Abstract
Background
Fluid infusion represents one of the cornerstones of resuscitation therapies in order to increase oxygen delivery during septic shock. Fluid overload as a consequence of excessive fluid administration seems to be linked to worse long-term outcome. However, its immediate effect on patient’s clinical state is poorly described. The goal of this study was to assess the impact of FO on SOFA score kinetics as a surrogate marker of organ dysfunction from day 0 to day 5.
Material and methods
Retrospective, multicenter, investigator-initiated study. All adult patients (> 18 years old) admitted from January 2012 to April 2017 in one of the three ICUs for septic shock, secondary to peritonitis or pulmonary infection and mechanically ventilated, were included. Univariate analysis was performed with Student’s t and chi-square test, for continuous and categorical variables, respectively. A multivariate linear regression model evaluated the impact of FO on delta SOFA score from day 0 to day 5. Secondly, a multivariate mixed-model accounting for repeated measures analyzed the impact of FO on SOFA score kinetics.
Results
One hundred twenty-nine patients met the inclusion criteria and were assigned into FO and no FO groups. FO occurred in 39% of the patients. The difference between SOFA score at day 0 and day 5 was more than twofold higher in the no FO group than in the FO group with a difference of 2.37 between the two groups (4.52 vs. 2.15; p = 0.001). Cumulative fluid intake at day 5 was higher in the FO group (2738 vs. 8715 ml, p < 0.001). In multivariate analysis, FO was associated with delta SOFA score: aRR = 0.15 (95% CI 0.03–0.63; p = 0.009). In mixed model, the regression coefficient for fluid overload status (r2 = 1.16; p = 0.014) indicated that the slope for SOFA score kinetic was less pronounced for patients with FO than for patients without FO.
Conclusions
FO patients had a more prolonged multi-organ failure according to SOFA score kinetics during septic shock from resuscitation phase to day 5.
Similar content being viewed by others
Background
Sepsis is still a major cause of mortality over the world [1, 2]. A recent systematic review reported an annual incidence of 256 hospital-treated sepsis cases per 100,000 person and per year [3]. It represents nearly 10% of intensive care unit (ICU) admissions, with an average ICU mortality of 18% and a 22% in-hospital mortality regardless of the source of infection [4]. Sepsis management-related cost accounted for 5.2% of total US health cost in 2011 [5].
Distinct phases of hemodynamic resuscitation have been described with different risks, goals, and challenges: resuscitation, optimization, stabilization, and de-escalation phases [6]. Fluid therapy represents one of the cornerstones of resuscitation treatments in order to increase oxygen delivery during circulatory failure [7]. During the salvation phase of septic shock, the current guidelines suggest that an aggressive fluid resuscitation is the best initial therapy [8]. During the optimization phase, the goal is to maintain adequate tissue perfusion and avoid the effects of fluid overload. During this phase, “liberal” or uncontrolled fluid therapy can induce an increased positive fluid balance with tissue fluid overload leading to potential harmful effects [9,10,11,12]. A restrictive fluid therapy strategy could be used to decrease fluid overload (FO) during the optimization phase in septic shock patients [13]. It is worth mentioning that inappropriate use of fluid therapy can induce its own side effects.
Therefore, a paradigm shift is currently occurring as concerns have been raised about the potential adverse effects of fluid therapy. Fluid overload is one of the major adverse effects reported and an independent factor of worse outcome in intensive care unit (ICU) patients [10]. It is now suggested that fluid administration should be conducted cautiously to avoid an unnecessary increase in fluid intake but ensure an adequate tissue perfusion. A patient goal-directed therapy may help to optimize fluid intake and avoid the deleterious effects of an increased fluid balance [14].
Most studies on FO or cumulative fluid balance have reported impact on in-hospital or 28-day mortality. To date, there is no data on the impact of FO on organ dysfunction. The aim of this pilot study was to describe the impact of FO on the kinetics of organ dysfunction assessed by the sequential organ failure (SOFA) score between day 0 and day 5 after the onset of septic shock.
Methods
Study design and population
We retrospectively analyzed data from three French ICUs at Brest and Morlaix Hospital (France): two units of a teaching hospital (medical ICU and surgical ICU) and one general ICU of a regional hospital. An approval of an institutional ethics committee was obtained before recruitment (approval number: ADZ/Avis no 2017-1). From institutional registries, we identified all adult patients (> 18 years) admitted from January 2012 to April 2017 in one of the three ICUs for septic shock, secondary to peritonitis or pulmonary infection and mechanically ventilated, were eligible for the study. In our cohort, septic shock was defined according to recommendations published in 2003 by Levy et al. [8]. We considered this definition because the more recent Sepsis-3 definition published in 2016 was not already used in daily practice from 2012 to 2017 the participating ICUs [9]. Thus, in our study, septic shock was defined by a suspected or documented infection, clinical/biological signs of systemic inflammatory response syndrome (SIRS), signs of organ dysfunction, and a persistent hypotension (defined as a systolic arterial pressure < 90 mmHg, mean arterial pressure < 65 mmHg, or a reduction in systolic arterial pressure of more than 40 mmHg from baseline) despite fluid challenge needed vasopressors. Diagnosis criteria of intra-abdominal and pulmonary infection used were the one reported in the most recent international guidelines [15,16,17]:
-
Intra-abdominal infection was suspected when the following criteria were met: digestive symptoms (acute abdominal pain, nausea, vomiting, anorexia), acute abdominal contracture or splinting, diffuse abdominal rigidity, and/or hyperthermia > 38.5 °C or hypothermia < 36 °C. Clinical suspicion was systematically confirmed by abdominal CT scan. Then, diagnostic laparoscopy confirmed peritonitis. Peritoneal fluid/tissue was systematically collected from the site of infection for microbiological analysis to identify pathogens [16]
-
Diagnosis of pulmonary infection was made using the following clinical criteria: presence of respiratory symptoms (cough, sputum, dyspnea, thoracic pain), hyperthermia > 38.5 °C or hypothermia < 36 °C, and signs of infection on chest radiography. Respiratory samples (sputum, endotracheal aspiration, or bronchoalveolar lavage) were performed to confirm diagnosis and identify pathogens
Patients included in our cohort required mechanical ventilation because they had:
-
An acute circulatory failure (defined by systolic arterial pressure < 90 mmHg or mean arterial pressure < 65 mmHg despite fluid challenge and needing of vasopressors > 0.3 μg/kg/min)
-
An alteration of consciousness (defined by a Glasgow Coma Scale < 8)
-
And/or an acute respiratory failure (defined by a respiratory rate > 35/min, SpO2 < 92% despite non-invasive support and signs of dyspnea)
Patients admitted for septic shock without catecholamine and/or mechanical ventilation were excluded. Patients firstly admitted for another reason were also excluded, even if they presented septic shock during their stay in ICU.
Data collection
All data were collected from medical records. For eligible patients, the following data were recorded: age, reason for ICU admission, source of infection, hemodynamic support, and respiratory devices used. For enrolled patients, demographic data, baseline body weight, and comorbidities were collected. We evaluated baseline severity with SOFA score and APACHE II score. Initial hemodynamic status (mean blood pressure, heart rate) and hemodynamic treatment received prior to admission (fluid challenge, amount of each catecholamine received) were also recorded. Relevant biological data were collected, especially data needed to obtain SOFA score and lactatemia.
Daily fluid intake from day 0 to day 10 (including fluid challenge, maintenance fluid, and nutrition), daily fluid output (including urine output, insensible losses, drain fluid, ultrafiltration rate, and estimated gastrointestinal losses), and daily fluid balance from day 1 to day 5 (calculated by subtracting the daily fluid output from daily fluid intake) were collected. Daily body weight was recorded. When the recorded body weight was 10% higher than the baseline one, FO was reported. Duration of FO was also recorded. In case of missing baseline or daily body weight, FO status could not be obtained and was marked as not available. All relevant clinical variables necessary to calculate daily SOFA score were collected. For neurological SOFA sub-score, Glasgow coma scale (GCS) was calculated with results of clinical examination. If daily GCS or neurological information were not available and if a patient was sedated, we considered the last GCS reported in the medical record. For non-survivors at day 5, delta SOFA score was calculated taking into account the last observation. We also recorded daily organ support (RRT, mechanical ventilation, amount of catecholamine), length of ICU stay, and mortality (28-day and 90-day).
Study outcomes
The main pre-specified objective of this study was the impact of FO on SOFA score kinetic from day 0 to day 5 following the onset of septic shock. The onset of septic shock was defined by the time of first antibiotic administration. We chose to evaluate SOFA score kinetics instead of other scores like MODS, SAPS II, and APACHE II because a sequential assessment of organ dysfunction with the SOFA score is robust and has been validated in ICU patients [18]. Several definitions of fluid overload (FO) were used. In some studies, FO was defined by dividing cumulative fluid balance (in liters) by patient’s baseline body weight; a cutoff value of 10% of fluid accumulation was used to define FO [19, 20]. Other studies suggested that a 10% increase in body weight is also clinically relevant [21]. In our study, we identified exposition to FO by dividing daily body weight by baseline body weight. A cutoff value of 10% of weight gain associated with peripheral edema was used to define FO. We hypothesized that FO increases the risk of persistent organ dysfunction during the first 5 days of septic shock.
We also analyzed the impact of patient’s baseline variables on SOFA score kinetics and the impact fluid overload on the following outcomes: 28-day and 90-day mortality, length of stay in ICU, number of ventilator-free days at day 28, and number of catecholamine-free days at day 10.
Statistical analysis
Population description was described as means and standard deviation for continuous variables and percentages for categorical variables. For any variable with less than 20% of data missing, multiple imputation was used with five iterations, except for categorical variables. Bivariate analysis was performed using student t test or Wilcoxon test for continuous variables and chi-square or Fisher’s exact test for categorical variables. Variables with significant bivariate relation (p < 0.1) to FO status were considered for multivariate analysis.
A multivariate linear regression model was performed to test the impact of FO on delta SOFA score from day 0 to day 5. The results were presented with risk ratio (RR), 95% confidence intervals (CI), and p value. Interactions between FO status and all variables were included in the multivariate model if required. Sensitivity analysis was performed: without outliers, without early dead patients (before day 5), and after being discarded to test assumption after bootstrap replication.
A mixed model was used to evaluate the impact of FO and duration of FO (0 to 1 day, 2 to 5 days, or more than 5 days) on SOFA score kinetics. A “subject” random effect was introduced into the model and was regarded as a fixed effect. “Time” variable was introduced into the model and was regarded as a fixed effect. The mixed model was adjusted for variables that were first in multivariate analysis and found significantly associated with delta SOFA score. Results were presented as coefficients and estimated p value (with Satterthwaite approximation method). For multivariate analysis, we considered two-tailed p values of less than 0.05 as significant. All statistical analysis was performed with R statistical software (version 3.3.2).
Results
Study population
From 1 January 2012 to 31 April 2017, 1209 patients were admitted in participating ICUs for severe sepsis or septic shock. During the study period, 275 eligible patients had septic shock with pneumonia or peritonitis. One hundred forty-six patients were excluded, 61 because they were admitted in ICU for another reason and 55 did not require mechanical ventilation. All included patients (129 over 275) were analyzed. Additional file 1: Figure S1 displays the flow of patients in the study.
Overall population characteristics and outcomes
Baseline characteristics of patients are summarized in Table 1. The first source of infection was pneumonia (75.2%), and the mean age was 65 years old. On ICU admission, the mean lactate level was 4.37 mmol/l (SD = 4.19) and leucocyte count was 13.97 G/l (SD = 10.85). The overall 28-day mortality was 34.1%, and the mean ICU length of stay was 17.15 days (SD = 19.06). About 39% of patients were exposed to FO during their stay in ICU. Percentages of FO exposure according to ICUs are presented in Additional file 2: Figure S2. For nine patients (7%), no weight was recorded during their stay and FO status was not available. Fluid intakes in the whole cohort were 2017 ml (SD = 2612), 13,320 ml (SD = 7018), and 24,307 ml (SD = 11,658) at day 0, day 5, and day 10 respectively. Daily fluid balance was 1,604 ml (SD = 2806) at day 1 and progressively decreased to 1263 ml (SD = 2955) at day 3 and 91 ml (SD = 1325) at day 5. The cumulative fluid balance at day 5 was 5041 ml (SD = 6789). All studied patients needed noradrenaline support during their ICU stay, 32 (25%) were treated with dobutamine, and 19 (14.8%) were treated with adrenaline at least 1 day. At day 1, the mean infusion rate was 0.4 μg/kg/min for noradrenaline and 5.6 μg/kg/min for dobutamine. At day 5, 25 and 5 patients needed noradrenaline and dobutamine infusion respectively. Mean catecholamine-free days at day 10 was 4.5 days (SD = 3.17).
Between-group differences according to fluid overload status
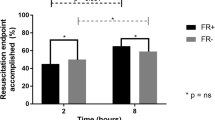
Patient’s characteristics according to fluid overload status are summarized in Table 1. Patients exposed to FO had more cardiovascular comorbidities than the non-exposed patients (59.7% vs. 34.8%, p = 0.014). At baseline, there was no difference in terms of leucocyte count and lactate level between the two groups. Baseline SOFA score (8.60 vs. 9.09, p = 0.39) and APACHE II score (25.3 vs. 25.1, p = 0.88) were not statistically different between the two groups. A number of patients treated with dobutamine (23.3% vs. 25.5%, p = 0.95) and adrenaline (15.1% vs. 12.8%, p = 0.932) were comparable between the two groups. The amount of norepinephrine infused was higher in patients with FO; however, this difference did not reach statistical significance. There was no difference in terms of dobutamine infusion. Fluid intake differences were respectively 1223 ml (p = 0.014), 5709 ml (p < 0.001), and 11,719 ml (p < 0.001) at day 0, day 5, and day 10. Daily fluid balance differences were respectively 954 ml (p = 0.075), 1245 ml (p < 0.001), 1867 ml (p = 0.001), 953 ml (p = 0.002), and 963 ml (p < 0.001) from day 1 to day 5. Cumulative fluid balance at day 5 was also more important in the FO group, with a between-group difference of 5977 ml (2738 versus 8715 ml, p < 0.001). Distribution of cumulative fluid balance and fluid intake at day 5 according to fluid overload status is represented in Fig. 1. There were more transfusions in the FO group (p = 0.007). Baseline measurement of SOFA score was similar in two groups (8.60 versus 9.09, p = 0.390).
Delta SOFA score, fluid intake, and cumulative FB at day 5 according to FO status. Delta SOFA score from day 0 to day 5 was higher in the group of patients without FO compared to the group of patients with FO (mean delta SOFA score: 4.52 (+/− 3.74) vs. 2.15 (+/− 3.50), p < 0.001). Fluid intake at day 5 was more important in the FO group, with a between-group difference of 5709 ml (11,171 ml vs. 16,880 ml, p < 0.001). Cumulative fluid balance at day 5 was more important in the FO group, with a between-group difference of 5977 ml (2738 vs. 8715 ml, p < 0.001)
Concerning clinical outcomes, patients without FO were more rapidly discharged from ICU compared to patients with FO with a between-group difference of 6 days (p < 0.001). The mean duration of mechanical ventilation was less important for patients without FO than FO patients (13.84 days vs. 7.19 days, p = 0.001). The mean duration of RRT was more important in patients without FO (5.21 days versus 3.85, p = 0.019). No between-group difference in catecholamine free-days was found. There was no statistically significant difference between groups in mortality at 28 days and 90 days. Bivariate analysis results are displayed in Table 1.
Daily SOFA score kinetics according to fluid overload status
The difference between SOFA score at day 0 and day 5 (delta SOFA score) was more than twofold higher in the no FO group than in the FO group with a difference of 2.37 between the two groups (p = 0.001). Delta SOFA score was also significantly different between the two groups at day 3 (no FO 2.52 +/− 3.39 vs FO 1.11 +/− 3.47; p = 0.034) and day 4 (no FO 3.74 +/− 3.39 vs FO 1.72 +/− 3.49; p = 0.003). Considering daily measurement of the SOFA score, the main differences occurred from day 3, with respectively a difference of 2.03 (p = 0.002), 2.69 (p < 0.001), and 2.83 (p < 0.001) in day 3, day 4, and day 5. Distribution of delta SOFA score according to fluid overload status is represented in Fig. 1. The mean daily SOFA score from day 0 to day 5 is presented in Additional file 3: Table S1.
We also analyzed the changes in daily SOFA score according to the length of FO. These results are presented in Fig. 2. There was an association between length of fluid overload and daily SOFA score from day 3 to day 5 (p < 0.001). The delta SOFA score was also higher in the group of patients without FO or 1 day of FO compared to the group of patients with 2 or more days of FO (p < 0.001).
Daily SOFA score from day 0 to day 5 according to the length of FO. Linear mixed model was performed to identify any differences at each time between three sub-groups: No fluid overload or 1 day of fluid overload, 2 to 5 days of fluid overload, more than 5 days. Regression coefficient for the length of FO of 2.1 (p = 0.008) for patients with 2 to 5 days of FO and 1.4 (p = 0.012) for patients with more than 5 days of FO. These results indicated that the slope for SOFA score kinetics was less pronounced for patients with more than 2 days of FO compared to patients without FO or 1 day of FO (*p < 0.05)
Impact of fluid overload on SOFA score kinetics
Fixed effect model analysis
A multivariate analysis was used to evaluate the impact of fluid overload on delta SOFA score. The independent variables which were imbalanced between FO and no FO patients were tested in the fixed effect model: FO, age, weight at admission, cardiovascular disease, chronic renal insufficiency, fluid intake at baseline, SOFA score at baseline, heart rate at baseline, and length of hydrocortisone infusion. In the unadjusted analysis, fluid overload status was significantly associated with changes in delta SOFA score (RR = 0.09; 95% CI 0.023–0.38, p = 0.001). The other variables significantly associated with delta SOFA score were age (p = 0.006), preexisting cardiovascular disease (p = 0.011), and SOFA score at baseline (p < 0.001). After adjustment, the association between FO status and delta SOFA score persisted (p = 0.009). Results of multivariate analysis are presented in Table 2. All sensitivity analysis confirmed this result (details are presented in Additional file 4: Table S2, Additional file 5: Table S3).
Linear mixed model with random effect
Variables associated with delta SOFA score in the adjusted analysis were analyzed in the mixed model after dichotomization (baseline SOFA score < or > 8, fluid overload or not, cardiovascular disease or not). The regression coefficient for FO status of 1.16 (p = 0.014) indicated that the slope for SOFA score kinetics was less pronounced for FO patients than for patients without FO whatever the baseline SOFA score was and preexisting cardiovascular comorbidity. The results of the mixed model are presented in Table 3.
Discussion
Our study investigates the association between FO and SOFA score kinetics in septic shock. The main findings of this study are as follows: (1) 40% of our septic shock patients experienced FO; (2) FO patients presented a more prolonged multi-organ failure during septic shock from resuscitation phase to day 5; and (3) the longer the duration of FO, the longer the duration of multi-organ failure.
Fluid therapy and the use of vasopressors are the cornerstones for hemodynamic management from the salvation phase to the de-escalation phase during septic shock [6]. After the resuscitation phase, the optimization phase should be characterized by a cautious titration of fluid administration with a serial reassessment of hemodynamic status [22]. “Liberal” fluid management has been shown to be deleterious for ICU and surgical patients [9, 23, 24]. The SOAP (Sepsis Occurrence in Acute ill Patients) study demonstrated that a positive fluid balance was associated with an increased mortality in patients with acute lung injury and acute renal failure whatever baseline severity and comorbidities were [23, 24]. Even if causality between fluid overload and mortality was not strictly proved, many studies confirmed the statistical association between positive fluid balance and mortality and reinforced this hypothesis [11, 12, 25,26,27,28]. In our study, there was no association between FO and 28-day mortality in the bivariate analysis. There is a trend of an increase of 90-day mortality in FO patients, but this result is not statistically significant. However, our study was not designed to test this hypothesis and therefore lack of statistical power.
To our knowledge, our study is the first report demonstrating FO early influence on multiple organ failure kinetic (measured with daily SOFA score). In our cohort, no therapeutic intervention other than fluid management was different between the two groups. In a prospective cohort, Sakr and colleagues identified an association between higher fluid balance (measured at 72 h) and higher mean/maximum SOFA score; however, the authors did not evaluate dynamic SOFA score evolution [11]. In our cohort, a length of fluid overload beyond 2 days seems to be an important determinant of the SOFA score kinetic as the longer FO duration, the longer lasting organ dysfunction. Serial SOFA score measurement is clinically meaningful and reflects global patient’s deterioration or improvement during the course of sepsis. Such association reinforced the link between FO and morbidity during sepsis. These findings are of interest as designing sepsis trial based on non-fatal outcomes (as short-term organ dysfunction) was recently encouraged by experts position paper [29].
In our study, given the longer ventilator-free days and shorter ICU stay, patients without FO had better short-term outcomes. These results were in line with several studies [30,31,32,33]. In a recent meta-analysis, considering a mixed population of septic and ARDS patients, Silversides et al. demonstrated that patients included in “liberal” fluid management group had higher mortality, length of stay in ICU, and length of mechanical ventilation [34]. The FACCT study tested in ARDS patients two fluid management strategies [30]. This study found a benefit in terms of ventilator free-days and ICU stay for patients included in the “conservative” strategy. On the other hand, compared to patients without FO, length of RRT was more important in the FO group despite a better SOFA score kinetic (p = 0.019). We hypothesized that the use of prolonged dialysis session helped clinicians to avoid FO in maintaining zero or negative net fluid balance. We cannot confirm this hypothesis because we did not collect specifically renal SOFA score and daily diuresis.
To our knowledge, only two pilot studies concluded that a protocolized fluid management can reduce fluid administration without harmful effects during septic shock [31, 32]. In the CLASSIC trial, Hjortrup et al. investigated a fluid restriction protocol compared to the standard care group [31]. Patients included in the interventional group received 250–500 ml of crystalloid boluses in case of severe hypoperfusion. In the standard group, patients received fluid as long as hemodynamic indices improved. Patients included in the fluid “restrictive” group received significantly less fluid during the first 5 days with a mean difference of − 1241 ml. Cumulative fluid balance was also less important: − 1148 ml. There were no differences in the 90-day mortality, but more patients had a new or worsening AKI and ischemic events in the “standard care” group [31]. Chen et al. evaluated a targeted fluid minimization protocol based on passive leg raising. Cumulative fluid balance at day 5 was less important in the interventional group. There were no statistically significant differences in the duration of mechanical ventilation, maximal dose of vasopressor, or in-hospital mortality [32].
Considering the target population of our study, we followed the recommendation underlined by a recent review which criticized the past design of trials focusing on septic patients [29]. In this review, Mebazaa et al. emphasized that designing sepsis trials without considering baseline risk resulted in lower event rates than expected and decreased statistical power. Therefore, we excluded low-risk patients (urosepsis, skin/soft tissue infection) and chose to enroll a homogeneous group of patients with a reported mortality and organ dysfunction consistent for between patients [35].
We acknowledge some limitations of this study. We chose to include septic shock patients according to the sepsis-2 definition because we considered that the more recent Sepsis-3 definition could not be implemented in daily practice in participating ICUs. This is a limitation of our cohort. Then, including only mechanically ventilated patients limits the external validity. Further studies are needed to confirm these results in patients without mechanical ventilation. Therefore, being observational and retrospective, our study results cannot be conclusive in regard to the correlation between FO and SOFA score kinetic. Then, the relatively small number of patients lowers the statistical power of the study. Finally, early death induced missing data in the collection of daily SOFA score. However, we performed a mixed modeling showing the same results, and a sensitivity analysis without missing data was performed. Results were consistent throughout the entire analysis.
Conclusions
This multicenter retrospective study is the first report of SOFA score kinetics determinants during septic shock. Fluid overload seems to be an independent determinant of SOFA score kinetic. Multi-organ dysfunction is more prolonged for patients with FO compare to patients without FO. This is a hypothesis-generating study; therefore, the results need further studies to be confirmed. Well-designed clinical trials aiming to demonstrate the benefit of using a targeted, pragmatic, and individualized hemodynamic management during the optimization phase are still needed to translate evidences into clinical practice.
Availability of data and materials
The dataset supporting the conclusions of this article is fully available. To have an access on it, please contact the corresponding author (O.H.)
Abbreviations
- aRR:
-
Adjusted relative risk
- CI:
-
Confidence interval
- FB:
-
Fluid balance
- FO:
-
Fluid overload
- ICU:
-
Intensive care unit
- RR:
-
Relative risk
- RRT:
-
Renal replacement therapy
- SOFA:
-
Sequential organ failure assessment
References
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:762–74.
Quenot J-P, Binquet C, Kara F, Martinet O, Ganster F, Navellou J-C, et al. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care Lond Engl. 2013;17:R65.
Fleischmann C, Scherag A, Adhikari N, Hartog C, Tsaganos T, Schlattmann P, et al. Global burden of sepsis: a systematic review. Crit Care. 2015;19:P21.
Kaukonen K, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–16.
Torio CM, Andrews RM. National inpatient hospital costs: the most expensive conditions by Payer, 2011: Statistical Brief #160. Healthc Cost Util Proj HCUP Stat Briefs. Rockville: Agency for Healthcare Research and Quality (US); 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK169005/. [cited 2016 Jul 4]
Vincent J-L, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–34.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228.
Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53.
Malbrain MLNG, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46:361–80.
Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, et al. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017;45:386–94.
Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;(5):625–632.
Vincent J-L. “Let’s give some fluid and see what happens” versus the “mini-fluid challenge.”. Anesthesiology. 2011;115:455–6.
Bellamy MC. Wet, dry or something else? Br J Anaesth. 2006;97:755–7.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111.
Sartelli M, Viale P, Catena F, Ansaloni L, Moore E, Malangoni M, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg WJES. 2013;8:3.
Montravers P, Dupont H, Leone M, Constantin J-M, Mertes P-M, Société française d’anesthésie et de réanimation (Sfar), et al. Guidelines for management of intra-abdominal infections. Anaesth Crit Care Pain Med. 2015;34:117–30.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent J-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
Vaara ST, Korhonen A-M, Kaukonen K-M, Nisula S, Inkinen O, Hoppu S, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care Lond Engl. 2012;16:R197.
Mitchell KH, Carlbom D, Caldwell E, Leary PJ, Himmelfarb J, Hough CL. Volume overload: prevalence, risk factors, and functional outcome in survivors of septic shock. Ann Am Thorac Soc. 2015;12:1837–44.
You JW1, Lee SJ, Kim YE, Cho YJ, Jeong YY, Kim HC, Lee JD, Kim JR, Hwang YS. Association between weight change and clinical outcomes in critically ill patients. J Crit Care. 2013;28(6):923–7. https://doi.org/10.1016/j.jcrc.2013.07.055. Epub 2013 Sep 24.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 43:2017, 304–377.
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care Lond Engl. 2008;12:R74.
Sakr Y, Vincent J-L, Reinhart K, Groeneveld J, Michalopoulos A, Sprung CL, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128:3098–108.
Shum HP, Lee FMH, Chan KC, Yan WW. Interaction between fluid balance and disease severity on patient outcome in the critically ill. J Crit Care. 2011;26:613–9.
Rosenberg AL, Dechert RE, Park PK, Bartlett RH, Network NIHNHLBIARDS. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35–46.
Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care Lond Engl. 2013;17:R246.
Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–9.
Mebazaa A, Laterre PF, Russell JA, Bergmann A, Gattinoni L, Gayat E, et al. Designing phase 3 sepsis trials: application of learned experiences from critical care trials in acute heart failure. J Intensive Care. 2016;4:24.
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network1. N Engl J Med. 2006;354:2564–75. https://doi.org/10.1056/NEJMoa062200.
Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettilä V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695–705.
Chen C, Kollef MH. Targeted fluid minimization following initial resuscitation in septic shock: a pilot study. Chest. 2015;148:1462–9.
Richard J-C, Bayle F, Bourdin G, Leray V, Debord S, Delannoy B, et al. Preload dependence indices to titrate volume expansion during septic shock: a randomized controlled trial. Crit Care Lond Engl. 2015;19:5.
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–70.
Jeganathan N, Yau S, Ahuja N, Otu D, Stein B, Fogg L, et al. The characteristics and impact of source of infection on sepsis-related ICU outcomes. J Crit Care. 2017;41:170–6.
Acknowledgements
No acknowledgement
Funding
No grant or funding was received to realize and conduct this study.
Author information
Authors and Affiliations
Contributions
XC and OH were involved equally in the conception of the study, hypothesis generation, and writing and revision of the article before submission. XC was involved in the acquisition of the data. XC, EV, and OH contributed equally to the data analysis. VV, P-YE, and GP contributed equally in the data collection. ZA was involved in the writing of the manuscript before submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An approval of an institutional ethics committee was obtained before recruitment.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Flow chart of the study. (PDF 47 kb)
Additional file 2:
Figure S2. Number of fluid overloaded patients in the three ICUs. (PDF 52 kb)
Additional file 3:
Table S1. Daily SOFA score evolution according to fluid overload status. (DOCX 47 kb)
Additional file 4:
Table S2. Results of multivariate linear regression model with delta SOFA score as outcome and fluid overload as principal independent covariate without early dead patients (n = 109). (DOCX 43 kb)
Additional file 5:
Table S3. Results of multivariate linear regression model with delta SOFA score as outcome and fluid overload as principal independent covariate without influential observations (n = 125). (DOCX 48 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chapalain, X., Vermeersch, V., Egreteau, PY. et al. Association between fluid overload and SOFA score kinetics in septic shock patients: a retrospective multicenter study. j intensive care 7, 42 (2019). https://doi.org/10.1186/s40560-019-0394-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-019-0394-0