZF-SPNPAs through the vitreous body to the retina for neuroprotection.
Anti-inflammatory Drugs
Inflammation is a common physiological protective response to injury. Inflammation is generally beneficial to the organism, promoting tissue survival, repair, and recovery, provided that it occurs for a limited period of time. Otherwise, it may be detrimental when extensive, prolonged, or unregulated [218]. In the central nervous system, the activation of astrocytes and microglia, which is indicative of inflammation, occurs in patients with Parkinson’s, Alzheimer’s, Huntington’s diseases, multiple sclerosis, and amyotrophic lateral sclerosis [219–221]. Hence, in the nervous system, prolonged inflammation may have a dual role, increasing neuronal loss or impeding regeneration. This justifies why anti-inflammatory drugs might also have a neuroprotective activity.
In glaucoma, the neuroinflammatory responses during RGC degeneration have not been elucidated. However, microglia are pleiotrophic immune cells and seem to take part in both neuroprotection and neurodegeneration of RGCs [222]. Thus, it is plausible that low-grade inflammation may occur in glaucoma in response to hypoxia, ischemia, vascular dysfunction, and elevated IOP. In fact, genes associated with cyclooxygenase 2 (COX-2)/PGE2 [223, 224], cytokines [225], tumor necrosis factor alpha (TNFα) in glial cells [226], and hypoxia inducible factor 1-alpha (HIFα) [227] are all upregulated in glaucomatous RGCs.
Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a polyphenol extracted from Curcuma longa [228]. It has been reported that this compound can beneficially modulate several biochemical processes involved in neurodegenerative diseases. For example, Dong et al. [229] showed that long-term (12-week) curcumin-supplemented diet increased hippocampal neurogenesis and cognitive function in aged rats. Similarly, Kim et al. [230] demonstrated the beneficial effects of low dose curcumin on mouse multi-potent neural progenitor cells, which means it can stimulate neural plasticity and repair. Furthermore, Belviranlı et al. [231] concluded that curcumin supplementation improves cognitive function in aged female rats by reducing the lipid peroxidation in brain tissue, which demonstrates its protective effect against neural oxidative stress.
Curcumin has been reported to protect RGCs and the microvasculature against ischemic damage via inhibition of NF-κB, signal transducer and activator of transcription 3 (STAT3), and monocyte chemotactic protein 1 (MCP-1; also known as C-C motif chemokine 2) overexpression [232]. Wang et al. conducted a study to investigate the ability of curcumin to inhibit retinal ischemia/reperfusion injury. Pretreatment with curcumin inhibited ischemia/reperfusion-induced cell loss in the ganglion cell layer. Also, 0.05% curcumin administered 2 days after the injury showed a vasoprotective effect [232]. On the basis of the hypothesis that local and systemic oxidative stress participate in the pathogenesis of glaucoma, Yue et al. [233] evaluated the antioxidant effects of curcumin both in vitro (BV-2 microglia cell line) and in vivo and found that curcumin may improve cell viability and decrease intracellular reactive oxygen species and apoptosis of RGCs.
Although the therapeutic potential of curcumin in ophthalmology is real, its poor water solubility [234], and low bioavailability [228, 235] are important limiting factors for clinical applicability. To surpass these limitations, Davis et al. [236] used a nanotechnology approach to provide a hydrophobic environment for a poorly soluble molecule such as curcumin, and to improve its bioavailability through the development of a nanocarrier suitable for utilization as a topical formulation. This nanoformulation was able to increase the solubility of curcumin by a factor of 400,000, which is more than enough to overcome natural ocular barriers. Topical application of curcumin-loaded nanocarriers twice-daily for 3 weeks, in in vivo models of ocular hypertension and partial optic nerve transection, significantly reduced RGC loss. These results suggest that topical curcumin nanocarriers have potential as a neuroprotective therapy in glaucoma.
Ketorolac is a synthetic pyrrolizine carboxylic acid derivative that belongs to the group of NSAIDs. Ketorolac is a non-selective inhibitor of the enzymes COX-1 and COX-2. The inhibition of COX-2, upregulated at sites of inflammation, prevents conversion of arachidonic acid to pro-inflammatory prostaglandins [237]. Cyclooxygenases are expressed by RGCs in the rodent retina [238] and are upregulated in the retina after optic nerve injury [239] and ischemia [240]. Nadal-Nicolás et al. [241] first described the neuroprotective effects of ketorolac on RGCs after optic nerve axotomy in rats. Two treatments were evaluated: intravitreal administration of ketorolac tromethamine solution and/or ketorolac-loaded PLGA microspheres, 1 week before the optic nerve lesion and intravitreal administration right after the optic nerve crush. In all treated groups there was a significant increase in the number of RGCs compared to all control groups, which confirmed that an NSAID can delay neuronal death in an acute model of axonal injury. Pretreatment (ketorolac solution administered pre-optic nerve lesion) resulted in 63% survival of RGCs, while simultaneous administration of ketorolac solution provided a 53% survival. However, in this study, microspheres did not show an additional effect, contrary to other investigations where intravitreal administration of the microparticle formulation was found to be advantageous [242].
Toxicity of Nanomaterials
Nanoparticles may generate cellular toxicity through oxidative stress, interaction with the cell membrane, and inflammation [243]. In contrast to chemical toxicity, where compound purity and drug concentration are the essential determinants, in nanotoxicity other characteristics should be taken into account, such as nanoparticle size, surface shape, and ionic charge [244]. In addition, there are a few toxicity forms that may be independent of the drug delivered, namely particle toxicity, excipient toxicity, contaminant toxicity, and inflammatory toxicity [243]. Each one must be independently investigated in vitro and in vivo.
Specific literature about the toxicity of therapeutic nanoparticles in the eye remains scarce [243]. Nanotoxicity is a subject of key importance to be determined in conjunction with the therapeutic potential of each nanoparticle [245, 246], particularly for those that require repeat-dose regimens [247]. All nanoparticles proposed to be utilized for therapeutic purposes must be thoroughly investigated in terms of local and systemic toxicity, before obtaining the full regulatory approval.
Conclusions and Perspectives
Glaucoma is a complex and multifactorial disease and truly effective treatments need to be developed and specifically targeted to the pathophysiological pathways that have been identified to date. We believe that, in the future, glaucoma treatment will include the use of hypotensive drugs associated with neuroprotective agents directed to the preservation of RGCs and the optic nerve.
Nanotechnology has been shown to be a robust and valuable modality for the treatment of ocular diseases, including glaucoma. In this review, we focused on the recent advances in the development of nanosystems for glaucoma treatment. Bearing in mind the desired therapeutic targets in glaucoma therapy, nanotechnology has the ability to meaningfully improve the efficacy of current treatment approaches. By improving the pharmacological characteristics of drugs, or the features of surgical devices, nanotechnology has remarkable potential in future glaucoma management. There is convincing experimental evidence that many nanoparticles may enhance the solubility of lipophilic drugs, which improves bioavailability, and promote sustained delivery owing to their optimized biodegradability and physicochemical characteristics. Nanomedicine formulations designed to lower IOP may not require frequent dosing and may reduce drug-related side effects, which ultimately will result in optimizing adherence. In addition, nanoformulated drugs have been shown to improve wound healing modulation following filtering surgery and at the same time reduce the toxicity to the conjunctiva and ciliary body. Nanoparticle-coated filtering drainage devices may also reduce the fibrotic reaction around the plate, increasing their long-term success.
Although promising, possible toxic effects of nanoparticles have to be thoroughly analyzed during preclinical evaluation. The detailed investigation of every possible biological repercussion is requisite to the future introduction of nanomaterials. Importantly, the vast majority of studies on the use of nanoparticles in glaucoma have been performed in animal models. Human studies are needed not only for the detailed determination of the actual cytotoxicity of each nanoparticle but also to investigate their tolerability and efficacy.
References
Thylefors B, Négrel AD. The global impact of glaucoma. Bull World Health Organ. 1994;72(3):323–6.
Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–51.
WHO. The global impact of glaucoma. Bull World Health Organ. 1994;72(3):323–6. http://www.who.int/blindness/publications/glaucoma/en/. Accessed 7 Dec 2019.
Stein JD, Lee PP. Screening for glaucoma. In: Yanoff M, Duker JS, editors. Ophthalmology. Amsterdan, The Netherlands: Elsevier Sanders; 2009, p. 1007.
Heiji A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–79.
The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration Am J Ophthalmol. 2000;130(4):429–40.
Medeiros FA, Weinreb RN. Medical backgrounders: glaucoma. Drugs Today (Barc). 2002;38(8):563–70.
Kumar H, Mansoori T, Warjri GB, et al. Lasers in glaucoma. Indian J Ophthalmol. 2018;66(11):1539–53.
Abrams KL. Medical and surgical management of the glaucoma patient. Clin Tech Small Anim Pract. 2001;16(1):71–6.
Levin LA, Crowe ME, Quigley HA. Lasker/IRRF initiative on astrocytes and glaucomatous neurodegeneration participants. Neuroprotection for glaucoma: requirements for clinical translation. Exp Eye Res. 2017;157:34–7.
Cetinel S, Montemagno C. Nanotechnology applications for glaucoma. Asia Pac J Ophthalmol (Phila). 2016;5(1):70–8.
Goyal G, Garg T, Rath G, et al. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 2014;31(5):365–405.
Cardigos J, Ferreira Q, Crisóstomo S, et al. Nanotechnology-ocular devices for glaucoma treatment: a literature review. Curr Eye Res. 2019;44(2):111–7.
Foldvari M. Noninvasive ocular drug delivery: potential transcorneal and other alternative delivery routes for therapeutic molecules in glaucoma. J Glaucoma. 2014;23(8 Suppl 1):S80–2.
Kim NJ, Harris A, Gerber A, et al. Nanotechnology and glaucoma: a review of the potential implications of glaucoma nanomedicine. Br J Ophthalmol. 2014;98(4):427–31.
Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–86.
Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–77.
Agarwal R, Gupta SK, Agarwal P, et al. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57(4):257–66.
Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20.
Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441–63.
Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83:711–6.
Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–6.
Loch C, Zakelj S, Kristl A, et al. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur J Pharm Sci. 2012;47:131–8.
Chiang CH, Schoenwald RD. Ocular pharmacokinetic models of clonidine-3H hydrochloride. J Pharmacokinet Biopharm. 1986;14(2):175–211.
Schoenwald RD. Ocular pharmacokinetics/pharmacodynamics. In: Mitra AK, editor. Ophthalmic drug delivery systems. 2nd ed. New York: Dekker, Inc.; 2003.
Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23(5):279–96.
Del Amo EM, Rimpela AK, Heikkinen E, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–85.
Shikamura Y, Yamazaki Y, Matsunaga T, et al. Hydrogel ring for topical drug delivery to the ocular posterior segment. Curr Eye Res. 2016;41:653–61.
Agrahari V, Mandal A, Agrahari V, et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6:735–54.
Pelkonen L, Tengvall-Unadike U, Ruponen M, et al. Melanin binding study of clinical drugs with cassette dosing and rapid equilibrium dialysis inserts. Eur J Pharm Sci. 2017;109:162–8.
Carreon TA, Edwards G, Wang H, et al. Segmental outflow of aqueous humor in mouse and human. Exp Eye Res. 2017;158:59–66.
Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: a review. Exp Eye Res. 2017;158:94–111.
Yücel YH. Discovery of lymphatics in the human eye and implications. Can J Ophthalmol. 2010;45(2):115–7.
Tomczyk-Socha M, Turno-Kręcicka A. A novel uveolymphatic drainage pathway-possible new target for glaucoma treatment. Lymphat Res Biol. 2017;15(4):360–3.
Yücel Y, Gupta N. Lymphatic drainage from the eye: a new target for therapy. Prog Brain Res. 2015;220:185–98.
Lee SJ, Kim SJ, Kim ES, et al. Trans-scleral permeability of Oregon Green 488. J Ocul Pharmacol Ther. 2008;24:579–86.
Del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: clinical predictability and quality of the published data. Exp Eye Res. 2015;137:111–24.
Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26:584–7.
Hamalainen KM, Kananen K, Auriola S, et al. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38:627–34.
Sponsel WE, Terry S, Khuu HD, Lam KW, Frenzel H. Periocular accumulation of timolol and betaxolol in patients with glaucoma under long-term therapy. Surv Ophthalmol. 1999;43(suppl 1):S210–3.
Holló G, Whitson JT, Faulkner R, et al. Concentrations of betaxolol in ocular tissues of patients with glaucoma and normal monkeys after 1 month of topical ocular administration. Invest Ophthalmol Vis Sci. 2006;47(1):235–40.
Lavik E, Kuehn MH, Kwon YH. Novel drug delivery systems for glaucoma. Eye (Lond). 2011;25:578–86.
Sena DF, Ramchand K, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. 2010;(2):CD006539.
Krupin T, Liebmann JM, Greenfield DS, et al. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671–81.
Quigley HA. Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol. 2012;23:144–54.
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age related macular degeneration. N Engl J Med. 2006;355:1419–31.
Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: cellular basis and development. Vision Res. 2017;139:123–37.
Liu G, Molas M, Grossmann GA, et al. Biological properties of poly-l-lysine-DNA complexes generated by cooperative binding of the polycation. J Biol Chem. 2001;276(37):34379–87.
Nagarwal RC, Kant S, Singh PN, et al. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136(1):2–13.
Singh Y, Meher JG, Raval K, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;28(252):28–49.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60.
Akbarzadeh A, Khalilov R, Mostafavi E, et al. Role of dendrimers in advanced drug delivery and biomedical applications: a review. Exp Oncol. 2018;40(3):178–83.
Cheng B, He H, Huang T, et al. Gold nanosphere gated mesoporous silica nanoparticle responsive to near-infrared light and redox potential as a theranostic platform for cancer therapy. J Biomed Nanotechnol. 2016;12(3):435–49.
Gao W, Zhang Y, Zhang Q, et al. Nanoparticle–hydrogel: a hybrid biomaterial system for localized drug delivery. Ann Biomed Eng. 2016;44(6):2049–61.
Lu Y, Qi J, Dong X, et al. The in vivo fate of nanocrystals. Drug Discov Today. 2017;22(4):744–50.
Zhang Y, Ren K, He Z, et al. Development of inclusion complex of brinzolamide with hydroxypropyl-β-cyclodextrin. Carbohydr Polym. 2013;98(1):638–43.
Chaudhary HM, Duttagupta AS, Jadhav KR, et al. Nanodiamonds as a new horizon for pharmaceutical and biomedical applications. Curr Drug Deliv. 2015;12(3):271–81.
Prajapati VD, Jani GK, Kapadia JR. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin Drug Deliv. 2015;12(8):1283–99.
Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;10(185):22–36.
Haidar MK, Eroglu H. Nanofibers: new insights for drug delivery and tissue engineering. Curr Top Med Chem. 2017;17(13):1564–79.
Talevi A, Gantner ME, Ruiz ME. Applications of nanosystems to anticancer drug therapy (Part I. Nanogels, nanospheres, nanocapsules). Recent Pat Anticancer Drug Discov. 2014;9(1):83–98.
Priwitaningrum DL, Blonde JG, Sridhar A, et al. Tumor stroma-containing 3D spheroid arrays: a tool to study nanoparticle penetration. J Control Release. 2016;244(Pt B):257–68.
Hornung A, Poettler M, Friedrich RP, et al. Treatment efficiency of free and nanoparticle-loaded mitoxantrone for magnetic drug targeting in multicellular tumor spheroids. Molecules. 2015;20(10):18016–30.
Yang L, Yin T, Liu Y, et al. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016;46:177–90.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl. 1):S131–55.
Tamboli V, Mishra GP, Mitrat AK. Polymeric vectors for ocular gene delivery. Ther Deliv. 2011;2:523–36.
Wang J, Huang Y, David AE, et al. Magnetic nanoparticles for MRI of brain tumors. Curr Pharm Biotechnol. 2012;13(12):2403–16.
Chen J, Patil S, Seal S, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–50.
Salem HF, Ahmed SM, Omar MM. Liposomal flucytosine capped with gold nanoparticle formulations for improved ocular delivery. Drug Des Devel Ther. 2016;13(10):277–95.
Gref R, Domb A, Quellec P, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev. 1995;19:215–33.
Venishetty VK, Komuravelli R, Kuncha M, et al. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomedicine. 2013;9(1):111–21.
Liu D, Lian Y, Fang Q, et al. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int J Biol Macromol. 2018;17(116):1026–36.
Jonas JB, Aung T, Boune RR, et al. Glaucoma. Lancet. 2017;390:2083–93.
Chrai SS, Robinson JR. Corneal permeation of topical pilocarpine nitrate in the rabbit. Am J Ophthalmol. 1974;77(5):735–9.
Lazare R, Horlington M. Pilocarpine levels in the eyes of rabbits following topical application. Exp Eye Res. 1975;21(3):281–7.
Plazoneet J, Grove M, Durr C, et al. Recent advances in pilocarpine delivery. In: Saettone MF, Bucci M, Speiser P, editors. Ophthalmic drug delivery: biopharmaceutical, technological and clinical aspects. Padova: Springer & Liviana; 1987. p. 122.
Pepic I, Jalsenjak N, Jalsenjak I. Micellar solutions of triblock copolymer surfactants with pilocarpine. Int J Pharm. 2004;272:57–64.
Jarho P, Järvinen K, Urtti A, et al. Modified beta-cyclodextrin (SBE7-beta-CyD) with viscous vehicle improves the ocular delivery and tolerability of pilocarpine prodrug in rabbits. J Pharm Pharmacol. 1996;48(3):263–9.
Vandamme TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102(1):23–38.
Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int J Pharm. 2000;198:29–38.
Aktaş Y, Unlü N, Orhan M, et al. Influence of hydroxypropyl beta-cyclodextrin on the corneal permeation of pilocarpine. Drug Dev Ind Pharm. 2003;29(2):223–30.
Lee CH, Li YJ, Huang CC, et al. Poly(ε-caprolactone) nanocapsule carriers with sustained drug release: single dose for long-term glaucoma treatment. Nanoscale. 2017;9(32):11754–64.
Casolaro M, Casolaro I, Lamponi S. Stimuli-responsive hydrogels for controlled pilocarpine ocular delivery. Eur J Pharm Biopharm. 2012;80(3):553–61.
Li J, Wu L, Wu W, et al. A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate. Int J Pharm. 2013;455(1–2):75–84.
Orasugh JT, Sarkar G, Saha NR, et al. Effect of cellulose nanocrystals on the performance of drug loaded in situ gelling thermo-responsive ophthalmic formulations. Int J Biol Macromol. 2019;1(124):235–45.
Kao HJ, Lin HR, Lo YL, et al. Characterization of pilocarpine-loaded chitosan/Carbopol nanoparticles. J Pharm Pharmacol. 2006;58(2):179–86.
Heel RC, Brogden RN, Speight TM, Avery GF. Timolol: a review of its therapeutic efficacy in the topical treatment of glaucoma. Drugs. 1979;17(1):38–55.
Fukuda M, Sasaki H. The transcorneal penetration of commercial ophthalmic formulations containing timolol maleate in rabbit eyes. J Ocul Pharmacol Ther. 2015;31(1):57–60.
Van Buskirk EM. Adverse reactions from timolol administration. Ophthalmology. 1980;87(5):447–50.
Maulvi FA, Patil RJ, Desai AR, et al. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: in vitro and in vivo evaluation. Acta Biomater. 2019;1(86):350–62.
Shokry M, Hathout RM, Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for timolol maleate: augmented in vivo efficacy and safe histological profile. Int J Pharm. 2018;545(1–2):229–39.
Jung HJ, Chauhan A. Extended release of timolol from nanoparticle-loaded fornix insert for glaucoma therapy. J Ocular Pharmacol Ther. 2013;29(2):229–35.
Shafaa MW, Sabra NM, Fouad RA. The extended ocular hypotensive effect of positive liposomal cholesterol bound timolol maleate in glaucomatous rabbits. Biopharm Drug Dispos. 2011;32(9):507–17.
Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290(1):155–9.
Gagandeep, Garg T, Malik B, Goyal AK. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 2014;53:10–6.
Fulgêncio Gde O, Viana FA, Ribeiro RR, et al. New mucoadhesive chitosan film for ophthalmic drug delivery of timolol maleate: in vivo evaluation. J Ocul Pharmacol Ther. 2012;28(4):350–8.
Siafaka PI, Titopoulou A, Koukaras EN, et al. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int J Pharm. 2015;495(1):249–64.
Bertram JP, Saluja SS, McKain J, et al. Sustained delivery of timolol maleate from poly(lactic-co-glycolic acid)/poly(lactic acid) microspheres for over 3 months. J Microencapsul. 2009;26:18–26.
Ilka R, Mohseni M, Kianirad M, et al. Nanogel-based natural polymers as smart carriers for the controlled delivery of timolol maleate through the cornea for glaucoma. Int J Biol Macromol. 2018;1(109):955–62.
Tan G, Yu S, Pan H, et al. Bioadhesive chitosan-loaded liposomes: a more efficient and higher permeable ocular delivery platform for timolol maleate. Int J Biol Macromol. 2017;94(Pt A):355–63.
Zhang HH, Luo QH, Yang ZJ, et al. Novel ophthalmic timolol meleate liposomal-hydrogel and its improved local glaucomatous therapeutic effect in vivo. Drug Deliv. 2011;18(7):502–10.
Zhao R, Li J, Wang J, et al. Development of timolol-loaded galactosylated chitosan nanoparticles and evaluation of their potential for ocular drug delivery. AAPS PharmSciTech. 2017;18(4):997–1008.
Yu S, Wang QM, Wang X, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm. 2015;480(1–2):128–36.
Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther. 1997;74(1):1–20.
Holló G. Carbonic anhydrase inhibitors. In: Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG, editors. Glaucoma. 2nd ed. Philadelphia: Elsevier Saunders; 2015. p. 559.
Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Retin Eye Res. 2000;19(1):87–112.
Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–93.
Carta F, Scozzafava A, Supuran CT. Sulfonamides: a patent review (2008–2012). Expert Opin Ther Pat. 2012;22(7):747–58.
Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat. 2000;10:575–600.
Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967;47(4):595–781.
Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem. 2014;75:349–59.
Ilies M, Supuran CT, Scozzafava A, et al. Carbonic anhydrase inhibitors: sulfonamides incorporating furan-, thiophene- and pyrrole-carboxamido groups possess strong topical intraocular pressure lowering properties as aqueous suspensions. Bioorg Med Chem. 2000;8(8):2145–55.
Winum JY, Casini A, Mincione F, et al. Carbonic anhydrase inhibitors: N-(p-sulfamoylphenyl)-alpha-d-glycopyranosylamines as topically acting antiglaucoma agents in hypertensive rabbits. Bioorg Med Chem Lett. 2004;14(1):225–9.
DeSantis L. Preclinical overview of brinzolamide. Surv Ophthalmol. 2000;44(Suppl 2):S119–29.
Kaur IP, Singh M, Kanwar M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int J Pharm. 2000;199(2):119–27.
Epstein DL, Grant WM. Carbonic anhydrase inhibitor side effects. Serum chemical analysis. Arch Ophthalmol. 1977;95(8):1378–82.
Kaur IP, Kapil M, Smitha R, et al. Development of topically effective formulations of acetazolamide using HP-beta-CD-polymer co-complexes. Curr Drug Deliv. 2004;1(1):65–72.
Sasaki H, Yamamura K, Mukai T, et al. Enhancement of ocular drug penetration. Crit Rev Ther Drug Carrier Syst. 1999;16(1):85–146.
Kaur IP, Smitha R, Aggarwal D, et al. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248(1–2):1–14.
Friedman Z, Allen RC, Raph SM. Topical acetazolamide and methazolamide delivered by contact lenses. Arch Ophthalmol. 1985;103(7):963–6.
Loftsson T, Frithriksdóttir H, Stefánsson E, et al. Topically effective ocular hypotensive acetazolamide and ethoxyzolamide formulations in rabbits. J Pharm Pharmacol. 1994;46(6):503–4.
El-Gazaierly O, Hikal AH. Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int J Pharm. 1997;158(2):121–7.
Aggarwal D, Pal D, Mitra AK, Kaur IP. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int J Pharm. 2007;338(1–2):21–6.
Duarte AR, Roy C, Vega-González A, Duarte CM, Subra-Paternault P. Preparation of acetazolamide composite microparticles by supercritical anti-solvent techniques. Int J Pharm. 2007;332(1–2):132–9.
Rathod LV, Kapadia R, Sawant KK. A novel nanoparticle impregnated ocular insert for enhanced bioavailability to posterior segment of eye: in vitro, in vivo and stability studies. Mater Sci Eng C Mater Biol Appl. 2017;1(71):529–40.
Singh J, Chhabra G, Pathak K. Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. Ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm. 2014;40(9):1223–32.
Bravo-Osuna I, Vicario-de-la-Torre M, Andrés-Guerrero V, et al. Novel water-soluble mucoadhesive carbosilane dendrimers for ocular administration. Mol Pharm. 2016;13(9):2966–76.
Mishra V, Jain NK. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm. 2014;461(1–2):380–90.
Morsi N, Ibrahim M, Refai H, et al. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci. 2017;15(104):302–14.
Verma P, Gupta RN, Jha AK, et al. Development, in vitro and in vivo characterization of Eudragit RL 100 nanoparticles for improved ocular bioavailability of acetazolamide. Drug Deliv. 2013;20(7):269–76.
Pfeiffer N. Dorzolamide: development and clinical application of a topical carbonic anhydrase inhibitor. Surv Ophthalmol. 1997;42(2):137–51.
Strahlman E, Tipping R, Vogel R. A double-masked, randomized 1-year study comparing dorzolamide (Trusopt), timolol, and betaxolol. International Dorzolamide Study Group. Arch Ophthalmol. 1995;113(8):1009–16.
Martens-Lobenhoffer J, Banditt P. Clinical pharmacokinetics of dorzolamide. Clin Pharmacokinet. 2002;41(3):197–205.
Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl 1):S57–8.
Shinde U, Ahmed MH, Singh K. Development of dorzolamide loaded 6-O-carboxymethyl chitosan nanoparticles for open angle glaucoma. J Drug Deliv. 2013;2013:562727.
Jansook P, Stefánsson E, Thorsteinsdóttir M, et al. Cyclodextrin solubilization of carbonic anhydrase inhibitor drugs: formulation of dorzolamide eye drop microparticle suspension. Eur J Pharm Biopharm. 2010;76(2):208–14.
Fu J, Sun F, Liu W, et al. Subconjunctival delivery of dorzolamide-loaded poly(ether-anhydride) microparticles produces sustained lowering of intraocular pressure in rabbits. Mol Pharm. 2016;13(9):2987–95.
Katiyar S, Pandit J, Mondal RS, et al. In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr Polym. 2014;15(102):117–24.
Kouchak M, Malekahmadi M, Bavarsad N, et al. Dorzolamide nanoliposome as a long action ophthalmic delivery system in open angle glaucoma and ocular hypertension patients. Drug Dev Ind Pharm. 2018;44(8):1239–42.
Gudmundsdottir BS, Petursdottir D, Asgrimsdottir GM, et al. γ-Cyclodextrin nanoparticle eye drops with dorzolamide: effect on intraocular pressure in man. J Ocul Pharmacol Ther. 2014;30(1):35–41.
Iester M. Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol. 2008;2(3):517–23.
Kadam RS, Jadhav G, Ogidigben M, et al. Ocular pharmacokinetics of dorzolamide and brinzolamide after single and multiple topical dosing: implications for effects on ocular blood flow. Drug Metab Dispos. 2011;39(9):1529–37.
Silver LH, the Brinzolamide Comfort Study Group. Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution. Results from two multicenter comfort studies. Surv Ophthalmol. 2000;44:141–5.
Tsukamoto H, Noma H, Mukai S, et al. The efficacy and ocular discomfort of substituting brinzolamide for dorzolamide in combination therapy with latanoprost, timolol, and dorzolamide. J Ocul Pharmacol Ther. 2005;21(5):395–9.
Wu W, Li J, Wu L, et al. Ophthalmic delivery of brinzolamide by liquid crystalline nanoparticles: in vitro and in vivo evaluation. AAPS Pharm Sci Tech. 2013;14(3):1063–71.
Tuomela A, Liu P, Puranen J, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1–2):34–41.
Ikuta Y, Aoyagi S, Tanaka Y, et al. Creation of nano eye-drops and effective drug delivery to the interior of the eye. Sci Rep. 2017;14(7):44229.
Mahboobian MM, Seyfoddin A, Rupenthal ID, et al. Formulation development and evaluation of the therapeutic efficacy of brinzolamide containing nanoemulsions. Iran J Pharm Res. 2017;16(3):847–57.
Wang F, Bao X, Fang A, et al. Nanoliposome-encapsulated brinzolamide-hydropropyl-β-cyclodextrin inclusion complex: a potential therapeutic ocular drug-delivery system. Front Pharmacol. 2018;13(9):91.
Salama HA, Ghorab M, Mahmoud AA, et al. PLGA nanoparticles as subconjunctival injection for management of glaucoma. AAPS PharmSciTech. 2017;18(7):2517–28.
Toris CB, Camras CB, Yablonski ME. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999;128(1):8–14.
Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3(2):275–87.
Konstas AG, Stewart WC, Topouzis F, et al. Brimonidine 0.2% given two or three times daily versus timolol maleate 0.5% in primary open-angle glaucoma. Am J Ophthalmol. 2001;131(6):729–33.
Prabhu P, Nitish KR, Koland M, et al. Preparation and evaluation of nano-vesicles of brimonidine tartrate as an ocular drug delivery system. J Young Pharm. 2010;2(4):356–61.
Bhagav P, Upadhyay H, Chandran S. Brimonidine tartrate-Eudragit long-acting nanoparticles: formulation, optimization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2011;12(4):1087–101.
Chiang B, Kim YC, Doty AC, et al. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release. 2016;28(228):48–57.
Ibrahim MM, Abd-Elgawad AH, Soliman OA, et al. Natural bioadhesive biodegradable nanoparticle-based topical ophthalmic formulations for management of glaucoma. Transl Vis Sci Technol. 2015;4(3):12.
El-Salamouni NS, Farid RM, El-Kamel AH, et al. Nanostructured lipid carriers for intraocular brimonidine localisation: development, in vitro and in vivo evaluation. J Microencapsul. 2018;35(1):102–13.
Pek YS, Wu H, Mohamed ST, et al. Long-term subconjunctival delivery of brimonidine tartrate for glaucoma treatment using a microspheres/carrier system. Adv Healthc Mater. 2016;5(21):2823–31.
Bean GW, Camras CB. Commercially available prostaglandin analogs for the reduction of intraocular pressure: similarities and differences. Surv Ophthalmol. 2008;53(Suppl 1):S69–84.
Tanna AP, Lin AB. Medical therapy for glaucoma: what to add after a prostaglandin analogs? Curr Opin Ophthalmol. 2015;26(2):116–20.
Holló G. The side effects of the prostaglandin analogues. Expert Opin Drug Saf. 2007;6(1):45–52.
Wong TT, Novack GD, Natarajan JV, et al. Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug Deliv Transl Res. 2014;4(4):303–9.
Giarmoukakis A, Labiris G, Sideroudi H, et al. Biodegradable nanoparticles for controlled subconjunctival delivery of latanoprost acid: in vitro and in vivo evaluation. Preliminary results. Exp Eye Res. 2013;112:29–36.
Natarajan JV, Ang M, Darwitan A, et al. Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int J Nanomed. 2012;7:123–31.
Cheng YH, Tsai TH, Jhan YY, et al. Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment. Carbohydr Polym. 2016;25(144):390–9.
Fahmy HM, Saad EAES, Sabra NM, et al. Treatment merits of latanoprost/thymoquinone—encapsulated liposome for glaucomatus rabbits. Int J Pharm. 2018;548(1):597–608.
Rodriguez-Aller M, Guinchard S, Guillarme D, et al. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur J Pharm Biopharm. 2015;95(Pt B):203–14.
Franca JR, Foureaux G, Fuscaldi LL, et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: in vitro and in vivo evaluation. PLoS One. 2014;9(4):e95461.
Di Trani N, Jain P, Chua CYX, et al. Nanofluidic microsystem for sustained intraocular delivery of therapeutics. Nanomedicine. 2019;16:1–9.
Lambert WS, Carlson BJ, van der Ende AE, et al. Nanosponge-mediated drug delivery lowers intraocular pressure. Transl Vis Sci Technol. 2015;4(1):1.
Aref AA, Gedde SJ, Budenz DL. Glaucoma drainage implant surgery. Dev Ophthalmol. 2017;59:43–52.
Ayyala RS, Duarte JL, Sahiner N. Glaucoma drainage devices: state of the art. Expert Rev Med Devices. 2006;3(4):509–21.
Chaudhry M, Grover S, Baisakhiya S, et al. Artificial drainage devices for glaucoma surgery: an overview. Nepal J Ophthalmol. 2012;4(2):295–302.
Epstein E. Fibrosing response to aqueous. Its relation to glaucoma. Br J Ophthalmol. 1959;43:641–7.
Hong CH, Arosemena A, Zurakowski D, et al. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005;50(1):48–60.
Ponnusamy T, Yu H, John VT, et al. A novel antiproliferative drug coating for glaucoma drainage devices. J Glaucoma. 2014;23(8):526–34.
Pan T, Brown JD, Ziaie B. An artificial nano-drainage implant (ANDI) for glaucoma treatment. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3174–7.
Harake RS, Ding Y, Brown JD, et al. Design, fabrication, and in vitro testing of an anti-biofouling glaucoma micro-shunt. Ann Biomed Eng. 2015;43(10):2394–405.
Popat KC, Desai TA. Poly(ethylene glycol) interfaces: an approach for enhanced performance of microfluidic systems. Biosens Bioelectron. 2004;19(9):1037–44.
Shokrollahi H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mater Sci Eng C Mater Biol Appl. 2013;33(5):2476–87.
Feynman R. “The Brownian Movement”. 1964. In: The Feynman Lectures of Physics, Volume I. pp. 41–1. http://www.feynmanlectures.caltech.edu/I_41.html. Accessed 7 Dec 2019.
Paschalis EI, Chodosh J, Sperling RA, et al. A novel implantable glaucoma valve using ferrofluid. PLoS One. 2013;8(6):e67404.
Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48:314–46.
Costa VP, Spaeth GL, Eiferman RA, Orengo-Nania S. Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:152–70.
Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005;19(4):CD002897.
The Fluorouracil Filtering Surgery Study Group. Three-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol. 1993;115:82–92.
Hou Z, Wei H, Wang Q, et al. New method to prepare mitomycin C loaded PLA-nanoparticles with high drug entrapment efficiency. Nanoscale Res Lett. 2009;4(7):732–7.
Gomes dos Santos AL, Bochot A, Doyle A, et al. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release. 2006;112(3):369–81.
Desai MA, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society in 2008. Ophthal Surg Lasers Imaging. 2011;42:202–8.
Occhiutto ML, Freitas FR, Lima PP, Maranhão RC, Costa VP. Paclitaxel associated with lipid nanoparticles as a new antiscarring agent in experimental glaucoma surgery. Invest Ophthalmol Vis Sci. 2016;57(3):971–8.
Duan Y, Guan X, Ge J, et al. Cationic nano-copolymers mediated IKKbeta targeting siRNA inhibit the proliferation of human Tenon’s capsule fibroblasts in vitro. Mol Vis. 2008;14:2616–28.
Ye H, Qian Y, Lin M, et al. Cationic nano-copolymers mediated IKKβ targeting siRNA to modulate wound healing in a monkey model of glaucoma filtration surgery. Mol Vis. 2010;26(16):2502–10.
Paula JS, Ribeiro VR, Chahud F, et al. Bevacizumab-loaded polyurethane subconjunctival implants: effects on experimental glaucoma filtration surgery. J Ocul Pharmacol Ther. 2013;29(6):566–73.
Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50(11):5217–25.
Grewal DS, Jain R, Kumar H, Grewal SPS. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study. Ophthalmology. 2008;115(12):2141–5.
Vandewalle E, Abegão Pinto L, Van Bergen T, et al. Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomised study. Br J Ophthalmol. 2014;98(1):73–8.
Han Q, Wang Y, Li X, et al. Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery. J Mater Sci Mater Med. 2015;26(8):225.
Gupta N, Yücel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18(2):110–4.
Brubaker RF. Delayed functional loss in glaucoma. LII Edward Jackson memorial lecture. Am J Ophthalmol. 1996;121:473–83.
Allen SJ, Watson JJ, Shoemark DK, et al. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138(2):155–75.
Frasson M, Picaud S, Léveillard T, et al. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci. 1999;40(11):2724–34.
Checa-Casalengua P, Jiang C, Bravo-Osuna I, et al. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J Control Release. 2011;156(1):92–100.
Ward MS, Khoobehi A, Lavik EB, et al. Neuroprotection of retinal ganglion cells in DBA/2 J mice with GDNF-loaded biodegradable microspheres. J Pharm Sci. 2007;96(3):558–68.
Jiang C, Moore MJ, Zhang X, et al. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007;24(13):1783–92.
García-Caballero C, Prieto-Calvo E, Checa-Casalengua P, et al. Six month delivery of GDNF from PLGA/vitamin E biodegradable microspheres after intravitreal injection in rabbits. Eur J Pharm Sci. 2017;30(103):19–26.
Clatterbuck RE. Cliliary neurotrophic factor prevents retrograde neuronal death in the adult central nervous system. Proc Natl Acad Sci USA. 1993;90:2222–6.
Emerich DF, Winn SR, Hantraye PM, et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature. 1997;386(6623):395–9.
Hagg T, Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci USA. 1993;90(13):6315–9.
Sendtner M, Schmalbruch H, Stockli KA, et al. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992;358(6386):502–4.
Nkansah MK, Tzeng SY, Holdt AM, et al. Poly(lactic-co-glycolic acid) nanospheres and microspheres for short- and long-term delivery of bioactive ciliary neurotrophic factor. Biotechnol Bioeng. 2008;100(5):1010–9.
Pease ME, Zack DJ, Berlinicke C, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50(5):2194–200.
Kitagawa K, Matsumoto M, Tagaya M, et al. Hyperthermia-induced neuronal protection against ischemic injury in gerbils. J Cereb Blood Flow Metab. 1991;11(3):449e52.
Caprioli J, Kitano S, Morgan JE. Hyperthermia and hypoxia increase tolerance of retinal ganglion cells to anoxia and excitotoxicity. Invest Ophthalmol Vis Sci. 1996;37(12):2376e81.
Barbe MF, Tytell M, Gower DJ, et al. Hyperthermia protects against light damage in the rat retina. Science. 1988;241(4874):1817e20.
Yin Y, Henzl MT, Lorber B, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–52.
Jeun M, Jeoung JW, Moon S, et al. Engineered superparamagnetic Mn0.5Zn0.5Fe2O4 nanoparticles as a heat shock protein induction agent for ocular neuroprotection in glaucoma. Biomaterials. 2011;32(2):387–94.
Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–30.
Pasinetti GM. Cyclooxygenase and inflammation in Alzheimer’s disease: experimental approaches and clinical interventions. J Neurosci Res. 1998;54:1–6.
Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–40.
Julien JP. Amyotrophic lateral sclerosis: unfolding the toxicity of the misfolded. Cell. 2001;104:581–91.
Mac Nair CE, Nickells RW. Neuroinflammation in glaucoma and optic nerve damage. Prog Mol Biol Transl Sci. 2015;134:343–63.
Kawano T, Anrather J, Zhou P, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–9.
Kolko M, DeCoster MA, de Turco EB, et al. Synergy by secretory phospholipase A2 and glutamate on inducing cell death and sustained arachidonic acid metabolic changes in primary cortical neuronal cultures. J Biol Chem. 1996;271:32722–8.
Li G, Luna C, Liton PB, et al. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–8.
Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000;20:8693–700.
Ergorul C, Ray A, Huang W, et al. Hypoxia inducible factor1alpha (HIF-1alpha) and some HIF-1 target genes are elevated in experimental glaucoma. J Mol Neurosci. 2010;42:183–91.
Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7.
Dong S, Zeng Q, Mitchell ES, et al. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 2012;7(2):e31211.
Kim DS, Kim JY, Han Y. Curcuminoids in neurodegenerative diseases. Recent Pat CNS Drug Discov. 2012;7(3):184–204.
Belviranlı M, Okudan N, Atalık KE, et al. Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology. 2013;14(2):187–96.
Wang L, Li C, Guo H, et al. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS One. 2011;6(8):e23194.
Yue YK, Mo B, Zhao J, et al. Neuroprotective effect of curcumin against oxidative damage in BV-2 microglia and high intraocular pressure animal model. J Ocul Pharmacol Ther. 2014;30(8):657–64.
Kaminaga Y, Nagatsu A, Akiyama T, et al. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555(2):311–6.
Gupta S, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218.
Davis BM, Pahlitzsch M, Guo L, et al. Topical curcumin nanocarriers are neuroprotective in eye disease. Sci Rep. 2018;8(1):11066.
National Cancer Institute Drug Dictionary. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/ketorolac. Accessed 7 Dec 2019.
Ju WK, Neufeld AH. Cellular localization of cyclooxygenase-1 and cyclooxygenase-2 in the normal mouse, rat, and human retina. J Comp Neurol. 2002;452:392–9.
Wang AG, Lee CM, Wang YC, et al. Up-regulation of cytochrome oxidase in the retina following optic nerve injury. Exp Eye Res. 2002;74:651–9.
Ju WK, Kim KY, Neufeld AH. Increased activity of cyclooxygenase-2 signals early neurodegenerative events in the rat retina following transient ischemia. Exp Eye Res. 2003;77:137–45.
Nadal-Nicolás FM, Rodriguez-Villagra E, Bravo-Osuna I, et al. Ketorolac administration attenuates retinal ganglion cell death after axonal injury. Invest Ophthalmol Vis Sci. 2016;57(3):1183–92.
Barcia E, Herrero-Vanrell R, Díez A, et al. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp Eye Res. 2009;89(2):238–45.
Prow TW. Toxicity of nanomaterials to the eye. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):317–33.
Voigt N, Henrich-Noack P, Kockentiedt S, et al. Toxicity of polymeric nanoparticles in vivo and in vitro. J Nanopart Res. 2014;16(6):2379.
De Matteis V, Rinaldi R. Toxicity assessment in the nanoparticle era. Adv Exp Med Biol. 2018;1048:1–19.
El-Ansary A, Al-Daihan S, Bacha AB, et al. Toxicity of novel nanosized formulations used in medicine. Methods Mol Biol. 2013;1028:47–74.
De Jong WH, Van Der Ven LT, Sleijffers A, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34(33):8333–43.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosures
Marcelo Luís Occhiutto, and Raul Cavalcante Maranhão have nothing to disclose. Vital Paulino Costa has received research funding from Allergan, Alcon, Novartis; honoraria from Allergan, Alcon, Novartis, Aerie, Genom, Ofta, Iridex; and congress expenses from Allergan, Alcon and Novartis. Anastasios-Georgios Konstas has received research funding from Allergan, Bayer and Santen; honoraria from Allergan, Mundipharma and Santen; and congress expenses from Vianex. Anastasios-Georgios Konstas is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10265768.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Occhiutto, M.L., Maranhão, R.C., Costa, V.P. et al. Nanotechnology for Medical and Surgical Glaucoma Therapy—A Review. Adv Ther 37, 155–199 (2020). https://doi.org/10.1007/s12325-019-01163-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01163-6