Abstract
Precursor B acute lymphoblastic leukemia (BCP-ALL), the most common childhood malignancy, arises from an expansion of malignant B cell precursors in the bone marrow. Epidemiological studies suggest that infections or immune responses to infections may promote such an expansion and thus BCP-ALL development. Nevertheless, a specific pathogen responsible for this process has not been identified. BCP-ALL cells critically depend on interactions with the bone marrow microenvironment. The bone marrow is also home to memory T helper (Th) cells that have previously expanded during an immune response in the periphery. In secondary lymphoid organs, Th cells can interact with malignant cells of mature B cell origin, while such interactions between Th cells and malignant immature B cell in the bone marrow have not been described yet. Nevertheless, literature supports a model where Th cells—expanded during an infection in early childhood—migrate to the bone marrow and support BCP-ALL cells as they support normal B cells. Further research is required to mechanistically confirm this model and to elucidate the interaction pathways between leukemia cells and cells of the tumor microenvironment. As benefit, targeting these interactions could be included in current treatment regimens to increase therapeutic efficiency and to reduce relapses.
Similar content being viewed by others
Introduction
Precursor B cell acute lymphoblastic leukemia (BCP-ALL) is the most common childhood malignancy and represents the leading cause of cancer-related death in children and young adults [1]. BCP-ALL arises from a monoclonal or oligoclonal expansion of malignant B cell precursors in the bone marrow. The malignant cells are characterized by chromosomal alterations leading to the expression of mutant proteins that confer survival and proliferation advantages. Nevertheless, these genetic lesions are not sufficient for BCP-ALL development. This is suggested by the fact that precursor B cells carrying such characteristic mutations are frequently found in newborns but the prevalence of leukemia is approximately a hundredfold lower [2, 3]. Based on these observations, a two-step model was proposed according to which the leukemia-initiating genetic lesion occurs in utero, followed by an event that promotes expansion of the pre-leukemic clone and eventually leads to the emergence of leukemia. Multiple causes for such a second event have been suggested, and most probably several of them account for the eventual transition to leukemia. Interestingly, epidemiological studies provide evidence that infections or immune responses to infections may represent a major trigger for the leukemia pathogenesis.
In the late 1980s, Kinlen noted a temporal increase in childhood leukemia in several occasions where previously isolated populations mingled [4,5,6,7,8]. At the same time, Mel Greaves postulated a “delayed infection” hypothesis, according to which the development of leukemia is partly caused by an abnormal immune reaction to a common infectious agent [9]. Thereafter, several large studies reported that children who attended a playgroup during their first year of life showed a significant protection from childhood ALL [10, 11]. Thus, similar to the hygiene hypothesis in allergy and asthma, a delayed exposure to common pathogens in developed societies may lead to abnormal or dysregulated immune responses that promote growth of the leukemic clone [12]. Most recently, this model received mechanistic support by an elegant investigation showing that experimental mice predisposed for BCP-ALL development due to a PAX5 mutation only developed BCP-ALL upon transfer from specific pathogen-free (SPF) environment to an environment containing common pathogens [13].
Immune responses to pathogens are typically composed of concerted actions by several types of immune cells. T helper (Th) cells play a central role in orchestrating immune responses by instructing and activating other immune cells. B cells, for example, depend on interaction with Th cells for their survival, proliferation, differentiation to plasma cells, hypermutation, class-switch recombination, adhesion, and migration [14]. While central in a functional immune system, such Th cell-B cell interactions, however, can also contribute to the pathogenesis of lymphoma and leukemia.
Malignantly transformed B cells often retain their capacity to interact with Th cells. As a consequence, malignant B cells seem to profit from Th cell help similar to healthy B cells. Such interactions between Th cells and malignant B cells with untoward effects have been described for several cancers arising from mature B cells, the main targets of Th cells. In contrast, the interaction of Th cells with malignant immature B cells such as BCP-ALL cells has not been studied extensively. In this article, we review the literature concerning the role of Th cells in mature B cell malignancies and summarize data hinting at a role of Th cells in BCP-ALL, i.e., in immature B cells, all in the context of the theory of an infectious etiology of BCP-ALL.
Review
Role of the microenvironment in BCP-ALL
The tumor microenvironment plays a key role in supporting survival and expansion of cancer cells [15,16,17]. In BCP-ALL, a variety of bone marrow stromal cells are believed to support survival and proliferation of BCP-ALL cells [18,19,20,21] and to confer drug resistance leading to treatment failure or disease relapse [22, 23]. Mesenchymal stromal cells [24], bone marrow endothelial cells [25], osteoblasts [26], and adipocytes [27] have all been shown to interact with BCP-ALL cells in mechanisms involving both soluble factors like cytokines, chemokines, and growth factors [28,29,30,31,32,33] as well as cell membrane-bound molecules such as Galectin-3 [34] or VE-cadherin [35]. These crosstalks between leukemic cells and cells of the tumor microenvironment include signaling pathways such as Notch signaling [36] or the wnt pathway [37]. While the microenvironment supports leukemia cells, the leukemia cells, in turn, shape the microenvironment according to their own benefit [38,39,40,41]. As a consequence, the bone marrow of leukemia patients exhibits substantial alterations that lead to support of the malignant cells and to impaired hematopoiesis [42].
The bone marrow is also home to mature Th cells [43,44,45]. These Th cells are derived from a past immune response in the periphery, where they have expanded and subsequently migrated to the bone marrow in order to provide long-term memory allowing for raising a rapid memory response upon re-challenge [46,47,48]. In addition, these bone marrow Th cells play a crucial role in normal hematopoiesis through the secretion of cytokines and chemokines [49,50,51].
Involvement of Th cells in B cell malignancies
Physiological T cell help for B cells takes place in germinal centers in peripheral lymphoid organs, where follicular Th cells interact with mature antigen-stimulated B cells. This interaction involves membrane-bound molecules like CD40 on the B cells and CD40L on the Th cells but also soluble factors like cytokines, chemokines or B cell-activating factor (BAFF) and Fms-related tyrosine kinase 3 (flt3) ligand. Besides providing a suitable environment for the interaction of Th cells and B cells, germinal centers are also the site where malignant transformation of B cells occurs most frequently. This has led to the hypothesis that Th cells may not only support normal germinal center B cells but also germinal center cell-derived malignant B cells. In fact, there is increasing evidence for supportive role of Th cells in mature B cell malignancies. Follicular lymphoma (FL) is a lymphoma of B cells residing in follicles of secondary lymph nodes. FL cells showed an increased survival when stimulated by CD40 crosslinking in vitro [52] as well as upon cognate interaction with CD4+ Th cells [53]. Support of FL cells by Th cells was also observed in vivo and seems to be mediated by follicular Th cell-derived CD40L and IL-4 [54]. Hodgkin lymphoma—another B cell malignancy presumably arising from germinal center B cells—is characterized by infiltration of Th cells. Even though the function of these infiltrating Th cells is unclear, the presence of certain Th cells subset has been correlated with reduced overall survival, suggesting that these infiltrating Th cells may support growth of the malignant B cells [55, 56].
Chronic lymphocytic leukemia (CLL) is a malignancy of mature B cells, although the precise cell of origin is unclear [57]. CLL cells proliferate in pseudofollicles in secondary lymphoid organs and in the bone marrow, where they receive support from the microenvironment. Recently, we demonstrated that peripheral blood and lymph nodes of CLL patients contained memory Th cells that were specific for endogenous CLL antigens and were able to interact with CLL cells in an antigen-dependent manner [58, 59]. These Th cells had a Th1-like phenotype and supported autologous CLL cell proliferation in vitro and in murine xenograft experiments. Furthermore, interaction of CLL cells with autologous Th cells led to an upregulation of the risk marker CD38 in an interferon (IFN)-γ-dependent mechanism [60]. Thus, while the support of normal mature B cells is central for the generation of an adaptive immune response, the same interaction between Th cells and malignant B cells seems to promote lymphoma or leukemia derived from malignantly transformed mature B cells.
A supportive role for Th cells in BCP-ALL?
Unlike the above-mentioned B cell malignancies that originate from mature B cells, BCP-ALL cells derive from precursor B cells, i.e., immature B cells. Surprisingly, little is known about the influence of Th cells on both normal and malignant precursor B cells in the bone marrow. Intriguingly, BCP-ALL cells as well as normal precursor B cells express all molecules required for cognate interaction with Th cells: CD40 [61], major histocompatibility complex (MHC) class II, adhesion and co-stimulatory molecules [62, 63], and receptors for cytokines [64,65,66,67,68,69,70,71] and BAFF [72, 73]. Thus, they seem to have the capacity to present antigen to Th cells and receive support through the classical pathways.
In fact, BCP-ALL cells are receptive for CD40L stimulation, which generally has an activating effect on BCP-ALL cells, inducing proliferation [74] and upregulation of surface molecules like CD70 [75] and the receptor for IL-3 [76], a cytokine with proliferative function in BCP-ALL. In addition, CD40L stimulation was shown to induce secretion of chemoattractants [77] and to upregulate antigen-processing machinery components [78]. This suggests that BCP-ALL cells may attract Th cells and activate them through presentation of antigens, thereby inducing a positive feedback loop.
BCP-ALL cells can also respond to Th cell cytokines. While IL-2 has been found to stimulate proliferation [66], the Th2 cytokines IL-4 and IL-13 instead inhibited growth [74, 79,80,81], and IL-4 as well as TGF-β induced BCP-ALL cell apoptosis [82,83,84]. More recently, a proliferative effect of the cytokines IL-17 and IL-21 on BCP-ALL cells has been reported [85]. The observations that cytokines may be involved in the pathogenesis of BCP-ALL are consistent with the highly inflammatory environment in the bone marrow of leukemia patients [40].
Further support for the ability of BCP-ALL cells to react to soluble and membrane-bound Th cell factors comes from a report where stimulation with activated allogenic Th cells induced activation and maturation of BCP-ALL cells [86]. Interestingly, BCP-ALL is associated with certain MHC class II haplotypes. This may be a further hint that antigen presentation to Th cells is involved in BCP-ALL, even though mechanistic evidence is not available yet [87, 88].
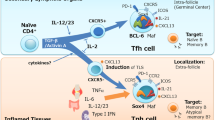
Model for Th cell contribution to BCP-ALL
While epidemiological as well as experimental animal studies suggest that infections contribute to the pathogenesis of BCP-ALL, a specific infectious agent has not been identified. A strong association of BCP-ALL with viral infections such as chicken pox, rubella, measles, and influenza was described in British children [89], but no incorporation of microbial genetic information into host DNA could be detected when 20 BCP-ALL cases where analyzed by representational difference analysis [90]. This makes it unlikely that an infectious agent contributes to BCP-ALL by direct transformation of the precursor B cells. Even though pathogens may stimulate proliferation of precursor B cells or BCP-ALL cells through Toll-like receptors (TLR), this seems rather unlikely, since BCP-ALL cells home to the bone marrow, whereas most pathogens are encountered in the periphery. Instead, Th cells that have expanded during an immune response—possibly due to a delayed first exposure to pathogens—may aberrantly interact with and support the pre-leukemic or leukemic precursor B cells upon (Fig. 1). This interaction may be antigen-independent; alternatively, pathogen-specific Th cells might cross-react with endogenous antigen expressed and presented by the BCP-ALL cells. The nature of such endogenous antigens remains highly speculative and elusive. Attractive candidates are the fusion proteins generated by the characteristic chromosomal translocations, since they are likely to be recognized as foreign as no immune tolerance against these novel proteins has been induced. Indeed, Th cell clones against the fusion proteins TEL/AML1 and BCR/ABL could be generated in vitro [91, 92]. Nevertheless, it remains to be determined whether BCP-ALL patients actually carry such fusion protein-specific Th cells in their bone marrow as expanded clones, and whether these Th cells are able to interact with and support BCP-ALL cells.
Model integrating the infectious etiology hypothesis with the potential role of Th cells in BCP-ALL pathogenesis. Precursor B cells develop in the bone marrow, where they may undergo chromosomal rearrangements. Cells harboring such translocations that confer survival advantages are often present as expanded clones at birth, but this does not necessarily lead to leukemia development (left side). Infections in early childhood induce expansion of Th cells, which home to the bone marrow after the infection has been cleared to take part in normal hematopoiesis and to rise a memory response upon re-challenge with the pathogen (left side). Th cells expanded during an aberrant immune response due to delayed pathogen exposure may aberrantly interact with precursor B cells or leukemia cells or both after migration to the bone marrow, supporting their growth and survival, which ultimately leads to leukemia (right side)
In our work on CLL, we found that the CLL-specific Th cells recognized an antigenic peptide within the CLL B cell receptor (BCR) [58]. While not all subsets of BCP-ALL cells express surface pre-BCR, most express components of the pre-BCR intracellularly. Indeed, epitopes within the variable regions of the pre-BCR are apt candidates to engage Th cells, since they are likely to be presented on MHCII, and the Th cells are presumably not tolerant to these peptides. Still, both the antigen-dependence of the interactions between Th cells and BCP-ALL cells as well as the antigenic source remain speculations.
Implications for therapeutic approaches
Although modern treatment has reached an excellent rate of success in western world, treatment success in high-risk groups such as children with BCR-ABL or MLL-AF4 translocations remains poor. Furthermore, relapse occurs in about 20% of the patients, and the cure rate of a recurrent disease is significantly lower. Survivors often suffer from severe chronic health problems due to the toxic effects current therapy still has [93]. Therefore, there is justified need for biologically targeted therapeutic strategies with less side effects. The tumor microenvironment plays a central role in supporting tumor cell survival, proliferation, and drug resistance. As a consequence, effective leukemia therapies also ought to target the malignant crosstalk between leukemia cells and the supporting cells. Should the leukemia supporting role of Th cells be confirmed, it will be of great importance to elucidate the mechanisms underlying this malignant collaboration, since key molecules of this interaction may be targeted by future therapies. If the interaction of Th cells and BCP-ALL cells is antigen-specific, the BCP-ALL-supporting Th cells are likely to be oligoclonal. Thus, TCR-specific therapeutic antibodies could be generated and used to specifically deplete the leukemia-promoting Th cells, while Th cells with other specificities will remain functional. Although it will not be feasible to apply such a personalized therapy in the initial phase of the treatment, the approach may be combined with the existing drugs in the initial regimen in order to prevent drug resistance and relapse.
Conclusions
The link between BCP-ALL and infections in early childhood was proposed decades ago, but the pathological mechanisms remain unclear. A direct transformation of B cell precursors by pathogens seems unlikely. Instead, immune cells activated and expanded in response to those pathogens may supportively interact with B cell precursors and thereby promote leukemia. Due to their role in the immune system and their presence in the bone marrow, Th cells are good candidates for such leukemia-supportive immune cells. Indeed, it has been reported that BCP-ALL cells are receptive for soluble and membrane-bound Th cell stimuli. Nevertheless, it is to date unclear if BCP-ALL cells are able to receive help from autologous Th cells, and whether such a supportive interaction actually takes place in BCP-ALL patients’ bone marrow. The tumor microenvironment plays a key role in supporting malignant cells. As a consequence, efficient anti-cancer treatment should include targeting the cells of the microenvironment. Thus, identification and characterization of malignant collaboration between Th cells and BCP-ALL cells or their precursors may provide mechanistic support of the infectious etiology hypothesis and thereby open for novel therapies aiming to target the tumor microenvironment.
Abbreviations
- BAFF:
-
B cell-activating factor
- BCP-ALL:
-
Precursor B acute lymphoblastic leukemia
- CLL:
-
Chronic lymphocytic leukemia
- FL:
-
Follicular lymphoma
- IFN:
-
Interferon
- MHC:
-
Major histocompatibility complex
- Th:
-
T helper
References
Pui CH, Robison LL, Look AT (2008) Acute lymphoblastic leukaemia. Lancet 371(9617):1030–1043
Ford AM, Bennett CA, Price CM, Bruin MC, Van Wering ER, Greaves M (1998) Fetal origins of the TEL-AML1 fusion gene in identical twins with leukemia. Proc Natl Acad Sci U S A 95(8):4584–4588
Mori H, Colman SM, Xiao Z, Ford AM, Healy LE, Donaldson C et al (2002) Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A 99(12):8242–8247
Kinlen L (1988) Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet 2(8624):1323–1327
Kinlen LJ, Clarke K, Hudson C (1990) Evidence from population mixing in British New Towns 1946-85 of an infective basis for childhood leukaemia. Lancet 336(8715):577–582
Kinlen LJ (1995) Epidemiological evidence for an infective basis in childhood leukaemia. Br J Cancer 71(1):1–5
Kinlen LJ, Hudson C (1991) Childhood leukaemia and poliomyelitis in relation to military encampments in England and Wales in the period of national military service, 1950-63. BMJ 303(6814):1357–1362
Kinlen LJ, Balkwill A (2001) Infective cause of childhood leukaemia and wartime population mixing in Orkney and Shetland, UK. Lancet 357(9259):858
Greaves MF (1988) Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia 2(2):120–125
Gilham C, Peto J, Simpson J, Roman E, Eden TO, Greaves MF et al (2005) Day care in infancy and risk of childhood acute lymphoblastic leukaemia: findings from UK case-control study. BMJ 330(7503):1294
Ma X, Buffler PA, Selvin S, Matthay KK, Wiencke JK, Wiemels JL et al (2002) Daycare attendance and risk of childhood acute lymphoblastic leukaemia. Br J Cancer 86(9):1419–1424
Greaves M (2006) Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer 6(3):193–203
Martin-Lorenzo A, Hauer J, Vicente-Duenas C, Auer F, Gonzalez-Herrero I, Garcia-Ramirez I et al (2015) Infection exposure is a causal factor in B-cell precursor acute lymphoblastic leukemia as a result of Pax5-inherited susceptibility. Cancer Discov 5:1328–1343
Crotty S (2015) A brief history of T cell help to B cells. Nat Rev Immunol 15(3):185–189
Sison EA, Brown P (2011) The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Expert Rev Hematol 4(3):271–283
Purizaca J, Meza I, Pelayo R (2012) Early lymphoid development and microenvironmental cues in B-cell acute lymphoblastic leukemia. Arch Med Res 43(2):89–101
Ayala F, Dewar R, Kieran M, Kalluri R (2009) Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia 23(12):2233–2241
Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D (1992) Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood 79(9):2370–2377
Manabe A, Murti KG, Coustan-Smith E, Kumagai M, Behm FG, Raimondi SC et al (1994) Adhesion-dependent survival of normal and leukemic human B lymphoblasts on bone marrow stromal cells. Blood 83(3):758–766
Mudry RE, Fortney JE, York T, Hall BM, Gibson LF (2000) Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood 96(5):1926–1932
Bradstock K, Bianchi A, Makrynikola V, Filshie R, Gottlieb D (1996) Long-term survival and proliferation of precursor-B acute lymphoblastic leukemia cells on human bone marrow stroma. Leukemia 10(5):813–820
Tesfai Y, Ford J, Carter KW, Firth MJ, O’Leary RA, Gottardo NG et al (2012) Interactions between acute lymphoblastic leukemia and bone marrow stromal cells influence response to therapy. Leuk Res 36(3):299–306
Duan CW, Shi J, Chen J, Wang B, Yu YH, Qin X et al (2014) Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell 25(6):778–793
Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D (2007) Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 117(4):1049–1057
Veiga JP, Costa LF, Sallan SE, Nadler LM, Cardoso AA (2006) Leukemia-stimulated bone marrow endothelium promotes leukemia cell survival. Exp Hematol 34(5):610–621
Krevvata M, Silva BC, Manavalan JS, Galan-Diez M, Kode A, Matthews BG et al (2014) Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 124(18):2834–2846
Ehsanipour EA, Sheng X, Behan JW, Wang X, Butturini A, Avramis VI et al (2013) Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Res 73(10):2998–3006
Kumagai M, Manabe A, Pui CH, Behm FG, Raimondi SC, Hancock ML et al (1996) Stroma-supported culture in childhood B-lineage acute lymphoblastic leukemia cells predicts treatment outcome. J Clin Invest 97(3):755–760
Polak R, de Rooij B, Pieters R, den Boer ML (2015) B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 126(21):2404–2414
Battula VL, Chen Y, Cabreira Mda G, Ruvolo V, Wang Z, Ma W et al (2013) Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood 122(3):357–366
Boyerinas B, Zafrir M, Yesilkanal AE, Price TT, Hyjek EM, Sipkins DA (2013) Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 121(24):4821–4831
Juarez J, Baraz R, Gaundar S, Bradstock K, Bendall L (2007) Interaction of interleukin-7 and interleukin-3 with the CXCL12-induced proliferation of B-cell progenitor acute lymphoblastic leukemia. Haematologica 92(4):450–459
Fragoso R, Pereira T, Wu Y, Zhu Z, Cabecadas J, Dias S (2006) VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood 107(4):1608–1616
Fei F, Joo EJ, Tarighat SS, Schiffer I, Paz H, Fabbri M et al (2015) B-cell precursor acute lymphoblastic leukemia and stromal cells communicate through Galectin-3. Oncotarget 6(13):11378–11394
O’Leary H, Akers SM, Piktel D, Walton C, Fortney JE, Martin KH et al (2010) VE-cadherin regulates Philadelphia chromosome positive acute lymphoblastic leukemia sensitivity to apoptosis. Cancer Microenviron 3(1):67–81
Nwabo Kamdje AH, Mosna F, Bifari F, Lisi V, Bassi G, Malpeli G et al (2011) Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood 118(2):380–389
Yang Y, Mallampati S, Sun B, Zhang J, Kim SB, Lee JS et al (2013) Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett 333(1):9–17
Mayani H (1996) Composition and function of the hemopoietic microenvironment in human myeloid leukemia. Leukemia 10(6):1041–1047
de Vasconcellos JF, Laranjeira AB, Zanchin NI, Otubo R, Vaz TH, Cardoso AA et al (2011) Increased CCL2 and IL-8 in the bone marrow microenvironment in acute lymphoblastic leukemia. Pediatr Blood Cancer 56(4):568–577
Vilchis-Ordonez A, Contreras-Quiroz A, Vadillo E, Dorantes-Acosta E, Reyes-Lopez A, del Quintela-Nunez Prado HM et al (2015) Bone marrow cells in acute lymphoblastic leukemia create a proinflammatory microenvironment influencing normal hematopoietic differentiation fates. Biomed Res Int 2015:386165
Conforti A, Biagini S, Del Bufalo F, Sirleto P, Angioni A, Starc N et al (2013) Biological, functional and genetic characterization of bone marrow-derived mesenchymal stromal cells from pediatric patients affected by acute lymphoblastic leukemia. PLoS One 8(11):e76989
Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA (2008) Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322(5909):1861–1865
Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ et al (2001) Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med 7(4):452–458
Dhodapkar MV, Krasovsky J, Osman K, Geller MD (2003) Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med 198(11):1753–1757
Herndler-Brandstetter D, Landgraf K, Jenewein B, Tzankov A, Brunauer R, Brunner S et al (2011) Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol 186(12):6965–6971
Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A et al (2002) Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood 100(13):4609–4614
Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M et al (2009) Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 30(5):721–730
Okhrimenko A, Grun JR, Westendorf K, Fang Z, Reinke S, von Roth P et al (2014) Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A 111(25):9229–9234
Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST (1994) Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood 84(8):2436–2446
Ho VT, Soiffer RJ (2001) The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 98(12):3192–3204
Monteiro JP, Benjamin A, Costa ES, Barcinski MA, Bonomo A (2005) Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood 105(4):1484–1491
Johnson PW, Watt SM, Betts DR, Davies D, Jordan S, Norton AJ et al (1993) Isolated follicular lymphoma cells are resistant to apoptosis and can be grown in vitro in the CD40/stromal cell system. Blood 82(6):1848–1857
Umetsu DT, Esserman L, Donlon TA, DeKruyff RH, Levy R (1990) Induction of proliferation of human follicular (B type) lymphoma cells by cognate interaction with CD4+ T cell clones. J Immunol 144(7):2550–2557
Pangault C, Ame-Thomas P, Ruminy P, Rossille D, Caron G, Baia M et al (2010) Follicular lymphoma cell niche: identification of a preeminent IL-4-dependent T(FH)-B cell axis. Leukemia 24(12):2080–2089
Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J et al (2005) Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 11(4):1467–1473
Muenst S, Hoeller S, Dirnhofer S, Tzankov A (2009) Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol 40(12):1715–1722
Chiorazzi N, Ferrarini M (2011) Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood 117(6):1781–1791
Os A, Burgler S, Ribes AP, Funderud A, Wang D, Thompson KM et al (2013) Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells. Cell Rep 4(3):566–577
Parente-Ribes A, Skanland SS, Burgler S, Os A, Wang D, Bogen B et al (2016) Spleen tyrosine kinase inhibitors reduce CD40L-induced proliferation of chronic lymphocytic leukemia cells but not normal B cells. Haematologica 101(2):e59–e62
Burgler S, Gimeno A, Parente-Ribes A, Wang D, Os A, Devereux S et al (2015) Chronic lymphocytic leukemia cells express CD38 in response to Th1 cell-derived IFN-gamma by a T-bet-dependent mechanism. J Immunol 194(2):827–835
Law CL, Wormann B, LeBien TW (1990) Analysis of expression and function of CD40 on normal and leukemic human B cell precursors. Leukemia 4(11):732–738
Mirkowska P, Hofmann A, Sedek L, Slamova L, Mejstrikova E, Szczepanski T et al (2013) Leukemia surfaceome analysis reveals new disease-associated features. Blood 121(25):e149–e159
Cardoso AA, Schultze JL, Boussiotis VA, Freeman GJ, Seamon MJ, Laszlo S et al (1996) Pre-B acute lymphoblastic leukemia cells may induce T-cell anergy to alloantigen. Blood 88(1):41–48
Wormann B, Anderson JM, Ling ZD, LeBien TW (1987) Structure/function analyses of IL-2 binding proteins on human B cell precursor acute lymphoblastic leukemias. Leukemia 1(9):660–666
Inoue K, Sugiyama H, Ogawa H, Yamagami T, Azuma T, Oka Y et al (1994) Expression of the interleukin-6 (IL-6), IL-6 receptor, and gp130 genes in acute leukemia. Blood 84(8):2672–2680
Touw I, Delwel R, Bolhuis R, van Zanen G, Lowenberg B (1985) Common and pre-B acute lymphoblastic leukemia cells express interleukin 2 receptors, and interleukin 2 stimulates in vitro colony formation. Blood 66(3):556–561
Gu L, Zhou M, Jurickova I, Yeager AM, Kreitman RJ, Phillips CN et al (1997) Expression of interleukin-6 receptors by pediatric acute lymphoblastic leukemia cells with the t(4;11) translocation: a possible target for therapy with recombinant IL6-Pseudomonas exotoxin. Leukemia 11(10):1779–1786
Kebelmann-Betzing C, Korner G, Badiali L, Buchwald D, Moricke A, Korte A et al (2001) Characterization of cytokine, growth factor receptor, costimulatory and adhesion molecule expression patterns of bone marrow blasts in relapsed childhood B cell precursor all. Cytokine 13(1):39–50
Reid GS, Terrett L, Alessandri AJ, Grubb S, Stork L, Seibel N et al (2003) Altered patterns of T cell cytokine production induced by relapsed pre-B ALL cells. Leuk Res 27(12):1135–1142
Wu S, Gessner R, von Stackelberg A, Kirchner R, Henze G, Seeger K (2005) Cytokine/cytokine receptor gene expression in childhood acute lymphoblastic leukemia: correlation of expression and clinical outcome at first disease recurrence. Cancer 103(5):1054–1063
Nakase K, Kita K, Miwa H, Nishii K, Shikami M, Tanaka I et al (2007) Clinical and prognostic significance of cytokine receptor expression in adult acute lymphoblastic leukemia: interleukin-2 receptor alpha-chain predicts a poor prognosis. Leukemia 21(2):326–332
Parameswaran R, Muschen M, Kim YM, Groffen J, Heisterkamp N (2010) A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res 70(11):4346–4356
Maia S, Pelletier M, Ding J, Hsu YM, Sallan SE, Rao SP et al (2011) Aberrant expression of functional BAFF-system receptors by malignant B-cell precursors impacts leukemia cell survival. PLoS One 6(6):e20787
Planken EV, Dijkstra NH, Bakkus M, Willemze R, Kluin-Nelemans JC (1996) Proliferation of precursor B-lineage acute lymphoblastic leukaemia by activating the CD40 antigen. Br J Haematol 95(2):319–326
Troeger A, Glouchkova L, Ackermann B, Escherich G, Hanenberg H, Janka G et al (2014) Significantly increased CD70 up regulation on TEL-AML positive B cell precursor acute lymphoblastic leukemia cells following CD40 stimulation. Klin Padiatr 226:332–337
Zhou M, Gu L, Holden J, Yeager AM, Findley HW (2000) CD40 ligand upregulates expression of the IL-3 receptor and stimulates proliferation of B-lineage acute lymphoblastic leukemia cells in the presence of IL-3. Leukemia 14(3):403–411
Ghia P, Transidico P, Veiga JP, Schaniel C, Sallusto F, Matsushima K et al (2001) Chemoattractants MDC and TARC are secreted by malignant B-cell precursors following CD40 ligation and support the migration of leukemia-specific T cells. Blood 98(3):533–540
Luczynski W, Kowalczuk O, Ilendo E, Stasiak-Barmuta A, Krawczuk-Rybak M (2007) Upregulation of antigen-processing machinery components at mRNA level in acute lymphoblastic leukemia cells after CD40 stimulation. Ann Hematol 86(5):339–345
Okabe M, Kuni-eda Y, Sugiwura T, Tanaka M, Miyagishima T, Saiki I et al (1991) Inhibitory effect of interleukin-4 on the in vitro growth of Ph1-positive acute lymphoblastic leukemia cells. Blood 78(6):1574–1580
Consolini R, Legitimo A, Cattani M, Simi P, Mattii L, Petrini M et al (1997) The effect of cytokines, including IL4, IL7, stem cell factor, insulin-like growth factor on childhood acute lymphoblastic leukemia. Leuk Res 21(8):753–761
Renard N, Duvert V, Banchereau J, Saeland S (1994) Interleukin-13 inhibits the proliferation of normal and leukemic human B-cell precursors. Blood 84(7):2253–2260
Manabe A, Coustan-Smith E, Kumagai M, Behm FG, Raimondi SC, Pui CH et al (1994) Interleukin-4 induces programmed cell death (apoptosis) in cases of high-risk acute lymphoblastic leukemia. Blood 83(7):1731–1737
Buske C, Becker D, Feuring-Buske M, Hannig H, Wulf G, Schafer C et al (1997) TGF-beta inhibits growth and induces apoptosis in leukemic B cell precursors. Leukemia 11(3):386–392
Buske C, Becker D, Feuring-Buske M, Hannig H, Griesinger F, Hiddemann W et al (1998) TGF-beta and its receptor complex in leukemic B-cell precursors. Exp Hematol 26(12):1155–1161
Bi L, Wu J, Ye A, Wu J, Yu K, Zhang S et al (2016) Increased Th17 cells and IL-17A exist in patients with B cell acute lymphoblastic leukemia and promote proliferation and resistance to daunorubicin through activation of Akt signaling. J Transl Med 14(1):132
Renard N, Lafage-Pochitaloff M, Durand I, Duvert V, Coignet L, Banchereau J et al (1996) Demonstration of functional CD40 in B-lineage acute lymphoblastic leukemia cells in response to T-cell CD40 ligand. Blood 87(12):5162–5170
Thompson P, Urayama K, Zheng J, Yang P, Ford M, Buffler P et al (2014) Differences in meiotic recombination rates in childhood acute lymphoblastic leukemia at an MHC class II hotspot close to disease associated haplotypes. PLoS One 9(6):e100480
Taylor GM, Hussain A, Verhage V, Thompson PD, Fergusson WD, Watkins G et al (2009) Strong association of the HLA-DP6 supertype with childhood leukaemia is due to a single allele, DPB1*0601. Leukemia 23(5):863–869
McKinney PA, Cartwright RA, Saiu JM, Mann JR, Stiller CA, Draper GJ et al (1987) The inter-regional epidemiological study of childhood cancer (IRESCC): a case control study of aetiological factors in leukaemia and lymphoma. Arch Dis Child 62(3):279–287
MacKenzie J, Greaves MF, Eden TO, Clayton RA, Perry J, Wilson KS et al (2006) The putative role of transforming viruses in childhood acute lymphoblastic leukemia. Haematologica 91(2):240–243
Yun C, Senju S, Fujita H, Tsuji Y, Irie A, Matsushita S et al (1999) Augmentation of immune response by altered peptide ligands of the antigenic peptide in a human CD4+ T-cell clone reacting to TEL/AML1 fusion protein. Tissue Antigens 54(2):153–161
Tanaka Y, Takahashi T, Nieda M, Masuda S, Kashiwase K, Ogawa S et al (2000) Generation of HLA-DRB1*1501-restricted p190 minor bcr-abl (e1a2)-specific CD4+ T lymphocytes. Br J Haematol 109(2):435–437
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT et al (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355(15):1572–1582
Acknowledgements
Simone Bürgler was supported by a grant of the Swiss cancer league (Krebsliga Schweiz, KLS-3189-02-2013) during the preparation of the manuscript.
Authors’ contributions
SB and DN wrote the manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bürgler, S., Nadal, D. Pediatric precursor B acute lymphoblastic leukemia: are T helper cells the missing link in the infectious etiology theory?. Mol Cell Pediatr 4, 6 (2017). https://doi.org/10.1186/s40348-017-0072-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-017-0072-z