Abstract
Vascular cognitive impairment (VCI) describes a wide spectrum of cognitive deficits related to cerebrovascular diseases. Although the loss of blood flow to cortical regions critically involved in cognitive processes must feature as the main driver of VCI, the underlying mechanisms and interactions with related disease processes remain to be fully elucidated. Recent clinical studies of cerebral blood flow measurements have supported the role of chronic cerebral hypoperfusion (CCH) as a major driver of the vascular pathology and clinical manifestations of VCI. Here we review the pathophysiological mechanisms as well as neuropathological changes of CCH. Potential interventional strategies for VCI are also reviewed. A deeper understanding of how CCH can lead to accumulation of VCI-associated pathology could potentially pave the way for early detection and development of disease-modifying therapies, thus allowing preventive interventions instead of symptomatic treatments.
Similar content being viewed by others
Introduction
A key missing piece in dementia research is the elucidation of the neurovascular basis of cognitive impairment [230, 258, 308]. The term vascular cognitive impairment (VCI) is used to describe a wide spectrum of conditions characterized by cerebrovascular disease ranging from subjective cognitive decline to vascular dementia (VaD) (Fig. 1). While VaD remains the second most common type of dementia worldwide after Alzheimer’s disease (AD), its prevalence may be underestimated—especially in populations with significant concomitant small vessel disease burden, such as those in Asia [42, 48, 49]. Vascular factors may also exacerbate the pathology of AD [221], giving rise to the argument that vascular pathology could even be the most common contributor to dementia in elderly populations [222]. Moreover, VaD is associated with a high mortality rate and rapid stepwise disease progression (Fig. 1) [4, 5, 116]. Therefore, the identification of interventions to potentially benefit VCI patients and reduce its socioeconomic burden is of critical importance.
Stepwise progression to VaD. The road from risk factors to disease manifestation in VCI is a complicated one, as the multiple demographics, lifestyle and comorbid disease risk and mitigating factors interact through the progression from asymptomatic vascular lesions, cognitive impairment, and finally to VaD. Furthermore, these complex interactions give rise to several distinct cerebrovascular diseases underlying different forms of vascular brain injuries, leading to the clinical heterogeneity of VaD. However, regardless of the specific nature of vascular injury (occlusive, thrombotic, etc.), a state of chronic cerebral hypoperfusion can be considered to be the common etiological link. *See Table 1 for details and summary of supporting research
The pathophysiology and clinical characteristics of VCI have been extensively reviewed [99, 281, 283, 304]. Briefly, VCI is characterized by brain lesions that occur due to vascular pathology which leads to diverse cognitive impairments. These lesions result in ischemic, hemorrhagic, and hypoperfusive states that can manifest as various clinical symptoms. Such vascular pathophysiological states in turn lead to a range of downstream effects and structural changes on the brain, including infarcts, lacunes, microbleeds, white matter injury and parenchymal lesions [108, 139, 140, 147, 228, 247]. VCI thus exists as a heterogeneous group of diseases which can be divided into various subtypes, such as multi-infarct dementia, post-stroke dementia and subcortical ischemic vascular dementia, each having unique features that may manifest clinically as dementia over time. While these disease subtypes provide an avenue to categorize the differing disease etiologies, they have a common set of risk factors, including demographic factors, lifestyle factors or presence of co-morbid conditions (summarized in Table 1). Each of these risk factors is known to independently contribute to the progression of cerebrovascular disease and is therefore associated with cognitive dysfunction and impact the progression to dementia. While there are several treatment options to manage VCI and their underlying risk factors as highlighted in Table 2, the root causes of the problem remain unclear. Hence, there remains several knowledge gaps in the understanding of VCI pathophysiology. Although several mechanisms have been reported to have roles in VCI progression, including neuroinflammation, oxidative stress-induced brain damage, neurodegeneration and brain atrophy [254], we still lack a thorough understanding of this complex disease, and even the aforementioned mechanisms have not been fully elucidated. Furthermore, the clinical signs and symptoms of VCI vary between patients given the heterogeneity of the severity and site of injury. Hence, a consensus on the underlying causes of VCI needs to be reached.
Chronic cerebral hypoperfusion (CCH) refers to chronically inadequate brain perfusion [56]. Given that CCH is intimately associated with various risk factors, pathophysiological processes and pathological lesions known to be involved in VCI (Fig. 1), we propose that CCH is the central underlying cause for the progression of VCI. We emphasize CCH as the underlying cause as it ties together some of the known mechanisms of VCI such as chronic inflammation, oxidative stress, neurodegeneration and brain atrophy. Age-related vascular changes lead to a state of global CCH and induce pathophysiological changes such as blood–brain barrier dysfunction, resulting in increased vulnerability to disease even in the absence of risk factors [278]. Recently, CCH was identified as the common feature observed in multiple subtypes of VCI [74, 283]. Furthermore, it was reported that global cerebral blood flow was significantly lower in VaD than in age-matched controls [227, 231] but significantly higher than in AD [227]. The reductions in cerebral blood flow is one of the earliest features observed from early VCI to VaD [130, 145, 224], and is consistently observed in different brain regions, such as a reported 31% decrease in cerebral blood flow in the frontal cortex and a 39% decrease in the parietal cortex [234]. Compromised cerebral blood flow in the deep white matter of the brain is also associated with hemodynamic ischemic injury, and therefore leads to a higher volume of white matter lesions (WMLs) [18, 286]. Moreover, cerebral hypoperfusion was shown to be a good predictor for WMLs in VCI patients [176, 198]. Given the above evidence, we postulate that CCH is a common driver of VCI pathologies such as WMLs, lacunes, infarcts, and subsequent cognitive impairment.
CCH is strongly associated with stepwise cognitive decline in VCI, where much of what is known about its clinical manifestation along the spectrum from normal to end-stage VCI comes from multiple longitudinal studies involving recruited subjects [128, 135, 200, 224, 268, 275, 286]. Neuropathological evidence, neuropsychological assessments and imaging are important adjuncts in many of these studies to ensure accurate study recruitment. In this review, we explore the concept that CCH is the main mediator of VCI pathology and cognitive impairment. We highlight the links between mechanisms and the development of structural neuropathological changes during VCI and provide a landscape of how each of these changes lead to the development of cognitive impairment in patients. Understanding how CCH drives VCI progression from pre-clinical to severe dementia is essential for both researchers and clinicians in diagnosing and developing novel therapeutics for early intervention.
Pathophysiology of chronic cerebral hypoperfusion (CCH)
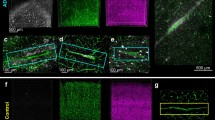
In VCI, isolated instances of vascular injury may accumulate into widespread damage that overcome intrinsic repair mechanisms in areas critical for cognitive functions. There are several possible mechanisms that govern the transition from a physiological to a pathological state in the brain during cerebral vascular disease. In order to understand how this transition occurs, animal studies employing CCH are commonly used to model the underlying pathology of VCI. Mouse CCH models are generally generated by manipulating the common carotid artery, for instance, via bilateral common carotid artery stenosis [242] or asymmetric common carotid artery surgery [114], both of which could lead to reduced cerebral blood flow, white matter rarefaction, glial activation, as well as subsequent cognitive impairment. A cascade of molecular and cellular events has been shown to be involved in the pathogenesis of CCH including energy imbalance, oxidative stress, endoplasmic reticulum stress, mitochondrial dysfunction and inflammation (Fig. 2).
Pathological drivers of CCH-associated VCI. Several CCH-induced pathological drivers have been long associated with the pathogenesis of VaD including energy imbalance, inflammation, endoplasmic reticulum (ER) stress, oxidative stress and mitochondrial dysfunction. Decreased ATP production impairs ATPase pumps, results in neuronal depolarization, and leads to a deregulation in the glutamate homeostasis at the synaptic cleft and excitotoxicity in the brain. Low cerebral blood flow triggers the brain to utilize anaerobic respiration to produce ATP and this results in the accumulation of lactate within the neurons, leading to acidosis. Neuronal death following CCH is primarily attributed to the increase in the pro-inflammatory cytokine release during the chronic inflammatory response. Danger associated molecular patterns (DAMPs) released by the brain cells can also trigger glial activation and leukocyte infiltration, both of which can also produce pro-inflammatory cytokines. At the cellular level, increase in the reactive oxidative species from various sources, including the mitochondria, induces the oxidative stress state. While the increase in reactive oxygen species can contribute to the redox dynamics and hemodynamics imbalance, it can also induce chronic ER stress. Persistent ER stress leads to an accumulation of misfolded proteins and can have fatal effects on neuronal survival and integrity via the terminal unfolded protein response (UPR) pathway, as well as contributing to Ca2+ homeostatic imbalance. Mitochondrial deterioration causes a further decrease in the ATP production, leading to proteosomal dysfunction, as well as contributing to the frequency of mutagenesis events at the mitochondrial DNA. In a chronic state of CCH, these drivers are pathological and ultimately pave the way for downstream disease mechanisms
Pathogenic mechanisms
Energy imbalance
During VCI, blood vessels in the brain including arterioles, veins and capillaries are partially occluded or hypoperfused [34, 145, 234]. Disruption to glucose and oxygen supply compromises the production of adenosine triphosphate (ATP) [118, 119]. The resultant state of energy imbalance impairs the function of ATP-dependent sodium–potassium pumps [82] which are critical for maintaining the resting membrane potential of neurons. As such, neurons spontaneously depolarize and release the excitatory neurotransmitter glutamate into the synaptic cleft. The excess accumulation of glutamate in the synaptic cleft is exacerbated by other defective ion pumps that fail to recycle the glutamate, leading to persistent depolarization and overstimulation of neighboring neurons [14, 38]. This excessive activation of glutamate receptors (i.e. NMDA and AMPA receptors) due to energy imbalance that results in neuronal dysfunction and death is called excitotoxicity, which has been reported to occur in chronic diseases such as VCI [265]. In order to compensate for the lack of glucose, the brain will begin to undergo anaerobic glycolysis, which produces lactate. Accumulation of lactate in the brain in turn leads to acidosis and acidotoxicity [143, 301].
Oxidative stress
Oxidative stress is defined as an environment where pro-oxidant species dominates over anti-oxidant species [95]. It is one of the central drivers of pathology in many diseases and it is implicated in the cognitive decline in VCI [26, 53, 166, 172]. Correspondingly, a reduction of circulatory antioxidant enzyme levels (e.g., superoxide dismutase, catalase) and antioxidant capacity (e.g., glutathione, ergothioneine) have been observed in VCI patients [80, 241, 297, 298]. In the brain, CCH causes a disruption in calcium (Ca2+) homeostasis which leads to acute and chronic production of reactive oxygen species [82, 166] from various sources, including electron transport chain, nicotinamide adenine dinucleotide phosphate oxidases (Nox) and nitric oxide synthase. In animal models of CCH, reduction of endothelial nitric oxide synthase expression [189] disrupts the vascular tone and exacerbates cerebral blood flow hypoperfusion [83]. CCH also increases Nox-1 expression in neurons, inducing apoptosis and contributing to cognitive impairment [53]. Oxidative stress also increases levels of circulating nitric oxide synthase inhibitor, reducing nitric oxide bioavailability, leading to vasodilation impairment as evident in cognitive impairment [67]. As the stiffness and pulsatility of the vessels increase, higher sheer stress is generated which disrupts normal continuous blood flow [19]. These vascular changes have been reported to be associated with reduced blood supply to white matter regions, thus precipitating the formation of white matter lesions and lacunes [266, 293].
Endoplasmic reticulum stress
Endoplasmic reticulum stress is emerging as a pathological mechanism in the etiology of VCI [195]. The endoplasmic reticulum is involved in the synthesis and post-translational modifications of molecules that are important in maintaining Ca2+ homeostasis [300]. Being the site of translation, protein folding and transport, disruptions to endoplasmic reticulum’s physiological function in the form of endoplasmic reticulum-calcium depletion, hypoxic conditions and oxidative stress, are known to result in misfolding and accumulation of unfolded integral proteins. Such stressors to endoplasmic reticulum activate an adaptive stress response pathway known as the unfolded protein response (UPR) [229]. This pathway involves three independent endoplasmic reticulum membrane-associated sensors which are protein kinase R-like endoplasmic reticulum kinase (PERK), inositol-requiring protein 1 (IRE1) and activating transcription factor 6 (ATF6) [300].
Under prolonged endoplasmic reticulum stress, cellular proteostasis becomes unsustainable, resulting in accumulation of misfolded proteins and activation of terminal UPR [120]. Attenuation of endoplasmic reticulum stress-induced apoptosis has been found to confer protection against ischemia and reperfusion injury [296]. More specifically, studies using neuronal models of vascular dementia have shown the contribution of zinc-induced neurotoxicity to its pathogenesis, upregulating endoplasmic reticulum stress-related genes like CCAAT-enhancer-binding protein homologous protein (CHOP) and growth-arrest- and DNA-damage-inducible gene 34 (GADD34) [263]. The same group also found that the endoplasmic reticulum stress pathway is involved in zinc-induced neurotoxicity thus implying its possible roles as both a cause and consequence in driving VaD [144].
Mitochondrial dysfunction
Given the high energy demands of the brain, mitochondria, as the ‘powerhouse of the cell’, play a central role in producing energy in the form of ATP. Mitochondria are also vital in regulating brain cell survival and death by controlling the movement of calcium ions between the cells and the extracellular surroundings. Reactive oxygen species produced by the mitochondrial energy-redox axis can signal for apoptosis when the cells are damaged [104, 159, 196, 218].
Mitochondrial dysfunction results in decreased energy production, thus altering cellular redox dynamics in the brain. Under these conditions, mitochondria begin producing an excess of \({\text{O}}_{2}^{ \cdot - }\) and H2O2 molecules in response to increased oxidation of proteins, phospholipids and DNA that pushes the redox equilibrium towards a pro-oxidative state [192]. There is also a global reduction in mitochondrial protein complexes over time [104, 159, 161, 310]. Therefore, it is not surprising that mitochondrial dysfunction has been observed in VaD [162]. In particular, mitochondrial damage such as increased mitochondrial bioenergetic deficits in the hippocampus plays important roles in the spatial learning and memory decline in both human patients and in CCH rodent models [16, 72, 162, 172]. Defects in mitochondrial metabolism lead to altered patterns in the mitochondrial respiratory rate, altered membrane potential, decreased pyruvate hydrogenase levels, increased oxidative stress as manifested by increased hydrogen peroxidase levels. However, mitochondrial dysfunction may not occur independently of other pathological processes but is commonly observed to overlap with other mechanisms such as oxidative stress and proteasome dysfunction [70].
Mitochondrial DNA contains genes that encode the cell’s mitochondrial energy production machinery, and defects or mutations in the mitochondrial DNA have been associated with age-related dementia and neuropathology [29, 31, 68, 102, 287]. The m.3316G > A mutation has been identified in early-onset VaD patients who did not manifest typical vascular symptoms [155]. Rather, the mutation causes a reduction in the activity of the respiratory chain complex I, and hence is associated with the well-established link that cerebrovascular damage increases when mitochondrial energy chain complexes are compromised [125].
Neuroinflammation
Inflammation involves a complex range of responses that are known to play a role in disease conditions. While inflammation is important in tissue repair and recovery, under disease conditions, chronic activation of inflammatory responses results in a destructive phenotype that is observed during disease development and progression [62]. Both acute and chronic inflammation have been implicated in cellular injury associated with a hypoxic state of VCI [136, 174, 217]. Under CCH, reduced blood supply disturbs cellular integrity, activates glial cells and recruits peripheral immune cells to the brain [23, 141, 309], causing death of neighboring cells and secondary tissue damage. Various molecular mechanisms such as activation of inflammatory pathways and inflammasome activation have been shown to play a role in inflammation during CCH.
Systemic inflammation serves as the initial signal of a stressed cell involving the release of damage associated molecular patterns (DAMPs), which are recognized by the pattern recognition receptors, namely Toll-like receptors (TLR) and NOD-like receptors (NLR) on neighboring cells, initiating an inflammatory response [82, 260]. Further studies have established the association of NLR family pyrin domain containing 3 (NLRP3) and Absent in melanoma-2 (AIM2), both of which are involved in inflammasome activation, with VaD and CCH [81, 177, 206, 207]. Regulatory pathways such as nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) are subsequently activated to upregulate a wide range of inflammatory proteins [105, 146] including interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF) [24, 317]. These inflammatory cytokines can cause cell death, oligodendrocyte damage and demyelination [264, 305]. Attenuation of IL-1β production was shown to ameliorate hypoperfusion-induced brain injury in mice [206]. Microglia and astrocytes also release adhesion molecules and chemokines, which activate and facilitate leukocyte infiltration [15, 20, 115]. In a mouse model of cerebral ischemia, genetic deletion of the chemokine CCL2 has been shown to reduce brain injury via modulation of inflammation. Other proinflammatory proteins such as c-reactive protein are also upregulated to facilitate cerebral inflammation in VCI patients [77, 215, 232].
The complement system has also been implicated in stroke [168]. Complement proteins promote inflammation via glial activation and induce neuronal injury through the C5 activating membrane attack complex (MAC). Formation and deposition of C5b-9/MAC complexes damages the myelin sheath [175, 233], and abrogation of C5 protein reduces glial activation and white matter ischemia under CCH [167]. The central effector protein in the system is the C3 convertase enzyme complex [28, 197, 240]. It has been demonstrated that under CCH, microglial cells aggravate white matter injury via the C3-C3aR pathway in rat brains [311]. Together, the entire inflammatory process facilitates astrogliosis and scar formation, oligodendrocyte and endothelial cell dysfunction and blood–brain barrier disruption [248, 282, 313], leading to neurodegeneration, neurovascular dissociation and eventually structural damage to the brain. Therefore, inflammation serves as a critical mechanism that drives subsequent pathological changes in CCH. In summary, the pathological mechanisms of CCH covered in the above section lay a foundation to comprehend the complex mechanistic underpinnings of the disease. The pathological mechanisms of CCH include the involvement of multiple molecules and signaling pathways, especially those related to inflammation and oxidative stress.
Neuropathological features of CCH
In this section, we will examine how the pathogenic mechanisms described above contribute to the neuropathological features that have been described in VCI.
Glial activation
The term neurovascular unit describes the structural and functional interactions between neurons, glial cells, pericytes, extracellular matrix components and endothelial cells in the brain. The neurovascular unit maintains homeostasis within the brain microenvironment ensuring optimal conditions for function of neurons and other cells. During CCH, the entire neurovascular unit is affected by the combined effects of the pathological mechanisms described above that can cause reduced integrity of the neurovascular unit [238]. This results in a homeostatic imbalance in the brain.
Glial cells, especially microglia, drive inflammatory responses by releasing proinflammatory molecules. Increased number of glial cells are commonly seen in VCI patients especially at the white matter regions [245, 269]. Mechanistic studies using animal models have shown that upon CCH, activated microglial cells participated in both systemic and complement-activated inflammation; whereas attenuation of microglial activity reduces proinflammatory cytokine levels, increases myelin density and eventually improves cognitive performance [138, 311].
Astrocytes are also involved in the process of inflammation during CCH. Astrogliosis has direct influence on blood–brain barrier integrity and induces damage when constitutively activated astrocytes form glial scars or swelling at the end feet processes [88, 211]. With CCH, a study reported decreased astrocyte polarity and structural support to the endothelial cells eventually contributing to blood–brain barrier damage [127].
In the white matter, oligodendrocytes are the predominant glial cell type, and produce the myelin sheath around myelinated axons. As CCH damages oligodendrocytes and white matter, repair mechanisms are often impaired due to inflammation and loss of growth factors released by neurons, microglia and astrocytes. The myelin-independent axonal support from oligodendrocyte is also affected, causing significant axonal loss [93, 181, 313]. Upon ischemia, oligodendrocytes also release inhibitory proteins Nogo-A and MMP-9, preventing neuronal remodeling, and initiating a deleterious cascade within white matter to cause blood–brain barrier damage [93, 181].
Together, dysfunction in each of the components within the neurovascular unit can result in the disruption of brain homeostasis, which can eventually lead to neuronal loss and white matter infarctions at the grey matter and the deep white matter territory [35, 252].
Activation of cell death
Programmed cell death is a critical role in animal development and tissue homeostasis. Abnormal regulation of programmed cell death is associated with various human diseases including neurodegeneration. Different forms of cell death such as apoptosis, pyroptosis and autophagy have been observed in cerebral ischemia and reperfusion injury [76, 84, 141]. Of these, there has been ample evidence in the literature implicating apoptosis in VCI. In postmortem studies of VCI patients, apoptotic vascular cells were identified in the basal ganglia and subcortical white matter regions [103]. Apoptotic neuronal cells were also observed at cortical layers 3 and 5; and extensive ischemic lesions and axonal damage were observed in severe dementia [103]. Furthermore, protein expression and proteomics studies have revealed a decreasing anti-apoptotic proteins expression pattern in the cortex of VCI patients compared to controls [64]. Within regions of leukoaraiosis, significant increases in apoptotic oligodendrocytes were observed compared to adjacent white matter [33]. Mirroring the evidence seen in human patients, animal models of CCH present similar results, with increased markers of apoptosis observed. Specific changes observed in these animal studies include increased visualization of apoptotic bodies, increased expression of apoptotic proteins such as caspase 3, and reduced expression of anti-apoptotic proteins such as Bcl-2 [194, 243, 257, 272, 290].
More recently, other forms of cell death such as pyroptosis have also been investigated in human patients [24] as well as rodent models of CCH [206, 312]. Autophagy has gained interest as well, having been shown to be upregulated specifically in VaD [41] and in CCH rodent models [47, 51, 126, 306].
These findings, in relation to cell death mechanisms being implicated in the pathophysiology of CCH, reinforce the concept of degeneration over the course of VCI progression, and may suggest that therapeutic interventions in cell death pathways may prove effective in curbing the pathological progression of VCI.
Blood–brain barrier dysfunction
The blood–brain barrier is a selectively permeable barrier that separates the circulating blood from the parenchymal tissue. The endothelial cells of the BBB are characterized by expression of tight junction proteins between adjacent cells, reduced rate of transcytosis and other transcellular movement across the barrier into or out of the brain. This property of blood–brain barrier establishes a finely tuned microenvironment for the brain by maintaining homeostasis and defending against pathogenic infections. In CCH, increased blood–brain barrier permeability has been observed [40, 191, 214, 277, 292], and is associated with neuronal loss and white matter degeneration during disease progression [271]. Blood–brain barrier damage can be induced through increased excitotoxicity, inflammation and oxidative stress, which can contribute to further brain injury via mechanisms such as increased leukocyte infiltration.
Excitotoxicity causes a persistent activation of endothelial cells causing cell death and uncontrolled movement of substance across the blood–brain barrier [6, 59]. Separately, high Ca2+ levels in the cytosol of the endothelial cells can also lead to activation of cell death mechanisms and the increased propagation of Ca2+ levels through the intracellular sources of Ca2+ such as mitochondria, and endoplasmic reticulum [92] can relocate the endothelial tight junction proteins [32]. Presence of proinflammatory cytokines can directly damage the blood–brain barrier, reducing its integrity by inducing endocytosis of the tight junction proteins thereby weakening the tight junction assembly [96, 307]. The internalised tight junction proteins are directed to lysosomal degradation, leading to long-term blood–brain barrier dysfunction [250, 279]. Reactive oxygen species have also been implicated in the progression of VaD and could possibly contribute to the breakdown of the blood–brain barrier [211]. Increased reactive oxygen species in endothelial cells downregulates epithelial cadherin levels [2] and bioavailability of nitric oxide, leading to endothelial and blood–brain barrier dysfunction [55, 89].
Blood–brain barrier dysfunction, oxidative stress [112] and inflammation induce matrix metalloproteinases (MMPs)-mediated proteolytic degradation of the extracellular matrix [63, 223, 250]. In both VaD and experimental CCH, increased levels of gelatinases (MMP-2 and MMP-9) have been reported [46, 223], which are associated with the degradation of basement membrane and tight junction proteins of the blood–brain barrier [160, 276]. Blood–brain barrier damage also increased the size of the perivascular spaces leading to cellular damage of pericytes within, a common observation in VaD. Association of pericyte damage with CCH, white matter damage, neuronal loss and cognitive impairment is evident [185] although a direct link between pericytes to VaD has yet to be demonstrated.
The blood–brain barrier regulates immune cell infiltration by maintaining low levels of leukocyte adhesion molecules on endothelial cells with inhibitory effects derived from pericytes [289]. In VaD, inflammation increases expression of adhesion molecules and chemokines such as intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule (VCAM) in endothelial cells [259], facilitating leukocyte infiltration [165, 171]. Upon crossing the damaged blood–brain barrier, activated leukocytes cause irreversible damage to the blood–brain barrier and contribute to further release of pro-inflammatory cytokines and reactive oxygen species, which forms a vicious feedback loop of activating endothelia [50]. Although increased ICAM levels have been reported in post-mortem studies of VaD patients [180], evidence showing specific temporal dynamics of leukocyte movement into the brain is still lacking. Overall, the establishment of endothelial cell activation upon CCH not just damages the blood–brain barrier, it also causes a reduction in the resting cerebral blood flow, and thus further contributes to a hypoperfused state within the brain. The biggest challenge here is the myriad aspects of blood–brain barrier damage and its downstream mechanisms in the context of VCI and other neuropathologies that remain unknown.
White matter lesions
One of the major pathological hallmarks of VCI is the formation of white matter lesions (WMLs) [18, 286]. The white matter functions to connect and preserve neural circuit signaling, thus implying the clinical importance of WMLs as markers of brain dysfunction due to cerebral vessel disease. Pathologically WMLs represent processes ranging from demyelination, astrogliosis, axonal loss and venular damage. These are in turn a consequence of the combined effects of increased oxidative stress and inflammation in the brain induced by CCH and blood–brain barrier breakdown [150, 163]. Our group has reported that disruption to the structural integrity of white matter can cause cognitive dysfunction [107, 123]. Anatomically, the white matter region comprises of numerous nerve fiber tracts that are surrounded by myelin. During disease progression, demyelination may occur due to various reasons. Excitotoxicity, oxidative stress and inflammation lead to oligodendrocyte damage through the loss of cellular function, mitochondrial dysfunction and production of pro-apoptotic signaling proteins, eventually contributing to their death and white matter injury [179, 255], and causing primary or secondary myelin destruction in white matter regions [178].
There is limited evidence regarding remyelination at the site of white matter injury following CCH. Remyelination is uncommon during CCH as the chronic hypoxic and pro-oxidative states block the ability of oligodendrocyte progenitor cells from being able to differentiate into newly matured oligodendrocytes [86, 91], a process further impeded by surrounding damaged endothelial cells and scar-formation during astrogliosis [10]. The age-dependent Wnt signaling pathway, which plays a role in the oligodendrocyte progenitor cells differentiation, is also compromised in VCI disease states leading to further remyelination dysfunction [130]. Nevertheless, despite being vulnerable to vascular injury, evidence showed restoration of oligodendrocyte progenitor cells and oligodendrocytes after prolonged CCH (i.e. 1 month of bilateral common carotid artery stenosis), suggesting their potential regeneration ability [181]. Studies suggested that oligodendrogenesis and regeneration are facilitated by reactive astrocytes, which secrete trophic factors such as brain-derived neurotrophic factor in response to white matter injury [169, 183, 184].
Epigenetic and genetic mechanisms
While VCI progression is mostly sporadic in nature, some forms of VCI are known to be influenced by the interplay of genetics and epigenetics. With technological advancements, researchers have improved access to diagnostic tools which carry out high throughput genomic-based investigations. The following section highlights recent research in the genetic factors of VCI as well as emerging interest in the epigenetics of VCI progression.
Epigenetics
Epigenetics refers to the alteration of gene expression without altering corresponding DNA sequences [170]. Epigenetic mechanisms are driven primarily by environmental stimuli including stress, diet and other behavioural factors. Given that most of the risk factors of VaD are associated with lifestyle-associated conditions such as hypertension and diabetes mellitus, the role of epigenetics seems to be critical in explaining the pathophysiology of the disease [203]. In fact, there are several lines of evidence for epigenetic contribution in the pathophysiology of dementia in general. These include studies where DNA methylation and hydroxymethylation were observed to be significantly reduced in the hippocampus, entorhinal cortex, cerebellum, and prefrontal cortex of AD patients compared to healthy controls [170]. Epigenetic modifications in AD neuropathology have been increasingly studied with the findings also implicated to other neurodegenerative diseases [79]. While there is limited evidence for the role of epigenetics specifically in VCI or CCH [203, 237, 299], this is likely due to the nascent nature of this topic, pointing to the need for further studies. Nevertheless, with epigenetic changes seen as drivers of pathological conditions, they may be regarded as biomarkers for early disease detection [199]. As such, further study of epigenetics would provide insights into VCI and perhaps aid in the stratification within VCI.
Genetic mutations
Certain forms of VCI onset and progression are known to have a familial component though the majority are sporadic cases [173]. Monogenic influences of tissue responses to VCI include NOTCH3 mutations causing cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a rare form of cerebrovascular disease. The Notch pathway is important in the regulation of cell fate [121]. In particular, the Notch 3 receptor-mediated pathway is involved in the vascular smooth muscle survival [173]. In CADASIL, the mutation of the NOTCH3 gene occurs within the epidermal growth factor—like repeat domains in the N terminal of the receptor. Brains of CADASIL patients manifest an aberrant oligomerization of mutant Notch 3 proteins, leading to altered protein–protein interactions [165]. The pathophysiology of CADASIL still remains unknown, but the NF-κB pathway has been reported to play an essential role in the inflammatory responses in the CADASIL-associated angiopathy. NF-κB promotes the expression of genes coding for cytokines that leads to an amplified vascular inflammation level and hence vascular dysfunction [149]. Notch 3 misfolding phenomenon can cause an increase in free radical production in the brain, although the levels produced may not be directly pathogenic [39].
There are also overlapping genes with AD which are known to be involved in the VCI pathogenesis, namely the presenilins, the amyloid precursor protein (APP), and the apolipoprotein E (APOE) [158, 220]. It has been reported that the presence of even a single allele of the APOE4 variant could be a potential risk factor for progression of VCI [220], thus providing evidence for a commensal interaction between AD and other CVD conditions.
Unravelling the potential of early detection and intervention strategies
Currently, the treatment options for VCI remain sparse. Understanding the underlying pathophysiology of VCI through CCH provides critical insights to the discovery of biomarkers and targets for disease-modifying treatments.
The identification of specific biomarkers for VCI will be critical for more specific and sensitive diagnosis. These biomarkers may allow for early detection of VCI in at-risk patients. While the diagnostic criteria for VCI is based primarily on neuroimaging, blood-based biomarkers are nevertheless useful as surrogate disease indicators. Many blood and cerebrospinal fluid biomarkers have been identified over the years. Several proinflammatory molecule, such as C-reactive protein, IL-1α and IL-6, have been proposed as potential biomarkers [57, 133, 288]. Given that the increase in plasma level of inflammatory proteins precedes cognitive impairment in VCI, the identification of proinflammatory proteins in early stages of the disease not only offers prognostic advantage but also possible therapeutic intervention [77]. Other than inflammation, the classic marker for blood–brain barrier dysfunction, matrix metalloproteinases, has also been reported multiple times in VCI patients and found to be an early biomarker for cognitive dysfunction [73, 78, 191]. While evidence for oxidative stress in VCI patients are limited, it has been shown that oxidative stress is increased in mild cognitive impairment and AD [44, 210]. Our team has contributed to the field in establishing several possible blood biomarkers for white matter hyperintensities and microinfarcts in clinical cohorts of VCI patients such as serum hepatocyte growth factors, IL-8 and growth differentiation factor-15 [45, 106, 122, 314, 315].
Beyond specific aspects of pathophysiology, several multi-purpose therapeutic interventions have been proposed. The basis of these therapeutic interventions is built upon the evidence that cerebrovascular injury is not always progressive but may instead be reversible. For instance, white matter hyperintensities which are indicative of white matter lesions may regress and be amendable to treatments [87]. Therefore, to the extent that cerebrovascular disease such as white matter hyperintensities is related to CCH and can contribute to the risk of VCI development, markers which allow for the early identification of these lesions may enable early mitigation of cerebrovascular disease and in turn, VCI development. In a similar vein, a deep understanding of the molecular underpinnings of CCH which are relevant to cerebrovascular disease allows for the identification of potential treatment markers as well as drug targets. In the latter case, this then facilitates a potential for development of disease-modifying treatments.
Given that chronic diseases such as VCI are linked to diet and lifestyle factors, interventions at this stage are important for managing the disease. Recent studies have found association of VCI with dietary habits, shedding light on using intermittent fasting as a possible treatment for VCI [201, 280]. Intermittent fasting has been shown to improve cognitive ability, neurotropic factor production, synaptic plasticity, mitochondrial biogenesis, and has also been shown to ameliorate vascular pathology and cognitive impairment in rodent VCI models [11, 85, 110, 213, 237, 273]. Additional systemic beneficial effects of intermittent fasting include attenuation of inflammation, oxidative stress, mitochondrial dysfunction and DNA damage [65]. Separately, in clinical studies on VaD, increasing physical activity has been suggested to reduce the risk of dementia manifestation, albeit not with entirely consistent results due to lack of standardized methods [1, 71, 249, 285].
These approaches are not only potentially disease-modifying at the pathophysiological level, but they also serve as a preventive strategy to mitigate an at-risk patient’s risk of disease. More studies are required for a more robust conclusion in order to delineate the role of intermittent fasting and exercise in VCI clearly [124, 156, 157, 235]. Notably, our team have been investigating the effect of multiple lifestyle interventions on the prevention of cognitive decline under the Singapore Geriatric Intervention Study to Reduce Cognitive Decline and Physical Frailty (SINGER) [52], which is an adaptation of the pioneering Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) [151]. Lifestyle interventions provide a promising potential in managing VCI and reframing the public health perspective of the disease.
Summary and future directions
VCI is now a widely accepted term introduced to embody the entire spectrum of vascular-related cognitive alterations or cerebrovascular disease-related burdens that can manifest into cognitive impairments [100]. Cognitive deficits associated with VCI include slower mental processing and impaired executive functioning such as poor planning, poor judgement and poor decision-making. Non-cognitive behavioural manifestations include apathy, anxiety and even depression are also common. VCI has been gaining interest in the field as it is potentially preventable, prior to reaching the end-stage dementia [132, 294]. Given such emphasis on early detection and diagnosis in the field, there is a need to better understand the pathophysiology of VCI. As reviewed above, current experimental evidence indicates that a chronic state of hypoperfusion in the brain drives the various pathophysiological mechanisms and structural changes in the brain. CCH therefore holds promise in shedding some light on the molecular and mechanistic underpinnings of VCI.
As reviewed above, there are several common mechanisms that occur during the progression of CCH-induced injury such as energy imbalance, oxidative stress, endoplasmic reticulum stress, mitochondrial dysfunction and inflammation (Fig. 2). These mechanisms drive the downstream structural neuropathological changes in the brain including glial activation, cell death activation, blood–brain barrier breakdown and white matter lesion formation. The pathological features begin from a hypoperfused state and can coexist and interact to adversely influence cognitive function as reported in animal models [25, 27, 43, 166]. This suggests that the effects of CCH on cognition are mediated by mechanistic drivers and structural changes in the brain. Indeed, CCH may be the earliest, insidious indicator of VCI, while brain atrophy and white matter lesions may occur downstream from CCH as more dynamic and detectable changes.
It is exciting to witness the field of VCI rapidly expanding and moving towards sharper definitions and deeper insights into underlying mechanisms. The heterogeneity of the disease is widely recognized to be due to the complex interactions between vascular injuries and risk factors that are involved prior to, and during disease manifestation. Across these subtypes, variations also exist at the clinical, neuroimaging, and pathological levels. Yet, a strong argument may be made that all subtypes of VCI include a CCH state, which we believe to be the main driver for subsequent pathological progression. Prolonged cerebral hypoperfusion may therefore serve as the transition from the at-risk state to the VCI state. The observational and experimental evidence from CCH models presented in this review help reinforce the importance of CCH as a critical feature in our efforts to unravel the underlying molecular mechanisms of VCI. Further identification of specific biomarkers of CCH may provide the rationale for the evaluation of these markers in the clinic which can bring us closer to detecting VCI at an early stage as well as introduce treatment options which may delay disease onset or slow disease progression.
References
Aarsland D, Saeedzadeh-Sardahaee F, Anderssen S, Ballard C (2010) Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health. https://doi.org/10.1080/13607860903586136
Abbruscato T, Davis T (1999) Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res 842:277–286. https://doi.org/10.1016/S0006-8993(99)01778-3
Abraham JM, Cho L (2010) The homocysteine hypothesis: still relevant to the prevention and treatment of cardiovascular disease? Cleve Clin J Med 77:911–918. https://doi.org/10.3949/ccjm.77a.10036
Aevarsson Ó, Svanborg A, Skoog I (1998) Seven-year survival rate after age 85 years: relation to Alzheimer disease and vascular dementia. Arch Neurol 55:1226–1232. https://doi.org/10.1001/ARCHNEUR.55.9.1226
Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B (1999) Mortality from dementia in advanced age: a 5-year follow-up study of incident dementia cases. J Clin Epidemiol 52:737–743. https://doi.org/10.1016/S0895-4356(99)00067-0
András IE, Deli MA, Veszelka S, Hayashi K, Hennig B, Toborek M (2007) The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab 27:1431–1443. https://doi.org/10.1038/SJ.JCBFM.9600445
Anjum I, Fayyaz M, Wajid A, Sohail W, Ali A (2018) Does obesity increase the risk of dementia: a literature review. Cureus 10:e2660. https://doi.org/10.7759/cureus.2660
Anstey KJ, von Sanden C, Salim A, O’Kearney R (2007) Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 166:367–378. https://doi.org/10.1093/aje/kwm116
Appleton JP, Scutt P, Sprigg N, Bath PM (2017) Hypercholesterolaemia and vascular dementia. Clin Sci 131:1561–1578. https://doi.org/10.1042/cs20160382
Arai K, Lo EH (2010) Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J Neurosci Res 88:758–763. https://doi.org/10.1002/jnr.22256
Arguin H, Dionne IJ, Sénéchal M, Bouchard DR, Carpentier AC, Ardilouze J-L, Tremblay A, Leblanc C, Brochu M (2012) Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause 19:870
Arnoldussen IA, Kiliaan AJ, Gustafson DR (2014) Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol 24:1982–1999. https://doi.org/10.1016/j.euroneuro.2014.03.002
Aronson MK, Ooi WL, Morgenstern H, Hafner A, Masur D, Crystal H, Frishman WH, Fisher D, Katzman R (1990) Women, myocardial infarction, and dementia in the very old. Neurology 40:1102–1106. https://doi.org/10.1212/wnl.40.7.1102
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34:325–337. https://doi.org/10.1016/S0143-4160(03)00141-6
Babcock AA, Kuziel WA, Rivest S, Owens T (2003) Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci 23:7922–7930. https://doi.org/10.1523/JNEUROSCI.23-21-07922.2003
Baik SH, Selvaraji S, Fann DY, Poh L, Jo DG, Herr DR, Zhang SR, Kim HA, Silva M, Lai MKP et al (2021) Hippocampal transcriptome profiling reveals common disease pathways in chronic hypoperfusion and aging. Aging (Albany NY) 13:14651–14674. https://doi.org/10.18632/aging.203123
Bakhru A, Erlinger TP (2005) Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med 2:e160. https://doi.org/10.1371/journal.pmed.0020160
Bakker SLM, de Leeuw FE, de Groot JC, Hofman A, Koudstaal PJ, Breteler MMB (1999) Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology 52:578–578. https://doi.org/10.1212/WNL.52.3.578
Barić D (2014) Why pulsatility still matters: a review of current knowledge. Croat Med J 55:609–620. https://doi.org/10.3325/cmj.2014.55.609
Barna BP, Pettay J, Barnett GH, Zhou P, Iwasaki K, Estes ML (1994) Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J Neuroimmunol 50:101–107. https://doi.org/10.1016/0165-5728(94)90220-8
Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA (2012) Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 69:493–498. https://doi.org/10.1001/archgenpsychiatry.2011.1481
Barreras A, Gurk-Turner C (2003) Angiotensin II receptor blockers. In: Baylor University Medical Center Proceedings, vol 16, pp 123–126. https://doi.org/10.1080/08998280.2003.11927893
Barreto G, White RE, Ouyang Y, Xu L, Giffard RG (2011) Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem 11:164–173. https://doi.org/10.2174/187152411796011303
Belkhelfa M, Beder N, Mouhoub D, Amri M, Hayet R, Nabila T, Bakhti S, Laimouche S, Azzouz D, Belhadj R et al (2018) The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia. J Neuroimmunol. https://doi.org/10.1016/j.jneuroim.2018.04.004
Ben Ari H, Lifschytz T, Wolf G, Rigbi A, Blumenfeld-Katzir T, Kreisel Merzel T, Koroukhov N, Lotan A, Lerer B (2019) White matter lesions, cerebral inflammation and cognitive function in a mouse model of cerebral hypoperfusion. Brain Res. https://doi.org/10.1016/j.brainres.2019.01.017
Bennett S, Grant MM, Aldred S (2009) Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J Alzheimers Dis 17:245–257. https://doi.org/10.3233/JAD-2009-1041
Bennett SA, Tenniswood M, Chen JH, Davidson CM, Keyes MT, Fortin T, Pappas BA (1998) Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. NeuroReport 9:161–166. https://doi.org/10.1097/00001756-199801050-00033
Bonifati DM, Kishore U (2007) Role of complement in neurodegeneration and neuroinflammation. Mol Immunol 44:999–1010. https://doi.org/10.1016/j.molimm.2006.03.007
Bowling AC, Mutisya EM, Walker LC, Price DL, Cork LC, Beal MH (1993) Age-dependent impairment of mitochondrial function in primate brain. J Neurochem 60:1964–1967. https://doi.org/10.1111/j.1471-4159.1993.tb13430.x
Breteler MM, Claus JJ, Grobbee DE, Hofman A (1994) Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam study. BMJ 308:1604–1608. https://doi.org/10.1136/bmj.308.6944.1604
Brown MD, Wallace DC (1994) Molecular basis of mitochondrial DNA disease. J Bioenerg Biomembr 26:273–289. https://doi.org/10.1007/BF00763099
Brown RC, Davis TP (2002) Calcium modulation of adherens and tight junction function. Stroke 33:1706–1711. https://doi.org/10.1161/01.STR.0000016405.06729.83
Brown WR, Moody DM, Thore CR, Challa VR (2000) Apoptosis in leukoaraiosis. Am J Neuroradiol 21:79–82
Brown WR, Thore CR (2011) Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 37:56–74. https://doi.org/10.1111/j.1365-2990.2010.01139.x
Brun A (1994) Pathology and pathophysiology of cerebrovascular dementia: pure subgroups of obstructive and hypoperfusive etiology. Dement Geriatr Cogn Disord 5:145–147. https://doi.org/10.1159/000106712
Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL et al (2010) Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm 7:433–437. https://doi.org/10.1016/j.hrthm.2009.12.004
Byers AL, Yaffe K (2011) Depression and risk of developing dementia. Nat Rev Neurol 7:323–331. https://doi.org/10.1038/nrneurol.2011.60
Camacho A, Massieu L (2006) Role of glutamate transporters in the clearance and release of glutamate during Ischemia and its relation to neuronal death. Arch Med Res 37:11–18. https://doi.org/10.1016/j.arcmed.2005.05.014
Campolo J, De Maria R, Mariotti C, Tomasello C, Parolini M, Frontali M, Inzitari D, Valenti R, Federico A, Taroni F et al (2013) Is the oxidant/antioxidant status altered in CADASIL patients? PLoS ONE 8:e67077–e67077. https://doi.org/10.1371/journal.pone.0067077
Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA (2011) Matrix metalloproteinases are associated with increased blood–brain barrier opening in vascular cognitive impairment. Stroke 42:1345–1350. https://doi.org/10.1161/STROKEAHA.110.600825
Castellazzi M, Patergnani S, Donadio M, Giorgi C, Bonora M, Bosi C, Brombo G, Pugliatti M, Seripa D, Zuliani G et al (2019) Autophagy and mitophagy biomarkers are reduced in sera of patients with Alzheimer’s disease and mild cognitive impairment. Sci Rep 9:20009–20009. https://doi.org/10.1038/s41598-019-56614-5
Catindig J-AS, Venketasubramanian N, Ikram MK, Chen C (2012) Epidemiology of dementia in Asia: insights on prevalence, trends and novel risk factors. J Neurol Sci 321:11–16
Cechetti F, Pagnussat AS, Worm PV, Elsner VR, Ben J, da Costa MS, Mestriner R, Weis SN, Netto CA (2012) Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull 87:109–116. https://doi.org/10.1016/j.brainresbull.2011.10.006
Cervellati C, Romani A, Seripa D, Cremonini E, Bosi C, Magon S, Bergamini CM, Valacchi G, Pilotto A, Zuliani G (2014) Systemic oxidative stress and conversion to dementia of elderly patients with mild cognitive impairment. Biomed Res Int 2014:309507–309507. https://doi.org/10.1155/2014/309507
Chai YL, Hilal S, Chong JR, Ng YX, Liew OW, Xu X, Ikram MK, Venketasubramanian N, Richards AM, Lai MKP et al (2016) Growth differentiation factor-15 and white matter hyperintensities in cognitive impairment and dementia. Medicine 95:e4566. https://doi.org/10.1097/MD.0000000000004566
Chai YL, Rajeev V, Poh L, Selvaraji S, Hilal S, Chen CP, Jo DG, Koo EH, Arumugam TV, Lai MK (2022) Chronic cerebral hypoperfusion alters the CypA-EMMPRIN-gelatinase pathway: implications for vascular dementia. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678x221146401
Che H, Yan Y, Kang X-H, Guo F, Yan M-L, Liu H-L, Hou X, Liu T, Zong D-K, Sun L-L et al (2017) MicroRNA-27a promotes inefficient lysosomal clearance in the hippocampi of rats following chronic brain hypoperfusion. Mol Neurobiol 54:2595–2610. https://doi.org/10.1007/s12035-016-9856-8
Chen C, Homma A, Mok VCT, Krishnamoorthy E, Alladi S, Meguro K, Abe K, Dominguez J, Marasigan S, Kandiah N (2016) Alzheimer’s disease with cerebrovascular disease: current status in the Asia–Pacific region. J Intern Med 280:359–374
Chen CPLH (2004) Transcultural expression of subcortical vascular disease. J Neurol Sci 226:45–47. https://doi.org/10.1016/j.jns.2004.09.010
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2017) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9:7204–7218. https://doi.org/10.18632/oncotarget.23208
Chen Y, Guo Z, Peng X, Xie W, Chen L, Tan Z (2018) Nimodipine represses AMPK phosphorylation and excessive autophagy after chronic cerebral hypoperfusion in rats. Brain Res Bull 140:88–96. https://doi.org/10.1016/j.brainresbull.2018.03.019
Chew KA, Xu X, Siongco P, Villaraza S, Phua AKS, Wong ZX, Chung CY, Tang N, Chew E, Henry CJ et al (2021) SINgapore GERiatric intervention study to reduce physical frailty and cognitive decline (SINGER)-pilot: a feasibility study. Alzheimers Dement (NY) 7:e12141. https://doi.org/10.1002/trc2.12141
Choi DH, Lee KH, Kim JH, Seo JH, Kim HY, Shin CY, Han JS, Han SH, Kim YS, Lee J (2014) NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal 21:533–550. https://doi.org/10.1089/ars.2012.5129
Choi JC (2015) Genetics of cerebral small vessel disease. J Stroke 17:7–16. https://doi.org/10.5853/jos.2015.17.1.7
Chrissobolis S, Banfi B, Sobey CG, Faraci FM (2012) Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J Appl Physiol 113:184–191. https://doi.org/10.1152/JAPPLPHYSIOL.00455.2012
Ciacciarelli A, Sette G, Giubilei F, Orzi F (2020) Chronic cerebral hypoperfusion: an undefined, relevant entity. J Clin Neurosci. https://doi.org/10.1016/j.jocn.2020.01.026
Cipollini V, Troili F, Giubilei F (2019) Emerging biomarkers in vascular cognitive impairment and dementia: from pathophysiological pathways to clinical application. Int J Mol Sci 20:2812–2812. https://doi.org/10.3390/ijms20112812
Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14:125–130. https://doi.org/10.1111/1467-9280.t01-1-01430
Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, Colgan SP (2002) Neutrophil-derived Glutamate regulates vascular endothelial barrier function. J Biol Chem 277:14801–14811. https://doi.org/10.1074/JBC.M110557200
Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH (2008) Prevalence of dementia after age 90: results from the 90+ study. Neurology 71:337–343. https://doi.org/10.1212/01.wnl.0000310773.65918.cd
Corraini P, Henderson VW, Ording AG, Pedersen L, Horváth-Puhó E, Sørensen HT (2017) Long-term risk of dementia among survivors of ischemic or hemorrhagic stroke. Stroke 48:180–186. https://doi.org/10.1161/strokeaha.116.015242
Courties G, Moskowitz MA, Nahrendorf M (2014) The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol 71:233–236. https://doi.org/10.1001/jamaneurol.2013.5026
Daneman R, Prat A (2015) The blood–brain barrier. Cold Spring Harb Perspect Biol 7:a020412. https://doi.org/10.1101/CSHPERSPECT.A020412
Datta A, Qian J, Chong R, Kalaria RN, Francis P, Lai MKP, Chen CP, Sze SK (2014) Novel pathophysiological markers are revealed by iTRAQ-based quantitative clinical proteomics approach in vascular dementia. J Proteom 99:54–67. https://doi.org/10.1016/j.jprot.2014.01.011
de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381:2541–2551. https://doi.org/10.1056/NEJMra1905136
de la Torre JC (2012) Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012:367516. https://doi.org/10.1155/2012/367516
De Silva TM, Faraci FM (2016) Microvascular dysfunction and cognitive impairment. Cell Mol Neurobiol 36:241–258. https://doi.org/10.1007/s10571-015-0308-1
De Vivo DC (1993) The expanding clinical spectrum of mitochondrial diseases. Brain Dev 15:1–22. https://doi.org/10.1016/0387-7604(93)90002-P
Del Ser T, Hachinski V, Merskey H, Munoz DG (1999) An autopsy-verified study of the effect of education on degenerative dementia. Brain 122(Pt 12):2309–2319. https://doi.org/10.1093/brain/122.12.2309
Ding Q, Dimayuga E, Keller JN (2006) Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal 8:163–172. https://doi.org/10.1089/ars.2006.8.163
Dong J, Zhao J, Lin Y, Liang H, He X, Zheng X, Sui M, Zhuang Z, Yan T (2018) Exercise improves recognition memory and synaptic plasticity in the prefrontal cortex for rats modelling vascular dementia. Neurol Res 40:68–77. https://doi.org/10.1080/01616412.2017.1398389
Du J, Ma M, Zhao Q, Fang L, Chang J, Wang Y, Fei R, Song X (2012) Mitochondrial bioenergetic deficits in the hippocampus of rats with chronic Ischemia-induced vascular dementia. Neuroscience. https://doi.org/10.1016/j.neuroscience.2012.11.062
Duits FH, Hernandez-Guillamon M, Montaner J, Goos JDC, Montañola A, Wattjes MP, Barkhof F, Scheltens P, Teunissen CE, van der Flier WM (2015) Matrix metalloproteinases in Alzheimer’s disease and concurrent cerebral microbleeds. J Alzheimer’s Dis 48:711–720. https://doi.org/10.3233/JAD-143186
Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria Raj N, Horsburgh K (2017) Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci 131:2451–2468. https://doi.org/10.1042/CS20160727
Duron E, Hanon O (2008) Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag 4:363–381. https://doi.org/10.2147/vhrm.s1839
Enciu A-M, Constantinescu SN, Popescu LM, Mureşanu DF, Popescu BO (2011) Neurobiology of vascular dementia. J Aging Res 2011:401604. https://doi.org/10.4061/2011/401604
Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JCM, Breteler MMB (2004) Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 61:668–672. https://doi.org/10.1001/archneur.61.5.668
Erhardt EB, Pesko JC, Prestopnik J, Thompson J, Caprihan A, Rosenberg GA (2018) Biomarkers identify the Binswanger type of vascular cognitive impairment. J Cereb Blood Flow Metab 39:1602–1612. https://doi.org/10.1177/0271678X18762655
Esposito M, Sherr GL (2019) Epigenetic modifications in Alzheimer’s neuropathology and therapeutics. Front Neurosci 13:476–476. https://doi.org/10.3389/fnins.2019.00476
Famulari AL, Marschoff ER, Llesuy SF, Kohan S, Serra JA, Domínguez RO, Repetto MG, Reides CG, de Lustig ES (1996) The antioxidant enzymatic blood profile in Alzheimer’s and vascular diseases. Their association and a possible assay to differentiate demented subjects and controls. J Neurol Sci 141:69–78
Fan Y, Ou X, Wang W, Tian X, Yan S, Hu N, Zhang X, Xing W (2017) Identification and analysis of toll like receptor 4 (TLR4) level changes in vascular dementia patients related type 2 diabetes mellitus. Biomed Res 28:4588
Fann DY-W, Lee S-Y, Manzanero S, Chunduri P, Sobey CG, Arumugam TV (2013) Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev 12:941–966. https://doi.org/10.1016/j.arr.2013.09.004
Faraci F (2006) Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol 100:739–743. https://doi.org/10.1152/japplphysiol.01044.2005
Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V (2012) Role of apoptosis in disease. Aging 4:330–349. https://doi.org/10.18632/aging.100459
Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A (2019) Effect of Ramadan fasting on weight and body composition in healthy non-athlete adults: a systematic review and meta-analysis. Nutrients 11:478–478. https://doi.org/10.3390/nu11020478
Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB et al (2006) White matter lesions in an unselected cohort of the elderly. Stroke 37:1391–1398. https://doi.org/10.1161/01.STR.0000221308.94473.14
Filley CM (2021) Cognitive dysfunction in white matter disorders: new perspectives in treatment and recovery. J Neuropsychiatry Clin Neurosci 33:349–355. https://doi.org/10.1176/appi.neuropsych.21030080
Fischer S, Wobben M, Kleinstück J, Renz D, Schaper W (2000) Effect of astroglial cells on hypoxia-induced permeability in PBMEC cells. Am J Physiol Cell Physiol 279:C935–C944. https://doi.org/10.1152/ajpcell.2000.279.4.C935
Förstermann U (2010) Nitric oxide and oxidative stress in vascular disease. Pflüg Arch Eur J Physiol 459(6):923–939. https://doi.org/10.1007/S00424-010-0808-2
Freiheit EA, Hogan DB, Eliasziw M, Patten SB, Demchuk AM, Faris P, Anderson T, Galbraith D, Parboosingh JS, Ghali WA et al (2012) A dynamic view of depressive symptoms and neurocognitive change among patients with coronary artery disease. Arch Gen Psychiatry 69:244–255. https://doi.org/10.1001/archgenpsychiatry.2011.1361
French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB (2009) Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res 87:3076–3087. https://doi.org/10.1002/jnr.22139
Friedman L (2007) CALCIUM: a role for neuroprotection and sustained adaptation. Mol Interv 6:315–329. https://doi.org/10.1124/mi.6.6.5
Fünfschilling U, Supplie L, Mahad D, Boretius S, Saab A, Edgar J, Brinkmann B, Kassmann C, Tzvetanova I, Möbius W et al (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. https://doi.org/10.1038/nature11007
Gannon OJ, Robison LS, Custozzo AJ, Zuloaga KL (2019) Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem Int 127:38–55. https://doi.org/10.1016/j.neuint.2018.11.014
Gemma C, Vila J, Bachstetter A, Bickford PC (2007) Oxidative stress and the aging brain: from theory to prevention. CRC Press/Routledge/Taylor & Francis Group, pp 353–374
González-Mariscal L, Tapia R, Chamorro D (2008) Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778:729–756. https://doi.org/10.1016/J.BBAMEM.2007.08.018
Gordon P, Flanagan P (2016) Smoking: a risk factor for vascular disease. J Vasc Nurs 34:79–86. https://doi.org/10.1016/j.jvn.2016.04.001
Gorelick PB (2004) Risk factors for vascular dementia and Alzheimer disease. Stroke 35:2620–2622. https://doi.org/10.1161/01.Str.0000143318.70292.47
Gorelick PB, Counts SE, Nyenhuis D (2016) Vascular cognitive impairment and dementia. Biochim Biophys Acta (BBA) Mol Basis Dis 1862:860–868. https://doi.org/10.1016/j.bbadis.2015.12.015
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D et al (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713. https://doi.org/10.1161/STR.0b013e3182299496
Graban A, Bednarska-Makaruk M, Bochyńska A, Lipczyńska-Łojkowska W, Ryglewicz D, Wehr H (2009) Vascular and biochemical risk factors of vascular dementia after lacunar strokes (S-VaD) and after multiinfarcts in strategic areas (M-VaD). J Neurol Sci 283:116–118. https://doi.org/10.1016/j.jns.2009.02.344
Graeber MB, Grasbon-Frodl E, Eitzen UV, Kösel S (1998) Neurodegeneration and aging: role of the second genome. J Neurosci Res 52:1–6. https://doi.org/10.1002/(SICI)1097-4547(19980401)52:1%3c1::AID-JNR1%3e3.0.CO;2-I
Gray F, Polivka M, Viswanathan A, Baudrimont M, Bousser M-G, Chabriat H (2007) Apoptosis in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol 66:597–607. https://doi.org/10.1097/nen.0b013e318093e574
Grimm A, Friedland K, Eckert A (2016) Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer’s disease. Biogerontology. https://doi.org/10.1007/s10522-015-9618-4
Grivennikov S, Karin M (2010) Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 21(1):11–19
Gyanwali B, Lai MKP, Lui B, Liew OW, Venketasubramanian N, Richards AM, Chen C, Hilal S (2021) Blood-based cardiac biomarkers and the risk of cognitive decline, cerebrovascular disease, and clinical events. Stroke 52:2275–2283. https://doi.org/10.1161/strokeaha.120.032571
Gyanwali B, Shaik MA, Tan BY, Venketasubramanian N, Chen C, Hilal S (2019) Risk factors for and clinical relevance of incident and progression of cerebral small vessel disease markers in an Asian memory clinic population. J Alzheimer’s Dis 67:1209–1219. https://doi.org/10.3233/JAD-180911
Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN et al (2006) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37:2220–2241. https://doi.org/10.1161/01.STR.0000237236.88823.47
Hainsworth AH, Yeo NE, Weekman EM, Wilcock DM (2016) Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta 1862:1008–1017. https://doi.org/10.1016/j.bbadis.2015.11.015
Halberg N, Henriksen M, Söderhamn N, Stallknecht B, Ploug T, Schjerling P, Dela F (2005) Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol 99:2128–2136. https://doi.org/10.1152/japplphysiol.00683.2005
Halling A, Berglund J (2006) Association of diagnosis of ischaemic heart disease, diabetes mellitus and heart failure with cognitive function in the elderly population. Eur J Gen Pract 12:114–119. https://doi.org/10.1080/13814780600881128
Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y (2007) Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood–brain barrier dysfunction. J Neurochem 101:566–576. https://doi.org/10.1111/j.1471-4159.2006.04393.x
Harter K, Levine M, Henderson SO (2015) Anticoagulation drug therapy: a review. West J Emerg Med 16:11–17. https://doi.org/10.5811/westjem.2014.12.22933
Hattori Y, Enmi J, Kitamura A, Yamamoto Y, Saito S, Takahashi Y, Iguchi S, Tsuji M, Yamahara K, Nagatsuka K et al (2015) A novel mouse model of subcortical infarcts with dementia. J Neurosci 35:3915–3928. https://doi.org/10.1523/jneurosci.3970-14.2015
Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME (1995) Production and function of monocyte chemoattractant protein-1 and other β-chemokines in murine glial cells. J Neuroimmunol 60:143–150. https://doi.org/10.1016/0165-5728(95)00064-9
Helmer C, Joly P, Letenneur L, Commenges D, Dartigues JF (2001) Mortality with dementia: results from a French prospective community-based cohort. Am J Epidemiol 154:642–648. https://doi.org/10.1093/aje/154.7.642
Hénon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D (2001) Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology 57:1216–1222. https://doi.org/10.1212/wnl.57.7.1216
Hertz L (2008) Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology 55:289–309. https://doi.org/10.1016/j.neuropharm.2008.05.023
Hertz L, Dienel GA (2002) Energy metabolism in the brain. In: Dwyer D (ed) Glucose metabolism in the brain. Academic Press, pp 1–102
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13:477–491. https://doi.org/10.1038/nrneurol.2017.99
High FA, Epstein JA (2008) The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9:49–61. https://doi.org/10.1038/nrg2279
Hilal S, Chai YL, van Veluw S, Shaik MA, Ikram MK, Venketasubramanian N, Richards AM, Biessels GJ, Chen C (2017) Association between subclinical cardiac biomarkers and clinically manifest cardiac diseases with cortical cerebral microinfarcts. JAMA Neurol 74:403–410
Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CLH (2017) Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 88:669–674. https://doi.org/10.1136/JNNP-2016-315324
Ho SC, Woo J, Sham A, Chan SG, Yu ALM (2001) A 3-year follow-up study of social, lifestyle and health predictors of cognitive impairment in a Chinese older cohort. Int J Epidemiol 30:1389–1396. https://doi.org/10.1093/ije/30.6.1389
Hsu M-J, Sheu J-R, Lin C-H, Shen M-Y, Hsu CY (2010) Mitochondrial mechanisms in amyloid beta peptide-induced cerebrovascular degeneration. Biochim Biophys Acta (BBA) Gen Subj 1800:290–296. https://doi.org/10.1016/j.bbagen.2009.08.003
Hu M, Liu Z, Lv P, Wang H, Zhu Y, Qi Q, Xu J (2017) Autophagy and Akt/CREB signalling play an important role in the neuroprotective effect of nimodipine in a rat model of vascular dementia. Behav Brain Res 325:79–86. https://doi.org/10.1016/j.bbr.2016.11.053
Huang J, Li J, Feng C, Huang X, Wong L, Liu X, Nie Z, Xi G (2018) Blood–brain barrier damage as the starting point of leukoaraiosis caused by cerebral chronic hypoperfusion and its involved mechanisms: effect of agrin and aquaporin-4. Biomed Res Int. https://doi.org/10.1155/2018/2321797
Huang J, Zhang Z, Hong X, Wang J, Wei J, Wen H (2009) Early cognitive predictors of vascular dementia: a population-based longitudinal study in Chinese elderly. J Exp Stroke Transl Med. https://doi.org/10.4172/1939-067x.1000103
Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR et al (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49:2063–2069. https://doi.org/10.2337/diabetes.49.12.2063
Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80:844–866. https://doi.org/10.1016/j.neuron.2013.10.008
Ikram MA, Bersano A, Manso-Calderón R, Jia JP, Schmidt H, Middleton L, Nacmias B, Siddiqi S, Adams HH (2017) Genetics of vascular dementia—review from the ICVD working group. BMC Med 15:48. https://doi.org/10.1186/s12916-017-0813-9
Ingles JL, Wentzel C, Fisk JD, Rockwood K (2002) Neuropsychological predictors of incident dementia in patients with vascular cognitive impairment, without dementia. Stroke 33:1999–2002. https://doi.org/10.1161/01.STR.0000024433.36590.1B
Jagtap A, Gawande S, Sharma S (2015) Biomarkers in vascular dementia: a recent update. Biomark Genom Med 7:43–56. https://doi.org/10.1016/j.bgm.2014.11.001
Jiang XL, Samant S, Lesko LJ, Schmidt S (2015) Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet 54:147–166. https://doi.org/10.1007/s40262-014-0230-6
Jørgensen IF, Aguayo-Orozco A, Lademann M, Brunak S (2020) Age-stratified longitudinal study of Alzheimer’s and vascular dementia patients. Alzheimers Dement 16:908–917. https://doi.org/10.1002/alz.12091
Juma W, Lira A, Marzuk A, Marzuk Z, Hakim A, Thompson C (2011) C-reactive protein expression in a rodent model of chronic cerebral hypoperfusion. Brain Res 1414:85–93. https://doi.org/10.1016/j.brainres.2011.07.047
Justin BN, Turek M, Hakim AM (2013) Heart disease as a risk factor for dementia. Clin Epidemiol 5:135–145. https://doi.org/10.2147/clep.S30621
Kakae M, Tobori S, Morishima M, Nagayasu K, Shirakawa H, Kaneko S (2019) Depletion of microglia ameliorates white matter injury and cognitive impairment in a mouse chronic cerebral hypoperfusion model. Biochem Biophys Res Commun 514:1040–1044. https://doi.org/10.1016/j.bbrc.2019.05.055
Kalaria RN (2016) Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol 131:659–685. https://doi.org/10.1007/s00401-016-1571-z
Kalaria RN (2018) The pathology and pathophysiology of vascular dementia. Neuropharmacology 134:226–239. https://doi.org/10.1016/j.neuropharm.2017.12.030
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7
Kang JH, Cook N, Manson J, Buring JE, Grodstein F (2007) Low dose aspirin and cognitive function in the women’s health study cognitive cohort. BMJ 334:987. https://doi.org/10.1136/bmj.39166.597836.BE
Katsura K-I, Kristián T, Smith M-L, Siesjö BK (1994) Acidosis induced by hypercapnia exaggerates ischemic brain damage. J Cereb Blood Flow Metab 14:243–250. https://doi.org/10.1038/jcbfm.1994.31
Kawahara M, Sadakane Y, Koyama H, Konoha K, Ohkawara S (2013) D-histidine and L-histidine attenuate zinc-induced neuronal death in GT1–7 cells. Metallomics Integr Biomet Sci 5(5):453–460
Kawamura J, Meyer JS, Terayama Y, Weathers S (1991) Leukoaraiosis correlates with cerebral hypoperfusion in vascular dementia. Stroke 22:609–614. https://doi.org/10.1161/01.STR.22.5.609
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Front Immunol 5:461
Khan A, Kalaria RN, Corbett A, Ballard C (2016) Update on vascular dementia. J Geriatr Psychiatry Neurol 29:281–301. https://doi.org/10.1177/0891988716654987
Kiliaan AJ, Arnoldussen IA, Gustafson DR (2014) Adipokines: a link between obesity and dementia? Lancet Neurol 13:913–923. https://doi.org/10.1016/s1474-4422(14)70085-7
Killeen MJ, Linder M, Pontoniere P, Crea R (2014) NF-κβ signaling and chronic inflammatory diseases: exploring the potential of natural products to drive new therapeutic opportunities. Drug Discovery Today 19:373–378. https://doi.org/10.1016/j.drudis.2013.11.002
Kim S, Choi SH, Lee YM, Kim MJ, Kim YD, Kim JY, Park JH, Myung W, Na HR, Han HJ et al (2015) Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatr 27:2069–2077. https://doi.org/10.1017/S1041610215001076
Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, Baker L, Belleville S, Brodaty H, Brucki SM (2020) World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement 16:1078–1094
Kokko JP (1984) Site and mechanism of action of diuretics. Am J Med 77:11–17. https://doi.org/10.1016/s0002-9343(84)80003-0
Kokmen E, Whisnant JP, O’Fallon WM, Chu CP, Beard CM (1996) Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984). Neurology 46:154–159. https://doi.org/10.1212/wnl.46.1.154
Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, Kawas C, Breitner JC, Fitzpatrick A, Dulberg C (2005) Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 64:1548–1552. https://doi.org/10.1212/01.Wnl.0000160115.55756.De
Lanza G, Cantone M, Musso S, Borgione E, Scuderi C, Ferri R (2018) Early-onset subcortical ischemic vascular dementia in an adult with mtDNA mutation 3316G>A. J Neurol 265:968–969. https://doi.org/10.1007/s00415-018-8795-x
Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 58:498–504. https://doi.org/10.1001/archneur.58.3.498
Lautenschlager NT, Cox K, Cyarto EV (2012) The influence of exercise on brain aging and dementia. Biochim Biophys Acta (BBA) Mol Basis Dis 1822:474–481. https://doi.org/10.1016/j.bbadis.2011.07.010
Leblanc GG, Meschia JF, Stuss DT, Hachinski V (2006) Genetics of vascular cognitive impairment. Stroke 37:248–255. https://doi.org/10.1161/01.STR.0000195177.61184.49
Lejri I, Grimm A, Eckert A (2018) Mitochondria, estrogen and female brain aging. Front Aging Neurosci 10:124–124. https://doi.org/10.3389/fnagi.2018.00124
Lenglet S, Montecucco F, Mach F, Schaller K, Gasche Y, Copin J-C (2017) Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke. Thromb Haemost 112:363–378. https://doi.org/10.1160/TH14-01-0007
Leuner K, Müller W, Reichert A (2012) From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol Neurobiol 46:186–193. https://doi.org/10.1007/s12035-012-8307-4
Li H, Liu Y, Lin LT, Wang XR, Du SQ, Yan CQ, He T, Yang JW, Liu CZ (2016) Acupuncture reversed hippocampal mitochondrial dysfunction in vascular dementia rats. Neurochem Int 92:35–42. https://doi.org/10.1016/j.neuint.2015.12.001
Lin J, Wang D, Lan L, Fan Y (2017) Multiple factors involved in the pathogenesis of white matter lesions. Biomed Res Int 2017:9372050. https://doi.org/10.1155/2017/9372050
Lindén T, Skoog I, Fagerberg B, Steen B, Blomstrand C (2004) Cognitive impairment and dementia 20 months after stroke. Neuroepidemiology 23:45–52. https://doi.org/10.1159/000073974
Ling C, Liu Z, Song M, Zhang W, Wang S, Liu X, Ma S, Sun S, Fu L, Chu Q et al (2019) Modeling CADASIL vascular pathologies with patient-derived induced pluripotent stem cells. Protein Cell 10:249–271. https://doi.org/10.1007/s13238-019-0608-1
Liu H, Zhang J (2012) Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci 122:494–499. https://doi.org/10.3109/00207454.2012.686543
Liu Q, He S, Groysman L, Shaked D, Russin J, Scotton TC, Cen S, Mack WJ (2013) White matter injury due to experimental chronic cerebral hypoperfusion is associated with C5 deposition. PLoS ONE 8:e84802. https://doi.org/10.1371/journal.pone.0084802
Ma Y, Liu Y, Zhang Z, Yang G-Y (2019) Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis 10:429–462. https://doi.org/10.14336/AD.2019.0119
Magami S, Miyamoto N, Ueno Y, Hira K, Tanaka R, Yamashiro K, Oishi H, Arai H, Urabe T, Hattori N (2019) The effects of astrocyte and oligodendrocyte lineage cell interaction on white matter injury under chronic cerebral hypoperfusion. Neuroscience 406:167–175. https://doi.org/10.1016/j.neuroscience.2019.03.004
Maloney B, Lahiri DK (2016) Epigenetics of dementia: understanding the disease as a transformation rather than a state. Lancet Neurol 15:760–774. https://doi.org/10.1016/S1474-4422(16)00065-X
Man S, Ubogu E, Ransohoff R (2007) Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol 17:243–250. https://doi.org/10.1111/j.1750-3639.2007.00067.x
Mancuso C, Scapagini G, Currò D, Stella AMG, Marco CD, Butterfield DA, Calabrese V (2007) Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. FBL 12:1107–1123
Markus HS, Schmidt R (2019) Genetics of vascular cognitive impairment. Stroke 50:765–772. https://doi.org/10.1161/STROKEAHA.118.020379
Masumura M, Hata R, Nagai Y, Sawada T (2001) Oligodendroglial cell death with DNA fragmentation in the white matter under chronic cerebral hypoperfusion: comparison between normotensive and spontaneously hypertensive rats. Neurosci Res 39:401–412. https://doi.org/10.1016/S0168-0102(01)00195-X
Mathey E, Park S, Hughes R, Pollard J, Armati P, Barnett M, Taylor B, Dyck P, Kiernan M, Lin C (2015) Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2014-309697
Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T (1994) Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients. Hypertension 23:565–568. https://doi.org/10.1161/01.HYP.23.5.565
Matsuyama H, Shindo A, Shimada T, Yata K, Wakita H, Takahashi R, Tomimoto H (2020) Chronic cerebral hypoperfusion activates AIM2 and NLRP3 inflammasome. Brain Res 1736:146779–146779. https://doi.org/10.1016/J.BRAINRES.2020.146779
Matute C (2011) Glutamate and ATP signalling in white matter pathology. J Anat 219:53–64. https://doi.org/10.1111/j.1469-7580.2010.01339.x
Matute C, Ransom BR (2012) Roles of white matter in central nervous system pathophysiologies. ASN Neuro 4:e00079–e00079. https://doi.org/10.1042/AN20110060
McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, Hortobagyi T, Ince P, Jellinger K, Gao J et al (2016) Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med 14:129–129. https://doi.org/10.1186/s12916-016-0676-5
McQueen J, Reimer M, Holland P, Manso Y, McLaughlin M, Fowler J, Horsburgh K (2014) Restoration of oligodendrocyte pools in a mouse model of chronic cerebral hypoperfusion. PLoS ONE 9:e87227–e87227. https://doi.org/10.1371/journal.pone.0087227
Mekaj YH, Daci FT, Mekaj AY (2015) New insights into the mechanisms of action of aspirin and its use in the prevention and treatment of arterial and venous thromboembolism. Ther Clin Risk Manag 11:1449–1456. https://doi.org/10.2147/tcrm.S92222
Miyamoto N, Magami S, Inaba T, Ueno Y, Hira K, Kijima C, Nakajima S, Yamashiro K, Urabe T, Hattori N (2020) The effects of A1/A2 astrocytes on oligodendrocyte linage cells against white matter injury under prolonged cerebral hypoperfusion. Glia 68:1910–1924. https://doi.org/10.1002/glia.23814
Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M et al (2015) Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci 35:14002–14008. https://doi.org/10.1523/jneurosci.1592-15.2015
Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A et al (2018) Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 24(3):326–337. https://doi.org/10.1038/nm.4482
Morris MC (2016) Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci 1367:31–37. https://doi.org/10.1111/nyas.13047
Morris MC (2012) Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc 71:1–13. https://doi.org/10.1017/s0029665111003296
Morris MC, Tangney CC (2014) Dietary fat composition and dementia risk. Neurobiol Aging 35(Suppl 2):S59-64. https://doi.org/10.1016/j.neurobiolaging.2014.03.038
Mracskó E, Hugyecz M, Institoris A, Farkas E, Bari F (2009) Changes in pro-oxidant and antioxidant enzyme levels during cerebral hypoperfusion in rats. Brain Res 1321:13–19. https://doi.org/10.1016/j.brainres.2009.11.080
Nagai M, Hoshide S, Kario K (2010) Hypertension and dementia. Am J Hypertens 23:116–124. https://doi.org/10.1038/ajh.2009.212
Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG et al (2019) Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25(2):270–276. https://doi.org/10.1038/s41591-018-0297-y
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–C686. https://doi.org/10.1152/ajpcell.00213.2006
Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH (2005) Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 53:1101–1107. https://doi.org/10.1111/j.1532-5415.2005.53360.x
Nishio K, Ihara M, Yamasaki N, Kalaria R, Maki T, Fujita Y, Ito H, Oishi N, Fukuyama H, Miyakawa T et al (2010) A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke J Cereb Circ 41:1278–1284. https://doi.org/10.1161/STROKEAHA.110.581686
Niu XL, Jiang X, Xu GD, Zheng GM, Tang ZP, Yin N, Li XQ, Yang YY, Lv PY (2018) DL-3-n-butylphthalide alleviates vascular cognitive impairment by regulating endoplasmic reticulum stress and the Shh/Ptch1 signaling-pathway in rats. J Cell Physiol. https://doi.org/10.1002/jcp.27332
Nunnari J, Suomalainen A (2012) Mitochondria: in sickness and in health. Cell 148:1145–1159. https://doi.org/10.1016/j.cell.2012.02.035
O’Barr S, Cooper NR (2000) The C5a complement activation peptide increases IL-1β and IL-6 release from amyloid-β primed human monocytes: implications for Alzheimer’s disease. J Neuroimmunol 109:87–94. https://doi.org/10.1016/S0165-5728(00)00291-5
O’Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SCR, Markus HS (2002) Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 59:321–326. https://doi.org/10.1212/WNL.59.3.321
Ogino S, Lochhead P, Chan AT, Nishihara R, Cho E, Wolpin BM, Meyerhardt JA, Meissner A, Schernhammer ES, Fuchs CS et al (2013) Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol Off J US Can Acad Pathol Inc 26:465–484. https://doi.org/10.1038/modpathol.2012.214
Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A (1995) Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. BMJ 310:970–973. https://doi.org/10.1136/bmj.310.6985.970
Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, Yonemoto K, Kitazono T, Kiyohara Y (2013) Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr 97:1076–1082. https://doi.org/10.3945/ajcn.112.045575
Paciaroni M, Bogousslavsky J (2013) Connecting cardiovascular disease and dementia: further evidence. J Am Heart Assoc 2:e000656. https://doi.org/10.1161/jaha.113.000656
Park J-M, Kim YJ, Song MK, Lee J-M, Kim Y-J (2018) Genome-wide DNA methylation profiling in a rat model with vascular dementia. Mol Med Rep 18:123–130. https://doi.org/10.3892/mmr.2018.8990
Peila R, Rodriguez BL, Launer LJ (2002) Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes 51:1256–1262. https://doi.org/10.2337/diabetes.51.4.1256
Phillips NA, Mate-Kole CC (1997) Cognitive deficits in peripheral vascular disease. A comparison of mild stroke patients and normal control subjects. Stroke 28:777–784. https://doi.org/10.1161/01.str.28.4.777
Poh L, Fann DY, Wong P, Lim HM, Foo SL, Kang S-W, Rajeev V, Selvaraji S, Iyer VR, Parathy N et al (2021) AIM2 inflammasome mediates hallmark neuropathological alterations and cognitive impairment in a mouse model of vascular dementia. Mol Psychiatry 26:4544–4560. https://doi.org/10.1038/s41380-020-00971-5
Poh L, Razak S, Lim HM, Lai MKP, Chen CL, Lim LHK, Arumugam TV, Fann DY (2021) AIM2 inflammasome mediates apoptotic and pyroptotic death in the cerebellum following chronic hypoperfusion. Exp Neurol 346:113856. https://doi.org/10.1016/j.expneurol.2021.113856
Portegies ML, Wolters FJ, Hofman A, Ikram MK, Koudstaal PJ, Ikram MA (2016) Prestroke vascular pathology and the risk of recurrent stroke and poststroke dementia. Stroke 47:2119–2122. https://doi.org/10.1161/strokeaha.116.014094
Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R (2002) The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology 58:1175–1181. https://doi.org/10.1212/wnl.58.8.1175
Praticò D, Clark CM, Liun F, Lee VYM, Trojanowski JQ (2002) Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol 59:972–976. https://doi.org/10.1001/archneur.59.6.972
Price BR, Wilcock DM, Weekman EM (2018) Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front Aging Neurosci 10:350–350. https://doi.org/10.3389/fnagi.2018.00350
Rafnsson SB, Deary IJ, Fowkes FG (2009) Peripheral arterial disease and cognitive function. Vasc Med 14:51–61. https://doi.org/10.1177/1358863x08095027
Rajeev V, Fann DY, Dinh QN, Kim HA, De Silva TM, Jo DG, Drummond GR, Sobey CG, Lai MKP, Chen CL et al (2022) Intermittent fasting attenuates Hallmark vascular and neuronal pathologies in a mouse model of vascular cognitive impairment. Int J Biol Sci 18:6052–6067. https://doi.org/10.7150/ijbs.75188
Rajeev V, Fann DY, Dinh QN, Kim HA, De Silva TM, Lai MKP, Chen CL, Drummond GR, Sobey CG, Arumugam TV (2022) Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics 12:1639–1658. https://doi.org/10.7150/thno.68304
Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, Mariani E, Licastro F, Patterson C (2007) Blood inflammatory markers and risk of dementia: the conselice study of brain aging. Neurobiol Aging 28:1810–1820. https://doi.org/10.1016/j.neurobiolaging.2006.08.012
Ravona-Springer R, Schnaider-Beeri M (2011) The association of diabetes and dementia and possible implications for nondiabetic populations. Expert Rev Neurother 11:1609–1617. https://doi.org/10.1586/ern.11.152
Reimer M, McQueen J, Searcy L, Scullion G, Desmazières A, Holland P, Smith J, Gliddon C, Wood E, Herzyk P et al (2011) Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion. J Neurosci Off J Soc Neurosci 31:18185–18194. https://doi.org/10.1523/JNEUROSCI.4936-11.2011
Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13:566–578. https://doi.org/10.1038/nrm3412
Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC (2010) Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging 31:1894–1902. https://doi.org/10.1016/j.neurobiolaging.2008.10.018
Rohn TT, McCarty KL, Love JE, Head E (2014) Is apolipoprotein E4 an important risk factor for dementia in persons with down syndrome? J Parkinson’s Dis Alzheimer’s Dis 1:7–7. https://doi.org/10.13188/2376-922x.1000004
Román GC (2003) Stroke, cognitive decline and vascular dementia: the silent epidemic of the 21st century. Neuroepidemiology 22:161–164. https://doi.org/10.1159/000069885
Román GC (2002) Vascular dementia may be the most common form of dementia in the elderly. J Neurol Sci 203:7–10. https://doi.org/10.1016/S0022-510X(02)00252-6
Rosenberg GA, Sullivan N, Esiri MM (2001) White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke 32:1162–1167. https://doi.org/10.1161/01.STR.32.5.1162
Ruitenberg A, Den Heijer T, Bakker SLM, Van Swieten JC, Koudstaal PJ, Hofman A, Breteler MMB (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 57:789–794. https://doi.org/10.1002/ANA.20493
Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimäki M, Batty GD (2013) Socioeconomic status as a risk factor for dementia death: individual participant meta-analysis of 86508 men and women from the UK. Br J Psychiatry 203:10–17. https://doi.org/10.1192/bjp.bp.112.119479
Rygiel K (2016) Can angiotensin-converting enzyme inhibitors impact cognitive decline in early stages of Alzheimer’s disease? An overview of research evidence in the elderly patient population. J Postgrad Med 62:242–248. https://doi.org/10.4103/0022-3859.188553
Sabayan B, Jansen S, Oleksik AM, van Osch MJP, van Buchem MA, van Vliet P, de Craen AJM, Westendorp RGJ (2012) Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev 11:271–277. https://doi.org/10.1016/j.arr.2011.12.009
Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer D, Chen C, Chui H (2014) Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 28:206–206
Saito A (2014) Physiological functions of endoplasmic reticulum stress transducer OASIS in central nervous system. Anat Sci Int 89:11–20. https://doi.org/10.1007/s12565-013-0214-x
Saridin FN, Hilal S, Villaraza SG, Reilhac A, Gyanwali B, Tanaka T, Stephenson MC, Ng SL, Vrooman H, van der Flier WM et al (2020) Brain amyloid β, cerebral small vessel disease, and cognition. Neurology 95:e2845–e2853. https://doi.org/10.1212/WNL.0000000000011029
Scheel P, Puls I, Becker G, Schöning M (1999) Volume reduction in cerebral blood flow in patients with vascular dementia. Lancet 354:2137–2137. https://doi.org/10.1016/S0140-6736(99)04016-7
Schmidt R, Schmidt H, Curb J, Masaki K, White L, Launer L (2002) Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia aging study. Ann Neurol 52:168–174. https://doi.org/10.1002/ana.10265
Schmitz T, Chew L-J (2008) Cytokines and myelination in the central nervous system. TheScientificWorldJOURNAL 8:1119–1147. https://doi.org/10.1100/tsw.2008.140
Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, Miller BL, Kramer JH, Jagust WJ, Chui HC et al (2009) Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 5:454–462. https://doi.org/10.1016/J.JALZ.2009.04.1233
Schuit AJ, Feskens MEJ, Launer LJ, Kromhout D (2001) Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc 33:772
Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J (2018) Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health 3:e124–e132. https://doi.org/10.1016/s2468-2667(18)30022-7
Selvaraji S, Efthymios M, Foo RSY, Fann DY, Lai MKP, Chen CLH, Lim KL, Arumugam TV (2022) Time-restricted feeding modulates the DNA methylation landscape, attenuates hallmark neuropathology and cognitive impairment in a mouse model of vascular dementia. Theranostics 12:3007–3023. https://doi.org/10.7150/thno.71815
Shabir O, Berwick J, Francis S (2018) Neurovascular dysfunction in vascular dementia, Alzhiemers and atherosclerosis. BMC Neurosci. https://doi.org/10.1186/s12868-018-0465-5
Sharp ES, Gatz M (2011) Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord 25:289–304. https://doi.org/10.1097/WAD.0b013e318211c83c
Shen Y, Halperin JA, Lee C-M (1995) Complement-mediated neurotoxicity is regulated by homologous restriction. Brain Res 671:282–292. https://doi.org/10.1016/0006-8993(94)01264-I
Shi G-X, Liu C-Z, Wang L-P, Guan L-P, Li S-Q (2012) Biomarkers of oxidative stress in vascular dementia patients. Can J Neurol Sci J Can Sci Neurol 39:65–68. https://doi.org/10.1017/S0317167100012701
Shibata M, Ohtani R, Ihara M, Tomimoto H (2004) White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 35:2598–2603. https://doi.org/10.1161/01.Str.0000143725.19053.60
Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, Ihara M, Takahashi R, Tomimoto H (2007) Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke 38:2826–2832. https://doi.org/10.1161/STROKEAHA.107.490151
Silva IVG, de Figueiredo RC, Rios DRA (2019) Effect of different classes of antihypertensive drugs on endothelial function and inflammation. Int J Mol Sci. https://doi.org/10.3390/ijms20143458
Simpson JE, Fernando MS, Clark L, Ince PG, Matthews F, Forster G, O’Brien JT, Barber R, Kalaria RN, Brayne C et al (2007) White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol 33:410–419. https://doi.org/10.1111/j.1365-2990.2007.00828.x
Skoog I (1998) Status of risk factors for vascular dementia. Neuroepidemiology 17:2–9. https://doi.org/10.1159/000026147
Skrobot OA, Attems J, Esiri M, Hortobágyi T, Ironside JW, Kalaria RN, King A, Lammie GA, Mann D, Neal J et al (2016) Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain 139:2957–2969. https://doi.org/10.1093/brain/aww214
Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420–a020420. https://doi.org/10.1101/cshperspect.a020420
Son H-H, Pt OhMS, Jung L, Pt PMS, Rae J, Pt PhD (2010) The effect of an exercise program on activities of daily living (ADL), balance and cognition in elderly individuals with Alzheimer’s disease and vascular dementia. J Korean Phys Ther 22:53–60
Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV (2016) Junctional proteins of the blood–brain barrier: new insights into function and dysfunction. Tissue Barriers. https://doi.org/10.1080/21688370.2016.1154641
Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH (1985) A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med 313:1044–1049. https://doi.org/10.1056/nejm198510243131703
Stanimirovic DB, Friedman A (2012) Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metabol 32:1207–1221. https://doi.org/10.1038/jcbfm.2012.25
Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271:1004–1010
Stewart R (2002) Vascular dementia: a diagnosis running out of time. Br J Psychiatry 180:152–156. https://doi.org/10.1192/bjp.180.2.152
Stys PK, Waxman SG, Ransom BR (1992) Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)–Ca2+ exchanger. J Neurosci 12:430–439. https://doi.org/10.1523/JNEUROSCI.12-02-00430.1992
Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM (2013) Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J Cereb Blood Flow Metab 33:708–715. https://doi.org/10.1038/jcbfm.2013.1
Sun Z-K, Ma XR, Jia YJ, Liu YR, Zhang JW, Zhang BA (2014) Effects of resveratrol on apoptosis in a rat model of vascular dementia. Exp Ther Med 7:843–848. https://doi.org/10.3892/etm.2014.1542
Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ (2019) Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimers Dement 15:158–167
Takeshita Y, Ransohoff RM (2012) Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev 248:228–239. https://doi.org/10.1111/j.1600-065X.2012.01127.x
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820. https://doi.org/10.1016/j.cell.2010.01.022
Tampi RR, Tampi DJ, Balachandran S, Srinivasan S (2016) Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis 7:229–245. https://doi.org/10.1177/2040622316658463
Tan R, Traylor M, Rutten-Jacobs L, Markus H (2017) New insights into mechanisms of small vessel disease stroke from genetics. Clin Sci 131:515–531. https://doi.org/10.1042/cs20160825
Tanaka K-I, Kawahara M (2017) Copper enhances zinc-induced neurotoxicity and the endoplasmic reticulum stress response in a neuronal model of vascular dementia. Front Neurosci 11:58–58. https://doi.org/10.3389/fnins.2017.00058
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29:347–364. https://doi.org/10.1038/s41422-019-0164-5
Tanovic A, Alfaro V (2006) Glutamate-related excitotoxicity neuroprotection with memantine, an uncompetitive antagonist of NMDA-glutamate receptor, in Alzheimer’s disease and vascular dementia. Rev Neurol 42:607–616. https://doi.org/10.33588/rn.4210.2005252
Tarumi T, Shah F, Tanaka H, Haley AP (2011) Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens 24:1108–1113. https://doi.org/10.1038/ajh.2011.101
Tasci I, Safer U, Naharci MI, Gezer M, Demir O, Bozoglu E, Doruk H (2018) Undetected peripheral arterial disease among older adults with Alzheimer’s disease and other dementias. Am J Alzheimers Dis Other Demen 33:5–11. https://doi.org/10.1177/1533317517724000
Tham W, Auchus AP, Thong M, Goh M-L, Chang H-M, Wong M-C, Chen CPLH (2002) Progression of cognitive impairment after stroke: one year results from a longitudinal study of Singaporean stroke patients. J Neurol Sci 203:49–52. https://doi.org/10.1016/S0022-510X(02)00260-5
Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, Kimura J (1996) Alterations of the blood–brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke 27:2069–2074. https://doi.org/10.1161/01.STR.27.11.2069
Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, Mahmood A, Fooks P, Singh-Manoux A, Mackay CE et al (2017) Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ 357:j2353. https://doi.org/10.1136/bmj.j2353
Toth P, Tarantini S, Csiszar A, Ungvari Z (2017) Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312:H1–H20. https://doi.org/10.1152/AJPHEART.00581.2016
Toyama K, Koibuchi N, Uekawa K, Hasegawa Y, Kataoka K, Katayama T, Sueta D, Ma MJ, Nakagawa T, Yasuda O et al (2014) Apoptosis signal-regulating kinase 1 is a novel target molecule for cognitive impairment induced by chronic cerebral hypoperfusion. Arterioscler Thromb Vasc Biol 34:616–625. https://doi.org/10.1161/ATVBAHA.113.302440
Tripolt NJ, Stekovic S, Aberer F, Url J, Pferschy PN, Schröder S, Verheyen N, Schmidt A, Kolesnik E, Narath SH et al (2018) Intermittent fasting (alternate day fasting) in healthy, non-obese adults: protocol for a cohort trial with an embedded randomized controlled pilot trial. Adv Ther 35:1265–1283. https://doi.org/10.1007/s12325-018-0746-5
Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH (2008) B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A 105:12474–12479. https://doi.org/10.1073/pnas.0805350105
Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ (2008) Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol 64:168–176. https://doi.org/10.1002/ana.21413
Truettner JS, Alonso OF, Dietrich WD (2005) Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab 25:1505–1516. https://doi.org/10.1038/SJ.JCBFM.9600150
Ueno M, Tomimoto H, Akiguchi I, Wakita H, Sakamoto H (2016) Blood–brain barrier disruption in white matter lesions in a rat model of chronic cerebral hypoperfusion. J Cereb Blood Flow Metab 22:97–104. https://doi.org/10.1097/00004647-200201000-00012
Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A (2010) Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65:1028–1041. https://doi.org/10.1093/gerona/glq113
Utech M, Mennigen R, Bruewer M (2010) Endocytosis and recycling of tight junction proteins in inflammation. J Biomed Biotechnol. https://doi.org/10.1155/2010/484987
van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC (2015) Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 6:154–168. https://doi.org/10.3945/an.114.007617
van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, Scheltens P (2018) Vascular cognitive impairment. Nat Rev Dis Primers 4(1):1–16. https://doi.org/10.1038/nrdp.2018.3
Varatharaj A, Galea I (2017) The blood–brain barrier in systemic inflammation. Brain Behav Immun 60:1–12. https://doi.org/10.1016/j.bbi.2016.03.010
Venkat P, Chopp M, Chen J (2015) Models and mechanisms of vascular dementia. Exp Neurol 272:97–108. https://doi.org/10.1016/J.EXPNEUROL.2015.05.006
Verbaten MN (2009) Chronic effects of low to moderate alcohol consumption on structural and functional properties of the brain: beneficial or not? Hum Psychopharmacol 24:199–205. https://doi.org/10.1002/hup.1022
Verdelho A, Madureira S, Ferro JM, Baezner H, Blahak C, Poggesi A, Hennerici M, Pantoni L, Fazekas F, Scheltens P et al (2012) Physical activity prevents progression for cognitive impairment and vascular dementia. Stroke 43:3331–3335. https://doi.org/10.1161/STROKEAHA.112.661793
Vernooij MW, Van der Lugt A, Ikram MA, Wielopolski PA, Vrooman HA, Hofman A, Krestin GP, Breteler MM (2007) Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam scan study. J Cereb Blood Flow Metab 28:412–419. https://doi.org/10.1038/SJ.JCBFM.9600526
Wallace DC (2001) A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp 235:247–263. https://doi.org/10.1002/0470868694.ch20
Wallin A, Kapaki E, Boban M, Engelborghs S, Hermann DM, Huisa B, Jonsson M, Kramberger MG, Lossi L, Malojcic B et al (2017) Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease—a consensus report. BMC Neurol 17:102–102. https://doi.org/10.1186/s12883-017-0877-3
Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K (2012) Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS ONE 7:e45499. https://doi.org/10.1371/journal.pone.0045499
Wang T, Liu CZ, Yu JC, Jiang W, Han JX (2009) Acupuncture protected cerebral multi-infarction rats from memory impairment by regulating the expression of apoptosis related genes Bcl-2 and Bax in hippocampus. Physiol Behav 96:155–161. https://doi.org/10.1016/j.physbeh.2008.09.024
Ward NC, Watts GF, Eckel RH (2019) Statin toxicity. Circ Res 124:328–350. https://doi.org/10.1161/circresaha.118.312782
Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Maniega SM, Farrall A, Sudlow C, Dennis M, Dhillon B (2009) Lacunar stroke is associated with diffuse blood–brain barrier dysfunction. Ann Neurol 65:194–202. https://doi.org/10.1002/ANA.21549
Webb AJS, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM (2012) Increased cerebral arterial pulsatility in patients with leukoaraiosis. Stroke 43:2631–2636. https://doi.org/10.1161/STROKEAHA.112.655837
Wentzel C, Rockwood K, MacKnight C, Hachinski V, Hogan DB, Feldman H, Østbye T, Wolfson C, Gauthier S, Verreault R et al (2001) Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology 57:714–716. https://doi.org/10.1212/WNL.57.4.714
Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH (2017) Beta-blockers for hypertension. Cochrane Database Syst Rev 1:Cd002003. https://doi.org/10.1002/14651858.CD002003.pub5
Wu C-x, Liu R, Gao M, Zhao G, Wu S, Wu C-f, Du G-h (2013) Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett 546:57–62. https://doi.org/10.1016/j.neulet.2013.04.060
Wu L-Y, Kan CN, Cheah IK, Chong JR, Xu X, Vrooman H, Hilal S, Venketasubramanian N, Chen CP, Halliwell B et al (2022) Low plasma ergothioneine predicts cognitive and functional decline in an elderly cohort attending memory clinics. Antioxidants 11:1717
Wu LY, Cheah IK, Chong JR, Chai YL, Tan JY, Hilal S, Vrooman H, Chen CP, Halliwell B, Lai MKP (2021) Low plasma ergothioneine levels are associated with neurodegeneration and cerebrovascular disease in dementia. Free Radic Biol Med 177:201–211. https://doi.org/10.1016/j.freeradbiomed.2021.10.019
Wu X, Sun J, Li L (2013) Chronic cerebrovascular hypoperfusion affects global DNA methylation and histone acetylation in rat brain. Neurosci Bull 29:685–692. https://doi.org/10.1007/s12264-013-1345-8
Xiang C, Wang Y, Zhang H, Han F (2017) The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 22:1–26. https://doi.org/10.1007/s10495-016-1296-4
Xiong Z-G, Zhu X-M, Chu X-P, Minami M, Hey J, Wei W-L, MacDonald JF, Wemmie JA, Price MP, Welsh MJ et al (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118:687–698. https://doi.org/10.1016/j.cell.2004.08.026
Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L (2009) Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 58:71–77. https://doi.org/10.2337/db08-0586
Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G (2003) Association between dementia and midlife risk factors: the radiation effects research foundation adult health study. J Am Geriatr Soc 51:410–414. https://doi.org/10.1046/j.1532-5415.2003.51117.x
Yang T, Sun Y, Lu Z, Leak RK, Zhang F (2017) The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev 34:15–29. https://doi.org/10.1016/j.arr.2016.09.007
Yang Y, Jiang G, Zhang P, Fan J (2015) Programmed cell death and its role in inflammation. Mil Med Res 2:12–12. https://doi.org/10.1186/s40779-015-0039-0
Yang Z, Zhang N, Shen H, Lin C, Lin L, Yuan B (2014) Microglial activation with reduction in autophagy limits white matter lesions and improves cognitive defects during cerebral hypoperfusion. Curr Neurovasc Res 11:223–229. https://doi.org/10.2174/1567202611666140520124407
Yarlagadda A, Alfson E, Clayton AH (2009) The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry 6:18–18
Yassi N, Hilal S, Xia Y, Lim YY, Watson R, Kuijf H, Fowler C, Yates P, Maruff P, Martins R (2020) Influence of comorbidity of cerebrovascular disease and amyloid-β on Alzheimer’s disease. J Alzheimers Dis 73:897–907
Yenari MA, Kauppinen TM, Swanson RA (2010) Microglial activation in stroke: therapeutic targets. Neurother J Am Soc Exp NeuroTher 7:378–391. https://doi.org/10.1016/j.nurt.2010.07.005
Yin F, Boveris A, Cadenas E (2014) Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal 20:353–371. https://doi.org/10.1089/ars.2012.4774
Zhang LY, Pan J, Mamtilahun M, Zhu Y, Wang L, Venkatesh A, Shi R, Tu X, Jin K, Wang Y et al (2020) Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 10:74–90. https://doi.org/10.7150/thno.35841
Zheng Y, Zhang J, Zhao Y, Zhang Y, Zhang X, Guan J, Liu Y, Fu J (2020) Curcumin protects against cognitive impairments in a rat model of chronic cerebral hypoperfusion combined with diabetes mellitus by suppressing neuroinflammation, apoptosis, and pyroptosis. Int Immunopharmacol 93:107422
Zhou Y, Zhang J, Wang L, Chen Y, Wan Y, He Y, Jiang L, Ma J, Liao R, Zhang X et al (2017) Interleukin-1β impedes oligodendrocyte progenitor cell recruitment and white matter repair following chronic cerebral hypoperfusion. Brain Behav Immun 60:93–105. https://doi.org/10.1016/j.bbi.2016.09.024
Zhu Y, Chai YL, Hilal S, Ikram MK, Venketasubramanian N, Wong B-S, Chen CP, Lai MKP (2017) Serum IL-8 is a marker of white-matter hyperintensities in patients with Alzheimer’s disease. Alzheimer’s Dement 7:41–47. https://doi.org/10.1016/j.dadm.2017.01.001
Zhu Y, Hilal S, Chai YL, Ikram MK, Venketasubramanian N, Chen CP, Lai MKP (2018) Serum hepatocyte growth factor is associated with small vessel disease in Alzheimer’s dementia. Front Aging Neurosci 10:8–8. https://doi.org/10.3389/fnagi.2018.00008
Zuccalà G, Onder G, Pedone C, Carosella L, Pahor M, Bernabei R, Cocchi A (2001) Hypotension and cognitive impairment: selective association in patients with heart failure. Neurology 57:1986–1992. https://doi.org/10.1212/wnl.57.11.1986
Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti A, Ble A, Fellin R (2007) Plasma cytokine profile in older subjects with late onset Alzheimer’s disease or vascular dementia. J Psychiatr Res 41:686–693. https://doi.org/10.1016/j.jpsychires.2006.02.008
Acknowledgements
The authors would like to thank the patients and their families for participating in the clinical studies cited in this review.
Funding
This work was supported by the National Medical Research Council of Singapore (MOH-000500-03, MOH-000707-01, MOH-001086-00, MOH-001086-00 to CPC, MKPL; NMRC-CBRG-0102/2016, NMRC/OFIRG/0036/2017 to TVA), Yong Loo Lin School of Medicine, National University of Singapore (Healthy Longevity Translational Research Programme HLTRP/2022/PS-01 to MKPL; Post-Doctoral Fellowship Award NUSMED/2021/PDF/05 to YLC), and La Trobe University (start-up grant to TVA).
Author information
Authors and Affiliations
Contributions
VR, TVA, CPC and MKPL developed the ideas for the review. DYF, DGJ, TMDS, GRD and CGS provided domain expertise. VR, YLC, LP and SS performed literature reviews. VR and YLC drafted the manuscript. All authors have read, edited and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rajeev, V., Chai, Y.L., Poh, L. et al. Chronic cerebral hypoperfusion: a critical feature in unravelling the etiology of vascular cognitive impairment. acta neuropathol commun 11, 93 (2023). https://doi.org/10.1186/s40478-023-01590-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-023-01590-1