Abstract
Introduction
In septic shock, pulse pressure or cardiac output variation during passive leg raising are preload dependence indices reliable at predicting fluid responsiveness. Therefore, they may help to identify those patients who need intravascular volume expansion, while avoiding unnecessary fluid administration in the other patients. However, whether their use improves septic shock prognosis remains unknown. The aim of this study was to assess the clinical benefits of using preload dependence indices to titrate intravascular fluids during septic shock.
Methods
In a single-center randomized controlled trial, 60 septic shock patients were allocated to preload dependence indices-guided (preload dependence group) or central venous pressure-guided (control group) intravascular volume expansion with 30 patients in each group. The primary end point was time to shock resolution, defined by vasopressor weaning.
Results
There was no significant difference in time to shock resolution between groups (median (interquartile range) 2.0 (1.2 to 3.1) versus 2.3 (1.4 to 5.6) days in control and preload dependence groups, respectively). The daily amount of fluids administered for intravascular volume expansion was higher in the control than in the preload dependence group (917 (639 to 1,511) versus 383 (211 to 604) mL, P = 0.01), and the same held true for red cell transfusions (178 (82 to 304) versus 103 (0 to 183) mL, P = 0.04). Physiologic variable values did not change over time between groups, except for plasma lactate (time over group interaction, P <0.01). Mortality was not significantly different between groups (23% in the preload dependence group versus 47% in the control group, P = 0.10). Intravascular volume expansion was lower in the preload dependence group for patients with lower simplified acute physiology score II (SAPS II), and the opposite was found for patients in the upper two SAPS II quartiles. The amount of intravascular volume expansion did not change across the quartiles of severity in the control group, but steadily increased with severity in the preload dependence group.
Conclusions
In patients with septic shock, titrating intravascular volume expansion with preload dependence indices did not change time to shock resolution, but resulted in less daily fluids intake, including red blood cells, without worsening patient outcome.
Trial registration
Clinicaltrials.gov NCT01972828. Registered 11 October 2013.
Similar content being viewed by others
Introduction
Fluid administration is the first-line component of hemodynamic support in septic shock treatment [1]. Preload optimization is a major issue in the treatment of these patients. However, observational studies found a strong association between intensive care unit (ICU) mortality and positive fluid balance [2,3], suggesting that aggressive fluid resuscitation may be harmful. While several hemodynamic algorithms have been evaluated in randomized controlled trials during the first hours of septic shock resuscitation [4-7], the evidence is relatively scarce regarding the practical modalities of fluid administration some hours later. A substantial amount of physiological studies [8,9] have demonstrated that static preload indices (such as central venous pressure (CVP)) may not be reliable to assess fluid responsiveness (cardiac output change in response to fluid administration), especially in septic patients. In contrast, dynamic preload indices such as pulse pressure variation (PPV) or stroke volume change during passive leg raising (PLR) are highly reliable to assess fluid responsiveness [10,11], should validity conditions for PPV accuracy be met. Driving intravascular volume expansion with dynamic preload indices should avoid unnecessary fluid administration, preventing pulmonary side effects, and select fluid-responsive patients. As a result, oxygen delivery should be optimized and organ failure shortened. However, while several studies have demonstrated a beneficial effect of volume expansion driven by preload dependence indices in the perioperative context [12-15], none has been performed in septic shock patients.
We conducted an exploratory randomized controlled study to explore whether preload dependence-driven fluid management in ICU patients with septic shock would reduce the duration of cardiovascular failure, as compared to CVP-driven fluid management.
Materials and methods
Study design
This trial was an open-label, controlled, parallel-group study, with balanced randomization, performed in a 15-bed medical ICU. The trial was registered at ClinicalTrials.gov under the number NCT01972828 and the study protocol was approved by the local ethics committee (Comité de Protection des Personnes Sud-Est III). Patients were enrolled between 1 July 2007 and 30 July 2013. The attending physician was responsible for enrolling the patients in the study, and following the protocol. Written informed consent was obtained from the patients themselves or their closest relatives, prior to their inclusion.
Patients
Eligible participants were adults aged 18 years or older with septic shock [16], who had received intravascular fluid loading of at least 25 mL.kg-1 body weight, with hypotension onset less than 6 hours before inclusion. An amendment to the original protocol extended the time from hypotension onset to 12 hours after inclusion of the first three patients.
Exclusion criteria were pregnancy, acute coronary syndrome, acute cerebral event during the previous 30 days, cardiogenic pulmonary edema, contraindication to central venous or femoral artery catheterization, uncontrolled hemorrhage, burn injury on more than 20% of the body surface, trauma, requirement for immediate surgery or radiological procedure, previous inclusion in present study, inclusion in another randomized controlled trial during the same ICU stay, advance directives to withhold or withdraw life-sustaining treatment, lack of written informed consent by patient or next of kin, lack of affiliation to social security as required by French regulation, patient under a legal protective measure.
Randomization
Patients were randomized into a control group, and a preload dependence group, with a computer-generated list using a 1:1 ratio. The allocation sequence was generated and concealed from the enrolling physician in sequentially numbered, opaque, and sealed envelopes, by a co-author (CG) who did not participate in the assessment of patient eligibility for the study.
Protocol description
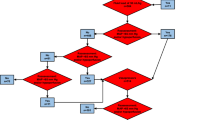
Jugular central venous and femoral arterial lines were connected to the Picco plus device (Pulsion Medical Systems, Munich, Germany) for the first 31 patients, or an Intellivue MP40 monitor equipped with the Picco-Technology module thereafter (Philips Healthcare, Andover, MA, USA). Calibration of cardiac output was performed by intravenous infusion of 15 mL serum saline in triplicate, every hour during the first 6 hours of the study, and every 4 hours thereafter. Hemodynamic treatments were administered according to an hemodynamic algorithm (Figure 1), run at each episode of hypotension (defined by mean arterial pressure (MAP) below 65 mm Hg), every hour during the first 6 hours after inclusion, and every 4 hours thereafter until vasopressor weaning (defined by the absence of vasopressor reintroduction for at least 24 hours). Intravascular volume expansion was performed using CVP in the control group, and using both PPV when applicable (invasive mechanical ventilation (MV) with no spontaneous respiratory movement (SRM), sinus rhythm (SR), tidal volume (VT) greater than 7 ml.kg-1 of predicted body weight (PBW), and absence of evidence for acute cor pulmonale (ACP)), or stroke volume change (ΔSV) during PLR in the preload dependence group. PLR was performed from the supine position, for 1 minute, and preload dependence was deemed present if stroke volume increased by at least 10% during the procedure. Intravascular volume expansion was administered by aliquots of 500 ml over 15 minutes, to achieve a CVP of at least 8 cm H2O in the control group, and a PPV below 13% or a stroke volume increase below 10% during PLR in the preload dependence group. The type of fluid used for intravascular volume expansion was left at the discretion of the attending physician. In both groups, MAP was maintained between 65 and 75 mm Hg using standardized vasopressors management. The same hematocrit (Ht) and cardiac index (CI) targets were used in both groups.
Treatment algorithm. *or hemoglobin <7 g.dL-1; †Ht ≤30% or hemoglobin ≤10 g.dL-1 in the first 6 hours following inclusion; ‡ml.kg-1 of predicted body weight. ACP, acute cor pulmonale; CI, cardiac index; CVP, central venous pressure; Ht, hematocrit; MAP, mean arterial pressure; MV, mechanical ventilation; PLR, passive leg raising test; PPV, pulse pressure variation; RBC, red blood cells; SR, sinus rhythm; SRM, spontaneous respiratory movements; ΔSV, stroke volume variation after fluid administration; VT, tidal volume.
Concomitant treatments were administered according to international guidelines at the time of study design [17] (antibiotherapy within the first hour of recognition of septic shock, source control, low-dose steroids for 7 days, MV in volume assist-control mode with VT 6 to 8 ml.kg-1 PBW and plateau pressure below or equal to 30 cm H2O, positive end-expiratory pressure (PEEP) and inspired oxygen fraction (FiO2) titrated using a PEEP-FIO2 table to achieve 88 to 95% pulse oximetry or 55 to 80 mm Hg partial pressure of arterial oxygen (PaO2) [18]). Sedation was reevaluated every day by the attending physician. Weaning from MV was performed daily by attending nurses, according to local protocol. Glucose control was performed by intravenous (IV) insulin targeting glycemia in the range 6 to 8.3 mmol.L-1. Diuretics use or fluid removal by hemodialysis was performed in case of fluid overload, and not recommended in the first 48 hours of septic shock.
Data collection and follow-up
The following variables were recorded at inclusion: demographic and anthropometric data, time of hospital and ICU admission, context for admission to the ICU, immunodeficiency, Charlson comorbidity score [19], vital signs, Simplified Acute Physiology Score (SAPS) II [20], delay from hypotension onset and inclusion, volume of fluids administered for intravascular volume expansion between hypotension onset and inclusion.
The following variables were recorded at time of inclusion, 6 and 12 hours later and daily during follow-up: Sequential Organ Failure Assessment (SOFA) score [21], vital signs, CVP, CI, extravascular lung water index, arterial lactate, arterial and venous blood gases, standard laboratory tests, need for MV or renal replacement therapy, dose of vasopressor (norepinephrine plus epinephrine) or dobutamine, volume and type of fluid used for volume expansion, red blood cell (RBC) transfusion requirement, fluid balance, PEEP level, FiO2 and VT if applicable, and other relevant therapeutic interventions.
The following variables were recorded at the time of availability: source of infection, results of microbiological culture, adequacy of initial empirical antibiotherapy (defined as an initial antimicrobial regimen with in vitro activity against one or more pathogens that were judged to be responsible for the infection), and time to successful extubation (defined as no reintubation within the 48 hours following extubation).
Hemodynamic data were reported by nurses at each assessment of the hemodynamic algorithm.
Patients were followed up until occurrence of one of the following events: vasopressor weaning and lactate normalization for at least 24 hours, 28 days after inclusion, or in case of patient death.
Study outcomes
The primary outcome was time to shock resolution, defined by vasopressor weaning. Secondary outcomes were the followings: ventilator-free days at day 28, number of days with greater than normal plasma lactate, pulmonary edema (that is extravascular lung water index (ELWI) >10 ml.kg-1 PBW) or organ system failure (that is SOFA ≥6) from inclusion to end of study, ICU length of stay, and mortality at day 28.
Assessment of data quality
All individual data were independently checked for accuracy by the Delegation à la Recherche Clinique des Hospices Civils de Lyon. Quality audits included validity control of the informed consent, compliance to the protocol, validity of data recorded in the case report form compared with the medical charts, validity of outcome assessment and accuracy of serious adverse events reporting. One of the co-authors (JCR) checked the adherence to hemodynamic and associated treatments protocols on a daily basis, and reported protocol violation if any.
Statistical analysis
The expected duration of shock in the control group was 9 ± 2 days [22]. We calculated that with a sample of 60 patients, the study would have an 80% power to detect an absolute reduction in shock duration of 1.5 days, using a two-sided test with a 0.05 type I error. The analysis was performed on an intention-to-treat basis, unless specifically stated. No imputation was performed for missing data. Median and interquartile range were reported for continuous variables, and number of patients in each category and corresponding percentages are given for categorical variables. Data were compared between groups with the chi-square or Fisher’s exact test for categorical variables and t test, ANOVA or Mann-Whitney U test for continuous or ordinal variables if indicated. The bias corrected and accelerated bootstrap method was used to compute confidence intervals for the difference in median times between groups [23]. Repeated physiological measurements over time were compared between groups with a linear mixed model using time as a continuous variable, group and their interaction as variables with fixed effects, and patient as variable with a random effect. The probability for remaining under vasopressor or to survive was analyzed with the Kaplan-Meier method, and compared between groups with the log-rank test. Multivariate analysis was performed using multiple linear regression and a backward selection algorithm. Statistical analyses were performed using R software [24], with packages nlme [25], simpleboot [26], survival [27] and prodlim [28].
Results
During the study inclusion period, 589 patients were admitted with septic shock, and 61 randomized (Figure 2). One patient subsequently withdrew consent, leaving 60 patients for final analysis. No patient was lost to follow-up.
Characteristics at inclusion
Both treatment arms were well balanced at both admission and inclusion (Tables 1 and 2). The total duration of the study (that is application of the hemodynamic algorithm) was 3.4 [2.6 to 4.5] and 3.4 [2.7 to 6.9] days in the control and preload dependence groups, respectively (P = 0.40).
Time course of hemodynamic parameters in both groups
CVP and CI were not significantly different between groups, without significant variation over time (Figure 3). In both groups, MAP significantly increased over time, while superior vena cava oxygenation saturation (ScvO2) and vasopressor dose significantly decreased, without any significant difference between groups (Figure 3). A significant interaction between time and group was found for plasma lactate, indicating that the lactate decline over time was greater in the experimental group than in the control group (Figure 4a). Similar findings were observed for lactate difference from baseline (Figure 4b).
Evolution of hemodynamic parameters over time. Symbols are mean parameter values over time (blue = control group, red = preload dependence group). Bars are standard deviation. CI, cardiac index; CVP, central venous pressure; MAP, mean arterial pressure; NS, non-statistically significant; ScvO2, superior vena cava venous oxygen saturation.
Fluid administration and fluid balance in both groups
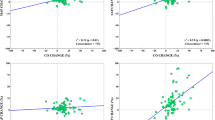
In the preload dependence group, intravascular volume expansion was indicated from PPV and PLR criteria in 4% and 96% of the cases, respectively. The reason for this difference is that those criteria required for PPV use were present in only 9% of the total number of hemodynamic algorithm sessions. The daily amount of fluids administered per protocol for intravascular volume expansion was significantly higher in the control group (917 [639 to 1,511] vs. 383 [211 to 604] mL.day-1, P = 0.01, Table 3). Additional data regarding fluid administration as a function of time from inclusion are provided in Additional file 1. The daily amount of RBC transfusion was significantly lower in the preload dependence group (P = 0.04), while hemoglobin levels were not different between groups (see Additional file 2). There was no significant difference in intake of other fluids, total fluid intake, total fluid output and fluid balance (Table 3). Volume expansion was performed almost exclusively using crystalloids, with the exception of one patient in the control, and two in the preload dependence group who received at least 500 mL of hydroxylethyl starch during the study (P = 1). A multivariate analysis of variables associated with the daily amount of intravascular volume expansion was performed using treatment arm, SAPS II, and the following variables at time of inclusion as predictors: SOFA score, lactates, body weight). SAPS II, treatment arm and their interaction were the only significant variables retained in the final model. As shown on Figure 5a, intravascular volume expansion was lower in the intervention group for patients with lower SAPS II, and the opposite was found for patients pertaining to the upper two SAPS II quartiles. Furthermore, the amount of intravascular volume expansion was unchanged in the control group whatever the quartile of severity (lack of significance of the quartile effect), while a stepwise increase was observed as severity increased in the preload dependence group (as a result of the significant interaction). Similar results were observed using the amount of fluid administered for intravascular volume expansion during the first 12 hours of the study (Figure 5b). This time point was chosen as the last time point with all included patients.
Amount of fluids administered for intravascular volume expansion as a function of treatment arm and SAPS II. (a) Daily amount of fluids. (b) Amount of fluid administered from H0 to H12 after inclusion. In both groups, patients were classified into four categories of severity at inclusion according to quartiles of SAPS II score [20]. Bars are mean values and error bars standard deviation. NS, non-statistically significant; SAPS II, Simplified Acute Physiology Score II.
Associated treatments
Norepinephrine was used as a first-line vasopressor agent in all patients (see Additional file 3). Epinephrine was used as a second-line vasopressor agent for refractory shock, in four (13%) and three (10%) patients in the control and preload dependence groups, respectively (P = 1). Use of other associated treatments for septic shock was similar in both groups.
Outcomes
There was no significant difference in time to shock resolution between groups (Table 4). The difference in median time to shock resolution between preload dependence and control groups was 0.3 days (95% confidence interval -0.8 to 2.1 days).
The probability of remaining under vasopressor until day 28 was not statistically different between groups (Figure 6a). Ventilator-free days tended to be higher in the preload dependence than in the control group (14 vs. 8, P = 0.35). Number of days with plasma lactate values higher than normal, pulmonary edema, or organ system failure and ICU length of stay were similar in both groups. Mortality was reduced by approximately 50% in the preload dependence group (23% vs. 47%), without reaching statistical significance (P = 0.10). Probability of survival until day 28 (Figure 6b) was not statistically significant (P = 0.07) between groups. There was no significant difference in cause of death or end-of-life decisions between groups (see Additional file 4).
Protocol violations
There was no significant difference between treatment arms regarding protocol violations related to intravascular volume expansion (see Additional file 5). The median number of protocol violations per day amounted to 0 in both groups (P = 0.23). Nine (30%) patients in the control and 13 (43%) patients in the preload dependence group were exposed to more than one protocol violation between inclusion and end of study (P = 0.42).
Discussion
This is the first randomized controlled study that investigates in medical ICU patients the effects of a strategy using preload dependence indices on both physiological end points and patient outcome. The main findings of this study are that titration of intravascular volume expansion by using preload dependence indices, as compared to CVP: (1) significantly reduces the daily amount of fluids intake and RBC transfusion, without adverse effect on outcome; (2) results in higher fluid administration in those patients with the most general severity score.
Limitations and strengths
Before discussing present results, some limitations should be acknowledged. First, patients were enrolled relatively late in the course of septic shock. It should, however, be stressed that this may decrease the probability of detecting an effect of the intervention being tested in the present study, while we observed beneficial effects regarding fluid requirement and lactate decrease. Second, expected shock duration was overestimated when computation of sample size was done, while we observed a mean ± standard deviation of shock duration amounting to 3.0 ± 3.8 days in the control group, making the study underpowered to detect significant difference in time to shock resolution. Nonetheless, based on the lower bound of the 95% confidence interval of the difference in median time to shock resolution between groups, a maximal reduction of 0.8 days of shock duration may be anticipated with the use of preload dependence indices, a difference that may not be clinically relevant. Third, one may question that the control group reflects the standard of care, as the surviving sepsis campaign now recommends CVP-guided fluid loading in the early phase of septic shock resuscitation and fluid challenge thereafter [1]. The target CVP level chosen in the present study is another questionable matter. A higher CVP target (that is 12 to 15 mm Hg) has been advocated in patients under mechanical ventilation or with preexisting decreased ventricular compliance [1]. Nevertheless, should this recommendation be applied in the present study, it would have increased the amount of fluids given to the control group, and hence increase the difference with the intervention group, regarding this parameter. Four, PLR was performed from the supine position in the present study, while one study suggested that starting from the semi-recumbent position increased the diagnosis performance of the PLR test [29]. However, a meta-analysis of nine studies did not find any effect of the starting position on the diagnosis accuracy of the PLR test [11]. Five, treatment arm blinding was not possible in the present study, and observer bias was hence uncontrolled, which may have influenced the main judgment criterion. Nevertheless, vasopressor weaning was strictly protocolled, performed by ICU nurses, and none of the investigators was involved in vasopressor tapering. Furthermore, no between-group differences in non-hemodynamic treatment could be documented (see Additional file 3). Six, external validity of the study may be questionable because of the relatively low inclusion to screening ratio (10%). Seven, evaluation of tissue hypoperfusion as an incentive to trigger intravascular volume expansion was not performed, since many clinical or biological variables may be used at the bedside (elevated lactate, low urine output, low ScvO2, tachycardia, mottling, metabolic acidosis, increased capillary refill time, low cardiac output, among others) and explicit implementation of these variables into an hemodynamic protocol to avoid co-intervention bias in a non-blinded study raises issues such as interobserver variability (mottling, capillary filling time), or explicit definition of cutoff values (cardiac rate, pH, cardiac output). Nevertheless, the lack of tissue perfusion evaluation before fluid bolus was applied in both study arms and may not interfere with the effect (or lack of effect) on outcome. Eight, the study was erroneously registered after the inclusion of the last patient, ending up in a theoretical selective outcome reporting bias. Finally, we observed a relatively high mortality in the control group. However, mortality of the present study was in the range of recently published randomized controlled studies on severe sepsis (see Additional file 6), while patient severity, comorbidities and rate of immunosuppressed patients were in the upper range, and exclusion criteria of patients with high expected mortality were less stringent in the present study.
Our study has, however, several strengths. First, the design, with protocolled hemodynamic management and associated treatments for septic shock, made intravascular volume expansion strategies the only distinct intervention between both treatments arms, allowing a direct comparison of the two fluid-loading strategies regarding their effect on patient outcome. Second, each hemodynamic strategy was applied for the whole duration of septic shock, from the early phase to the vasopressor withdrawal, contrary to previous studies that tested hemodynamic interventions applied in the first 6 to 8 hours of management [4-7]. Third, we observed a strong adherence to the hemodynamic protocol during the whole study period. Fourth, the study population was characterized with a high prevalence of bacterial documentation and raised arterial lactate level at inclusion,
Effect of preload dependence-driven intravascular volume expansion on plasma lactate
The beneficial effect on lactate decrease, that is the faster rate of decline observed in the preload dependence group, is in line with previous studies using dynamic preload indices performed in the perioperative setting [12-15]. In contrast to some of the aforementioned studies [12,14,15], but in line with another one [13], the effect on lactate decrease was achieved in the present study with less intravascular fluids administration. However, this effect was identified on post hoc time-dependant analysis, while the predefined criterion (number of days with greater than normal plasma lactate) was not statistically significant. Therefore, these results should be addressed with caution and only viewed as exploratory for future studies.
Effect of preload dependence-driven intravascular volume expansion on fluid administration
An important finding of the present study is that driving intravascular fluid administration with preload dependance indices reduced the daily amount of administered fluids with, at most, no detrimental effect on outcome. The significant decrease in RBC transfusion requirement in the preload dependence arm may be a consequence of the lower amount of fluid administered in this group since hemoglobin levels were not significantly different between groups. Another important finding of this study is that preload dependence-driven fluid resuscitation resulted in a stepwise increase in the amount of administered fluids as patient severity increased (Figure 5), as opposed to CVP-driven resuscitation. This suggests that such strategy may help to tailor fluid administration, by selecting the most severe patients for aggressive fluid resuscitation, and emphasizes the lack of efficacy of CVP-driven resuscitation for that purpose.
Effects on outcomes
The present study did not find any significant effect of the intervention arm on shock duration, or any end point related to outcome. The interaction between patient severity and fluid administration may explain this finding since half of the intervention arm population was exposed to a more restrictive fluid resuscitation with no expected effect on shock duration and an expected beneficial effect on outcome criteria related to volume overload (the less severe patients), while the opposite was true in the more severe patients (Figure 5), making the study underpowered to detect significant differences.
Clinical relevance of PPV
While we originally planned a combined use of PPV and PLR test to trigger fluid administration since many clinical scenarios preclude the use of PPV to test fluid responsiveness [30-33], we observed that PPV validity criteria were marginally present in the study patients, mainly because of the strong adherence to low VT ventilation (see Additional file 3), suggesting that PPV may be of little clinical use in the management of septic shock patients.
Conclusions
To sum up, in patients with septic shock titrating intravascular volume expansion with preload dependence indices did not change time to shock resolution but resulted in less daily fluids intake, including red blood cells, without worsening patient outcome.
Key messages
-
In septic shock patients, titrating intravascular volume expansion with preload dependence indices may have no effect on time to shock resolution.
-
This strategy is, however, associated with a decrease in both the daily amount of intravascular fluids and red blood cell transfusion, with no outcome penalty.
Abbreviations
- ACP:
-
acute cor pulmonale
- CI:
-
cardiac index
- CVP:
-
central venous pressure
- ELWI:
-
extravascular lung water index
- FiO2 :
-
inspired oxygen fraction
- Ht:
-
hematocrit
- ICU:
-
intensive care unit
- IV:
-
intravenous
- MAP:
-
mean arterial pressure
- MV:
-
mechanical ventilation
- NS:
-
non-statistically significant
- PaO2 :
-
partial pressure of arterial oxygen
- PBW:
-
predicted body weight
- PEEP:
-
positive end-expiratory pressure
- PLR:
-
passive leg raising
- PPV:
-
pulse pressure variation
- RBC:
-
red blood cell
- SAPS II:
-
simplified acute physiology score II
- ScvO2 :
-
superior vena cava oxygenation saturation
- SOFA:
-
sequential organ failure assessment
- SR:
-
sinus rhythm
- SRM:
-
spontaneous respiratory movements
- ΔSV:
-
stroke volume variation after fluid administration
- VT :
-
tidal volume
References
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228.
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53.
Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61.
Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–46.
Investigators PCESS, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93.
Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–81.
Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–8.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–7.
Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, De Waure C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475–83.
Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14:R118.
Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111:910–4.
Lopes MR, Oliveira MA, Pereira VOS, Lemos IPB, Auler Jr JOC, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100.
Goepfert MS, Richter HP, Eulenburg CZ, Gruetzmacher J, Rafflenbeul E, Roeher K, et al. Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: a prospective, randomized controlled trial. Anesthesiology. 2013;119:824–36.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Chest. 1992;101:1644–55.
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–73.
The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Jacobs S, Price Evans DA, Tariq M, Al Omar NF. Fluconazole improves survival in septic shock: a randomized double-blind prospective study. Crit Care Med. 2003;31:1938–46.
Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82:171–85.
R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013.
Pinheiro J, Bates D, Debroy S, Sarkar D, The R Development Core Team. Nlme: linear and nonlinear mixed effects models. R package version 3.1-97. 2013.
Peng RD. Simpleboot: simple bootstrap routines. R package version 1.1-3. 2008.
Therneau TM. A package for survival analysis in S. R package version 2.37-7. 2014.
Gerds TA. Prodlim: Product-limit estimation. Kaplan-Meier and Aalen-Johansson method for censored event history (survival) analysis. R package version 1.4.2. 2014.
Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009;35:85–90.
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–8.
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent J-L. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–23.
Heenen S, De Backer D, Vincent JL. How can the response to volume expansion in patients with spontaneous respiratory movements be predicted? Crit Care. 2006;10:R102.
Renner J, Gruenewald M, Quaden R, Hanss R, Meybohm P, Steinfath M, et al. Influence of increased intra-abdominal pressure on fluid responsiveness predicted by pulse pressure variation and stroke volume variation in a porcine model. Crit Care Med. 2009;37:650–8.
Acknowledgments
This study was founded by the following grant: Appel d’offre jeune chercheur 2005 des Hospices Civils de Lyon, Lyon France.
We wish to thank the nurses of our ICU whose help was invaluable to conduct the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JCR made substantial contributions to the study design, acquisition, analysis, and interpretation of data; drafted the manuscript; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. FB made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GB made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. VL made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SD made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. BD made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AS made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. FW made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. HY made substantial contributions to acquisition and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CG made substantial contributions to the study design, analysis, and interpretation of data; revised the manuscript for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final version.
Additional files
Additional file 1:
Fluid administration.
Additional file 2:
Evolution of hemoglobin levels over time.
Additional file 3:
Associated treatments.
Additional file 4:
Causes of death and limitation of treatment.
Additional file 5:
Protocol violations regarding intravascular volume expansion.
Additional file 6:
Severe sepsis randomized controlled trials published since 2010.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Richard, JC., Bayle, F., Bourdin, G. et al. Preload dependence indices to titrate volume expansion during septic shock: a randomized controlled trial. Crit Care 19, 5 (2015). https://doi.org/10.1186/s13054-014-0734-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-014-0734-3