Abstract
The role of platelets extends beyond hemostasis. The pivotal role of platelets in inflammation has shed new light on the natural history of conditions associated with acute or chronic inflammation. Beyond the preservation of vascular integrity, platelets are essential to tissue homeostasis and platelet-derived products are already used in the clinics. Unanticipated was the role of platelets in the adaptative immune response, allowing a renewed conceptual approach of auto-immune diseases. Platelets are also important players in cancer growth and dissemination. Platelets fulfill most of their functions through the expression of still incompletely characterized membrane-bound or soluble mediators. Among them, CD154 holds a peculiar position, as platelets represent a major source of CD154 and as CD154 contributes to most of these new platelet attributes. Here, we provide an overview of some of the new frontiers that the study of platelet CD154 is opening, in inflammation, tissue homeostasis, immune response, hematopoiesis and cancer.
Similar content being viewed by others
Introduction
Platelets are cytoplasmic fragments released in the bloodstream during the fragmentation of polyploid megakaryocytes (MK), a phenomenon critically dependent on thrombopoietin [1-3]. The mammalian platelet is thought to result from a phylogenic trend to ensure hemostasis under high vascular shear forces; indeed, it can specifically form arterial thrombi sustaining high shear stress [4]. It is thought that the platelet coopted attributes of a nucleated cell ancestor endowed with a multifunctional role in coagulation, inflammation and defense against infections [5,6]. Platelets have a short lifespan, of around 7 days; mechanisms responsible for their clearance are ill-understood; lectin-carbohydrate recognition of aged and damaged platelets by splenic and liver macrophages and hepatocytes is emphasized [7]. The best-defined function of platelets is hemostasis. Disruption of the endothelial cell (EC) lining leads to platelet activation, platelet adherence and aggregation which temporarily plug the damaged vessel. In this process, platelets also drive and confine coagulation at sites of tissue damage. Indeed, deficiencies in platelet production or function are associated to bleeding disorders, while increases in platelet number or gain of function are associated to thrombosis. The role of platelets in health and disease extends beyond hemostasis; non-hemostatic platelet functions include inflammation, innate and adaptative immune responses and tissue homeostasis (Figure 1). Decisive advances in understanding platelet function have been made through the characterization of platelet receptors and their ligands and platelet-derived mediators [8]. Among platelet mediators, CD154, the ligand of CD40, has attracted specific attention as it orchestrates many of these new platelet attributes.
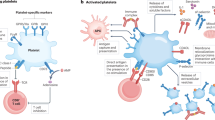
Platelets have a pleiotropic range of biological roles that extend beyond hemostasis. The breaching of tissue homeostasis leads to platelet activation, a common event in various causes of tissue injury, traumatic, infectious, ischemic, autoimmune… Platelet activation, apart from its essential role in bleeding arrest, is the source of a flow of information that fuels the inflammatory reaction. Platelets represent host defense machines against infection, via the clearing of pathogens and the expression of membrane-bound and soluble signals that regulate the innate and adaptative arms of the immune response. Pathways activated in inflammation, coagulation, vascular/tissue repair and host defense are connected via soluble and cell-mediated signals, providing a coherent biological response aiming at arresting bleeding, curing infection and reestablishing tissue homeostasis. CD154 interfaces with many of these pathways (see Figures 2 and 3); activated platelets express a membrane-bound form of CD154 and release a soluble form (sCD154). Platelet derived microparticles (PMPs) recapitulate several of activated platelet functions (see text for details). Only some relevant molecules have been depicted. Small circles symbolize secreted molecules, large circles membrane-associated molecules. Abbreviations: CAMs, cell adhesion molecules; Fg, fibrinogen; Fn, fibronectin; ECM, extracellular matrix; NET ind., neutrophil extracellular traps induction; PAF, platelet activating factor; ROS, reactive oxygen species; Vn, vitronectin; vWF, von Willebrand factor.
CD154
CD154, the CD40 ligand, a member of the Tumor Necrosis Factor (TNF) family, is central to the immune response [9,10]. CD154 was discovered as mediating humoral immunity and was originally considered to be restricted to activated helper T cells. The CD154/CD40 interaction drives B cell proliferation, antibody production and isotype switching and is involved in thymic selection. This interaction is required for B memory cell generation and germinal center formation. Accordingly, CD154 deficiency is associated with an impairment of the humoral immune response to T-cell dependent antigens, including defective immunoglobulin class switching; patients with the X-linked hyper-IgM syndrome caused by mutations of the CD154 gene, generally present low serum IgG and IgA, but normal or increased serum IgM, and are susceptible to opportunistic infections. Mice with a disrupted Cd154 gene fail to undergo isotype switching to T-cell dependent antigens while normally responding to T-cell independent antigens. In line with its regulatory role on the adaptative immune response, the CD40/CD154 interaction contributes to autoimmune disorders in a number of animal models [11-15]. Manipulation of the CD154/CD40 interaction has been used in efforts to develop novel strategies in autoimmune diseases, results in animal models being encouraging [13]. Clinical trials have been launched with humanized anti-CD154 monoclonal antibodies. Clinical interest of this strategy remains mixed, and is strongly limited by thrombotic complications [12-14].
Apart from B cells, CD40 is expressed by various cells, including dendritic cells (DC), monocytes, T lymphocytes, EC, a variety of epithelial cells, smooth muscle cells, fibroblasts; its expression is low in basal conditions and is stimulated by inflammatory mediators [16-19]. CD40 expression is increased by CD154, however it is not known whether this induction is direct or indirect [20,21]. CD40 is not the sole receptor for CD154; alternative receptors have been described, such as integrins α5β1, αIIbβ3 and αMβ2; CD154 binding depends on their activation states [22-25]. These additional receptors are of significance in the pathophysiology of atherogenesis and are important to consider when comparing CD40- and CD154-deficient mouse phenotypes.
CD154 is a transmembrane protein and a proteolytic soluble form, sCD154, which keeps the CD40-binding domain, is released by a partially understood mechanism. The release of sCD154 was first documented in activated T-lymphocytes [26]. CD154 has a trimeric configuration, required for functional activity [27-30]. A complex signaling cascade is triggered by CD40 ligation, involving TNF receptor-associated factors (TRAF) as proximal transducing signal initiators [10,20]. Several signaling pathways, including nuclear factor-κB (NF-κB), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase pathways, are activated by CD40 ligation; however, there is a differential outcome depending upon which TRAF member binds preferentially, and which cell/conditions are involved [31]; the binding of TRAF-6 is critical in vascular inflammation and metabolic complications associated with obesity [32,33].
CD154 expression is also observed in natural killer cells, DC, cells of the monocyte/macrophage lineage, endothelial, smooth muscle and epithelial cells [20]. Basal CD154 expression is very low, or undetectable, as in EC and epithelial cells for example [34], and is increased by a variety of stimuli, most notably inflammatory cytokines [20]. This suggests that CD154 expression may mostly have relevance when induced, as in inflammation. CD154 is also expressed by blood platelets, being cryptic in unstimulated platelets and rapidly exposed at the platelet surface following platelet activation [35].
CD154 expression by platelets
The distribution of CD154 in platelets is partly understood. CD154 was found in α-granules, as shown by immunoelectron microscopy or quantitative immunofluorescence approaches [36,37]. Accordingly, patients presenting a Gray-platelet syndrome, are characterized by platelets that lack α-granules, and do not release CD154 upon activation [37]. CD154 is highly coclustered with insulin growth factor in α-granules, the signification of which is unknown [36]. One question is whether CD154 is also cytosolic, as found in resting platelets [38].
Pre-mRNAs and mature mRNAs are present in platelets and a functional spliceosome and translational apparatus allow platelets to process them, in response to platelet-activating signals [39,40]. Detecting CD154 mRNA by RT-PCR in platelets is challenging because of purity issues. However, CD154 mRNA was evidenced in mouse platelets, introducing other potential regulatory layers of CD154 expression by platelets [34].
When activated, platelets express a membrane form and release a soluble form of CD154
Platelets are activated by immobilized or soluble agonists. The activation-driven secretion of granule content is a primary phenomenon [41-46]. Platelets also synthetize mediators, including interleukin-1β, tissue factor (TF), fibrinogen, thrombospondin, von Willebrand Factor, αIIbβ3, through a translational-dependent pathway triggered by platelet activation [47,48].
Soluble CD154 is released by an activation-driven proteolytic mechanism. Agonists, including thrombin, thrombin receptor-agonist peptide, ADP or collagen, stimulate CD154 expression at the platelet membrane and the release of sCD154; long-term platelet activation leads to complete conversion of CD154 to sCD154 [38,49-53]. A matrix metalloproteinase (MMP)-dependent proteolytic event is involved. The involvement of MMPs, MMP-2 and/or MMP-9, [51,54-57], differs from the release of sCD154 by activated T-cells, which involves ADAM10 and 17 [58]. A role for αIIb/β3 has been put forward, as αIIb/β3 antagonists inhibit sCD154 release and as Glanzmann platelets show reduced sCD154 release rate [53,54,59]. An interaction between αIIb/β3 and MMP-2 is involved [57]. The roles of NADPH activation and reactive oxygen species (ROS) generation as well as CD154 binding to platelet CD40 have been underlined [50,60]. The particularity of sCD154 release may explain its specific response to agonists and secretion kinetics [38,53]; however, how sCD154 is released remains be fully understood, as shown for example by the effects of inhibitors added after platelet activation, suggesting complex, intra-platelet mechanisms [53]. A debate remains about the parallel biological activities of platelet-derived soluble and membrane-associated CD154; recombinant soluble forms, particularly trimeric forms, are active [50,61-63]. Finally, sCD154 activates platelets by itself, suggesting feed-back amplification of its secretion [64,65].
The megakaryocytic origin of platelet CD154
The assembly and loading of granules mainly occur in MK; granules are distributed in proplatelets via a microtubule-dependent mechanism [2,66,67]. The main origin of platelet CD154 is likely to be the MK that express CD154 mRNA, as shown in MK derived by differentiation of human and mouse hematopoietic progenitor cells and in MK of immune thrombocytopenic purpura (ITP) patients [68,69]. CD154 mRNA expression is increased upon MK differentiation [69]. CD154 protein is also found in MK cell lines and in MK from ITP patients [38,68,69]. As for T cells, the calcium-dependent activation of nuclear factor of activated T cells-c2 and the early growth response transcription factor EGR-1 contribute to CD154 gene activation in MK [69,70].
Translation from endogenous mRNAs contributes to platelet content. Its significance in quiescent platelets is unclear. However, pre-mRNA processing and mRNA translation are driven by platelet activation [40,48,71]. The contribution of such mechanism in CD154 expression during platelet lifespan is unknown.
Platelets also carry mediators present in plasma and possibly concentrated and/or modified within platelets [72,73]. Fibrinogen, albumin, immunoglobulins, amino acids, inflammatory and angiogenic mediators including vascular endothelial growth factor (VEGF), histamine or serotonin, are among them. Soluble CD154 is not detected in platelets, making unlikely its uptake from plasma.
Platelets are a significant reservoir of CD154 in the organism
Platelets carry approximately 5 ng of CD154/mL of blood [52]. Correlation studies suggest a link between platelet count and plasma or serum sCD154 [37,52,74-78]. Such a correlation is also found in experimental ITP [78]. In ITP, albeit platelet CD154 is elevated [68], plasma sCD154 is reduced [78], again suggesting relationship between the platelet count and circulating sCD154. However, there are contrasting studies, and a correlation between the platelet count and sCD154 is not always found [79,80].
Importantly, platelet activation is associated to elevated sCD154 and, indeed, platelet activation markers correlate with sCD154 in blood [81-83]. For this reason, serum seems inappropriate to evaluate circulating sCD154; in fact, sCD154 levels are higher in serum than in plasma, clotting resulting in increased sCD154 generation [52,79,80,84-88]. Hence the importance of a preanalytical standardization of blood samples processing, conditions such as temperature, length of storage, centrifugation, interfering with measurement [84,89]. Further, plasma/serum sCD154 may correspond to a pool of free soluble and microparticle-bound CD154 [84] and ELISA may not discriminate between sCD154 and platelet microparticles (PMP)-associated CD154 [90]. Circulating sCD154 is linked to platelet activation state; in patients with recent thrombotic events, plasma sCD154 correlates with platelet count, but this correlation is not found in patients with non-thrombotic, non-inflammatory conditions [84]. Finally, in patients with cardiovascular conditions, commonly used drugs such as statins, interfere with sCD154 releasing, a point that has also to be considered [91-93]. The baseline presence of sCD154 in the plasma of healthy subjects may be secondary to basal platelet activation, as in high shear stress flow areas [94]. PMP are released upon platelet activation [95]. A functional CD154 is expressed by PMP [63,96]. The importance of the contribution of PMP-bound CD154, in comparison with the “true” soluble CD154, to plasma sCD154 has been emphasized [90]. Questions also remain on the fate and half-life of sCD154 in blood and how the CD154 information can be delivered at distance from platelet activation sites.
Platelet CD154: a critical mediator of the inflammatory reaction
Platelets orchestrate a subtle balance between tissue injury and repair; they are a key source of material for reestablishing tissue homeostasis but they also contribute to tissue injury. CD154 mediates several platelet functions in tissue homeostasis (Figure 2).
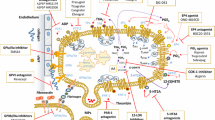
CD154 is a universal contributor to platelet functions. Activated platelets display CD154 at their membrane and release a soluble form (sCD154). Platelet CD154, directly or indirectly, is a molecular driver of inflammation, coagulation, tissue remodeling, and host defense, processes that intersect at multiple levels. Endothelium is a primary target. Platelet CD154 induces tissue factor (TF) expression and activity, thereby contributing to thrombin generation and upregulates urokinase plasminogen activator receptor which is at the interface between fibrinolysis/inflammation/tissue remodeling. MMP-9 and MT1-MMP induction contribute to regulate endothelium proteolytic activity [97]. Platelet CD154 also induces pro-inflammatory and chemotactic (dotted semicircles) molecule expression, and adhesion molecule expression (CD62e, CD54, CD106,…), leading to leukocyte recruitment and activation. Once activated, target cells recruit and activate other cells through multiple inputs; several amplification loops are thus generated including platelet activation by sCD154 itself (blue arrow). Platelet CD154 also activates cellular effectors of the innate and adaptative immune responses, polymorphonuclear cells (PMN), monocytes (MNC), macrophages (MΦ), dendritic cells (DC); how platelet CD154 contributes to host defense is schematized in Figure 3. CD154-expressing platelet microparticles (PMPs) share most of these functions. Depicted molecules do not comprehensively represent the range of platelet-derived mediators that are controlled by platelet CD154, and other interfaces, such as with endothelin-1, continue to be identified. Magenta arrows depict interaction with CD40. Dotted line for thrombomodulin (ThMod) represents inhibition; full line for others represents stimulation. Dotted semicircles symbolize chemotaxis. Abbreviations: Ly, lymphocytes; MMP, matrix metalloproteinases.
Platelet CD154 and inflammation
Regardless of its cause, the inflammatory milieu is rich in platelet-activating material, including chemokines [98]. The dialog between EC and platelets in inflammation has been widely studied as EC are primary platelet partners. Upon CD40 ligation, EC switch to an activated phenotype, expressing molecules that contribute to an inflammatory and thrombotic scenario, including cytokines/chemokines, adhesion molecules, and tissue factor [16,20,99]. Platelets/EC reciprocal activation is critical in atherosclerosis and cardiovascular conditions [100-103]. The pathogenic role of platelet CD154 is a major theme in atherosclerosis and cardiovascular diseases [25,62,74,100-109].
The role of platelet CD154 in inflammation extends beyond the dialog with EC, as activated platelets interact with various CD40 expressing-cells. Platelets are brought to inflammatory sites via vascular injury/permeability, attachment to activated leukocytes, and also chemotactic recruitment [110]. CD40 ligation on inflammatory cells at sites of tissue injury is a potent stimulus for the expression of a variety of proinflammatory mediators including cytokines, chemokines, eicosanoids, products of the proteolytic cascades, ROS generation, and of adhesion molecules [49,111], making platelet CD154 a versatile fuel for inflammation. The platelet contribution in many inflammation-associated disorders, including rheumatic, lung, gastrointestinal, neuro-inflammatory and metabolic diseases is actively studied [112-120] and the specific pathogenic role played by platelet CD154 in these disorders is a recently opened frontier. Soluble CD154 levels were found to correlate with disease activity as in systemic lupus erythematosus [121]; whether sCD154 could represent a potential useful marker in inflammation-associated disorders is an interesting question. PMP also contribute to inflammatory disorders [122-128]; the specific role of PMP-associated CD154 remains however to be fully understood.
Platelet CD154 and tissue repair
The effectors of inflammation are orchestrated to cure infection and restore tissue integrity [129-131]. At various steps of tissue repair, platelets are a source of relevant material, including growth factors, pro- and anti-apoptotic mediators, matrix and matrix remodeling proteins [132-135] (Figure 1). Platelets contribute to maintain resting and injured endothelium integrity [136]. On injured endothelium, platelets provide EC growth-promoting and anti-apoptotic mediators, attractants for progenitor cells endowed with vascular healing properties [135]. They contribute to restoring the vascular network, by secreting regulators of angiogenesis [137-139]. Beyond endothelium, a remarkable role for platelets in organ regeneration has been substantiated. Platelets contribute to liver regeneration, serotonin being essential [140-142]. It is tempting to speculate that platelets will be found to have a broader role in organ regeneration by providing key mitogenic signals in various organs, such as for example fibroblast growth factor or platelet-derived growth factor that contribute to muscle or brain repair [143,144]. This is also in line with the known ability of platelet lysates to sustain the growth of primary cell cultures. PMP also contribute to vascular integrity [145-148] and promote tissue repair [128,149]. Platelet products have already found various applications in the clinics [150-154].
The specific role of CD154 has been mainly studied in EC. CD154 promotes EC survival, proliferation and migration, capillary-like tube formation in vitro and angiogenesis in vivo. Mechanisms include activation of the phosphatidylinositol-3 kinase/Akt pathway, induction of angiogenic mediators and matrix remodeling protein production [155-157]. CD40 signaling contributes to neointima repair, TRAF6 signaling intermediate being critical [32,158,159]. However, platelet CD154 was shown to inhibit the VEGF-induced EC migration via increased ROS generation, and sCD154 to inhibit VEGF-induced angiogenesis [160]. Soluble CD154 also promotes oxidative stress in endothelial outgrowth cells (EOC), reducing their viability and proliferation [161], while promoting endothelial repair via increased production of MMP-9 by EOC [162]. These findings may be context-dependent; they emphasize the importance of platelet CD154 in vascular homeostasis and the complexity of its biological interfaces. Other tissues for which platelet CD154 is likely to show importance for repair are skin and bone. CD40 ligation stimulates keratinocyte differentiation, suggesting contribution to skin wound repair [163]. Regulation of osteoclastogenesis by CD154 is suggested by the reduced bone mineral density together with elevated urine markers of osteoclast activity in patients with the X-linked hyper-IgM syndrome, and the reduced bone mineral density in CD154 deficient mice [164,165]. CD40 is expressed by osteoblastic cells and CD154 is anti-apoptotic in these cells [166]. Therefore, much remains to be found about the role of platelet CD154 in tissue repair. As CD40 is largely distributed, platelet CD154 could be conjectured to be generally involved, to one degree or another, in tissue repair.
Platelet CD154 as a mediator of tissue injury
The model of platelets promoting tissue repair is to be compared to their deleterious role in acute and chronic tissue injury. Difficult points are raised by this friend or foe facet, implicating balanced therapeutic approaches [119]. Ischemia/reperfusion (I/R) underscores platelet deleterious role, and the importance to control platelet activation in this context. In I/R, platelet activation in the microcirculation vascular bed leads to tissue injury, as shown in lung, liver or kidney. Platelet depletion or antiplatelet treatments are protective in several experimental I/R models [167-169]; CD154 is contributing: mice deficient in CD154 are protected from I/R-mediated injury in brain, lung, liver or intestine; in lung I/R-mediated injury platelet CD154 is specifically contributing [170-172].
Platelet CD154 and the immune response: unanticipated new frontiers
Platelets participate to the control of infection via direct and indirect mechanisms [6,173-178]. The significance of platelet Toll-like receptors (TLR) has been emphasized; TLR ligation activates platelet secretion of mediators regulating the immune response, including sCD154 [6,179-184]. Platelets also regulate several steps of the adaptative immune response [6,182-194]. Moreover, platelets can present antigen [195]; they express MHC class I molecules and T cell costimulatory molecules, including CD86 and CD40 and harbor a functional proteasome [196-199]. Among platelet mediators, CD154 proved to be critical in linking platelet and immunity (Figure 3).
Platelet CD154 contributes to the host defense against infections. Infection triggers inflammation and coagulation. The interaction with pathogens, pathogen-derived molecules such as lipopolysaccharide (LPS), inflammation and coagulation concur to activate platelets, leading to CD154 display at the platelet membrane and the release of soluble CD154 (sCD154). Multiple inputs amplify the platelet activation scenario, including soluble and cellular effectors of the inflammatory network. Platelet CD154 targets several immune response effectors, including contribution to the chemotactic recruitment (dotted semicircles symbolize chemotaxis) of leukocytes to sites of infection, e.g. through the induction of adhesion molecules on EC (CD62e, CD54, CD106) and activation/upregulation of integrins such as αMβ2 on neutrophils [158,200]. CD40 triggering is a major inducer of pathogen-killing mechanisms by phagocytic cells. These responses are amplified by inflammatory mediators generated upon CD40 ligation; this schematic representation does not represent all interfaces that are directly or indirectly regulated by platelet CD154. Platelet CD154 influences the adaptative immune response, through several mechanisms, including the activation/maturation of antigen presenting cells (see text for details). Magenta arrows depict interaction with CD40. Abbreviations: PAMPs, pathogen-associated molecular patterns; PRR, pathogen recognition receptors; TLR, Toll-like receptors.
Although much remains to be understood, particularly with reference to the innate immune response, the specific role of platelet CD154 in immunity is strengthening. Several pathogen-clearing mechanisms are stimulated by CD154, including platelet aggregation [173], phagocytosis and production of defense proteins, such as complement proteins and interferon-α, by cells of the innate immune system [6,20,201]. CD40 contributes to the regulation of innate immune response, including induction of TLR expression, cooperation in TLR-mediated B cell activation, engagement in the crosstalk between intracellular MHC class II molecules and TLR signaling pathway [202-204]. The specific role of platelet CD154 in these mechanisms remains to be precised. However, it is now appreciated that platelet CD154 controls many facets of the interface between innate and adaptive immune responses [173,187,191,205]. Platelet CD154 induces DC maturation, can activate B cells, antibody production and isotype switching, contributes to germinal center formation, and enhances CD8+ T cell responses [188,206-213]. Platelet CD154 helps mounting a protective cytotoxic T cell immune response to viral or bacterial challenge [206,214]. Platelet CD154 may promote the immune response in the context of low antigen challenge by lowering the antigen threshold, and improve B cell response in regulatory T-cell limiting settings [210,215]. Further, sCD154 per se induces cardiac allograft rejection [212]. Many questions remain. How platelet CD154 enters the draining lymph nodes to regulate the adaptive immune response machinery is not known; PMP may convey this information, as CD154 associated to PMP is functional: it enhances DC activation, germinal center formation, B cell proliferation and IgG production [63,216]. Several questions are also raised with reference to platelet CD154 in autoimmunity; this “dark side” [14,217] feature of platelet CD154 is a recently opened frontier. Platelet CD154 is competent to increase production of antiplatelet antibodies in immune thrombocytopenic purpura [68] and, in systemic lupus erythematosus, platelet CD154 activates antigen presenting cells contributing to enhanced interferon-α production [218].
Platelet CD154: a new hematopoietic regulator?
Hematopoiesis can be adapted in response to inflammation/infection by signals generated at bone marrow distal sites [219-224]. Platelets are activated at sites of inflammation/infection and are a major source of circulating sCD154. Could platelets deliver a CD154 signal, through sCD154, platelet- or PMP-associated CD154 that regulates hematopoiesis? Platelet mediators enhance hematopoietic stem cell proliferation and platelet-derived signals may contribute to CD34+ cell mobilization [225,226]. Several studies have demonstrated CD154 involvement in hematopoiesis. CD154 regulation of early B cell lymphopoiesis is suggested by the sCD154-induced increased number of B cell progenitors (BCP) in mice after bone marrow transplantation (BMT) [227]. CD40 is expressed on BCP, and a positive effect of CD40 ligation on BCP proliferation can be observed on pre- and immature B cells in human and pro-B cells in the mouse [228,229]. In the mouse, there is clear experimental evidence for a positive role of CD154 in B cell hematopoiesis and, particularly in stress conditions, as after BMT [229]. However, normal numbers of circulating B cells in patients with X-linked hyper-IgM syndrome would rule out an absolute requirement for the CD154/CD40 signaling in early B cell development. CD154 may therefore mostly play a significant role in emergency B cell hematopoiesis [229]. More is known about CD154 regulation of the lymphoid system maturation, which has been fully reviewed [230]. A role for platelet CD154 on myelopoiesis is suggested by the sCD154-mediated increased granulocyte and platelet recovery after BMT in the mouse and by the neutropenia and thrombocytopenia observed in patients with X-linked hyper-IgM syndrome [227]. In vitro, sCD154 promotes the differentiation of CD34+ cells towards the granulocytic/monocytic and megakaryocytic lineages in CD34+/stromal cell cocultures. The mode of action of sCD154 appears to be essentially indirect, through the induction of hematopoietic cytokines by bone marrow stromal cells [231,232]. Platelet CD154 may therefore play a role in regulating emergency hematopoiesis. However, many questions remain unsolved, particularly which and how platelet CD154 signals could be delivered and interact with bone marrow stem/progenitor cells.
Platelet CD154 and cancer: a rapidly expanding frontier
There is strong evidence for the involvement of platelets in cancer progression; mechanisms are multiple [233-240]. Platelets are activated in the tumor environment and bind tumor cells. Mediators released upon platelet activation are key to tumor angiogenesis [241,242] and are likely to contribute to the tumor-supporting inflammatory environment [243,244]. Platelets play a positive role in metastasis [234,238,245-249]. However, this may not be true for all organs [250]. In hematogenous dissemination, platelet/cancer cell microthrombi provide protection, including shielding from shear flow, or immune evasion; during the arrest and extravasation phases, platelet mediators facilitate tumor cell arrest on EC, extravasation, survival and growth after seeding [251]. Platelet MPs are also contributing [124,252,253].
Many tumor cells express CD40. The outcomes of CD40 ligation on tumor cells are ambivalent depending on the models studied. In one hand, CD40 ligation promotes anti-tumor immune surveillance through a variety of mechanisms including antigen-presenting cell activation, restoration of malignant cell immune recognition, activation of tumoricidal-infiltrating macrophages, immunostimulatory cytokine production. CD40 ligation also induces tumor growth arrest and sensitization to apoptotic signals. On the other hand, CD40 ligation has positive consequences on tumor growth, survival and resistance to chemotherapy and metastatic potential. The interpretation of CD154 effects on cancer cells is made complex, first by the existence of several receptors for CD154, potentially explaining variable outcomes of CD154 treatment of tumor cells, and second, by the difficulty in assessing direct versus indirect effects. The contribution of the CD40 signaling in cancer, and prospects offered by targeting the CD40 signaling for cancer treatment have recently been underlined and reviewed [254-258]. However, the specific role played by platelet CD154 remains a new important frontier. If platelet activation is likely to result in expression of CD154 and generation of sCD154 in the tumor cell environment, this study is made complex as there are extra platelet sources of CD154.
Conclusion
There have been recent and rapid advances in our current knowledge of the non-hemostatic functions of platelets, placing them in the middle of the spectrum of mechanisms that maintain homeostasis, and highlighting their role in a variety of inflammatory and immune disorders. However, platelets store and release such a wide diversity of biologically active mediators that major gaps remain in our understanding of which and how these mediators collectively fulfill these functions. Platelet CD154 has attracted considerable attention as it recapitulates several of non-hemostatic platelet attributes. Considering the large number of different cells expressing CD40, the complex signaling cascade and the wide range of effectors activated by the CD154/CD40 interaction, it can be anticipated that future investigations will further extend the contribution of platelet CD154 in health and disease. For example, recent publications on the CD154/CD40 dyad have pointed to its role in obesity and hepatic steatosis [259-263], and it is tempting to speculate that platelet CD154 contributes to metabolic homeostasis. In the same direction, the number of physiological or pathological conditions associated with platelet activation is enlarging. For example, platelet activation has been found associated to aging, to emotional or environmental stresses…; platelet CD154 might represent a significant link between these conditions and accompanying pathologies, such as cardiovascular events [264]. However, platelet CD154 is always acting in a multicytokine context, including inhibitors and activators released at the same time by platelets; understanding how this complexity is tuned and evidencing the specific role of platelet CD154 remains a difficult challenge.
References
Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–47.
Thon JN, Italiano JE. Platelet formation. Semin Hematol. 2010;47(3):220–6.
Machlus KR, Italiano Jr JE. The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201(6):785–96.
Schmaier AA, Stalker TJ, Runge JJ, Lee D, Nagaswami C, Mericko P, et al. Occlusive thrombi arise in mammals but not birds in response to arterial injury: evolutionary insight into human cardiovascular disease. Blood. 2011;118(13):3661–9.
Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1(9):1897–905.
Semple JW, Italiano Jr JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–74.
Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Curr Opin Hematol. 2010;17(6):585–9.
Coller BS. Historical perspective and future directions in platelet research. J Thromb Haemost. 2011;9 Suppl 1:374–95.
Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35.
van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17.
Howard LM, Miller SD. Immunotherapy targeting the CD40/CD154 costimulatory pathway for treatment of autoimmune disease. Autoimmunity. 2004;37(5):411–8.
Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37(6–7):457–64.
Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv Exp Med Biol. 2009;647:8–36.
Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21(5):293–300.
Alaaeddine N, Hassan GS, Yacoub D, Mourad W. CD154: an immunoinflammatory mediator in systemic lupus erythematosus and rheumatoid arthritis. Clin Dev Immunol. 2012;2012:490148.
Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, et al. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995;182(1):33–40.
Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc Natl Acad Sci U S A. 1995;92(10):4342–6.
Yellin MJ, Brett J, Baum D, Matsushima A, Szabolcs M, Stern D, et al. Functional interactions of T cells with endothelial cells: the role of CD40L-CD40-mediated signals. J Exp Med. 1995;182(6):1857–64.
Schonbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89(12):1092–103.
Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58(1):4–43.
Delmas Y, Viallard JF, Solanilla A, Villeneuve J, Pasquet JM, Belloc F, et al. Activation of mesangial cells by platelets in systemic lupus erythematosus via a CD154-dependent induction of CD40. Kidney Int. 2005;68(5):2068–78.
Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, et al. CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med. 2002;8(3):247–52.
Leveille C, Bouillon M, Guo W, Bolduc J, Sharif-Askari E, El-Fakhry Y, et al. CD40 ligand binds to alpha5beta1 integrin and triggers cell signaling. J Biol Chem. 2007;282(8):5143–51.
Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007;115(12):1571–80.
Hassan GS, Merhi Y, Mourad WM. CD154 and its receptors in inflammatory vascular pathologies. Trends Immunol. 2009;30(4):165–72.
Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek RA. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25(6):1749–54.
Peitsch MC, Jongeneel CV. A 3-D model for the CD40 ligand predicts that it is a compact trimer similar to the tumor necrosis factors. Int Immunol. 1993;5(2):233–8.
Fanslow WC, Srinivasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994;6(5):267–78.
Karpusas M, Hsu YM, Wang JH, Thompson J, Lederman S, Chess L, et al. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure. 1995;3(10):1031–9.
Pietravalle F, Lecoanet-Henchoz S, Blasey H, Aubry JP, Elson G, Edgerton MD, et al. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J Biol Chem. 1996;271(11):5965–7.
Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–51.
Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, et al. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 2008;111(9):4596–604.
Chatzigeorgiou A, Seijkens T, Zarzycka B, Engel D, Poggi M, van den Berg S, et al. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proc Natl Acad Sci U S A. 2014;111(7):2686–91.
Horrillo A, Fontela T, Arias-Salgado EG, Llobat D, Porras G, Ayuso MS, et al. Generation of mice with conditional ablation of the Cd40lg gene: new insights on the role of CD40L. Transgenic Res. 2014;23(1):53–66.
Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–4.
Kamykowski J, Carlton P, Sehgal S, Storrie B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet alpha-granules. Blood. 2011;118(5):1370–3.
Charafeddine AH, Kim EJ, Maynard DM, Yi H, Weaver TA, Gunay-Aygun M, et al. Platelet-derived CD154: ultrastructural localization and clinical correlation in organ transplantation. Am J Transplant. 2012;12(11):3143–51.
Hermann A, Rauch BH, Braun M, Schror K, Weber AA. Platelet CD40 ligand (CD40L)–subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 2001;12(2):74–82.
Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122(3):379–91.
Rowley JW, Schwertz H, Weyrich AS. Platelet mRNA: the meaning behind the message. Curr Opin Hematol. 2012;19(5):385–91.
Reed GL, Fitzgerald ML, Polgar J. Molecular mechanisms of platelet exocytosis: insights into the “secrete” life of thrombocytes. Blood. 2000;96(10):3334–42.
Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31(4):381–92.
Ren Q, Ye S, Whiteheart SW. The platelet release reaction: just when you thought platelet secretion was simple. Curr Opin Hematol. 2008;15(5):537–41.
Koseoglu S, Flaumenhaft R. Advances in platelet granule biology. Curr Opin Hematol. 2013;20(5):464–71.
Wijten P, van Holten T, Woo LL, Bleijerveld OB, Roest M, Heck AJ, et al. High precision platelet releasate definition by quantitative reversed protein profiling–brief report. Arterioscler Thromb Vasc Biol. 2013;33(7):1635–8.
Golebiewska EM, Poole AW. Secrets of platelet exocytosis - what do we really know about platelet secretion mechanisms? Br J Haematol. 2013;165(2):204–16.
Lindemann S, Gawaz M. The active platelet: translation and protein synthesis in an anucleate cell. Semin Thromb Hemost. 2007;33(2):144–50.
Weyrich AS, Schwertz H, Kraiss LW, Zimmerman GA. Protein synthesis by platelets: historical and new perspectives. J Thromb Haemost. 2009;7(2):241–6.
Aukrust P, Muller F, Ueland T, Berget T, Aaser E, Brunsvig A, et al. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation. 1999;100(6):614–20.
Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98(4):1047–54.
Jin Y, Nonoyama S, Morio T, Imai K, Ochs HD, Mizutani S. Characterization of soluble CD40 ligand released from human activated platelets. J Med Dent Sci. 2001;48(1):23–7.
Nannizzi-Alaimo L, Rubenstein MH, Alves VL, Leong GY, Phillips DR, Gold HK. Cardiopulmonary bypass induces release of soluble CD40 ligand. Circulation. 2002;105(24):2849–54.
Otterdal K, Pedersen TM, Solum NO. Release of soluble CD40 ligand after platelet activation: studies on the solubilization phase. Thromb Res. 2004;114(3):167–77.
Furman MI, Krueger LA, Linden MD, Barnard MR, Frelinger 3rd AL, Michelson AD. Release of soluble CD40L from platelets is regulated by glycoprotein IIb/IIIa and actin polymerization. J Am Coll Cardiol. 2004;43(12):2319–25.
Menchen L, Marin-Jimenez I, Arias-Salgado EG, Fontela T, Hernandez-Sampelayo P, Rodriguez MC, et al. Matrix metalloproteinase 9 is involved in Crohn’s disease-associated platelet hyperactivation through the release of soluble CD40 ligand. Gut. 2009;58(7):920–8.
Reinboldt S, Wenzel F, Rauch BH, Hohlfeld T, Grandoch M, Fischer JW, et al. Preliminary evidence for a matrix metalloproteinase-2 (MMP-2)-dependent shedding of soluble CD40 ligand (sCD40L) from activated platelets. Platelets. 2009;20(6):441–4.
Choi WS, Jeon OH, Kim DS. CD40 ligand shedding is regulated by interaction between matrix metalloproteinase-2 and platelet integrin alpha(IIb)beta(3). J Thromb Haemost. 2010;8(6):1364–71.
Yacoub D, Benslimane N, Al-Zoobi L, Hassan G, Nadiri A, Mourad W. CD154 Is Released from T-cells by a Disintegrin and Metalloproteinase Domain-containing Protein 10 (ADAM10) and ADAM17 in a CD40 Protein-dependent Manner. J Biol Chem. 2013;288(50):36083–93.
Nannizzi-Alaimo L, Alves VL, Phillips DR. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation. 2003;107(8):1123–8.
Pignatelli P, Sanguigni V, Lenti L, Ferro D, Finocchi A, Rossi P, et al. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110(10):1326–9.
Mazzei GJ, Edgerton MD, Losberger C, Lecoanet-Henchoz S, Graber P, Durandy A, et al. Recombinant soluble trimeric CD40 ligand is biologically active. J Biol Chem. 1995;270(13):7025–8.
Anand SX, Viles-Gonzalez JF, Badimon JJ, Cavusoglu E, Marmur JD. Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb Haemost. 2003;90(3):377–84.
Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111(10):5028–36.
Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92(9):1041–8.
Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003;100(21):12367–71.
King SM, Reed GL. Development of platelet secretory granules. Semin Cell Dev Biol. 2002;13(4):293–302.
Schulze H, Shivdasani RA. Mechanisms of thrombopoiesis. J Thromb Haemost. 2005;3(8):1717–24.
Solanilla A, Pasquet JM, Viallard JF, Contin C, Grosset C, Dechanet-Merville J, et al. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood. 2005;105(1):215–8.
Crist SA, Sprague DL, Ratliff TL. Nuclear factor of activated T cells (NFAT) mediates CD154 expression in megakaryocytes. Blood. 2008;111(7):3553–61.
Crist SA, Elzey BD, Ahmann MT, Ratliff TL. Early growth response-1 (EGR-1) and nuclear factor of activated T cells (NFAT) cooperate to mediate CD40L expression in megakaryocytes and platelets. J Biol Chem. 2013;288(47):33985–96.
Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95(10):5556–61.
Maguire PB, Fitzgerald DJ. Platelet proteomics. J Thromb Haemost. 2003;1(7):1593–601.
Gnatenko DV, Perrotta PL, Bahou WF. Proteomic approaches to dissect platelet function: Half the story. Blood. 2006;108(13):3983–91.
Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106(8):896–9.
Viallard JF, Solanilla A, Gauthier B, Contin C, Dechanet J, Grosset C, et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99(7):2612–4.
Nagasawa M, Zhu Y, Isoda T, Tomizawa D, Itoh S, Kajiwara M, et al. Analysis of serum soluble CD40 ligand (sCD40L) in the patients undergoing allogeneic stem cell transplantation: platelet is a major source of serum sCD40L. Eur J Haematol. 2005;74(1):54–60.
Feng X, Scheinberg P, Wu CO, Samsel L, Nunez O, Prince C, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96(4):602–6.
Feng X, Scheinberg P, Samsel L, Rios O, Chen J, McCoy Jr JP, et al. Decreased plasma cytokines are associated with low platelet counts in aplastic anemia and immune thrombocytopenic purpura. J Thromb Haemost. 2012;10(8):1616–23.
Fan Y, Ge Y, Zhu H, Wang Y, Yang B, Zhuang Y, et al. Characterization and application of two novel monoclonal antibodies against CD40L: epitope and functional studies on cell membrane CD40L and studies on the origin of soluble serum CD40L. Tissue Antigens. 2004;64(3):257–63.
Mason PJ, Chakrabarti S, Albers AA, Rex S, Vitseva O, Varghese S, et al. Plasma, serum, and platelet expression of CD40 ligand in adults with cardiovascular disease. Am J Cardiol. 2005;96(10):1365–9.
Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, et al. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation. 2002;106(4):399–402.
Riondino S, Martini F, La Farina F, Spila A, Guadagni F, Ferroni P. Increased plasma levels of soluble CD40 ligand correlate with platelet activation markers and underline the need for standardized pre-analytical conditions. Clin Biochem. 2010;43(7–8):666–70.
Burdess A, Michelsen AE, Brosstad F, Fox KA, Newby DE, Nimmo AF. Platelet activation in patients with peripheral vascular disease: reproducibility and comparability of platelet markers. Thromb Res. 2012;129(1):50–5.
Ahn ER, Lander G, Jy W, Bidot CJ, Jimenez JJ, Horstman LL, et al. Differences of soluble CD40L in sera and plasma: implications on CD40L assay as a marker of thrombotic risk. Thromb Res. 2004;114(2):143–8.
Thom J, Gilmore G, Yi Q, Hankey GJ, Eikelboom JW. Measurement of soluble P-selectin and soluble CD40 ligand in serum and plasma. J Thromb Haemost. 2004;2(11):2067–9.
Varo N, Nuzzo R, Natal C, Libby P, Schonbeck U. Influence of pre-analytical and analytical factors on soluble CD40L measurements. Clin Sci (Lond). 2006;111(5):341–7.
Weber M, Rabenau B, Stanisch M, Elsaesser A, Mitrovic V, Heeschen C, et al. Influence of sample type and storage conditions on soluble CD40 ligand assessment. Clin Chem. 2006;52(5):888–91.
Weber M, Rabenau B, Stanisch M, Nef HM, Mollmann H, Elsasser A, et al. Influence of sample type on soluble CD40 ligand assessment in patients with acute coronary syndromes. Thromb Res. 2007;120(6):811–4.
Ivandic BT, Spanuth E, Haase D, Lestin HG, Katus HA. Increased plasma concentrations of soluble CD40 ligand in acute coronary syndrome depend on in vitro platelet activation. Clin Chem. 2007;53(7):1231–4.
Mobarrez F, Sjovik C, Soop A, Hallstrom L, Frostell C, Pisetsky DS et al. CD40L expression in plasma of volunteers following LPS administration: A comparison between assay of CD40L on platelet microvesicles and soluble CD40L. Platelets. 2014:1–5. [Epub ahead of print]
Schonbeck U, Gerdes N, Varo N, Reynolds RS, Horton DB, Bavendiek U, et al. Oxidized low-density lipoprotein augments and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors limit CD40 and CD40L expression in human vascular cells. Circulation. 2002;106(23):2888–93.
Semb AG, van Wissen S, Ueland T, Smilde T, Waehre T, Tripp MD, et al. Raised serum levels of soluble CD40 ligand in patients with familial hypercholesterolemia: downregulatory effect of statin therapy. J Am Coll Cardiol. 2003;41(2):275–9.
Li J, Zhao SP, Peng DQ, Xu ZM, Zhou HN. Early effect of pravastatin on serum soluble CD40L, matrix metalloproteinase-9, and C-reactive protein in patients with acute myocardial infarction. Clin Chem. 2004;50(9):1696–9.
Tamura N, Yoshida M, Ichikawa N, Handa M, Ikeda Y, Tanabe T, et al. Shear-induced von Willebrand factor-mediated platelet surface translocation of the CD40 ligand. Thromb Res. 2002;108(5–6):311–5.
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–9.
Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30(5):450–9.
May AE, Kälsch T, Massberg S, Herouy Y, Schmidt R, Gawaz M. Engagement of glycoprotein IIb/IIIa (aIIbb3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cells. Circulation. 2002;106(16):2111–7.
Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10(3–4):335–50.
Dechanet J, Grosset C, Taupin JL, Merville P, Banchereau J, Ripoche J, et al. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells. J Immunol. 1997;159(11):5640–7.
Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–84.
Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–94.
Projahn D, Koenen RR. Platelets: key players in vascular inflammation. J Leukoc Biol. 2012;92(6):1167–75.
Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112(11):1506–19.
Mach F, Schonbeck U, Libby P. CD40 signaling in vascular cells: a key role in atherosclerosis? Atherosclerosis. 1998;137(Suppl):S89–95.
Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394(6689):200–3.
Danese S, Fiocchi C. Platelet activation and the CD40/CD40 ligand pathway: mechanisms and implications for human disease. Crit Rev Immunol. 2005;25(2):103–21.
Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009;54(8):669–77.
Lievens D, Eijgelaar WJ, Biessen EA, Daemen MJ, Lutgens E. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb Haemost. 2009;102(2):206–14.
Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116(20):4317–27.
Czapiga M, Gao JL, Kirk A, Lekstrom-Himes J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp Hematol. 2005;33(1):73–84.
Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155(10):4917–25.
Danese S, de la Motte C, Sturm A, Vogel JD, West GA, Strong SA, et al. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124(5):1249–64.
Kornerup KN, Page CP. The role of platelets in the pathophysiology of asthma. Platelets. 2007;18(5):319–28.
Tabuchi A, Kuebler WM. Endothelium-platelet interactions in inflammatory lung disease. Vascul Pharmacol. 2008;49(4–6):141–50.
Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009;15(8):1245–55.
Ripoche J. Blood platelets and inflammation: their relationship with liver and digestive diseases. Clin Res Hepatol Gastroenterol. 2011;35(5):353–7.
Boilard E, Blanco P, Nigrovic PA. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012;8(9):534–42.
Santilli F, Vazzana N, Liani R, Guagnano MT, Davi G. Platelet activation in obesity and metabolic syndrome. Obes Rev. 2012;13(1):27–42.
Gasparyan AY, Ayvazyan L, Pretorius E, Kitas GD. Platelets in Rheumatic Diseases: Friend or Foe? Curr Pharm Des. 2014;20(4):552–66.
Langer HF, Chavakis T. Platelets and neurovascular inflammation. Thromb Haemost. 2013;110(5):888–93.
Kato K, Santana-Sahagùn E, Rassenti LZ, Weisman MH, Tamura N, Kobayashi S, et al. The soluble CD40 ligand sCD154 in systemic lupus erythematosus. J Clin Invest. 1999;104(7):947–55.
Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34(6):392–401.
Tan KT, Lip GY. The potential role of platelet microparticles in atherosclerosis. Thromb Haemost. 2005;94(3):488–92.
Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov Med. 2009;8(43):237–41.
Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–3.
Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8(11):2358–68.
Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond). 2013;124(7):423–41.
Burnouf T, Goubran HA, Chou ML, Devos D, Radosevic M. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014;28(4):155–66.
Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–52.
Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008;118(2):413–20.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–35.
Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–7.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21.
Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105 Suppl 1:S13–33.
Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122(15):2550–4.
Ho-Tin-Noe B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9 Suppl 1:56–65.
Verheul HM, Jorna AS, Hoekman K, Broxterman HJ, Gebbink MF, Pinedo HM. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood. 2000;96(13):4216–21.
Brill A, Elinav H, Varon D. Differential role of platelet granular mediators in angiogenesis. Cardiovasc Res. 2004;63(2):226–35.
Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835–42.
Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312(5770):104–7.
Markiewski MM, DeAngelis RA, Lambris JD. Liver inflammation and regeneration: two distinct biological phenomena or parallel pathophysiologic processes? Mol Immunol. 2006;43(1–2):45–56.
Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, et al. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45(2):369–76.
Doukas J, Blease K, Craig D, Ma C, Chandler LA, Sosnowski BA, et al. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol Ther. 2002;5(5 Pt 1):517–27.
Norazit A, Nguyen MN, Dickson CG, Tuxworth G, Goss B, Mackay-Sim A, et al. Vascular endothelial growth factor and platelet derived growth factor modulates the glial response to a cortical stab injury. Neuroscience. 2011;192:652–60.
Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124(3):376–84.
Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67(1):30–8.
Italiano Jr JE, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17(6):578–84.
Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Muller-Newen G, et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122(5):495–506.
Hayon Y, Shai E, Varon D, Leker RR. The role of platelets and their microparticles in rehabilitation of ischemic brain tissue. CNS Neurol Disord Drug Targets. 2012;11(7):921–5.
Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15.
Langer HF, Gawaz M. Platelets in regenerative medicine. Basic Res Cardiol. 2008;103(4):299–307.
Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–48.
Burnouf T, Goubran HA, Chen TM, Ou KL, El-Ekiaby M, Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27(2):77–89.
Textor J. Platelet-Rich Plasma (PRP) as a Therapeutic Agent: Platelet Biology, Growth Factors and a Review of the Literature. In: Andrade Santana MH, Dias Belangero W, Malheiros Luzo AC, editors. Lana JFSD. Springer Berlin Heidelberg: Platelet-Rich Plasma. Lecture Notes in Bioengineering; 2014. p. 61–94.
Mach F, Schonbeck U, Fabunmi RP, Murphy C, Atkinson E, Bonnefoy JY, et al. T lymphocytes induce endothelial cell matrix metalloproteinase expression by a CD40L-dependent mechanism: implications for tubule formation. Am J Pathol. 1999;154(1):229–38.
Melter M, Reinders ME, Sho M, Pal S, Geehan C, Denton MD, et al. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96(12):3801–8.
Deregibus MC, Buttiglieri S, Russo S, Bussolati B, Camussi G. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J Biol Chem. 2003;278(20):18008–14.
Li G, Sanders JM, Bevard MH, Sun Z, Chumley JW, Galkina EV, et al. CD40 ligand promotes Mac-1 expression, leukocyte recruitment, and neointima formation after vascular injury. Am J Pathol. 2008;172(4):1141–52.
Song Z, Jin R, Yu S, Nanda A, Granger DN, Li G. Crucial role of CD40 signaling in vascular wall cells in neointimal formation and vascular remodeling after vascular interventions. Arterioscler Thromb Vasc Biol. 2012;32(1):50–64.
Urbich C, Dernbach E, Aicher A, Zeiher AM, Dimmeler S. CD40 ligand inhibits endothelial cell migration by increasing production of endothelial reactive oxygen species. Circulation. 2002;106(8):981–6.
Hristov M, Gumbel D, Lutgens E, Zernecke A, Weber C. Soluble CD40 ligand impairs the function of peripheral blood angiogenic outgrowth cells and increases neointimal formation after arterial injury. Circulation. 2010;121(2):315–24.
Bou Khzam L, Boulahya R, Abou-Saleh H, Hachem A, Zaid Y, Merhi Y. Soluble CD40 ligand stimulates the pro-angiogenic function of peripheral blood angiogenic outgrowth cells via increased release of matrix metalloproteinase-9. PLoS One. 2013;8(12):e84289.
Peguet-Navarro J, Dalbiez-Gauthier C, Moulon C, Berthier O, Reano A, Gaucherand M, et al. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J Immunol. 1997;158(1):144–52.
Lopez-Granados E, Temmerman ST, Wu L, Reynolds JC, Follmann D, Liu S, et al. Osteopenia in X-linked hyper-IgM syndrome reveals a regulatory role for CD40 ligand in osteoclastogenesis. Proc Natl Acad Sci U S A. 2007;104(12):5056–61.
Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian W, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–48.
Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144(5):1761–9.
Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol. 2009;40(2):123–34.
Hu H, Batteux F, Chereau C, Kavian N, Marut W, Gobeaux C, et al. Clopidogrel protects from cell apoptosis and oxidative damage in a mouse model of renal ischaemia-reperfusion injury. J Pathol. 2011;225(2):265–75.
Dixon JT, Gozal E, Roberts AM. Platelet-mediated vascular dysfunction during acute lung injury. Arch Physiol Biochem. 2012;118(2):72–82.
Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111(13):1690–6.
Ke B, Shen XD, Gao F, Tsuchihashi S, Farmer DG, Briscoe D, et al. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation. 2005;79(9):1078–83.
Lapchak PH, Ioannou A, Kannan L, Rani P, Dalle Lucca JJ, Tsokos GC. Platelet-associated CD40/CD154 mediates remote tissue damage after mesenteric ischemia/reperfusion injury. PLoS One. 2012;7(2):e32260.
Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25(9):489–95.
Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4(6):445–57.
Flaujac C, Boukour S, Cramer-Borde E. Platelets and viruses: an ambivalent relationship. Cell Mol Life Sci. 2010;67(4):545–56.
Speth C, Loffler J, Krappmann S, Lass-Florl C, Rambach G. Platelets as immune cells in infectious diseases. Future Microbiol. 2013;8(11):1431–51.
Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12(11):1764–75.
Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12(6):426–37.
Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22(9):913–22.
Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, et al. Expression of Toll-like receptors on human platelets. Thromb Res. 2004;113(6):379–85.
Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141(1):84–91.
Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67(4):499–511.
Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34(1):5–30.
Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35(3):254–61.
Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273(5272):252–5.
Diacovo TG, Catalina MD, Siegelman MH, von Andrian UH. Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J Exp Med. 1998;187(2):197–204.
Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238(1):1–9.
Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83(5):1069–78.
McNicol A, Israels SJ. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008;8(2):99–117.
Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759–66.
Sowa JM, Crist SA, Ratliff TL, Elzey BD. Platelet influence on T- and B-cell responses. Arch Immunol Ther Exp (Warsz). 2009;57(4):235–41.
Qu Z, Chaikof EL. Interface between hemostasis and adaptive immunity. Curr Opin Immunol. 2010;22(5):634–42.
Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, et al. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Adv Hematol. 2012;2012:384685.
Garraud O, Hamzeh-Cognasse H, Pozzetto B, Cavaillon JM, Cognasse F. Bench-to-bedside review: Platelets and active immune functions - new clues for immunopathology? Crit Care. 2013;17(4):236.
Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, Yui K, et al. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189(2):916–23.
Kao KJ, Cook DJ, Scornik JC. Quantitative analysis of platelet surface HLA by W6/32 anti-HLA monoclonal antibody. Blood. 1986;68(3):627–32.
Yukawa M, Sakon M, Kambayashi J, Shiba E, Kawasaki T, Ariyoshi H, et al. Proteasome and its novel endogeneous activator in human platelets. Biochem Biophys Res Commun. 1991;178(1):256–62.
Gupta N, Li W, Willard B, Silverstein RL, McIntyre TM. Proteasome proteolysis supports stimulated platelet function and thrombosis. Arterioscler Thromb Vasc Biol. 2014;34(1):160–8.
Zufferey A, Schvartz D, Nolli S, Reny JL, Sanchez JC, Fontana P. Characterization of the platelet granule proteome: Evidence of the presence of MHC1 in alpha-granules. J Proteomics. 2014;101:130–40.
Jin R, Yu S, Song Z, Zhu X, Wang C, Yan J, et al. Soluble CD40 ligand stimulates CD40-dependent activation of the beta2 integrin Mac-1 and protein kinase C zeda (PKCzeta) in neutrophils: implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS One. 2013;8(6):e64631.
Suttles J, Stout RD. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin Immunol. 2009;21(5):257–64.
Hassan GS, Mourad W. An unexpected role for MHC class II. Nat Immunol. 2011;12(5):375–6.
Jain S, Chodisetti SB, Agrewala JN. CD40 signaling synergizes with TLR-2 in the BCR independent activation of resting B cells. PLoS One. 2011;6(6):e20651.
Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol. 2011;12(5):416–24.
von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100(1):27–40.
Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19(1):9–19.
Kaneider NC, Kaser A, Tilg H, Ricevuti G, Wiedermann CJ. CD40 ligand-dependent maturation of human monocyte-derived dendritic cells by activated platelets. Int J Immunopathol Pharmacol. 2003;16(3):225–31.
Czapiga M, Kirk AD, Lekstrom-Himes J. Platelets deliver costimulatory signals to antigen-presenting cells: a potential bridge between injury and immune activation. Exp Hematol. 2004;32(2):135–9.
Martinson J, Bae J, Klingemann HG, Tam Y. Activated platelets rapidly up-regulate CD40L expression and can effectively mature and activate autologous ex vivo differentiated DC. Cytotherapy. 2004;6(5):487–97.
Elzey BD, Grant JF, Sinn HW, Nieswandt B, Waldschmidt TJ, Ratliff TL. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol. 2005;78(1):80–4.
Solpov A, Shenkman B, Vitkovsky Y, Brill G, Koltakov A, Farzam N, et al. Platelets enhance CD4+ lymphocyte adhesion to extracellular matrix under flow conditions: role of platelet aggregation, integrins, and non-integrin receptors. Thromb Haemost. 2006;95(5):815–21.
Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J Clin Invest. 2006;116(3):769–74.
Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogne M, Richard Y, et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007;35(9):1376–87.
Iannacone M, Sitia G, Isogawa M, Whitmire JK, Marchese P, Chisari FV, et al. Platelets prevent IFN-alpha/beta-induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proc Natl Acad Sci U S A. 2008;105(2):629–34.
Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B, et al. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood. 2008;111(7):3684–91.
Nomura S, Fujita S, Nakanishi T, Yokoi T, Shimamoto K, Miyamoto R, et al. Platelet-derived microparticles cause CD154-dependent activation of dendritic cells. Platelets. 2012;23(1):81–2.
Elzey BD, Ratliff TL, Sowa JM, Crist SA. Platelet CD40L at the interface of adaptive immunity. Thromb Res. 2011;127(3):180–3.
Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, et al. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2(47):47ra63.
Metcalf D. Hematopoietic cytokines. Blood. 2008;111(2):485–91.
Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2):57–65.
Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119(13):2991–3002.
Schuettpelz LG, Link DC. Regulation of hematopoietic stem cell activity by inflammation. Front Immunol. 2013;4:204.
Libregts SF, Nolte MA. Parallels between immune driven-hematopoiesis and T cell activation: 3 signals that relay inflammatory stress to the bone marrow. Exp Cell Res. 2014;329(2):239–47.
Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14(5):302–14.
Foss B, Bruserud O, Hervig T. Platelet-released supernatants enhance hematopoietic stem cell proliferation in vitro. Platelets. 2008;19(2):155–9.
de Boer HC, van Oeveren-Rietdijk AM, Rotmans JI, Dekkers OM, Rabelink TJ, van Zonneveld AJ. Activated platelets correlate with mobilization of naive CD34(+) cells and generation of CD34(+) /KDR(+) cells in the circulation. A meta-regression analysis. J Thromb Haemost. 2013;11(8):1583–92.
Funakoshi S, Taub DD, Anver MR, Raziuddin A, Asai O, Reddy V, et al. Immunologic and hematopoietic effects of CD40 stimulation after syngeneic bone marrow transplantation in mice. J Clin Invest. 1997;99(3):484–91.
Larson AW, LeBien TW. Cross-linking CD40 on human B cell precursors inhibits or enhances growth depending on the stage of development and the IL costimulus. J Immunol. 1994;153(2):584–94.
Carlring J, Altaher HM, Clark S, Chen X, Latimer SL, Jenner T, et al. CD154-CD40 interactions in the control of murine B cell hematopoiesis. J Leukoc Biol. 2011;89(5):697–706.
Seijkens T, Engel D, Tjwa M, Lutgens E. The role of CD154 in haematopoietic development. Thromb Haemost. 2010;104(4):693–701.
Solanilla A, Dechanet J, El Andaloussi A, Dupouy M, Godard F, Chabrol J, et al. CD40-ligand stimulates myelopoiesis by regulating flt3-ligand and thrombopoietin production in bone marrow stromal cells. Blood. 2000;95(12):3758–64.
Mavroudi I, Papadaki V, Pyrovolaki K, Katonis P, Eliopoulos AG, Papadaki HA. The CD40/CD40 ligand interactions exert pleiotropic effects on bone marrow granulopoiesis. J Leukoc Biol. 2011;89(5):771–83.
Honn KV, Tang DG, Chen YQ. Platelets and cancer metastasis: more than an epiphenomenon. Semin Thromb Hemost. 1992;18(4):392–415.
Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11(3–4):325–51.
Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3(7):425–30.
Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10(5):355–62.
Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30(12):2362–7.
Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–34.
Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013;24(5):393–400.
Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231–69.
Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352(9142):1775–7.
Sabrkhany S, Griffioen AW, Oude Egbrink MG. The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta. 2011;1815(2):189–96.
Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–83.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968;61(1):46–52.
Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81(4):1012–9.
Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A. 2001;98(6):3352–7.
Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–36.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90.
Coupland LA, Chong BH, Parish CR. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012;72(18):4662–71.
Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24(1):130–7.
Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752–60.
Varon D, Hayon Y, Dashevsky O, Shai E. Involvement of platelet derived microparticles in tumor metastasis and tissue regeneration. Thromb Res. 2012;130 Suppl 1:S98–9.
Tong AW, Stone MJ. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther. 2003;10(1):1–13.
Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13(4):1083–8.
Loskog AS, Eliopoulos AG. The Janus faces of CD40 in cancer. Semin Immunol. 2009;21(5):301–7.
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–6.
Korniluk A, Kemona H, Dymicka-Piekarska V. Multifunctional CD40L: pro- and anti-neoplastic activity. Tumour Biol. 2014;35(10):9447–57.
Villeneuve J, Lepreux S, Mulot A, Berard AM, Higa-Nishiyama A, Costet P, et al. A protective role for CD154 in hepatic steatosis in mice. Hepatology. 2010;52(6):1968–79.
Poggi M, Engel D, Christ A, Beckers L, Wijnands E, Boon L, et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler Thromb Vasc Biol. 2011;31(10):2251–60.
Wolf D, Jehle F, Ortiz Rodriguez A, Dufner B, Hoppe N, Colberg C, et al. CD40L deficiency attenuates diet-induced adipose tissue inflammation by impairing immune cell accumulation and production of pathogenic IgG-antibodies. PLoS One. 2012;7(3):e33026.
Guo CA, Kogan S, Amano SU, Wang M, Dagdeviren S, Friedline RH, et al. CD40 deficiency in mice exacerbates obesity-induced adipose tissue inflammation, hepatic steatosis, and insulin resistance. Am J Physiol Endocrinol Metab. 2013;304(9):E951–63.
Wolf D, Jehle F, Michel NA, Bukosza EN, Rivera J, Chen YC, et al. Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation. 2014;129(23):2414–25.
Franchini M, Mannucci PM. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood. 2011;118(9):2405–12.
Acknowledgments
A.T. acknowledges support from the Amadeus LabEx, Université de Bordeaux. J.V. acknowledges support from a Marie Curie international outgoing fellowship within the 7th European community framework program. The support of the Association pour la Recherche en Néphrologie is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed to the writing of the manuscript. All authors read and approved the manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.