Abstract
Background
Helminths are endemic in more than half of the world’s countries, raising serious public health concerns. Accurate diagnosis of helminth infection is crucial to control strategies. Traditional parasitological methods, serological tests and PCR-based assays are the major means of the diagnosis of helminth infection, but they are time-consuming and/or expensive, and sometimes provide inaccurate results. Loop mediated isothermal amplification (LAMP) assay, a sensitive, simple and rapid method was therefore developed for detection of helminths. This study aims to discuss the current status of application of LAMP on helminths detection and to make a comprehensive evaluation about this updated technology and its future outlook by comparing with several other diagnostic methods.
Main body
This review summarizes LAMP assay applied for helminth detection and helminthiasis surveillance. The basic principle of LAMP is introduced to help better understand its characteristics and each reported assay is assessed mainly based on its detection sensitivity, specificity and limitations, in comparison with other common diagnostic tests. Moreover, we discuss the limitations of the assays so as to clarify some potential ways of improvement.
Conclusions
Here, we summarize and discuss the advantages, disadvantages and promising future of LAMP in heliminth detection, which is expected to help update current knowledge and future perspectives of LAMP in highly sensitive and specific diagnosis and surveillance of helminthiasis and other parasitic diseases, and can contribute to the elimination of the diseases from endemic areas.
Similar content being viewed by others
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the five official working languages of the United Nations.
Background
Helminths, including trematodes (flukes), nematodes (roundworms) and cestodes (tapeworms), are associated with substantial morbidity and economic losses worldwide [1,2,3]. Approximately one-sixth of the worlds’ population is infected with helminths [4], with an estimated 15 billion individuals, particularly in low socio-economic regions, suffered from soil-transmitted helminth (STH) infections [5, 6]. Although most of helminths have been well investigated epidemiologically [7], actual distributions of them are still unknown and accurate diagnosis is urgently needed because of their generally non-specific and similar symptoms (nausea and/or vomiting, diarrhoea, abdominal pain, and fever) between the causative species [8, 9].
The approaches to clinical diagnosis and epidemiological surveillance of helminthiasis vary according to the samples, infectious stages, life cycle, morphological characteristics of helminths. Although the methods are diversified, there is not an ideal and reliable point-of-care (POC) diagnostic method that can eminently meet the World Health Orgnization (WHO)’s expectation of characteristics of affordable, sensitive, specific, user-friendly, rapid and equipment-delivered (ASSURED) [10, 11]. Though the simple and cost-effective morphological identification of parasites has been commonly employed in clinical diagnosis and field survey, it shows poor sensitivity in low-density parasite infections [12,13,14,15,16]. Furthermore, with respect to the discernment of parasite eggs that are morphologically similar, it will lose its specificity [12,13,14,15,16]. In addition, its prerequisite for a considerable quality and quantity of manpower also makes it unadaptable as a POC tool [17]. To avoid misdiagnosis and missed diagnosis, particularly in low-grade infections and in low-intensity regions, enzyme-linked immunosorbent assay (ELISA), as a representative of serological tests, has been applied [18, 19]. However, the major drawbacks with the use of ELIAS are clear due to its inability to distinguish between past and present infections, relatively high false-positive rate, and cross reactions [16, 19, 20]. Alternatively, a series of polymerase chain reaction (PCR)-based techniques, which are both specific and sensitive, started a new era for nucleic acid-based molecular detection of helminths. The 1990s witnessed the inception of various amplification techniques, e.g., nucleic acid sequence-based amplification [21], strand displacement amplification [22], and rolling circle amplification [23]. But none of these methods manages to conquer the inherent weakness of heavy dependence on a particular instrument or elaborate detection methods [24, 25]. As a result, their application is restricted where they are urgently needed, such as in primary medical institutions, underdeveloped areas and field studies [16, 26, 27]. As LAMP, a nucleic acid amplification method with extremely high sensitivity and specificity, appears to promise an appealing resolution for almost all of issues mentioned above, this review investigates the recent research progress in use of LAMP in helminth detection and make an comprehensive evaluation about this updated technology and highlights the future perspectives regarding the possible applications of LAMP in diagnosis of parasitic diseases, comparing with etiological detection, serological tests and other molecular assay.
In the present paper, we reviewed published studies between 2001 and 2018 to identify studies exploiting LAMP in helminth detection. A comprehensive search strategy was developed in PubMed, proper key words and free text terms employed. Search terms were “(helminth” [All fields] OR nematode [All fields] OR cestode [All fields] OR trematode [All fields]) AND (“loop-mediated isothermal amplification” [All fields] OR “LAMP” [All fields]). In brief, information was collected and analyzed from 54 articles in Chinese or English.
Main text
Principle of LAMP
Using the sophisticated mechanism of auto-cycling strand displacement DNA synthesis, LAMP was developed as a novel method requiring minimal instrumentation [25]. An inner primer, termed forward inner primer (FIP), containing sequences corresponding to the sense and antisense sequences of the target DNA, initiates the reaction [25]. An outer primer primes the subsequent strand displacement DNA synthesis [25]. As a result, a single-stranded DNA molecule is released, serving as the template for similar DNA synthesis primed by another set of primers at the other end of the target DNA [25]. In the initial step, dumbbell-like DNA strands with a stem-loop structure are produced (Fig. 1) [25]. In the following cycling step, DNA synthesis is triggered by an inner primer hybridizing to the loop on the product, which produces an identical stem-loop structure [25]. Released by the strand displacement reaction, the 3′ end of the original stem-loop DNA molecule is able to complete the self-primed DNA synthesis, yielding a new stem-loop DNA molecule with the stem twice length as the original one [25]. The above reactions circularly repeat during the entire cycling step (Fig. 2) [25].
Principle of LAMP. The initiative stage of LAMP assay: Besides the target DNA, the reaction system in (a) contains a set of inner primers—BIP and FIP, and a set of outer primers –F3 and B3 primer. An inner primer initiates the reaction in (b-g) by replacing the template strand with the help of polymerase with strand-displacement activity such as Bst DNA polymerase. An outer primer working, a single strand DNA is released, serving as the template of the following reaction. The similar strand-displacing DNA synthesis proceeding in the other end, yields the dumbbell-like DNA strands with a stem-loop structure in (g), which take part in the auto-cycling stage
Principles of LAMP assay. The auto-cycling stage: After the self-hybridizing reaction dissociate the stem-loop structure in the 5′ end, an inner primer hybridized to the stem-loop in the 3′ end, initiating the auto-cycling stage. The newly synthesized 3′ end continues its self-hybridizing reaction, producing a stem-loop DNA essentially identical with the initial one and a new one with the stem twice as the original one. Inner primers hybridizes, elongating new strands once there is a stem free thus to repeat the aforementioned reaction. The final products in (g), namely stem-loop DNAs of varied sizes and cauliflower-like structures with multiple loops, accumulates as long as the reaction circularly continues
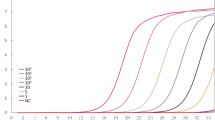
Without a thermocycler [28], target DNA is amplified by employing Bst DNA polymerase under a constant temperature of 60–65 °C, and accumulated 109 copies of target DNA in less than an hour, with a detection limit of a few copies [24, 25, 29]. Properly designed primers are given [30], as four different primers recognize 6 distinct sequences in a target DNA. The process will be blocked once non-specific recognition occurs, hence high selectiveness [29]. If supplemented with loop primers, stem primers and swarm primers, an even higher reaction speed can be expected [31,32,33]. The final products of the LAMP reaction are stem-loop DNAs inverted with a large amount of repeats of the target and cauliflower-like structures with multiple loops. The approaches of endpoint monitoring differ according to varied purposes. Sometimes agarose gel electrophoresis is employed as the gold standard, but it is not always compulsory [25, 34, 35]. And turbidity determination is more suitable for field research [24]. As a pyrophosphate ion is released once a nucleotide is added to the DNA strands, a large number of target DNA will be accumulate by the end of the assay, forming visible white precipitates of magnesium pyrophosphate, which is used to determine whether the target nucleic acid was amplified or not [36]. Based on the principle mentioned above, LAMP is characteristically able to meet the ASSURED needs, since it is a one-step process running within 1 h when there is Bst polymerase and a simple heating block, and the result can be read by the naked eyes. Furthermore, LAMP has also reported to be more tolerant than PCR for some biological inhibitors. Therefore, it can detect DNA in some specific clinical samples, such as swabs, without DNA extraction [28].
For further improvement, the fluorescent probe calcein, DNA-binding dye SYBR Green I, DNA-functionalized gold nanoparticles, etc., are then added to reach a higher sensitivity [37, 38]. To achieve the analysis of minute quantities of nucleic acid, real-time turbidimetry [39] is used, followed by the introduction of cationic polymers, e.g., poly-ethylenimine, which makes it feasible for use on a conventional UV illuminator [40]. Further progress is based on colorimetry with a hydroxy naphthol blue (HNB) indicator, which changes colour without affecting the amplification reactions and can be performed in a microtiter plate [35, 41], which empowers its development as a portable tool in field surveys. Combined with several biotechnology tools, LAMP has been widely applied in recent years, e.g., LAMP-chromatographic lateral flow dipsticks [42] and LAMP-ELISA [43, 44]. Moreover, simultaneous amplification of multiple targets has been achieved, termed multiplex LAMP (mLAMP), and has currently attracted much attention in the biomedical applications [45, 46].
Nevertheless, as disadvantages are always accompanied by advantages, LAMP assays still have a long way to go until their robustness, performance and utility are validated [47]. As mentioned above, primer design is a prerequisite and critical part of the LAMP assay [48], but it is also a major drawback plaguing researchers, even helpful software can be freely acquired [49,50,51]. The introduction of multiple primers theoretically promotes specificity, whereas it may increase the risk of primer-primer hybridizations, giving rise to template-free amplification at the same time [52]. The chances of false-positive outcomes, however, need further evaluation [45]. To avoid the foregoing situation, redesign of the primers should be taken into consideration [45]. Another chief obstacle is the unintended carryover contamination caused by its extremely high efficacy [48, 53, 54]. An isolated room and a closed reaction system for the test, e.g., agar dye capsule [55], or the pre-addition of dye, hydroxynaphthol blue dye (HNB), are recommended [45]. Another prominent resolution is the emerging lab-on-chip technique, which allows all the analytical steps to be processed on a single chip [56, 57]. Due to the lack of a thermocycler and convenience in sample extraction and endpoint determination, LAMP may prompt the development of lab-on-chip techniques [58, 59]. In combination with LAMP, mLAMP will manifest notable superiority of high-throughput screening, high sensitivity and lower risk of cross-contamination, which shows momentum in multiple target screening and determination of pathogens with frequent gene mutation [46].
Detection of helminths by LAMP
The impressive progress currently made in the LAMP assay for helminths includes trematodes of Clonorchis sinensis [12, 26, 60], Opisthorchis viverrini [14, 61, 62], Amphimerus spp. [63, 64], Paragonimus westermani [15], Fasciola hepatica [65,66,67], F. gigantica [65], Schistosoma japonicum [16, 27, 68,69,70], S. mansoni [13, 71,72,73,74,75,76,77], S. haematobium [51, 71, 72, 76]; nematodes of Necator americanus [78, 79], Ascaris lumbricoides [17, 79], Trichuris trichiura [79], Toxocara canis [80] and T. cati [81], Strongyloides stercoralis [52, 82], Onchocerca volvulus [83,84,85,86], Wuchereria bancrofti [86, 87], Brugia malayi [86, 88], B. tomori [88], Loa loa [89,90,91], Dirofilaria repens [92], Angiostrongylus cantonensis [93, 94], Trichinella spiralis [95, 96], Bursaphelenchus xylophilus [97], and Haemonchus contortus [98, 99]; cestodes of T. solium [44, 100,101,102,103], T. saginata [44, 100,101,102,103], T. asiatica [44, 100,101,102,103], T. hydatigena [104], T. multiceps [104], T. pisiformis [104] and T. crassiceps [104], Echinococcus granulosus [104,105,106], E. multilocularis [104, 107], E. equinus [108], E. canadensis [108], E. felidi [108], E. ortleppi [108, 109] and E. shiquicus [104], have been covered in this review for further insight into its adoption for clinical diagnosis, field surveys and surveillance of helminths. The sensitivity and specificity of detection of helminths by LAMP are shown in Table 1.
Detection of trematodes by LAMP
Foodborne trematode infections remain a serious global health burden, resulting in 2 million disability-adjusted life years lost annually [110, 111].
Clonorchiasis and opisthorchiasis, being mainly prevalent in Asia and Europe, are characterised by significant pathological hepatobiliary changes caused by C. sinensis, O. viverrini and O. felineus [110, 112]. Both C. sinensis and O. viverrini, classified as class one carcinogens of human cholangiocarcinoma by the International Agency for Research on Cancer, are cancerogenic after years of infestation in bile ducts of the host [112, 113]. As developed as biotechnology tools are, microscopic egg counting in stool samples continues to be the routine method of diagnosis, which is simple but lacks sensitivity in early and light infections [112, 114, 115]. How to accurately differentiate between liver flukes and intestinal flukes in areas where they coexist remains an unsolved problem [116]. In endemic areas where residents become infected by consuming raw fish with metacercariae, the epidemiological investigation of C. sinensis infection in freshwater fish is an important part of clonorchiasis supervision. The current epidemiological method in fish partly depends on the labour-intensive microscopic inspection of fish muscle, which may lead to missed detection of low worm burden or cross-border contamination [117, 118]. Hence, LAMP, as an innovative technique that is sensitive and convenient, will help to solve these problems. The LAMP assay has been devised to detect DNA of C. sinensis and O. viverrini in freshwater snails [12], the second intermediate fish hosts [14, 60, 61] and patient faeces [26, 61, 62].
In the detection of C. sinensis infection in fish, the respective detection limit of LAMP and PCR were 10− 8 ng/μL and 10− 6 ng/μL, respectively, demonstrating that LAMP was 100-fold more sensitive than PCR [60]. When the true positive and negative results of LAMP were in 100% agreement with the conventional microscopic examination, this approach shows the potential to replace the conventional method in the investigation of fluke invasion in the fish industry [14, 60, 61]. In addition, LAMP is sensitive enough to examine up to 0.0002 cercariae in a snail, and it is promising to be a prominent figure in epidemiological surveillance for snail control intervention [12]. In human faecal samples, LAMP-based technology was established to detect C. sinensis with infection intensity as low as 1 egg per 100 mg. Further evaluation of the LAMP-based diagnosis test showed a sensitivity of 97.1% and specificity of 100% as confirmed by the Kato-Katz (KK) method as well as real-time PCR (RT-PCR) [26]. However, it also perceived five additional positive samples of 13 microscopically negative samples in O. viverrini determination [61]. Future studies are expected to assess the valid detection limit of this method in comparison with the KK method and RT-PCR as well as its feasibility as a routine standard method [26]. Similar LAMP assays were also developed in O. viverrini, with the variation of sensitivity and specificity relating to the repetition of different target genes when detecting copro-DNA [14, 61, 62]. For example, LAMP is highly sensitive when targeting internal transcribed spacer 1 (ITS1) of O. viverrini, but specificity cannot be guaranteed for ITS1 cross-amplifying genes from O. felineus, F. gigantica and Haplorchoihoides spp. [61, 62]. When amplifying the mitochondrial gene nad1 of O. viverrini in 100% specificity, the sensitivity for LAMP was between 1 petagram (pg) and 100 femtograms (fg), whereas it was 10 pg for PCR [62].
Amphimeriasis, caused by Amphimerus spp., has been recently reported as an emerging zoonotic fish-borne trematodisasis affecting indigenous inhabitants and domestic animals in the tropical Pacific side of Ecuador [119]. To date, a novel LAMP assay (namely LAMPhimerus) is devised for the first time to detect internal transcribed spacer 2 (ITS2) of Amphimerus spp. DNA in patient faecal samples, with detection limit (1 pg) identical to conventional PCR [63]. LAMPhimerus was more sensitive than traditional parasitological techniques, including direct microscopy detection, formalin-ether concentration, simple sedimentation technique, Kato-Katz technique, fecal egg count [63]. Of 44 human stool samples, the LAMPhimerus method achieved 76.67% sensitivity; 80.77% specificity; 82.14% positive predict value (PPV) and 75.00% negative predict value (NPV) [63]. As the current scarce genomic information of Amphimerus spp. is scarce, further enhancement of the assay could be based on the exploitation of different DNA target [63]. The procedure, in combination with the air-dried faecal specimens on common filter paper as source of DNA, is superior in feasible collection, long-term preservation and transportation, and potentially applicable as an effective diagnostic or epidemiological tool in amphimeriasis-endemic regions [64]. Furthermore, the system ‘air-dried stool sample on filter paper’-LAMP assay would be practical in large-scale molecular investigation of the other helminthiasis [64].
Given the infection of the genus Fasciola, fascioliasis mainly affects ruminants and only occasionally humans, raising public health and economic concerns due to a reduction in output [120,121,122]. Triclabendazole-resistant F. hepatica, an emerging problem, calls for reliable assessment of efficacy or resistance after deworming therapy [122]. Serological ELISA is applied in the detection of cattle and sheep, but it is unreliable for species distinction and the effectiveness of drug therapy [123]. Coproantigen ELISA is appropriate for monitoring adult infection, whereas it is insufficient correlation with larval stage invasion until 6 weeks post treatment [124]. LAMP targeting ribosomal intergenic spacer seems to be an optional detection method that overcomes the difficulty in taxonomical classification of F. hepatica and F. gigantica. It can amplify genes from adults, eggs and juvenile stages with a sensitivity 10 000-fold higher than PCR, while running an hour faster in the laboratory [65]. Other LAMP-based assays amplifying sequences of the second internal transcribed spacer (ITS2) show their inability to distinguish between the two Fasciola species, F. hepatica and F. gigantica [66, 67]. Under field conditions, the LAMP assay can identify infected sheep in the first week post-infection and 30 days post-therapy, while ELISA cannot detect infections until 6 weeks and is insufficient to discriminate current and past infections, indicating the practical and applicable determination of drug efficacy or resistance [66]. In contrast, M.I. Arifin et al. reported poor performance of LAMP and PCR in comparison with other conventional methods for the diagnosis of F. hepatica in naturally infected sheep and cattle in the field. Of the 64 animals examined, LAMP and PCR had low sensitivities of 17.9 and 10.7%, respectively, and high specificities of 97.2 and 100%, respectively, with faecal egg count (FEC) and coproantigen ELISA as composite reference standards. The failure of LAMP and PCR may be due to factors including insufficiency of DNA sample, possibly in relation to the choice of DNA extraction method, amount of faeces substantially used, and uneven egg distribution in faeces of different host species [67]. If promoted in the future, such a test is still suitable for early diagnosis, thus reducing veterinary costs and the loss of livestock due to fascioliasis [65,66,67]. To the best of our knowledge, LAMP has not yet been used for the detection of human fascioliasis.
Paragonimiasis, also known as lung fluke disease, is a pulmonary inflammation caused by Paragonimus species [125, 126], of which P. westermani is the most epidemiologically relevant in Asia and sporadically in American and African countries [127]. The conventional immunological diagnosis method is sensitive in human paragonimiasis but unsustainable in epidemiological surveys when intermediate hosts are detected [128]. A LAMP assay has successfully amplified the gene sequence of P. westermani eggs in sputum and pleura fluid from patients, as well as metacercariae in freshwater crabs and crayfish. With a detection limit of 1 × 10− 8 ng/μL, LAMP is close to 100 times more sensitive than PCR. The LAMP method also yields positive and negative results coinciding with those from parasitology tests, acting as an excellent candidate for field surveys and clinical diagnoses of paragonimiasis [15].
Schistosomiasis ranks on the list of neglected tropical diseases (NTD) for its impacts on an estimated number of over 200 million individuals in more than 70 countries [126, 129, 130]. Of the five Schistosoma spp. that usually cause human schistosomiasis, S. japonicum is prevalent in Asia, while S. mansoni and S. haematobium are mainly concurrent in Africa and the Middle East [130]. Currently, infection and reinfection continue to be global challenges, particularly in poverty-stricken and insanitary communities [131, 132] and in other regions due to transmission by tourists and immigrants who come into contact with infested water [130, 132]. Meanwhile, low-density infection remains after deworming programmes, which still demands an affordable diagnostic approach for pre-patent infection and massive epidemiological surveillance despite current parasitological, immunological and molecular diagnostic methods [131,132,133,134]. The KK method is the current mainstay of schistosomiasis diagnosis, and its drawback of day-to-day variation is inevitable in massive surveillance [9, 130, 131, 134]. In addition, it is of great importance to overcome the limitation of serological methods and their incapacity to discriminate between past and present infections due to the persistent existence of circular antibodies in the patient even after an effective cure [135].
As the control of intermediate host snails considerably contributes to the monitoring of schistosomiasis [126], LAMP assays were established to detect S. japonicum in Oncomelania hupensis [27, 68], S. mansoni in Biomphalaria spp. [13, 71, 72, 75] and S. haematobium in other snails [71, 72]. LAMP assays are sensitive and specific in pooled samples, with a detection limit of up to one positive in 100 negative O. hupensis (expecting for a larger sample) [68] as well as one snail infected with S. mansoni in 1000 normal snails [13]. In addition, a snail invaded by a single miracidium can be detected only 1 day after exposure [68, 72, 132]. Therefore, LAMP was used to construct the risk map of schistosomiasis based on infected O. hupensis in a field survey and readily adapted to predict the prevalence tendency [27]. What’s more, there is another work of LAMP (named SmMIT-LAMP) assessing not only infected snails but also human stool in low-transmission area of S. mansoni in Brazil, where the incidence was corresponded to what has been reported, ascertaining the foci of schistosomiasis transmission and helping build risk maps of schistosomiasis [77]. Furthermore, LAMP was developed to detect S. japonicum in rabbit models [16, 69, 70] and S. mansoni in murine models [71, 73, 74]. This approach detected positive results as early as 1 week [16, 69], and even 3 days, after low-intensity infection in rabbit models [70], tested negative as late as 12 weeks post treatment, which is consistent with PCR in early diagnosis, and tested negative 2 weeks later than PCR [70], thereby possessing potential in early diagnosis, treatment and assessment of the efficacy after chemotherapy [16, 69, 70]. LAMP is also readily adopted in the clinical determination of S. japonicum in human serum samples [16, 70], S. mansoni in stool samples [77], as well as S. mansoni and S. haematobium in urine samples [51, 76]. In human sera with light to mediate infection, LAMP achieves the sensitivity, specificity, PPV and NPV of 95.5, 100, 100 and 89.4%, respectively, whereas those for S. mansoni and S. haematobium in urine sample are 90–100% [76]. Additionally, the sensitivity (92.86%), specificity (80.11%), and NPV (99.33%) of SmMIT-LAMP in human stool samples are overall acceptable, but the PPV is 26.00%, which can be explained by the higher sensibility of LAMP over the reference standard (KK), especially in patient with low infection levels [77]. In addition, without any need for costly laboratory instrumentation and highly skilled personnel, the refinement of DNA extraction (i.e., LAMPellet, NaOH and heat lysis [51]), the harness of a portable plasma separator [136] and the utility of a user-friendly chip [74] fulfil the requirements of the POC test and are estimated to have a competitive per-person cost, with less than $7.25 for the circulating cathodic antigen test and no more than $7.00 for a single KK test [74]. Accordingly, further evaluation is required for POC use in endemic areas [51, 74, 76].
Detection of nematodes by LAMP
Nemathelminthiasis, caused by nematodes, is a globally rampant parasitic disease. The pathogenic nematode infecting human includes STH, S. stercoralis, Toxocara spp., filariae, and other nematodes with distinctive life cycles, namely, A. cantonensis and Trichinella. Nematodes in veterinary and agricultural fields are also included.
STH, including A. lumbricoides, hookworms, and whipworms, mainly occur in tropical and subtropical regions [137]. The KK method is currently the most common method in STH diagnosis and is recommended by the WHO to conduct STH surveys [17, 78, 79, 138]. However, for the false negative results brought about by the reduction of egg production after chemotherapy or the hatching of eggs due to the delay of examination [139, 140], it is actually a suboptimal choice in a mass drug administration (MDA) programme where post-chemotherapy evaluation is needed. In contrast, the LAMP assay is superior to the parasitological and unspecific serological approaches in that it tests positive when there is merely a single ovum [17], without cross reactivity or non-template positive [17, 78, 79]. In terms of the quantity of DNA, the SmartAmp2 assay amplifies the STH β-tubulin gene provided that there is one pg of DNA [79], and hookworm detection targeting the ITS-2 gene can even succeed with 0.4 fg of DNA [78]. None of the false positives is observed in these LAMPs, which is important, as multiple helminthiases may coexist in individuals in endemic areas [17]. In simulated clinical samples, the LAMP assays exhibit great agreement with the KK method in which the kappa coefficient is calculated to be 0.72 for A. lumbricoides determination targeting ITS-1 [79] and 0.9 for hookworm measuring targeting ITS-2 [17, 78]. In the SmartAmp2 assay, the pre-addition of HNB dye achieves even better accuracy by providing a closed system to avoid contamination in post-reaction manipulation using SYBR Green [79]. Bovine serum albumin was added, and it performs well in crudely prepared stool samples despite the presence of inhibitors, which is undoubtedly a competitive advantage for a POC tool, though it still needs further comparison [79]. However, the vulnerability of HNB to pH changes may be a challenge for its stability but can be resolved by standardizing reaction conditions [79].
S. stercoralis, acting as one of the opportunistic nematodes transmitted by soil, is the causative agent of human strongyloidiasis. It usually contributes to asymptomatic infection but is a deadly uncontrolled hyperinfection syndrome in immunocompromised patients [141,142,143,144,145], with a mortality rate of up to 87% [146, 147]. There is no single gold standard for its detection, as the microscopic examination of larvae in stool samples is insufficiently sensitive even when supplemented with enrichment techniques. Serological tests are sensitive but lack specificity [148,149,150,151]. PCR-based techniques, though sufficiently specific, are not diagnostically superior to parasitological techniques because of their unsatisfactory sensitivity, which is presumably attributed to the irregular larval output in chronic strongyloidiasis, the uneven distribution in stool specimens, the DNA extraction process, the existence of inhibitors in stool samples, etc. [151]. Generally, the definitive diagnosis of strongyloidiasis is made by parasitological examinations based on clinical symptoms, serological evidence, etc. [52, 82]. Compared with morphological examination, nucleic acid tests are advantageous in that they can detect specimens where parasites had been killed [52]. In 2014, the LAMP assay for S. stercoralis was first reported to be capable of amplifying less than ten 0 DNA copies of larvae per reaction, or 10− 2 dilution of one spiked larva in stool samples, comparable to the results of PCR [52]. Unfortunately, the foregoing factors that may influence PCR-based techniques, e.g., the DNA extraction process, also may impact it [52]. Aiming at surmounting the shortcomings of common stool samples, urine samples from rodent models were used in a novel LAMP assay named Strong-LAMP [82]. The creative introduction of urine samples may possess predominant advantages in collection, storage and processing over stool samples. Furthermore, when employing urine samples of the rodent model, Strong-LAMP shows positive results from 5 days after infection of 40 third-stage (L3) infective larvae (1 day earlier than employing stool samples) to 3 days after infection of 400 or 4000 L3 infective larvae (2 days earlier than employing stool samples). Nevertheless, since requests for urine samples in S. stercoralis detection are rare, its clinical value in latent infection of humans needs further study [82].
The larvae of T. canis and T. cati are responsible for human toxocariasis. Children specifically tend to acquire these kinds of telluric zoonosis and saprozoonosis by environmental exposure to Toxocara spp. [152], which makes it one of the most common cosmopolitan helminthiases [153]. The prevention of its transmission depends on the condition of the environmental contamination levels and the accurate determination of its sources [81]. However, Toxocara identification by traditional microscopy of stools from pets or environmental samples remains a methodological concern due to its insensitivity in low-burden cases and its difficulty in distinguishing T. canis from T. cati eggs [80, 81]. PCR assays have been designed to discern Toxocara spp. in stools [154] or environmental samples [155] and to distinguish between T. canis and T. cati in soil samples [156]. The species-specific LAMP assay targeting ITS-2 was validated by two groups and found to be ten-fold more sensitive than PCR without cross reactivity in the laboratory between Toxocara spp. and is applied in domesticated dogs and sand samples [80, 81]. In the context of environmental specimens, LAMP manifests a detection limit of 3 eggs/10 g of sand and less than 3 eggs/30 g of stools, compared with the 6 eggs/10 g of sand and more than 2 eggs/30 g of stools detection limit of PCR [80, 81]. In a field survey of soil contamination, LAMP yield a positive rate of 42.7% versus 7.7% of PCR [157]. In another field study, even LAMP fails to identify very low contamination, which is a pitfall that may be attributed to the crude processing of DNA extraction in LAMP compared with that of PCR [81], the LAMP assay successfully decreased the standard examination time by 50% compared to that of PCR [81].
As one of the most debilitating infectious diseases in the world, lymphatic filariasis, which is caused by brugian filariae and W. bancrofti, is also regarded as a serious public health concern for 856 million people in 52 countries around the world [158]. The WHO MDA programme effectively reduces morbidity, raising new concerns about diagnosis and surveillance in the control areas and determination of the treatment endpoint in the post-MDA stage [8, 83, 87, 88, 159]. So far, the diagnosis largely counts on the microfilaraemia test, which employs night blood samples [86, 88] and is recommended by the WHO to conduct a transmission assessment survey (TAS) where Brugia spp. is endemic. It is used as the minimum in TAS but suffers from the reduction of sensitivity in response to the prevalence decrease in the post-MDA era. Simultaneously, more accurate methods, such as antibody tests and PCR, are restricted by their inherent shortcomings. The antigenaemia tests recommended to map W. bancrofti endemicity, namely, immunochromatography card test and filariasis test strip [160, 161], are unavailable for brugian filariae and may cross react with Loa loa [160, 162, 163]. Alternatively, as a competitive candidate in the present study, LAMP assays manifest cheerful outcomes in both laboratory and clinical tests [87, 88]. For instance, the W. bancrofti LAMP test, with a determination limit of 0.1 pg per reaction equivalent to that of PCR, costs over $1.38 less than the latter [87]. It is estimated that there is approximately 200 pg and 100 pg of DNA inside a single microfilaria of W. bancrofti or Brugia spp., respectively [164]; that is to say, the detection limit of the LAMP assay exceeds the theoretical detection limit of microfilariae per ml via microscopic inspection [165]. Furthermore, compared with the serological tests that are inadequately specific, almost all the LAMP assays for lymphatic filaria diagnosis are species-specific, except one detecting brugian filariae for both B. timori and B. malayi [86,87,88].
A similar methodological handicap is used to eliminate O. volvulus, another major public health concern mostly rampant in sub-Saharan Africa [83, 166]. Following the impediment to onchocerciasis transmission, the challenge emerges in that the conventional diagnostic method of skin snip microscopy and the primary diagnostic antibody test, the Ov-16 rapid diagnostic test, is losing its sensitivity in low-prevalence settings [167, 168]. Alternatively, nucleic acid-based assays can be employed in both diagnosis and xenomonitoring with extreme sensitivity and specificity. O-150 PCR, therefore, is recommended by the WHO to undertake vector surveillance but is limited in resource-limited areas [84, 169]. Using the economical LAMP assay as a diagnostic option manifests sensitivity just slightly lower than the utmost sensitive qPCR when targeting cox1 but is ten times higher than conventional PCR in O-150 assay at the same time [84, 85]. In terms of specificity, the cox1 assay is reported to cross react with O. chengi, a sympatric cattle parasite transmitted by black flies, or rather, the cox1 assay can be used only in clinical diagnosis using skin biopsy samples unless significant progress is made to improve specificity [85]. However, whether the other set of primers designed for O-150 can amplify the heterologous sequence from O. chengi remains to be determined [84], as the PCR targeting O-150 has been proven to cross react with O. chengi unless a specific DNA probe is added [170]. In addition, an elaborate comparison is designed between the HNB and neutral red dyes, and the latter improves the sensitivity 10-fold, which sheds light on a new approach for parasite LAMP amelioration, maximizing its usefulness in a world with a changing global landscape of infection [84].
In contrast to other parasites, in the post-MDA surveillance of filariae, the exploitation of samples from mosquito vectors is considered timelier, more operationally feasible and more ethically accepted than detection using specimens from humans [8, 159, 168, 169, 171]. As entomological inspection via field dissension is expensive, time consuming and unable to distinguish O. volvulus from O. chengi, O-150 PCR using vector samples is currently widely accepted to determine the interruption of filariae [8, 87, 159, 167,168,169]. LAMP can also act as an excellent surrogate for PCR in this case. As shown in O. volvulus detection targeting OvGST1a, without crossreactivity with O. chengi or other filariae, LAMP tests positive with merely 0.01 ng of DNA spiked in 200 insects, which is more sensitive than PCR, which tests positive in 0.01 ng/50 insects [83]. Based on the conventional LAMP assays, an improved non-instrumented nucleic acid-LAMP was developed, devised as a single portable electricity free device with comparable or even higher sensitivity than a normal assay, demonstrating that it is more suited for field surveys [86]. Whereas the existing LAMP assays for vector monitoring are designed to utilize the DNA extracted from infective-stage larvae (L3), there are great hurdles in xenomonitoring, where the DNA test cannot identify DNA from L3 larvae from immature stage parasites (L1 or L2) in vectors, which actually distinguishes xenomonitoring from entomological monitoring of transmission [159]. As the discrimination between infectious and immature parasites will clarify whether the positive result is due to adult filariae not responding to drug treatment or recent infection indicating active transmission, it is taking on increasing significance in the assessment after large-scale drug treatment [8, 171]. For O. volvulus, in which the infectious stage parasites are located in the head capsule isolated from the immature stage larvae in the abdomen and thoracic muscle, the obstacle can be overcome by the separation of the head and body and therefore provide accurate evaluation of transmission [159, 172]. On the other hand, although there are specific L3-stage RT-PCR tests that are capable of indirectly determining the infection potential and transmission dynamics of lymphatic filariae via RNA [173, 174], dissection remains more common for the detection of infectious stage lymphatic filariae [159]. However, it can be expected that the development of RT-LAMP in parasitology may favour this technique to replace RT-PCR and conventional dissection to precisely predict the transmission potential even in low-resource areas.
Loa loa is a long-neglected filariae that is reported to cause deadly serious adverse events after ivermectin treatment [86, 89,90,91, 175, 176] at a low threshold of microfilaria (mf) burden [175], where determination of the mf burden before MDA programme is especially important. Unfortunately, the routine diagnosis and quantification in remote areas rely on microscopic inspection of midday blood samples, which requires expertise and processing of a considerable number of samples and is unqualified to serve as a POC or a large-scale screening tool. Among the existing LAMPs, one amplifies the LL3M9 gene and exhibits the lowest detection limit of 0.5 ag/reaction, far lower than the formerly reported 0.1 pg/reaction for W. bancrofti [87, 90]. Considering the practical significance of Loa loa mf burden quantitation in MDA practice, Loa loa LAMP targeting LLMF72 was assessed for its potential for semi-quantitation. As a result, a correlation was observed between the time to LAMP reaction positivity (minutes) and the mf concentration in the blood, allowing the naked-eye determination of whether the mf burden is above or below the specific threshold. For example, the run time to positivity is 15 min at the threshold of > 30 000 mf/mL, 20 min at threshold of > 5000 mf/mL, and 25 min at the threshold of >v100 mf/mL, which is promising for application in the Loa loa microfilaraemia assessment before ivermectin treatment and thus facilitating the elimination of filariasis [89]. Since the LL3M9 comprises multiple copies of a simple nematode-conserved repeat, and LLMF72 is a single copy gene, which may exert an impact on the sensitivity and specificity, a new bioinformatic pipeline is designed to mine a new species-specific sequence that is more suitable for MDA practice. Consequently, RF4 is a new biomarker with specificity; however, it lacks sensitivity compared with the LL3M9 or LLMF72 assays. Nevertheless, the bioinformatic pipeline remains a creative and robust method to further explore the potential of LAMP [91].
Dirofilariasis caused by D. repens, another species of mosquito-borne filariae [177], is regarded as an emerging zoonotic disease calling for more accurate diagnosis. The traditional diagnostic method relies on microscopic examination of blood from the hosts [178]. Serological screenings [179] and PCR tests have been designed [180, 181]. The LAMP assay targeting the COI gene was devised as 2 versions for further evaluation. With respect to sensitivity, the detection limits of reverse transcriptase LAMP (RT-LAMP) and propidium iodide LAMP (PI-LAMP) are 0.15 fg and 10 fg, respectively, versus the detection limit of 15 fg for conventional PCR. With a lower limit, the LAMP assays yield amplicons within approximately 40 min, while conventional PCR takes 2 h. Generally, both versions of LAMP prevail over conventional PCR in both sensitivity and efficiency, while all of them are species-specific in the current study. Considering practical value, while RT-LAMP employs a RT-PCR instrument, PI-LAMP, by introducing propidium iodide, permits visualization of the amplification as UV fluorescence, meriting more widespread application in field surveys and clinical diagnoses [92]. Because of its combination of sensitivity, specificity, rapidity and convenience, it may be a promising ancillary tool in dirofilariasis surveillance and prevention, such as large-scale travelling animal quarantine inspection or culicid mosquito screening.
A. cantonensis infects people on the Pacific islands and Southeast Asia. It is the major cause of an eosinophilic meningitis in humans in endemic areas [182]. The lack of standardization of a diagnostic procedure and the current situation of being overlooked in accounts for the use of a presumptive diagnosis, which is primarily based on the combination of patient history and clinical criteria, e.g., morphological examination of adult worms or larvae in cerebrospinal fluid, of which the positive rate is between 2%~ 12% [183], are unable to meet the expectation of either clinical diagnosis or large-scale surveillance [184, 185]. In an effort to help establish a surveillance system, two LAMP assays were developed to detect the L3 larvae in molluscan hosts. One amplifying the ITS-1 gene manifests a detection limit of 1 fg/reaction [94]. The other test targeting the 18S rRNA gene is inferior, with a detection limit of 10 pg/reaction [93], while both have higher sensitivity than PCR, which can detect DNA > 100 pg/reaction [93, 94]. In a similar field survey, the ITS-1 LAMP assay demonstrates detection rates of 6.7 and 4.4% higher than the standard digestion method and PCR, respectively [94]. In summary, all of the above information exhibits considerable potential and superiority in replacing existing approaches in large-scale field surveys and clinical diagnoses [93, 94].
Trichinellosis is a significant zoonotic disease caused by the ingestion of raw or insufficiently cooked meat containing Trichinella spp., for which the inadequacy of veterinary control is one factor to blame. There had been no detailed and systematic reports of the sensitivity and conditions of the assays for Trichinella determination by 2012, when 2 LAMP assays were designed [95, 96], amplifying mitochondrial large ribosomal subunit DNA (mt-lsrDNA) and a 1.6 kb repetitive sequence from the larvae, respectively. Both assays manifest sensitivity 10-fold stronger than conventional PCR [95, 96], but the one targeting mt-lsrDNA turns out to be 10-fold less sensitive than RT-PCR [96]. Further exploration could be made to improve the sensitivity of LAMP to make it an optimal methodology for trichinellosis detection in practice, e.g., meat quarantine or field survey.
In addition to the human medical nematoda mentioned above, the application of LAMP has spread to the veterinary [98, 99] and agricultural fields [97], which makes it a promising detection tool shared by all fields of bioscience.
Detection of cestodes by LAMP
Taenia species (T. solium, T. saginata and T. asiatica), the causative pathogens of taeniasis, can be sympatrically endemic in Asia, such as in China and Thailand [186]. T. solium, normally transmitted between pigs and humans, results in neurocysticercosis with a range of manifestations, especially epilepsy and seizures [7]. Conventional proglottid examination, as a common diagnostic method for taeniasis, fails to morphologically differentiate the eggs of Taenia species. Multiplex PCR and nested PCR open the door for characteristic discrimination [187, 188] but are unrealistically applied in field surveys for high expense and time considerations. Therefore, a LAMP assay with the cytochrome c oxidase subunit 1 (cox1) primer set was developed for the differentiation of Taenia spp. at the species level in the laboratory and in the field, managing to detect eggs in traditional faecal samples in epidemiological surveys with high specificity and even higher sensitivity than PCR [100,101,102,103]. Ranging from five to ten eggs per gram (EPG) of faeces, the detection limit of LAMP is comparable to that of five EPG and 40 EPG of multiplex PCR and nested PCR, respectively [100, 187, 188]. The specificity is approximately 100%, with only two in 76 (2.6%) T. saginata recognized as T. asiatica in faecal samples [100]. Out of 51 proglottids expelled from 35 carriers, consistent results were obtained by LAMP under field conditions and in the laboratory, except for one sample [102]. Thus, the tedious procedure of simultaneously identifying Taenia species is expected to be simplified to reduce the possibility of cross contamination and to save time, while the handy copro-DNA extraction method is expected to take the place of centrifugation. Remarkably, the modification of mLAMP combined with dot-ELISA has succeeded in specific amplification in a single tube, demonstrating an easier and more practical POC diagnostic method for real-time human Taenia species confirmation [44].
Widely distributed in pastoral areas worldwide but often neglected, echinococcosis, especially cystic echinococcosis and alveolar echinococcosis, attracts enormous attention by posing as a threat to both humans and animals and results in economic loss [189,190,191,192,193]. An on-site approach is expected to replace the ethically-challenged post-mortem inspection as the gold standard in susceptible Echinococcus-infected definitive canid hosts [189, 193]. In addition, a more practical and available tool is sought to solve the problem of copro-ELISA lacking sensitivity in latent infection monitoring [194] and to sustain reliability of copro-PCR while reducing the expense [195, 196] in epidemiological surveillance in endemic areas at the same time. LAMP was exploited to detect E. granulosus s. s. (G1-G3) copro-DNA in dogs [104,105,106] and then cysts in camels and humans [109]. It stands out for its high sensitivity in detecting infection in copro-samples from definitive hosts 22 days after exposure, which is equivalent to 3 days, 4 days and 47 days earlier than ELISA, conventional PCR and light microscopy, respectively [106]. A similar advance in E. multilocularis determination depicts LAMP as a substantial alternative for field surveillance of AE in areas of endemicity [107]. LAMP was also applied in other cestodes of veterinary relevance, including E. equinus (G4), E. canadensis (G6-G10), E. felidi (lion strain), E. ortleppi (G5) [108], E. shiquicus, T. hydatigena, T. multiceps, T. pisiformis and T. crassiceps [104]. Furthermore, it was sensitive enough to distinguish different Echinococcus species, achieving sensitivity down to 2% of a single protoscolex or egg per reaction [104, 108], but failed to discriminate at the genotype level [108]. There are not yet inadequate data to relate intrastrain genetic variants to different life cycles, pathogenicity or any other practical relative features [191, 192, 194, 197, 198]. Subsequently, LAMP has great potential to become a new tool for future perspectives on molecular epidemiology in echinococcosis surveillance at this stage. Additionally, the real-time LAMP assay gave 100% concordance with the results obtained by nested RT-PCR when testing parasite DNA extracted from hydatid cysts from domestic animals and humans, which highlights a brilliant future in clinical diagnosis of CE [108, 109]. Recently, LAMP was first reported to determine Taenia species in an epidemiological survey in Mongolia [199]. Above all, the rapid, sensitive and accurate LAMP is sufficient to facilitate a large-scale epidemiological survey.
Application of LAMP in field research
As discussed above, the LAMP assay is a robust and versatile tool that is capable of meeting the WHO’s requirements for ideal POC tools of ASSURED and possesses the potential to become an appealing option for field research, which was substantiated by a series of laboratory and diagnostic tests.
From the perspective of field application, major achievements were made for LAMP assays for malaria and tuberculosis [200, 201]; in both cases, scientists worked extensively with the WHO for the implementation of the tests in field, and their standardized reagent kits have been used in developing countries as patient side tools [202]. For protozoa, bacteria and fungi, several commercial reagent kits have been put on the market and have performed excellently [203, 204]. With respect to helminths, considerable significance is attached to filariae. LAMP assays for the detection filariae have already come to MDA management practice in Guinea, Nigeria and Southeast Asia [205,206,207]. In a recent epidemiological survey in Mongolia, LAMP also played a significant part [199].
Conclusions
To sum up, though presently in its infancy, the LAMP assay is a groundbreaking DNA amplification technique with prominent advantages. Its ASSURED characteristics and its versatility in adapting to various circumstances make it an ideal POC tool and friendly to field surveys. The chief shortcoming of LAMP is the false-positive result caused by primer-primer reaction and contamination. The former needs further evaluation, and the latter can be solved by the amelioration of the reaction system, detection approaches, etc. Another handicap in LAMP development is the difficulty in primer design. However, its merits outweigh its weakness, and LAMP has blossomed in the detection of microorganisms and protozoa detection and has already entered into the market and epidemiological surveys. Overall, the methodology will be improved in the future, and the active role of LAMP in clinical and epidemiological practice is foreseeable.
Abbreviations
- ASSURED:
-
Affordable, Sensitive, Specific, User-friendly, Rapid and equipment-delivered
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPG:
-
Egg (or eggs) per gram
- FIP:
-
Forward inner primer
- HNB:
-
Hydroxy naphthol blue
- KK:
-
Kato-Katz
- LAMP:
-
Loop-mediated isothermal amplification
- MDA:
-
Mass drug administration
- mf:
-
Microfilariae
- mLAMP:
-
Multiplex LAMP
- NPV:
-
Negative predict value
- NTD:
-
Neglected tropical diseases
- PCR:
-
Polymerase chain reaction
- PEI:
-
Poly-ethylenimine
- POC:
-
Point-of-care
- PPV:
-
Positive predict value
- RT-PCR:
-
Real-time PCR
- STH:
-
Soil-transmitted helminth
- TAS:
-
Transmission assessment survey
References
Cox FE. History of human parasitology. Clin Microbiol Rev. 2002;15:595–612.
Botelho MC, Machado JC, Brindley PJ, Correia da Costa JM. Targeting molecular signaling pathways of Schistosoma haemotobium infection in bladder cancer. Virulence. 2014;2:267–79.
Cardoso R, Alves H, Richter J, Botelho MC. Parasites in forensic science: a historic perspective. Ann Parasitol. 2017;63:235–41.
World Health Organization: Soil-transmitted helminth infections. 2017. http://www.who.int/mediacentre/factsheets/fs366/en/, Accessed 23 Feb 2018.
World Health Organization. Parasitic Hazards. In: World Health Organization, editor. WHO estimates the global burden of foodborne disease. Swiss francs: WHO Press; 2015. p. 35–38.
Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870.
Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5:e1152.
Okorie PN, de Souza DK. Prospects, drawbacks and future needs of xenomonitoring for the endpoint evaluation of lymphatic filariasis elimination programs in Africa. Trans R Soc Trop Med Hyg. 2016;110:90–7.
Spear RC, Seto EY, Carlton EJ, Liang S, Remais JV, Zhong B, et al. The challenge of effective surveillance in moving from low transmission to elimination of schistosomiasis in China. Int J Parasitol. 2011;41:1243–7.
Njiru ZK. Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl Trop Dis. 2012;6:e1572.
Mabey D, Peeling RW, Ustianowski A, Perkins MD. Diagnostics for the developing world. Nat Rev Microbiol. 2004;2:231–40.
Chen Y, Wen T, Lai DH, Wen YZ, Wu ZD, Yang TB, et al. Development and evaluation of loop-mediated isothermal amplification (LAMP) for rapid detection of Clonorchis sinensis from its first intermediate hosts, freshwater snails. Parasitology. 2013;140:1377–83.
Caldeira RL, Jannotti-Passos LK, Dos Santos Carvalho O. Use of molecular methods for the rapid mass detection of Schistosoma mansoni (Platyhelminthes: Trematoda) in Biomphalaria spp. (Gastropoda: Planorbidae). J Trop Med. 2017;2017:8628971.
Arimatsu Y, Kaewkes S, Laha T, Sripa B. Specific diagnosis of Opisthorchis viverrini using loop-mediated isothermal amplification (LAMP) targeting parasite microsatellites. Acta Trop. 2015;141:368–71.
Chen MX, Ai L, Zhang RL, Xia JJ, Wang K, Chen SH, et al. Sensitive and rapid detection of Paragonimus westermani infection in humans and animals by loop-mediated isothermal amplification (LAMP). Parasitol Res. 2011;108:1193–8.
Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int J Parasitol. 2010;40:327–31.
Shiraho EA, Eric AL, Mwangi IN, Maina GM, Kinuthia JM, Mutuku MW, et al. Development of a loop mediated isothermal amplification for diagnosis of Ascaris lumbricoides in fecal samples. J Parasitol Res. 2016;2016:7376207.
Jin Y, Kim EM, Choi MH, Oh MD, Hong ST. Significance of serology by multi-antigen ELISA for tissue helminthiases in Korea. J Korean Med Sci. 2017;32:1118–23.
Doenhoff MJ, Chiodini PL, Hamilton JV. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol. 2004;20:35–9.
Xu X, Zhang Y, Lin D, Zhang J, Xu J, Liu YM, et al. Serodiagnosis of Schistosoma japonicum infection: genome-wide identification of a protein marker, and assessment of its diagnostic validity in a field study in China. Lancet Infect Dis. 2014;14:489–97.
Compton J. Nucleic acid sequence-based amplification. Nature. 1991;350:91–2.
Walker GT, Fraiser MS, Schram JL, Little MC, Nadeau JG, Malinowski DP. Strand displacement amplification--an isothermal, in vitro DNA amplification technique. Nucleic Acids Res. 1992;20:1691–6.
Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward DC. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet. 1998;19:225–32.
X-e F, Li J, Chen Q. One new method of nucleic acid amplification—loop-mediated isothermal amplification of DNA. Virol Sin. 2008;23:167–72.
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63-e.
Rahman SMM, Song HB, Jin Y, Oh JK, Lim MK, Hong ST, et al. Application of a loop-mediated isothermal amplification (LAMP) assay targeting cox1 gene for the detection of Clonorchis sinensis in human fecal samples. PLoS Negl Trop Dis. 2017;11:e0005995.
Tong QB, Chen R, Zhang Y, Yang GJ, Kumagai T, Furushima-Shimogawara R, et al. A new surveillance and response tool: risk map of infected Oncomelania hupensis detected by loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Trop. 2015;141:170–7.
Kaneko H, Kawana T, Fukushima E, Suzutani T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J Biochem Biophys Methods. 2007;70:499–501.
Li Y, Fan P, Zhou S, Zhang L. Loop-mediated isothermal amplification (LAMP): a novel rapid detection platform for pathogens. Microb Pathog. 2017;107:54–61.
Chang CC, Chen CC, Wei SC, Lu HH, Liang YH, Lin CW. Diagnostic devices for isothermal nucleic acid amplification. Sensors (Basel). 2012;12:8319–37.
Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16:223–9.
Gandelman O, Jackson R, Kiddle G, Tisi L. Loop-mediated amplification accelerated by stem primers. Int J Mol Sci. 2011;12:9108–24.
Martineau RL, Murray SA, Ci S, Gao W, Chao SH, Meldrum DR. Improved performance of loop-mediated isothermal amplification assays via swarm priming. Anal Chem. 2017;89:625–32.
Zhang X, Lowe SB, Gooding JJ. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens Bioelectron. 2014;61:491–9.
Abdul-Ghani R, Al-Mekhlafi AM, Karanis P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: would it come to clinical reality as a point-of-care test? Acta Trop. 2012;122:233–40.
Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289:150–4.
Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3:877–82.
Seetang-Nun Y, Jaroenram W, Sriurairatana S, Suebsing R, Kiatpathomchai W. Visual detection of white spot syndrome virus using DNA-functionalized gold nanoparticles as probes combined with loop-mediated isothermal amplification. Mol Cell Probes. 2013;27:71–9.
Mori Y, Kitao M, Tomita N, Notomi T. Real-time turbidimetry of LAMP reaction for quantifying template DNA. J Biochem Biophys Methods. 2004;59:145–57.
Mori Y, Hirano T, Notomi T. Sequence specific visual detection of LAMP reactions by addition of cationic polymers. BMC Biotechnol. 2006;6:3.
Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–72.
Lalle M, Possenti A, Dubey JP, Pozio E. Loop-mediated isothermalamplification-lateral-flowdipstick (LAMP-LFD) to detect Toxoplasma gondii oocyst in ready-to-eat salad. Food Microbiol. 2018;70:137–42.
Ravan H, Yazdanparast R. Development and evaluation of a loop-mediated isothermal amplification method in conjunction with an enzyme-linked immunosorbent assay for specific detection of Salmonella serogroup D. Anal Chim Acta. 2012;733:64–70.
Nkouawa A, Sako Y, Okamoto M, Ito A. Simple identification of human Taenia species by multiplex loop-mediated isothermal amplification in combination with dot enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 2016;94:1318–23.
Wong YP, Othman S, Lau YL, Radu S, Chee HY. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J Appl Microbiol. 2018;124:626–43.
Lin WH, Zou BJ, Song QX, Zhou GH. Progress in multiplex loop-mediated isothermal amplification technology. Yi Chuan. 2015;37:899–910.
Han ET. Loop-mediated isothermal amplification test for the molecular diagnosis of malaria. Expert Rev Mol Diagn. 2013;13:205–18.
Dhama K, Karthik K, Chakraborty S, Tiwari R, Kapoor S, Kumar A, et al. Loop-mediated isothermal amplification of DNA (LAMP): a new diagnostic tool lights the world of diagnosis of animal and human pathogens: a review. Pak J Biol Sci. 2014;17:151–66.
Biswas G, Sakai M. Loop-mediated isothermal amplification (LAMP) assays for detection and identification of aquaculture pathogens: current state and perspectives. Appl Microbiol Biotechnol. 2014;98:2881–95.
Law JW, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2014;5:770.
Gandasegui J, Fernandez-Soto P, Carranza-Rodriguez C, Perez-Arellano JL, Vicente B, Lopez-Aban J, et al. The rapid-heat LAMPellet method: a potential diagnostic method for human urogenital schistosomiasis. PLoS Negl Trop Dis. 2015;9:e0003963.
Watts MR, James G, Sultana Y, Ginn AN, Outhred AC, Kong F, et al. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. Am J Trop Med Hyg. 2014;90:306–11.
Bi A, Nakajima C, Fukushima Y, Tamaru A, Sugawara I, Kimura A, et al. A rapid loop-mediated isothermal amplification assay targeting hspX for the detection of Mycobacterium tuberculosis complex. Jpn J Infect Dis. 2012;65:247–51.
Fischbach J, Xander NC, Frohme M, Glokler JF. Shining a light on LAMP assays--a comparison of LAMP visualization methods including the novel use of berberine. Biotechniques. 2015;58:189–94.
Karthik K, Rathore R, Thomas P, Arun TR, Viswas KN, Dhama K, et al. New closed tube loop mediated isothermal amplification assay for prevention of product cross-contamination. MethodsX. 2014;1:137–43.
Kopp MU, AJd M. Manz a. Chemical amplification: continuous-flow PCR on a chip. Science. 1998;280:1046–8.
Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–73.
Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015;53:1–5.
Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother. 2009;15:62–9.
Cai XQ, Xu MJ, Wang YH, Qiu DY, Liu GX, Lin A, et al. Sensitive and rapid detection of Clonorchis sinensis infection in fish by loop-mediated isothermal amplification (LAMP). Parasitol Res. 2010;106:1379–83.
Arimatsu Y, Kaewkes S, Laha T, Hong SJ, Sripa B. Rapid detection of Opisthorchis viverrini copro-DNA using loop-mediated isothermal amplification (LAMP). Parasitol Int. 2012;61:178–82.
Le TH, Nguyen NT, Truong NH, De NV. Development of mitochondrial loop-mediated isothermal amplification for detection of the small liver fluke Opisthorchis viverrini (Opisthorchiidae; Trematoda; Platyhelminthes). J Clin Microbiol. 2012;50:1178–84.
Cevallos W, Fernandez-Soto P, Calvopina M, Fontecha-Cuenca C, Sugiyama H, Sato M, et al. LAMPhimerus: a novel LAMP assay for detecting Amphimerus sp. DNA in human stool samples. PLoS Negl Trop Dis. 2017;11:e0005672.
Cevallos W, Fernandez-Soto P, Calvopina M, Buendia-Sanchez M, Lopez-Aban J, Vicente B, et al. Diagnosis of amphimeriasis by LAMPhimerus assay in human stool samples long-term storage onto filter paper. PLoS One. 2018;13:e0192637.
Ai L, Li C, Elsheikha HM, Hong SJ, Chen JX, Chen SH, et al. Rapid identification and differentiation of Fasciola hepatica and Fasciola gigantica by a loop-mediated isothermal amplification (LAMP) assay. Vet Parasitol. 2010;174:228–33.
Martinez-Valladares M, Rojo-Vazquez FA. Loop-mediated isothermal amplification (LAMP) assay for the diagnosis of fasciolosis in sheep and its application under field conditions. Parasit Vectors. 2016;9:73.
Arifin MI, Hoglund J, Novobilsky A. Comparison of molecular and conventional methods for the diagnosis of Fasciola hepatica infection in the field. Vet Parasitol. 2016;232:8–11.
Kumagai T, Furushima-Shimogawara R, Ohmae H, Wang TP, Lu S, Chen R, et al. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am J Trop Med Hyg. 2010;83:542–8.
Wang C, Chen L, Yin X, Hua W, Hou M, Ji M, et al. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasit Vectors. 2011;4:164.
Xu J, Guan ZX, Zhao B, Wang YY, Cao Y, Zhang HQ, et al. DNA detection of Schistosoma japonicum: diagnostic validity of a LAMP assay for low-intensity infection and effects of chemotherapy in humans. PLoS Negl Trop Dis. 2015;9:e0003668.
Abbasi I, King CH, Muchiri EM, Hamburger J. Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: identification of infected snails from early prepatency. Am J Trop Med Hyg. 2010;83:427–32.
Hamburger J, Abbasi I, Kariuki C, Wanjala A, Mzungu E, Mungai P, et al. Evaluation of loop-mediated isothermal amplification suitable for molecular monitoring of schistosome-infected snails in field laboratories. Am J Trop Med Hyg. 2013;88:344–51.
Fernandez-Soto P, Gandasegui Arahuetes J, Sanchez Hernandez A, Lopez Aban J, Vicente Santiago B, Muro A. A loop-mediated isothermal amplification (LAMP) assay for early detection of Schistosoma mansoni in stool samples: a diagnostic approach in a murine model. PLoS Negl Trop Dis. 2014;8:e3126.
Song J, Liu C, Bais S, Mauk MG, Bau HH, Greenberg RM. Molecular detection of schistosome infections with a disposable microfluidic cassette. PLoS Negl Trop Dis. 2015;9:e0004318.
Gandasegui J, Fernandez-Soto P, Hernandez-Goenaga J, Lopez-Aban J, Vicente B, Muro A. Biompha-LAMP: a new rapid loop-mediated isothermal amplification assay for detecting Schistosoma mansoni in Biomphalaria glabrata snail host. PLoS Negl Trop Dis. 2016;10:e0005225.
Lodh N, Mikita K, Bosompem KM, Anyan WK, Quartey JK, Otchere J, et al. Point of care diagnosis of multiple schistosome parasites: species-specific DNA detection in urine by loop-mediated isothermal amplification (LAMP). Acta Trop. 2017;173:125–9.
Gandasegui J, Fernandez-Soto P, Muro A, Simoes Barbosa C, Lopes de Melo F, Loyo R, et al. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: assessment in human and snail samples. PLoS Negl Trop Dis. 2018;12:e0006314.
Mugambi RM, Agola EL, Mwangi IN, Kinyua J, Shiraho EA, Mkoji GM. Development and evaluation of a loop mediated isothermal amplification (LAMP) technique for the detection of hookworm (Necator americanus) infection in fecal samples. Parasit Vectors. 2015;8:574.
Rashwan N, Diawara A, Scott ME, Prichard RK. Isothermal diagnostic assays for the detection of soil-transmitted helminths based on the SmartAmp2 method. Parasit Vectors. 2017;10:496.
Khoshakhlagh P, Spotin A, Mahami-Oskouei M, Shahbazi A, Ozlati M. Loop-mediated isothermal amplification as a reliable assay for Toxocara canis infection in pet dogs. Parasitol Res. 2017;116:2591–7.
Macuhova K, Kumagai T, Akao N, Ohta N. Loop-mediated isothermal amplification assay for detection and discrimination of Toxocara canis and Toxocara cati eggs directly from sand samples. J Parasitol. 2010;96:1224–7.
Fernandez-Soto P, Sanchez-Hernandez A, Gandasegui J, Bajo Santos C, Lopez-Aban J, Saugar JM, et al. Strong-LAMP: A LAMP Assay for Strongyloides spp detection in stool and urine samples Towards the diagnosis of human strongyloidiasis starting from a rodent model. PLoS Negl Trop Dis. 2016;10:e0004836.
Alhassan A, Makepeace BL, LaCourse EJ, Osei-Atweneboana MY, Carlow CK. A simple isothermal DNA amplification method to screen black flies for Onchocerca volvulus infection. PLoS One. 2014;9:e108927.
Alhassan A, Osei-Atweneboana MY, Kyeremeh KF, Poole CB, Li Z, Tettevi E, et al. Comparison of a new visual isothermal nucleic acid amplification test with PCR and skin snip analysis for diagnosis of onchocerciasis in humans. Mol Biochem Parasitol. 2016;210:10–2.
Lagatie O, Merino M, Batsa Debrah L, Debrah AY, Stuyver LJ. An isothermal DNA amplification method for detection of Onchocerca volvulus infection in skin biopsies. Parasit Vectors. 2016;9:624.
Poole CB, Li Z, Alhassan A, Guelig D, Diesburg S, Tanner NA, et al. Colorimetric tests for diagnosis of filarial infection and vector surveillance using non-instrumented nucleic acid loop-mediated isothermal amplification (NINA-LAMP). PLoS One. 2017;12:e0169011.
Takagi H, Itoh M, Kasai S, Yahathugoda TC, Weerasooriya MV, Kimura E. Development of loop-mediated isothermal amplification method for detecting Wuchereria bancrofti DNA in human blood and vector mosquitoes. Parasitol Int. 2011;60:493–7.
Poole CB, Tanner NA, Zhang Y, Evans TC Jr, Carlow CK. Diagnosis of brugian filariasis by loop-mediated isothermal amplification. PLoS Negl Trop Dis. 2012;6:e1948.
Drame PM, Fink DL, Kamgno J, Herrick JA, Nutman TB. Loop-mediated isothermal amplification for rapid and semiquantitative detection of Loa loa infection. J Clin Microbiol. 2014;52:2071–7.
Fernandez-Soto P, Mvoulouga PO, Akue JP, Aban JL, Santiago BV, Sanchez MC, et al. Development of a highly sensitive loop-mediated isothermal amplification (LAMP) method for the detection of Loa loa. PLoS One. 2014;9:e94664.
Poole CB, Ettwiller L, Tanner NA, Evans TC Jr, Wanji S, Carlow CK. Genome filtering for new DNA biomarkers of Loa loa infection suitable for loop-mediated isothermal amplification. PLoS One. 2015;10:e0139286.
Raele DA, Pugliese N, Galante D, Latorre LM, Cafiero MA. Development and application of a loop-mediated isothermal amplification (LAMP) approach for the rapid detection of Dirofilaria repens from biological samples. PLoS Negl Trop Dis. 2016;10:e0004789.
Chen R, Tong Q, Zhang Y, Lou D, Kong Q, Lv S, et al. Loop-mediated isothermal amplification: rapid detection of Angiostrongylus cantonensis infection in Pomacea canaliculata. Parasit Vectors. 2011;4:204.
Liu CY, Song HQ, Zhang RL, Chen MX, Xu MJ, Ai L, et al. Specific detection of Angiostrongylus cantonensis in the snail Achatina fulica using a loop-mediated isothermal amplification (LAMP) assay. Mol Cell Probes. 2011;25:164–7.
Li X, Liu W, Wang J, Zou D, Wang X, Yang Z, et al. Rapid detection of Trichinella spiralis larvae in muscles by loop-mediated isothermal amplification. Int J Parasitol. 2012;42:1119–26.
Lin Z, Cao J, Zhang H, Zhou Y, Deng M, Li G, et al. Comparison of three molecular detection methods for detection of Trichinella in infected pigs. Parasitol Res. 2013;112:2087–93.
Kikuchi T, Aikawa T, Oeda Y, Karim N, Kanzaki N. A rapid and precise diagnostic method for detecting the pinewood nematode Bursaphelenchus xylophilus by loop-mediated isothermal amplification. Phytopathology. 2009;99:1365–9.
Melville L, Kenyon F, Javed S, McElarney I, Demeler J, Skuce P. Development of a loop-mediated isothermal amplification (LAMP) assay for the sensitive detection of Haemonchus contortus eggs in ovine faecal samples. Vet Parasitol. 2014;206:308–12.
Yang X, Qi MW, Zhang ZZ, Gao C, Wang CQ, Lei WQ, et al. Development and evaluation of a loop-mediated isothermal amplification (LAMP) assay for the detection of Haemonchus contortus in goat fecal samples. J Parasitol. 2017;103:161–7.
Nkouawa A, Sako Y, Nakao M, Nakaya K, Ito A. Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species. J Clin Microbiol. 2009;47:168–74.
Nkouawa A, Sako Y, Li T, Chen X, Wandra T, Swastika IK, et al. Evaluation of a loop-mediated isothermal amplification method using fecal specimens for differential detection of Taenia species from humans. J Clin Microbiol. 2010;48:3350–2.
Nkouawa A, Sako Y, Li T, Chen X, Nakao M, Yanagida T, et al. A loop-mediated isothermal amplification method for a differential identification of Taenia tapeworms from human: application to a field survey. Parasitol Int. 2012;61:723–5.
Sako Y, Nkouawa A, Yanagida T, Ito A. Loop-mediated isothermal amplification method for a differential identification of human Taenia tapeworms. Methods Mol Biol. 2013;1039:109–20.
Feng K, Li W, Guo Z, Duo H, Fu Y, Shen X, et al. Development of LAMP assays for the molecular detection of taeniid infection in canine in Tibetan rural area. J Vet Med Sci. 2017;79:1986–93.
Salant H, Abbasi I, Hamburger J. The development of a loop-mediated isothermal amplification method (LAMP) for Echinococcus granulosus [corrected] coprodetection. Am J Trop Med Hyg. 2012;87:883–7.
Ni XW, McManus DP, Lou ZZ, Yang JF, Yan HB, Li L, et al. A comparison of loop-mediated isothermal amplification (LAMP) with other surveillance tools for Echinococcus granulosus diagnosis in canine definitive hosts. PLoS One. 2014;9:e100877.
Ni X, McManus DP, Yan H, Yang J, Lou Z, Li H, et al. Loop-mediated isothermal amplification (LAMP) assay for the identification of Echinococcus multilocularis infections in canine definitive hosts. Parasit Vectors. 2014;7:254.
Wassermann M, Mackenstedt U, Romig T. A loop-mediated isothermal amplification (LAMP) method for the identification of species within the Echinococcus granulosus complex. Vet Parasitol. 2014;200:97–103.
Ahmed ME, Eldigail MH, Elamin FM, Ali IA, Grobusch MP, Aradaib IE. Development and evaluation of real-time loop-mediated isothermal amplification assay for rapid detection of cystic echinococcosis. BMC Vet Res. 2016;12:202.
World Health Organization: Foodborne trematodiases. 2018. http://www.who.int/news-room/fact-sheets/detail/foodborne-trematodiases, Accessed 8 Feb 2018.
World Health Orgnization. Expert consultation to accelerate control of foodborne trematode infections, taeniasis and cysticercosis, Seoul, Republic of Korea, 17–19 May 2017.2017.
Saijuntha W, Sithithaworn P, Kaitsopit N, Andrews RH, Petney TN. Liver flukes: Clonorchis and Opisthorchis. In: Toledo R, Fried B, editors. Digenetic Trematodes. New York: Springer New York; 2014. p. 153–99.
IARC working group on the evaluation of carcinogenic risk to humans. Biological agents. In: A review of human carcinogens, vol. 100 B. Lyon (FR): International agency for research on cancer; 2012. p. 341–70.
Rim HJ. Clonorchiasis: an update. J Helminthol. 2005;79:269–81.
Hong ST, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol Int. 2012;61:17–24.
Traub RJ, Macaranas J, Mungthin M, Leelayoova S, Cribb T, Murrell KD, et al. A new PCR-based approach indicates the range of Clonorchis sinensis now extends to Central Thailand. PLoS Negl Trop Dis. 2009;3:e367.
Furst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210–21.
Chen D, Chen J, Huang J, Chen X, Feng D, Liang B, et al. Epidemiological investigation of Clonorchis sinensis infection in freshwater fishes in the Pearl River Delta. Parasitol Res. 2010;107:835–9.
Calvopina M, Cevallos W, Kumazawa H, Eisenberg J. High prevalence of human liver infection by Amphimerus spp. flukes, Ecuador. Emerg Infect Dis. 2011;17:2331–4.
Mas-Coma S, Valero MA, Bargues MD. Fascioliasis. In: Toledo R, Fried B, editors. Digenetic trematodes. New York: Springer New York; 2014. p. 77–114.
El-Tahawy AS, Bazh EK, Khalafalla RE. Epidemiology of bovine fascioliasis in the Nile Delta region of Egypt: its prevalence, evaluation of risk factors, and its economic significance. Vet World. 2017;10:1241–9.
Fairweather I. Reducing the future threat from (liver) fluke: realistic prospect or quixotic fantasy? Vet Parasitol. 2011;180:133–43.
Salimi-Bejestani MR, McGarry JW, Felstead S, Ortiz P, Akca A, Williams DJ. Development of an antibody-detection ELISA for Fasciola hepatica and its evaluation against a commercially available test. Res Vet Sci. 2005;78:177–81.
George SD, Vanhoff K, Baker K, Lake L, Rolfe PF, Seewald W, et al. Application of a coproantigen ELISA as an indicator of efficacy against multiple life stages of Fasciola hepatica infections in sheep. Vet Parasitol. 2017;246:60–9.
Blair D, Xu Z-B, Agatsuma T. Paragonimiasis and the genus Paragonimus. Adv Parasitol. 1999;42:113–222.
Lu XT, Gu QY, Limpanont Y, Song LG, Wu ZD, Okanurak K, et al. Snail-borne parasitic diseases: an update on global epidemiological distribution, transmission interruption and control methods. Infect Dis Poverty. 2018;7:28.
Yoshida A, Matsuo K, Moribe J, Tanaka R, Kikuchi T, Nagayasu E, et al. Venison, another source of Paragonimus westermani infection. Parasitol Int. 2016;65:607–12.
Lee JS, Lee J, Kim SH, Yong TS. Molecular cloning and characterization of a major egg antigen in Paragonimus westermani and its use in ELISA for the immunodiagnosis of paragonimiasis. Parasitol Res. 2007;100:677–81.
World Health Organization: Preventive chemotherapy (PC) data portal. 2017. http://apps.who.int/gho/cabinet/pc.jsp, Accessed 8 Jun 2018.
World Health Organization: Schistosomiasis. 2018. http://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis, Accessed 8 Jun 2018.
Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19:196–205.
Savioli L, Fenwick A, Rollinson D, Albonico M, Ame SM. An achievable goal: control and elimination of schistosomiasis. Lancet. 2015;386:739.
Ross AG, Sleigh AC, Li Y, Davis GM, Williams GM, Jiang Z, et al. Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st century. Clin Microbiol Rev. 2001;14:270–95.
Ross AG, Chau TN, Inobaya MT, Olveda RM, Li Y, Harn DA. A new global strategy for the elimination of schistosomiasis. Int J Infect Dis. 2017;54:130–7.
Cai YC, Xu JF, Steinmann P, Chen SH, Chu YH, Tian LG, et al. Field comparison of circulating antibody assays versus circulating antigen assays for the detection of schistosomiasis japonica in endemic areas of China. Parasit Vectors. 2014;7:138.
Liu C, Liao SC, Song J, Mauk MG, Li X, Wu G, et al. A high-efficiency superhydrophobic plasma separator. Lab Chip. 2016;16:553–60.
WHO/Department of control of neglected tropical diseases. Soil-transmitted helminthiases. In: Holmes P, editor. Investing to overcome the global impact of neglected tropical diseases. Swiss franc: WHO Press; 2015. p. 161–7.
WHO/Department of control of neglected tropical diseases. Assessing the epidemiology of STH during a TAS. In: Montresor A, editor. Assessing the epidemiology of soil-transmitted helminths during a transmission assessment survey (TAS). Rome: WHO Press; 2015. p. 1–16.
Dacombe RJ, Crampin AC, Floyd S, Randall A, Ndhlovu R, Bickle Q, et al. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans R Soc Trop Med Hyg. 2007;101:140–5.
Phuphisut O, Yoonuan T, Sanguankiat S, Chaisiri K, Maipanich W, Pubampen S, et al. Triplex polymerase chain reaction assay for detection of major soil-transmitted helminths, Ascaris lumbricoides, Trichuris trichiura, Necator americanus, in fecal samples. Southeast Asian J Trop Med Public Health. 2014;45:267–75.
Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:967–72.
Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–7.
Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7:e2288.
Montes M, Sawhney C, Barros N. Strongyloides stercoralis: there but not seen. Curr Opin Infect Dis. 2010;23:500–4.
Paula FM, Costa-Cruz JM. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology. 2011;138:1331–40.
Marcos LA, Terashima A, Dupont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102:314–8.
Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep. 2011;13:35–46.
Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002.
Campo Polanco L, Gutierrez LA, Cardona AJ. Diagnosis of Strongyloides stercoralis infection: meta-analysis on evaluation of conventional parasitological methods (1980-2013). Rev Esp Salud Publica. 2014;88:581–600.
Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2014;8:e2640.
Buonfrate D, Requena-Mendez A, Angheben A, Cinquini M, Cruciani M, Fittipaldo A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection-a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12:e0006229.
Gawor J, Borecka A, Zarnowska H, Marczynska M, Dobosz S. Environmental and personal risk factors for toxocariasis in children with diagnosed disease in urban and rural areas of Central Poland. Vet Parasitol. 2008;155:217–22.
Fillaux J, Magnaval JF. Laboratory diagnosis of human toxocariasis. Vet Parasitol. 2013;193:327–36.
Jacobs DE, Zhu X, Gasser RB, Chilton NB. PCR-based methods for identification of potentially zoonotic ascaridoid parasites of the dog, fox and cat. Acta Trop. 1997;68:191–200.
Borecka A, Gawor J. Modification of gDNA extraction from soil for PCR designed for the routine examination of soil samples contaminated with Toxocara spp. eggs. J Helminthol. 2008;82:119–22.
Fogt-Wyrwas R, Jarosz W, Mizgajska-Wiktor H. Utilizing a polymerase chain reaction method for the detection of Toxocara canis and T. cati eggs in soil. J Helminthol. 2007;81:75–8.
Ozlati M, Spotin A, Shahbazi A, Mahami-Oskouei M, Hazratian T, Adibpor M, et al. Genetic variability and discrimination of low doses of Toxocara spp. from public areas soil inferred by loop-mediated isothermal amplification assay as a field-friendly molecular tool. Vet World. 2016;9:1471–7.
World Health Organization: Lymphatic filariasis. 2018. http://www.who.int/en/news-room/fact-sheets/detail/lymphatic-filariasis, Accessed 26 May 2018.
Pilotte N, Unnasch TR, Williams SA. The current status of molecular xenomonitoring for lymphatic filariasis and onchocerciasis. Trends Parasitol. 2017;33:788–98.
WHO/Department of control of neglected tropical diseases. Comparison of tests for lymphatic filariasisantigen. In: King DJ, editor. Strengthening the assessment of lymphatic filariasis transmission and documenting the achievement of elimination. France: WHO Press; 2016. p. 8–15.
WHO/Department of control of neglected tropical diseases. Responding to failed transmission assessment surveys. Report of an ad hoc meeting. Washington: WHO Press; 2016.
Weil GJ, Lammie PJ, Weiss N. The ICT Filariasis test: a rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today. 1997;13:401–4.
McCarthy JS, Lustigman S, Yang GJ, Barakat RM, Garcia HH, Sripa B, et al. A research agenda for helminth diseases of humans: diagnostics for control and elimination programmes. PLoS Negl Trop Dis. 2012;6:e1601.
Rao RU, Atkinson LJ, Ramzy RMR, Helmy H, Farid HA, Bockarie MJ, et al. A real-time PCR-based assay for detection of Wcuhereria bancrofti DNA in blood and mosquitoes. Am J Trop Med Hyg. 2006;74:826–32.
Melrose WD. Lymphatic filariasis: new insights into an old disease. Int J Parasitol. 2002;32:947–60.
World Health Organization: Onchocerciasis. 2018. http://www.who.int/news-room/fact-sheets/detail/onchocerciasis. Accessed 18 August 2018.
World Health Organization: Report of the 1st meeting of the WHO Onchocerciasis technical advisory subgroup. Edited by Cantey DP. Geneva: World Health Organization; 2017.
Unnasch TR, Golden A, Cama V, Cantey PT. Diagnostics for onchocerciasis in the era of elimination. Int Health. 2018;10:i20–i6.
WHO/Department of control of neglected tropical diseases. Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis. Geneva: WHO Press; 2016.
Merriweather A, Unnasch TR. Onchocerca volvulus: development of a species specific polymerase chain reaction-based assay. Exp Parasitol. 1996;83:164–6.
Alhassan A, Li Z, Poole CB, Carlow CK. Expanding the MDx toolbox for filarial diagnosis and surveillance. Trends Parasitol. 2015;31:391–400.
World Health Organization. Defining the roles of vector control and xenomonitoring in the Global Programme to Eliminate Lymphatic Filariasis: report of the informal consultation WHO/HQ, Geneva, 29–31 January 2002. In: IRIS: World Health Organization. p. 2002. http://apps.who.int/iris/handle/10665/67340. Accessed 4 Jan 2019.
Laney SJ, Buttaro CJ, Visconti S, Pilotte N, Ramzy RMR, Weil GJ, et al. A reverse transcriptase-PCR assay for detecting filarial infective larvae in mosquitoes. PLoS Negl Trop Dis. 2008;2:e251.
Laney SJ, Ramzy RM, Helmy HH, Farid HA, Ashour AA, Weil GJ, et al. Detection of Wuchereria bancrofti L3 larvae in mosquitoes: a reverse transcriptase PCR assay evaluating infection and infectivity. PLoS Negl Trop Dis. 2010;4:e602.
Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22.
Chippaux JP, Boussinesq M, Gardon J, Gardon-Wendel N, Ernould JC. Severe adverse reaction risks during mass treatment with ivermectin in loiasis-endemic areas. Parasitol Today. 1996;12:448–50.
Pampiglione S, Rivasi F, Angeli G, Boldorini R, Incensati RM, Pastormerlo M, et al. Dirofilariasis due to Dirofilaria repens in Italy, an emergent zoonosis: report of 60 new cases. Histopathology. 2001;38:344–54.
Magnis J, Lorentz S, Guardone L, Grimm F, Magi M, Naucke TJ, et al. Morphometric analyses of canine blood microfilariae isolated by the Knott’s test enables Dirofilaria immitis and D. repens species-specific and Acanthocheilonema (syn. Dipetalonema) genus-specific diagnosis. Parasit Vectors. 2013;6:48.
Tasić-Otašević SA, Gabrielli SV, Tasić AV, MiladinovićTasić NL, Kostić JT, Ignjatović AM, et al. Seroreactivity to Dirofilaria antigens in people from different areas of Serbia. BMC Infect Dis. 2014;14:68.
Latrofa MS, Montarsi F, Ciocchetta S, Annoscia G, Dantas-Torres F, Ravagnan S, et al. Molecular xenomonitoring of Dirofilaria immitis and Dirofilaria repens in mosquitoes from North-Eastern Italy by real-time PCR coupled with melting curve analysis. Parasit Vectors. 2012;5:76.
Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Dominguez Alpizar JL. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol. 2006;135:303–14.
Alicata JE. Angiostrongyliasis cantonensis (eosinophilic meningitis): historical events in its recognition as a new parasitic disease of man. J Wash Acad Sci. 1988;78:38–46.
Rodpai R, Intapan PM, Thanchomnang T, Sanpool O, Sadaow L, Laymanivong S, et al. Angiostrongylus cantonensis and A. malaysiensis broadly overlap in Thailand, Lao PDR, Cambodia and Myanmar: a molecular survey of larvae in land snails. PLoS One. 2016;11:e0161128.
Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, et al. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology. 2016;143:1087–118.
Murphy GS, Johnson S. Clinical aspects of eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis, the rat lungworm. Hawaii J Med Public Health. 2013;72(Suppl 2):35–40.
Li T, Craig PS, Ito A, Chen X, Qiu D, Qiu J, et al. Taeniasis/cysticercosis in a Tibetan population in Sichuan Province. China Acta Trop. 2006;100:223–31.
Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, et al. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–53.
Mayta H, Gilman RH, Prendergast E, Castillo JP, Tinoco YO, Garcia HH, et al. Nested PCR for specific diagnosis of Taenia solium taeniasis. J Clin Microbiol. 2008;46:286–9.
Mag JE, Meslin F-X, Pawłowski ZS, World Health Organization. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. In: IRIS: World Health Organization; 2001. https://apps.who.int/iris/handle/10665/42427. Accessed 30 Jan 2019.
McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–304.
Tigre W, Deresa B, Haile A, Gabriel S, Victor B, Pelt JV, et al. Molecular characterization of Echinococcus granulosus s.L. cysts from cattle, camels, goats and pigs in Ethiopia. Vet Parasitol. 2016;215:17–21.
Roinioti E, Papathanassopoulou A, Theodoropoulou I, Simsek S, Theodoropoulos G. Molecular identification of Echinococcus granulosus isolates from ruminants in Greece. Vet Parasitol. 2016;226:138–44.
Eckert J. Predictive values and quality control of techniques for the diagnosis of Echinococcus multilocularis in definitive hosts. Acta Trop. 2003;85:157–63.
Ito A, Nakao M, Lavikainen A, Hoberg E. Cystic echinococcosis: future perspectives of molecular epidemiology. Acta Trop. 2017;165:3–9.
Bowles J, Blair D, McManus D. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54:165–73.
Huttner M, Nakao M, Wassermann T, Siefert L, Boomker JD, Dinkel A, et al. Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: Taeniidae) from the African lion. Int J Parasitol. 2008;38:861–8.
Romig T, Ebi D, Wassermann M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet Parasitol. 2015;213:76–84.
Avila HG, Santos GB, Cucher MA, Macchiaroli N, Perez MG, Baldi G, et al. Implementation of new tools in molecular epidemiology studies of Echinococcus granulosus sensu lato in South America. Parasitol Int. 2017;66:250–7.
Davaasuren A, Dorjsuren T, Yanagida T, Sako Y, Nakaya K, Davaajav A, et al. Recent situation of taeniasis in Mongolia (2002-2012). Korean J Parasitol. 2014;52:211–4.
World Health Organization. The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis: policy guidance. Geneva: WHO Press; 2016.
Patel JC, Lucchi NW, Srivastava P, Lin JT, Sug-Aram R, Aruncharus S, et al. Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, RealAmp, for the diagnosis of malaria in Thailand and India. J Infect Dis. 2014;210:1180–7.
Mansour SM, Ali H, Chase CC, Cepica A. Loop-mediated isothermal amplification for diagnosis of 18 world Organization for Animal Health (OIE) notifiable viral diseases of ruminants, swine and poultry. Anim Health Res Rev. 2015;16:89–106.
Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother. 2013;19:404–11.
Niessen L. Current state and future perspectives of loop-mediated isothermal amplification (LAMP)-based diagnosis of filamentous fungi and yeasts. Appl Microbiol Biotechnol. 2015;99:553–74.
Kouassi BL, de Souza DK, Goepogui A, Narh CA, King SA, Mamadou BS, et al. Assessing the presence of Wuchereria bancrofti in vector and human populations from urban communities in Conakry. Guinea Parasit Vectors. 2015;8:492.
Pam DD, de Souza DK, D'Souza S, Opoku M, Sanda S, Nazaradden I, et al. Is mass drug administration against lymphatic filariasis required in urban settings? The experience in Kano. Nigeria PLoS Negl Trop Dis. 2017;11:e0006004.
Dorkenoo MA, de Souza DK, Apetogbo Y, Oboussoumi K, Yehadji D, Tchalim M, et al. Molecular xenomonitoring for post-validation surveillance of lymphatic filariasis in Togo: no evidence for active transmission. Parasit Vectors. 2018;11:52.
Acknowledgements
We sincerely appreciate that Prof. Heinz Mehlhorn polished the revised manuscript.
Funding
This work was supported by grants from the Project of Basic Platform of National Science and Technology Resources of the Ministry of Sciences and Technology of China (grant no. TDRC-2017-22), the National Key Research and Development Program of China (grant no. 2016YFC1202003, 2016YFC1202005 and 2016YFC1200500), the National Natural Science Foundation of China (grant No. 81371836, 81572023 and 81271855), the Guangdong Natural Science Foundation (grant No. 2014A030313134), the Science and Technology Planning Project of Guangdong Province (grant No. 2016A050502008), the Science and Technology Planning Project of Guangzhou (grant No. 201607010029), the “111” Project (grant No. B12003), the Undergraduates Innovation Training Program of Guangdong Province (grant No. 201410558274 and 201601084) and the Teaching Reform Project of Sun Yat-sen University (grant No. 2016012).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
DMH, ZLY and KO conceived and drafted the review; YN and LZY revised the subsequent version of the review. All authors read and approved the final version of the review.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional file
Additional file 1:
Multilingual abstract in the five official working languages of the United Nations. (PDF 590 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Deng, MH., Zhong, LY., Kamolnetr, O. et al. Detection of helminths by loop-mediated isothermal amplification assay: a review of updated technology and future outlook. Infect Dis Poverty 8, 20 (2019). https://doi.org/10.1186/s40249-019-0530-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-019-0530-z