Abstract
Background
Barberry fruit is a good source of natural antioxidants and various functional compounds. Different concentrations of maltodextrin (10, 13, and 16% w/w) were used to produce spray-dried barberry juice powder and the powders (50, 60, and 70%) were applied to create effervescent tablets.
Results
The results showed that by increasing the amount of maltodextrin concentration, moisture, and water activity decreased (p < 0.05), but antioxidant activity increased. The barberry powder prepared with 13% (w/w) maltodextrin showed appropriate flowability, color, high antioxidant activity, and phenol content. The presence of high amounts of barberry powder in the tablet increased the disintegration time (1.02–4.03 min).
Conclusions
The tablet containing 60% barberry powder was selected as the best sample. Based on the results, barberry tablets with good color, high antioxidant activity, and phenolic compounds can be used as a ‘ready-to-drink’ product.

Similar content being viewed by others
Background
Fruits contain health-promoting compounds such as phytochemicals [1]. Barberry or Berberis vulgaris is a plant of Berberidaceae family that grows in parts of Asia and Europe. Iranian seedless barberry fruit (Berberis integerrima ‘Bidaneh’) grows in Khorasan Razavi Province, Iran. Dried barberry fruits are applied as a food additive, and fresh fruits are used in the production of juices, sauces, syrups, jellies, fruit concentrates, carbonated beverages, and jams [2]. Barberry is a unique plant with different functional compounds [3,4,5,6,7,8,9,10,11]. They have a lot of valuable compounds, such as flavonoids, berberine, vitamins, chlorogenic acid, and caffeic acid, which can affect creating a pleasant taste and red color in products [9, 12]. The fruit and roots of barberry contain berberine (isoquinoline alkaloid), which has functional properties [13, 14]. The barberry fruits with these high nutritional compounds can be applied to develop new products.
Fruit powder is used in beverages and as a secondary ingredient in candies, baby foods, and fruit yogurt drinks. Various studies were conducted to develop fruit powder such as sugarcane Juice [15], apple juice concentrate [16], sour cherry juice concentrate [17], and grape syrup [18] powders. Therefore, the production of barberry juice powder can develop the application of barberry fruit juice as a ready-to-drink product. There are several methods for producing fruit powders, among which spray-drying is the easiest and most widespread method used in food industry [19,20,21,22]. Maltodextrin is the most popular polysaccharide as a carrier in the food industry because of its very good water solubility and low cost [23]. Its higher water solubility significantly reduces the apparent viscosity of feed dispersion in favor of the atomization and drying of the liquid feed [24].
However, fruit powder is dehydrated and hygroscopic, so it imposes high costs of storage and transportation. To this end, compressing fruit powder and producing tablets are considered as a unique technique [20, 25]. Tableting stabilizes physicochemical properties and increases the shelf life of the final product. The other benefits of tablet production are the reduction of transportation and storage costs, better appearance, and high consumer acceptance [26].
Consumers usually prefer to dissolve fruit tablets in water and consume them in the form of juice, so fruit tablets can dissolve very quickly are very acceptable. The solubility of fruit powder tablets is poor, so for rapid dissolution of the tablet, disintegrates or effervescent agents are necessary during tableting [27, 28]. Previous studies showed that effervescent tablets were prepared from pineapple [28], barberry fruit pulp [29], pitaya [30], mango [27], guava [20], and watermelon fruits [31].
This investigation is mostly concerned with barberry powder characteristics and tablet dissolution behavior in water. The effect of maltodextrin as wall material on physical and bioactive characteristics of the barberry powders was examined (antioxidant activity, total phenolic content, anthocyanin content, color properties, density, repose angle, sensory properties, powder cohesiveness and compressibility index). Then, different percentages of best-prepared powder were applied to produce barberry effervescent tablets (color, mechanical strength, disintegration time, antioxidant activity, total phenolic content, and anthocyanin content).
Materials and methods
Materials
Fresh barberry was prepared from Ghaen Zereshk Company (Ghaen, Khorasan Razavi, Iran). Reagent 2-diphenyl-1–1-picrylhydrazyl (DPPH) and Folin–Ciocalteu reagent were prepared from Sigma. Sodium carbonate was and gallic acid prepared from Merck Company. Maltodextrin (DE of 18–20) was prepared from Foodchem Company (China).
Preparation of juice and spray-drying
Concentrations of 10%, 13%, and 16% maltodextrin were used to prepare the solution for spray-drying, which were named M10, M13, and M16, respectively. Barberry juice was mixed with maltodextrin powder on a stirrer. This solution was used as the feed for spray-drying (190B model, Buchi Company, Switzerland). The dimensions of the drying chamber were 150 cm in cylindrical height and 80 cm in diameter. The drying process was performed at constant feed speed and the input temperature of 150 °C.
Preparation of barberry effervescent tablets
Production of effervescent tablets from barberry powder was performed at different levels of barberry powder percentages (50, 60, and 70%, which were named BP50%, BP60%, and BP70%, respectively). First, dry barberry granules were produced from barberry powder. In the formulation of effervescent tablets, the active ingredient (barberry powder), sodium bicarbonate, citric acid, tartaric acid, and magnesium stearate were applied. Then, the tablets were compressed using a tablet compressor with an equal force. The effervescent tablets had 25 mm in diameter and 3 mm thickness. After preparing the tablets, various tests, including hardness, disintegration time, total phenolic content, anthocyanin content, and antioxidant activity, were carried out.
Product yield (PY)
The ratio of the dry content of the barberry powder to the dry content of the feeding solution was used to calculate product yield (Eq. 1) [32]:
Encapsulation efficiency (EE)
Encapsulation efficiency was analyzed according to the total anthocyanin content in the barberry juice before and after encapsulation based on Eq. 2 [33]:
Moisture content and water activity
The moisture content of the samples was obtained according to AOAC (1998) standard by drying the samples in an oven (UF55 MEMMERT) at 70 °C until reaching a constant weight.
Water activity was determined using a water activity meter (Novasina ms1-aw, Axair Ltd, Switzerland).
Determination of antioxidant activity, total phenolic, and anthocyanin content
The antioxidant activity of barberry powder was determined by measuring the activity of radical scavenging 2,2-diphenyl-1-picrylhydrazyl [34].
The total phenolic content was measured by the Folin–Ciocalteu method. The absorbance of gallic acid at the wavelength of 750 nm was applied as the standard compound for the calibration curve [35].
The total monomeric anthocyanin concentration was analyzed by the pH differential method, according to Muzaffar et al. (2016). The samples were diluted with two buffer solutions at pH 1 and pH 4.5. The absorbance was analyzed at 520 and 700 nm. Total anthocyanin content was determined based on the molar extinction coefficient of cyanidin-3-glucoside (cyd-3-glu) (26,900 L/cm mol). Results were reported as milligrams of cyd-3-glu/100 g of dry matter [36].
Color
The powder sample images were captured by a scanner (Scanjet G2710 HP, China), and their color parameters were investigated in the color space of L* a* b* by Image J software (Version 1.45 s).
Solubility of powders
One gram of the prepared powder was added to 100 ml of distilled water and stirred on a magnetic stirrer at 600 rpm for 5 min. The dispersed powder was then poured into centrifuge tubes and centrifuged at 4000g for 10 min. The supernatant was dried at 105 °C until it reached a constant weight. The solubility percentage of the powder was calculated from the difference between the weight of the primary and secondary dry matters [37].
Flow behavior properties
Bulk density
Bulk density was defined by measuring the volume of a known mass of powder sample in a graduated glass cylinder. Two grams of the powder was gently poured into a graduated cylinder and shaken slightly to smooth the surface of the powder. Finally, the density of the mass was obtained according to Eq. (3) [38]:
where m indicates the weight of the powder (gr), and V is the volume of sample (ml).
Tapped density
To obtain the tapped density, after determining the bulk density, were repeatedly tapped until powder volume changes in the cylinder stop. Finally, tapped density was calculated as m/V (g.ml) [39].
Angle of repose
Ten grams of the powder was passed through a funnel at a constant height with an outer diameter of 12 mm. The powder was poured on a flat horizontal surface. The angle of repose was calculated from the angle formed by the slope of the mass relative to the base surface [40].
Flowability and cohesiveness
Flowability and cohesiveness of the powders were determined according to the Carr index (CI) and Hausner ratio (HR), respectively. The Carr index and the Hausner ratio were calculated from the bulk density and tapped density as Eqs. 4 and 5, respectively. The Carr index also shows the compressibility of the powder, where higher values of the Carr index represent poor flowability and high compressibility. The Hausner ratio is calculated from the ratio between the tapped and bulk densities of the powder. HR > 1.2 indicates low cohesiveness, HR > 2.4 shows moderate cohesiveness, and powders with HR < 1.4 represents high cohesiveness [41, 42]:
where TD, and BD are tapped density, and bulk density, respectively.
Particle size of the powder
In order to study particle size, Zetasizer made by Malvern (England) was used.
Morphology of microparticles
The morphology of the particles obtained by spray-drying was observed by scanning electron microscopy (SEM). For this purpose, a drop of the particle sample suspension was placed on a carbon film grade, and after drying at ambient temperature, it was imaged using SEM (ZEISS LEO 912 AB, Germany).
Zeta potential
To determine the zeta potential of particles, a zeta sizer (CAD, France) was used. The particles were diluted with deionized water in a constant ratio, injected into the device, and tested at least four times. The preparation of nanoparticles was performed in three replications.
Sensory test
Sensory properties including color, flavor, solubility, agglomeration, and general acceptance of powder were investigated by 20 trained panelists in the format of 9-point Hedonic Scale. The panelists were selected from the age range of 24 to 30 years from graduate students of food science and technology.
Mechanical strength tests
Tablet hardness was measured by a hardness tester (Electrofarmed, Iran).
Tablet disintegration time test
The tablet disintegration time was measured using a dissolution device (Electrofarmed, Iran) in distilled water at 37 °C.
Statistical analysis
The statistical analysis was conducted based on a complete randomized design (SPSS, ver. 11.0) according to a one-way analysis of variance (ANOVA). The significant differences were determined by Duncan’s multiple range test at the 95% confidence level.
Results and discussion
Moisture content and water activity
The stability, flow properties, morphology, and particle size of powders are the major properties affected by moisture content. In general, the moisture content of powders produced by spray-drying is between 2 and 5%, which reduces oxidative decomposition and microbial activity. Less moisture reduces powder adhesion and provides more surface contact with water during regeneration. The moisture content in the samples was between 2.51 to 3.50 (Table 1). The effect of the amount of maltodextrin on the moisture content of the spray-dried barberry powder was significant (p< 0.05). Increasing the amount of maltodextrin decreased moisture content (p < 0.05). Fazaeli et al. reported the reduction of moisture content in black mulberry juice powder with an increase in carrier concentration [43].
The water activity of all samples was between 0.24 and 0.25, which confirmed the powder is stable in terms of microbial activity (water activity < 0.6). The results obtained from the water activity test showed that 10 M had the highest water activity (p < 0.05), and 13 M and 16 M had the lowest water activity (Table 1). It can be stated that increasing the amount of maltodextrin in the coating of barberry juice powder causes a decrease in water activity. Maltodextrin can bind with water and decrease the free water in the system.
Yusof et al. (2012) investigated the compaction and dissolution properties of fruit mixture tablets prepared from guava, and pitaya fruit powders. The moisture content in the powder of pitaya, guava, and their mixture was 5.09%, 5.31%, and 5.56%, respectively [30]. Pushkar et al. (2012) dried watermelon juice using different methods (under sunlight, under vacuum, spraying, and lyophilization) and reported the moisture content of the obtained powder in the range of 1.62–9.26% [31].
Product yield and encapsulation efficiency
The results showed that different maltodextrin concentrations had a significant effect (p˂0.05) on the percentage of microencapsulation efficiency (Table 1). 13 M and 16 M samples had the highest encapsulation efficiencies (89.53 and 89.40%, respectively) and the lowest encapsulation efficiency related to 10 M (p < 0.05). Increasing the concentration of maltodextrin in the wall of barberry juice powder increased the encapsulation efficiency (p < 0.05).
Peng et al. (2013) studied the effect of maltodextrin addition on the product yield of spray-dried purple sweet potato powder. The addition of 30% maltodextrin enhanced the product yield from 23.32 to 39.85%, but further addition showed no significant effect [44]. Different factors can lead to loss of powder during spray-drying such as fine particle loss by the outlet air filter, residue on the dryer walls, and losses because of hand manipulation [45].
Functional properties of barberry powder
According to Table 1, the lowest amount of anthocyanin was obtained in 10 M (49.31 mg /100 g). The highest levels of anthocyanin were observed in the 16 M and 13 M (56.36 and 56.28 mg/ 100 mg, respectively) (p < 0.05). The microencapsulation effect of maltodextrin, which acts as a wall for anthocyanin compounds and protects them against external factors.
The antioxidant capacity and amount of phenolic compounds of barberry juice powder are reported in Table 1. In addition, the results showed that the amount of total phenolic compounds increased by the increment of maltodextrin concentration. The ability of free radical scavenging increased from 69.34% in 10 M sample to 75.46% and 73.99% in 13 M and 16 M samples, respectively. There was no significant difference between the antioxidant activity of 13 M and 16 M samples (p > 0.05).
As shown in Table 1, two samples of 16 M and 13 M barberry juice powder were at a higher level in terms of antioxidant activity, total phenolic content, and anthocyanin compared with the 10-M sample.
Color properties of barberry juice powder
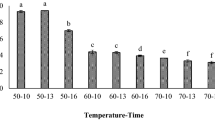
The color of the produced powders determines the color of the reconstituted juice and plays a pivotal role in the acceptance of the product. Color evaluation of the samples showed that the effect of maltodextrin on L* (lightness) was not significant (p > 0.05) (Fig. 1). a* parameter represents the red color in all samples. Figure 1 shows that increasing the amount of maltodextrin from 13 to 16% resulted in lower a* values (p > 0.05). It may be attributed to the improved encapsulation efficiency of anthocyanin due to the increase in maltodextrin concentration. + b* represents the yellow, and -b* shows the blue color of the product. The b* value significantly decreased with increasing the maltodextrin levels from 10 (4.12) to 16% (2.46) (p < 0.05). According to the results obtained from the functional properties of the samples, it seems that the 13 M barberry juice powder has more stability against 10 M and 16 M.
Particle size
The largest particle size was obtained in the 16 M, and the smallest particle size was observed 10 M (Table 2). By increasing the maltodextrin level from 10 to 16%, the particle size significantly improved from 2.33 μm to 4.27 μm value (p < 0.05). It can be caused by the sticking of some particles together and forming a network. These results are consistent with the results obtained in the repose angle (Table 3). The particle size obtained from spray dryers depends on the physical properties, and concentration of the feed input to the system. The larger particle size is produced by increasing the viscosity of the input feed solution. So increasing the concentration of maltodextrin has resulted in higher viscosity values, and therefore, larger particles have been produced.
Seyfollah et al. (2016) evaluated and compared the dissolution profiles of effervescent tablets of natural fruit powders. The particle size values of pitaya, pineapple, guava, mango, stevia, citric acid, sodium carbonate were reported to be 25.072, 38.30, 29.36, 079, 74.25, 285.71, and 421.36 µm, respectively [46]. Yusof et al. (2012) investigated the compaction and dissolution properties of fruit mixture tablets prepared from guava and pitaya fruit powders. The particle size of pitaya, and guava powders, and a mixture of these two fruits were 85.09, 158.10, and 167.11 µm, respectively [30].
Zeta potential
The results showed that the surface electric charge of all barberry juice solutions containing different maltodextrin levels was negative and ranged from − 4.62 to − 13.53 (Table 2). The data show that as the concentration of maltodextrin increases, the negative zeta potential of the product also increases (p < 0.05). In a solution of particles with high zeta potential, the particles will repel each other. As a consequence, the solution will be well dispersed [47].
Flow characteristics of the powders
The Hausner ratio can be used as a measure of the transient from free flow to adhesion of the powders. This makes it possible to predict relatively stable operating and processing points in terms of particle size and relative humidity. The Hausner ratio is measured to determine the compressibility and free flow of the powders. The importance of Hausner index is more related to the properties of handling and transport than the static state of the powder [48].
Powders with Hausner ratio 1.1 to 1.25 have good flowability and particles with Hausner 1.25 to 1.95 have poor flowability and powders with Hausner number 1.25 are considered as moderate. In this study, the cohesiveness value of the produced barberry powders was between 1.14 and 1.23, which indicates the relatively good flow behavior of the samples. Increasing the concentration of maltodextrin did not influence the degree of cohesion significantly. According to the cohesion index results, the powder particles containing maltodextrin have established strong bonds with each other, and a similar cohesion can be observed. These strong and stable bonds will be effective in preserving nutrients such as anthocyanins.
The compressibility index in barberry juice powders ranged from 12.5 to 14.19, and at higher concentrations of maltodextrin as wall material, the compressibility index significantly increased (p < 0.05). Thus, the lowest compressibility and the highest flow properties were observed in 10 M barberry juice powders.
The repose angle of different samples is shown in Table 3. The values are between 25 and 31 degrees, which indicates the good flowability of the produced powders. The repose angle of the barberry juice powder remained constant (25°) by increasing the amount of maltodextrin from 10 to 13% (p > 0.05) while increasing the amount of maltodextrin from 13 to 16%, the repose angle changed significantly from 0.25 to 0.31° (p < 0.05). It indicated the higher flowability of 10 M and 13 M compared to 16 M. Increasing the concentrations of maltodextrin resulted in higher repose angle values, which indicates a decrease in flowability due to the surface adhesion under moisture.
Increasing the carrier concentration improves the flowability of powder by increasing the viscosity and size of the particles, and reducing the contact surface, reactions, and adhesion of adjacent particles [49]. However, this trend was not observed in this study.
Bulk density is a measure of the compaction of particulate solids and it indicates the amount of space between the powder particles, so it depends on the particle density and the arrangement of particles in the powder bed [50, 51]. The results showed that using maltodextrin at levels of 10, 13, and 16% in microencapsulation of barberry juice did not significantly influence the amount of trapped air and consequently the density of the mass (p > 0.05).
The results showed that increasing the maltodextrin concentration led to the reduction of tapped density from 0.39 in 10 M to 0.31 and 0.29 in 13 M, and 16 M, respectively (p < 0.05). Samples containing fine particles significantly showed higher tapped density and less porosity than those without fine particles, and this effect is probably due to the occupation of the pores between the large particles by the fine particles [52]. Zea et al. (2013) examined the compaction and dissolution properties of mixed fruit tablets made from guava and pitaya fruit powder. Tapped densities of pitaya, guava, and a mixture of these two fruits were reported to be 571, 818, and 850 (kg/m3), respectively [20].
Solubility of powder
Powder solubility is an important functional property that affects the behavior of the powder when reconstituted in water. This feature is of particular importance to manufacturers and consumers. The data in Table 3 show that the concentration of the carrier has a significant effect on the solubility of barberry juice powders (p < 0.05). Therefore, the solubility was 92.65, 96.39, and 97.99% for 10 M, 13 M, and 16 M samples, respectively. It might be attributed to the increase in the size and spaces between particles and subsequently the facilitated penetration of moisture into the structure of powders. Fazaeli et al. [43] investigated the effect of spray-drying conditions and the coating compounds on the properties of blackberry extract powder. The results showed a mixture of 2% maltodextrin with 6% dextrose and 6% gum arabic had the highest solubility (about 87%) [43].
Microscopic images
Based on scanned electron microscopic images (Fig. 2), uniform and spherical maltodextrin particles can be detected. These findings are similar to the observations of Manickavasagan et al. [53]. They reported that date powders produced with maltodextrin carriers showed relatively uniform, smooth, spherical particles with large agglomerates [53].
The increment of maltodextrin concentration resulted in larger porosities between the powder particles (Fig. 2). Increasing the carrier concentration, by decreasing the particle adhesion leads to the production of more separated particles. As the amount of maltodextrin increased, more porosity and shrinkage were observed on the surface of powder particles.
By increasing the carrier concentration due to preventing the rapid exit of moisture from the particles during drying, an increase in shrinkage was created in the particles. At the level of 16% maltodextrin, a broad particle size distribution of particles with non-uniform shapes (smooth, wrinkled, spherical, or polygonal surfaces) can be observed. Viscosity and viscoelastic behavior of wall materials also affect the surface structure and morphology of spray-dried particles. High concentrations of maltodextrin increased the surface shrinkage of particles. It is probably due to the difficult dispersion of water molecules among the larger molecules of maltodextrin.
Barberry effervescent tablets
The spray-dried barberry powder prepared with 13% (w/w) maltodextrin showed appropriate flowability, good color, high antioxidant activity, and total phenolic content. Therefore, 13 M was selected for the preparation of barberry effervescent tablets. Different percentages (50, 60, and 70%) of 13 M barberry powder were used to prepare effervescent tablets.
Physico-chemical properties of barberry effervescent tablets
The resistance of the tablet to breakage in storage, transport, and transfer conditions before use depends on its hardness. The tablet hardness is one of the effective and important factors in the process of its disintegration, and it can be considered as a reflection of the density of the tablet ingredients [48]. The hardness of barberry effervescent tablets was 45–39 N (Table 4). In BP50% and BP60% barberry juice powder, the hardness was 40 and 39 N, respectively, which were not significantly different from each other. By increasing the amount of barberry juice powder to 70%, the hardness of the tablet significantly increased (45 N).
The wetting or water absorption ability of capsules is one of the most important physical properties related to the regenerative properties of tablets, which is directly affected by molecular reactions between solid and liquid phases. In this study, the disintegration time varied between 1.02 and 4.03 min. These values indicate the fast integration process of tablets at lower levels of barberry juice powder in tablet formulation. In other words, the higher degree of amorphous levels increases the solubility of the powder in water. By increasing the amount of barberry juice powder from 60 to 70%, the disintegration time of the tablet increased from 2.66 to 4.03 min. These results were consistent with the hardness of the tablet (Table 4). Saifullah et al. [46] evaluated and compared the dissolution profiles of effervescent tablets of natural fruit powder. They reported the dissolution time of pita, pineapple, guava, mango, stevia, citric acid, sodium carbonate in water and saliva in the range of (2–10) and (4–12) minutes, respectively [46].
As expected, the total phenolic content and anthocyanin of barberry tablets increased significantly by the increment of barberry juice powder in the tablets (p < 0.05). The highest total phenolic content and anthocyanin were observed in BP70%, which were 70.66 (mg GAE/100 g) and 30.32 (mg/100 g), respectively. The antioxidant activity also increased noticeably by the increment of barberry juice powder from 50 to 70%. This contributes to its higher total phenolic and anthocyanin content as they are considered as the most active antioxidant compounds. From these results can be concluded that barberry juice effervescent tablets could be considered good antioxidant products.
Color parameters of barberry effervescent tablets
Table 5 shows with increasing the amount of barberry water powder used at the level of 70% compared to the level of 50%, the L* value of effervescent tablets significantly increased (p < 0.05). The BP50% effervescent tablet with its predominant pink color had the lowest value of a* (+ 36.55). The addition of reddish-pink powder of barberry juice has made significant changes in a* color parameter of the effervescent tablets, so the red color has increased. In BP60% and BP70% tablets, the value of a* parameter were 38.44 and 40.87, respectively. In other words, with increasing the concentration of barberry powder at 70% level compared to 50% level, the red color of the drink (a*) has significantly increased (p < 0.05). The addition of barberry juice powder in effervescent tablets did not significantly change the b*color parameter. As can be seen in Table 5, the values of b* in BP60% and BP70% samples were 4.11 and 4.49, respectively, which did not show any significant difference with BP50% sample (3.08) (p < 0.05).
Chromatic features of barberry drink
L* value did not change significantly with increasing the amount of barberry juice powder in the effervescent tablet in the drink (p < 0.05) (Table 6). The BP50% had the lowest a* value (+ 41.4). It is obvious that with the addition of reddish-pink powder of barberry juice, significant changes in a* color parameter of the drink were observed. In drinks containing BP60% and BP70% samples, the value of a* parameter was 7.67 and 11.70, respectively. In other words, with increasing the concentration of barberry powder at the levels of 60 and 70% compared to BP50%, the red color of the drink significantly increased. At higher levels of reddish-pink barberry juice powder in the drink, b* parameter significantly changed. As can be seen in Table 6, the value of b* in the BP50% (2.91) was significantly different from the other samples (p < 0.05). The b* parameter in the drink increased by the increment of the effective material (barberry powder) in the tablet.
Sensory characteristics of barberry drink
The sensory characteristics of barberry drinks are reported in Table 7. In the evaluation of aroma score, the BP70% did not significantly differ from BP60% and scored 5.39 against 4.91 points, but had significantly more odor than the BP50% sample. The taste of different samples did not differ significantly and varied between 3.15 and 5.74. In evaluating of color score, the BP70% sample was not considerably different from the sample of BP60%, and scored 6.42 and 5.98 points, respectively, but the aroma was significantly higher in the sample of BP70%. There was no significant difference between the time of disintegration of BP60% and BP70% tablets, and scored 3.10 and 4.78 points, respectively. BP50% sample with the most integration time scored 5.62. According to the results of sensory tests, BP60% and BP70% tablets revealed the highest acceptance score (p < 0.05).
Conclusion
The results of producing barberry juice powder by spray-drying using different percentages of maltodextrin showed that by increasing the amount of wall (maltodextrin), moisture, and water activity decreased, and better preservation of phenolic, antioxidant, and anthocyanin compounds occurred. But the red color of the powder decreased with the increasing percentage of maltodextrin. The production and measurement efficiency of barberry juice in water with concentrations of maltodextrin showed a significant influence on encapsulation efficiency. Barberry effervescent tablets with a diameter of 25 mm and a thickness of 3 mm with a hardness of 39–45 N were obtained. The time required for the tablet particles to disintegrate varied between 1.02 and 4.03 min. With increasing the concentration of barberry juice powder from 50 to 70%, the red color of the drink (value of parameter a*) increased significant. According to the results of sensory tests, BP60% tablet obtained the highest overall acceptance point and the appropriate disintegration time. According to the results, BP60% tablet can be a good candidate as a ‘ready-to-drink’ product with respect to high antioxidant activity and overall acceptance score to term the tablet.
Availability of data and materials
The datasets used and, or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Carr index
- EE:
-
Encapsulation efficiency
- HR:
-
Hausner ratio
- PY:
-
Product yield
- SEM:
-
Scanning electron microscopy
References
Sakooei-Vayghan R, Peighambardoust SH, Hesari J, Soltanzadeh M, Peressini D. Properties of dried apricots pretreated by ultrasound-assisted osmotic dehydration and application of active coatings. Food Technol Biotechnol. 2020;58(3):249–59.
Alemardan A, Asadi W, Rezaei M, Tabrizi L, Mohammadi S. Cultivation of Iranian seedless barberry (Berberis integerrima ‘Bidaneh’): A medicinal shrub. Ind Crops Prod. 2013;50:276–87.
Sarraf M, Beig Babaei A, Naji‐Tabasi S. Investigating functional properties of barberry species: an overview. J Science Food Agriculture. 2019. 99(12):5255-69
Kosalec I, Gregurek B, Kremer D, Zovko M, Sanković K, Karlović K. Croatian barberry (Berberis croatica Horvat): a new source of berberine—analysis and antimicrobial activity. World J Microbiol Biotechnol. 2009;25(1):145–50.
Aliakbarlu J.; Ghiasi S.; Bazargani-Gilani B. Effect of extraction conditions on antioxidant activity of barberry (Berberis vulgaris L.) fruit extracts. in Veterinary Research Forum. Faculty of Veterinary Medicine. Urmia, Iran: Urmia University; 2018.
Hassanpour H, Alizadeh S. Evaluation of phenolic compound, antioxidant activities and antioxidant enzymes of barberry genotypes in Iran. Sci Hortic. 2016;200:125–30.
Hemmati M, Zohoori E, Mehrpour O, Karamian M, Asghari S, Zarban A, Nasouti R. Anti-atherogenic potential of jujube, saffron and barberry: anti-diabetic and antioxidant actions. EXCLI J. 2015;14:908.
Ardestani SB, Sahari MA, Barzegar M, Abbasi S. Some physicochemical properties of Iranian native barberry fruits (abi and poloei): Berberis integerrima and Berberis vulgaris. J Food Pharmac Sci. 2013;1(3):60–7.
Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22(8):999–1012.
Mortazavi SA, Sharifi A, Maskooki A, Niakousari M, Elhamirad AH. Optimisation of bioactive compounds extraction from barberry fruit (Berberis vulgaris) using response surface methodology. Res Innovation Food Sci Technol. 2014;3(1):24–11.
Sarraf M, Beig-babaei A, Naji-Tabasi S. Optimizing extraction of berberine and antioxidant compounds from barberry by maceration and pulsed electric field-assisted methods. J Berry Res. 2020. (Preprint): p. 1–16.
Zarei A, Changizi-Ashtiyani S, Taheri S, Ramezani M. A quick overview on some aspects of endocrinological and therapeutic effects of Berberis vulgaris L. Avicenna J Phytomed. 2015;5(6):485.
Safari Z, Farrokhzad A, Ghavami A, Fadel A, Hadi A, Rafiee S, Mokari-Yamchi A, Askari G. The effect of barberry (Berberis vulgaris L.) on glycemic indices: A systematic review and meta-analysis of randomized controlled trials. Complementary Therapies Med. 2020: p. 102414.
Khosroukhavar R, Ahmadiani A, Shamsa F. Antihistaminic and anticholinergic activity of methanolic extract of barberry fruit (Berberis vulgaris) in the guinea-pig ileum. 2010;9(35):99–105.
Largo Avila E, Cortes Rodríguez M, Ciro Velásquez HJ. Influence of maltodextrin and spray drying process conditions on sugarcane juice powder quality. Revista Facultad Nacional de Agronomía Medellín. 2015;68(1):7509–20.
Sarabandi K, Peighambardoust S, Mahoonak AS, Samaei S. Effect of different carriers on microstructure and physical characteristics of spray dried apple juice concentrate. J Food Sci Technol. 2018;55(8):3098–109.
Sarabandi K, Peighambardoust SH, Mahoonak AS, Samaei SP. Effect of carrier types and compositions on the production yield, microstructure and physical characteristics of spray dried sour cherry juice concentrate. J Food Measurement Characterization. 2017;11(4):1602–12.
Sarabandi K, Peighambardoust SH, Shirmohammadi M. Physical properties of spray dried grape syrup as affected by drying temperature and drying aids. Int J Agriculture Crop Sci. 2014;7(12):928.
Fernandes FA, Rodrigues S, Law CL, Mujumdar AS. Drying of exotic tropical fruits: a comprehensive review. Food Bioprocess Technol. 2011;4(2):163–85.
Zea LP, Yusof YA, Aziz MG, Ling CN, Amin NAM. Compressibility and dissolution characteristics of mixed fruit tablets made from guava and pitaya fruit powders. Powder Technol. 2013;247:112–9.
Peighambardoust S, Tafti AG, Hesari J. Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food Sci Technol. 2011;22(5):215–24.
Tafti AG, Peighardoust S, Behnam F, Bahrami A, Aghagholizadeh R, Ghamari M, Rafat SA. Effects of spray-dried sourdough on flour characteristics and rheological properties of dough. Czech J Food Sci. 2013;31(4):361–7.
Du J, Ge Z-Z, Xu Z, Zou B, Zhang Y, Li C-M. Comparison of the efficiency of five different drying carriers on the spray drying of persimmon pulp powders. Drying Technol. 2014;32(10):1157–66.
Vidović SS, Vladić JZ, Vaštag ŽG, Zeković ZP, Popović LM. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014;258:209–15.
Saifullah M, Yusof Y, Chin N, Aziz M, Mohammed M, Aziz N. Tableting and dissolution characteristics of mixed fruit powder. Agriculture Agricultural Sci Procedia. 2014;2:18–25.
Yousefi S, Emam-Djomeh Z, Mousavi S. Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica granatum L.). J Food Science Technol. 2011;48(6):677–84.
Ong M, Yusof Y, Aziz M, Chin N, Amin NM. Characterisation of fast dispersible fruit tablets made from green and ripe mango fruit powders. J Food Eng. 2014;125:17–23.
Saifullah M, Yusof Y, Chin NL, Aziz M, Mohammed M, Aziz N. Dissolution profiling and its comparison of natural fruit powder effervescent tablets. J Food Eng. 2016;178:60–70.
Naji-Tabasi S, Emadzadeh B, Shahidi-Noghabi M, Abbaspour MR, Akbari E. Investigation of physicomechanical and nutritional properties of powder and compressed tablets based on barberry fruit pulp. J Food Measurement Characterization. In press, 2020.
Yusof Y, Mohd Salleh F, Chin N, Talib R. The drying and tabletting of pitaya powder. J Food Process Eng. 2012;35(5):763–71.
Pushkar S, Sachan NK, Ghosh S. Pharmacotechnical Assessment of Processed Watermelon Flesh as Novel Tablet Disintegrant. In: Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives. Springer; 2012. p. 159–63.
Elez Garofulić I, Zorić Z, Pedisić S, Dragović-Uzelac V. Optimization of sour cherry juice spray drying as affected by carrier material and temperature. Food Technol Biotechnol. 2016;54(4):441–9.
Murali S, Kar A, Mohapatra D, Kalia P. Encapsulation of black carrot juice using spray and freeze drying. Food Sci Technol Int. 2015;21(8):604–12.
Khalafi R, Goli SAH, Behjatian M. Characterization and classification of several monofloral Iranian honeys based on physicochemical properties and antioxidant activity. Int J Food Prop. 2016;19(5):1065–79.
Sharifi F, Poorakbar L. The survey of antioxidant properties of phenolic compounds in fresh and dry hybrid Barberry fruits (Berberis integerrima× vulgaris). Cumhuriyet Sci J. 2015;36(3):1609–17.
Muzaffar K, Dinkarrao BV, Kumar P. Optimization of spray drying conditions for production of quality pomegranate juice powder. Cogent Food Agriculture. 2016;2(1):1127583.
Caparino O, Tang J, Nindo C, Sablani S, Powers J, Fellman J. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. J Food Engineering. 2012;111(1):135–48.
Mahdavi SA, Jafari SM, Assadpoor E, Dehnad D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int J Biol Macromol. 2016;85:379–85.
Goula AM, Adamopoulos KG. Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: II Powder properties. Drying Technol. 2008;26(6):726–37.
Bhandari BR, Datta N, D’Arcy BR, Rintoul GB. Co-crystallization of honey with sucrose. LWT-Food Science and Technology. 1998;31(2):138–42.
Carr RL. Evaluating flow properties of solids. Chem Eng. 1965;18:163–8.
Hausner HH. Friction conditions in a mass of metal powder. Los Angeles: Polytechnic Inst. of Brooklyn. Univ. of California; 1967.
Fazaeli M, Emam-Djomeh Z, Ashtari AK, Omid M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod Process. 2012;90(4):667–75.
Peng Z, Li J, Guan Y, Zhao G. Effect of carriers on physicochemical properties, antioxidant activities and biological components of spray-dried purple sweet potato flours. LWT-Food Sci Technol. 2013;51(1):348–55.
Fang Z, Bhandari B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011;129(3):1139–47.
Saifullah M, Yusof Y, Chin N, Aziz M. Physicochemical and flow properties of fruit powder and their effect on the dissolution of fast dissolving fruit powder tablets. Powder Technol. 2016;301:396–404.
Rydström C. Nanoparticles in Food-with a focus on the toxicity of titanium dioxide. Uppsala: Uppsala Universitet; 2012.
Patel SG, Siddaiah M. Formulation and evaluation of effervescent tablets: a review. J Drug Delivery Therapeutics. 2018;8(6):296–303.
Goula AM, Adamopoulos KG. A new technique for spray drying orange juice concentrate. Innov Food Sci Emerg Technol. 2010;11(2):342–51.
Phisut N. Spray drying technique of fruit juice powder: some factors influencing the properties of product. Int Food Res J. 2012. 19(4):1297.
Tafti AG, Peighambardoust SH, Hesari J, Bahrami A, Bonab ES. Physico-chemical and functional properties of spray-dried sourdough in breadmaking. Food Sci Technol Int. 2013;19(3):271–8.
de Barros Fernandes RV, Borges SV, Botrel DA. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohyd Polym. 2014;101:524–32.
Manickavasagan A, Thangavel K, Dev SR, Delfiya DA, Nambi E, Orsat V, Raghavan G. Physicochemical characteristics of date powder produced in a pilot-scale spray dryer. Drying Technol. 2015;33(9):1114–23.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
It is not applicable.
Consent for publication
The authors confirm that the content of the manuscript has not been submitted or published elsewhere.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Naji-Tabasi, S., Emadzadeh, B., Shahidi-Noghabi, M. et al. Physico-chemical and antioxidant properties of barberry juice powder and its effervescent tablets. Chem. Biol. Technol. Agric. 8, 23 (2021). https://doi.org/10.1186/s40538-021-00220-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-021-00220-z