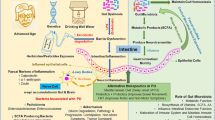
Abstract
Parkinson’s disease is characterized by dopaminergic neuron loss and intracellular inclusions composed mainly of alpha synuclein (α-syn), but the mechanism of pathogenesis is still obscure. In recent years, more attention has been given to the gut as a key player in the initiation and progression of PD pathology. Several studies characterizing changes in the microbiome, particularly the gut microbiome, have been conducted. Although many studies found a decrease in the bacterial family Prevotellaceae and in butyrate-producing bacterial genera such as Roseburia and Faecalibacteria, and an increase in the genera Akkermansia many of the studies reported contradictory findings. In this review, we highlight the findings from the different studies and reflect on the future of microbiome studies in PD research.
Similar content being viewed by others
Background
Introduction
With diseases where genetic factors and identified environmental causes account only for a minority of the cases, unidentified environmental factors are believed to play a key role in the initiation and progression of the disease. Such is the case with Parkinson’s disease (PD) wherein 90% of cases are of idiopathic nature [1]. In PD, dopaminergic neurons of the substantia nigra pars compacta degenerate and the protein alpha-synuclein (α-syn) misfolds and aggregates into Lewy bodies (LBs) and Lewy neurites (LNs). As Braak identified that α-syn inclusions appear in the dorsal motor nucleus of the vagus (DMNV) at very early stages of PD, he postulated that the pathology could be initiated in the gut or nose [1, 2]. Additionally, direct evidence of α-syn pathology propagating from the GI tract to the DMNV via the vagal nerve has been provided by two different studies in rodents [3, 4]. There is also a general consensus that a full truncal vagotomy decreases the risk of developing PD later in life [5, 6]. Moreover, up to 80% of PD patients experience constipation and delayed gastric emptying, often many years before motor symptoms appear [7]. To date, it is still of debate whether PD patients are at a higher risk of developing IBD [8,9,10,11]. A recent study showed that intestinal infection could trigger PD-like symptoms in genetically predisposed mice [12]. Other studies, which identified α-syn inclusions in intestinal biopsies years prior to PD diagnosis [13], have provided further evidence for this hypothesis. The gut, which harbors a large portion of our microbiome, acts as an intermediary between us and the environment and also plays pivotal roles in the priming and development of our immune system. That the microbiota in the GI system would play a role in synucleinopathies is supported by findings of bacterial substances modulating α-syn aggregation: both lipopolysaccharide [14, 15] and the different subunits of the E. coli amyloid protein Curli [16,17,18] have been shown to alter α-syn aggregation kinetics and produce fibrils with distinct toxicities compared to ‘pure’ fibrils. What concerns the intestinal microbiome, several studies have shown that germ-free mice display attenuated pathology and behavioral deficits in several models [19]. In a landmark study, Sampson et al. demonstrated that the microbiota itself could trigger or delay motor symptom onset in mice: Thy-1 α-syn mice colonized with microbiota from PD patients showed enhanced α-syn pathology load and microglial activation compared to mice colonized with microbiota from healthy individuals. Of note is that the study also found short chain fatty acids (SCFAs), metabolites of certain bacteria, to be sufficient in inducing α-syn pathology and microglial activation in the α-syn-overexpressing mice [19]. Of interest is that inflammation is a central component of PD pathology. Treatment with immunosuppressants has been shown to ameliorate PD pathology in mouse models [20] and mice lacking CD4+ T-cells have an attenuated response to MPTP with markedly decreased dopaminergic neuronal loss [21]. As emerging studies have found links between microbiome alterations, particularly gut dysbiosis, and a myriad of inflammation-driven diseases, including but not limited to inflammatory bowel disease (IBD), arthritis, liver disease, and obesity [22], it should come as no surprise that recent studies have aimed to identify changes in the microbiome of PD patients compared to healthy controls. In this review, we will summarize the findings from mostly human studies but also a few studies in animal models, discuss reasons behind the often contradictory findings, and relate the findings to studies in other disease conditions.
The microbiome
Even though most studies have centered on the gut, the microbiome is not limited to the gastrointestinal (GI) system but rather also includes the microbiota in the nose epithelium, the skin, genitals, all mucosal surfaces and any other number of tissues naturally inhabited by microorganisms. Distinct body parts show distinct microbiomes. The development of the microbiome begins in utero although the actual birth marks the first major colonization of the child with studies showing marked differences in the gut microbiome in children born vaginally compared to those born via caesarean section (C-section) [23]. The microbiome of children born vaginally is characterized by Prevotella and Lactobacillus taxa whereas that of children born via C-section is dominated by Staphylococcus and Streptococcus species [24]. Children born via C-section more often carry Kliebsella and Enterobacter [25]. Additionally, colonization with Bifidobacteria and Bacteroides is delayed in children born via C-section [25,26,27]. These differences seem to have effects persisting into adulthood with children born vaginally being at lower risk of developing allergies, asthma, IBD and obesity compared to those born via C-section [23]. During the first 3 years of life, the microbiome is constantly changing but stabilizes afterwards and has been shown to be relatively constant throughout life. A study of monozygotic and dizygotic twins reported heritability of members of the Ruminococcaceae and Lachnospiraceae families and also of methanogens from the archaeal domain. Contrarily, Bacteroidetes were found to be mostly environmentally determined [28]. Factors such as lifestyle, diet and antibiotic use play considerable roles in shaping the microbiome [22]. Antibiotic usage has been shown in several studies, to both acutely and permanently perturb the gut microbiome, with a loss of diversity and a shift in community composition [29,30,31]. Long-lasting perturbations to the gut microbiome are also caused by infections with the pathogen Clostridium difficile [32]. It seems that ageing, too, affects gut microbial composition. Several studies have now shown that ageing in healthy adults is correlated with an increase in the diversity of microbial species, known as alpha-diversity [33]. Although there have been differences in the microbial species examined and the results reported, most studies have found Bifidobacteria and certain Firmicutes to be decreased in stool from healthy aged adults compared with younger subjects [33,34,35]. Finally, although it is difficult to assess whether the differences reported reflect the true situation within the intestines or is an artifact from sample collection, a fast transmission time has been correlated with a decrease in microbial diversity and an abundance of fast growing species and mucosal renewal [36,37,38].
Main text
The microbiome in PD
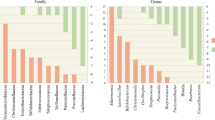
Several studies have now aimed at identifying microbiota differences in PD patients compared to healthy controls. While most of these have used fecal samples as a proxy for the microbiota composition in the distal colon, some have examined mucosal biopsies or even salivary and nasal mucosa samples [39,40,41]. A summary of the findings from stool microbiome studies in PD patients can be found in Table 1 and a more comprehensive compilation of the findings in Additional file 1: Table S1. While only a few of the studies found significant differences in the alpha-diversity, that is the community richness and evenness within a sample, of the fecal microbiota from PD patients and controls [39, 42, 43, 45], most did find statistically significant differences in the beta-diversity, the community differences between the study groups [39, 41,42,43,44, 46, 47, 55]. Unsurprisingly, many of the studies found bacteria from the phyla Firmicutes and Bacteroidetes to dominate in the feces of both PD patients and controls [39, 46, 52]. From the phylum Bacteroidetes, the family Prevotellaceae, which has been implicated in the pathogenesis of IBD [56] was found to be altered in most of the studies: six studies found a dramatic decrease of Prevotellaceae in the feces of PD patients [43,44,45,46,47,48, 51, 55] with Scheperjans et al. even finding such a strong correlation between low abundance of Prevotellacea and PD phenotype that it could be used as a classifier with over 90% specificity [46]. Minato et al. found Prevotellacea to decrease in the feces of PD patients two years after the first sample collection [57]. Keshavarzian et al., however, found no such difference in the feces of PD patients and controls but did see a reduction by approximately 50% of Prevotellacea in the sigmoid mucosa of PD patients, although this did not reach statistical significance [39]. Additionally, Pereira et al. found a decrease of Prevotellaceae in the oral cavity of PD patients compared to healthy controls [41]. Interestingly, Qian et al. found a higher abundance of Paraprevotella, a member of the Prevotellaceae family, in PD patients compared to controls [42]. A study has found a five-fold decrease of Paraprevotella in the feces of multiple system atrophy (MSA) patients compared to healthy subjects [58]. Additionally, chronic exposure to rotenone resulted in a decrease of Paraprevotella in the feces of mice already one week post initiation of treatment [59]. In the case of Bifidobacteriaceae, the findings in different studies have been contradictory: Bedarf et al. reported a lower abundance of Bifidobacteriaceae in PD patients [44], Keshavarzian et al. found a lower abundance of Bifidobacteriaceae in both the mucosa and feces of PD patients compared to controls [39] whereas Hopfner et al., Li et al. and Lin et al. described the opposite [47, 49, 54]. Hill-Burns et al. also found an increased abundance of Bifidobacteriaceae in the feces of PD patients compared to controls but this difference disappeared once they accounted for medication, specifically the use of COMT inhibitors, as a confounding factor [50]. Minato et al., however, found that patients with lower counts of Bifidobacteriaceae had a more severe worsening of symptoms 2 years later compared to those with originally higher counts [57]. Mihaila et al. found an increased abundance of Bifidobacteriaceae in the saliva of PD patients compared to controls [60]. In a rotenone-induced mouse model of PD, Bifidobacteriaceae from the ceacal content and mucosa was reduced compared to control mice [61]. Enterobacteriacea were found to be more abundant in PD patients by four studies [39, 49, 51, 53], decreased in one [44] and unchanged in one [48]. Lactobacillaceae were found to be more abundant in PD patients’ stool in six studies [43, 46, 47, 49, 50, 53] and lower in two [42, 44]. Mihaila et al. found an increased abundance of Lactobacillaceae in the saliva of PD patients compared to controls [60]. Erysipelotrichaceae is another bacterial family which 3 studies found to be increased in PD stools [40, 42, 43] but 3 others found to be decreased or unchanged compared to healthy controls [39, 44, 49]. The butyrate producing Roseburia [39, 44, 50], Blautia [39, 44, 49, 50], and Faecalibacterium [39, [43,44,44,45], 49, 51] and Dorea [39, 44, 45] have been reported to be less abundant in PD patients’ stool. Interestingly, a study has shown that infection with Clostridium difficile results in dysbiosis characterized by an over-abundance of Lactobacillaceae and a decrease of Roseburia, Blautia, Faecalibacterium, and Dorea [32]. Akkermansia was consistently found to be more abundant in PD stool samples [39, 40, 43, 44, 50,51,52]. Akkermansia muciniphila is a recently identified mucin-dwelling species of the genus Akkermansia. A. muciniphila has been shown to mediate glucose tolerance via an IFNγ dependent mechanism and protect against obesity in mice [62]. Additionally, reduced levels of A. muciniphila in gut microbiota were reported for children with asthma [63]. A recent study showed that A. mucniphila can induce different T-cell responses based on the other species present in a given microbiota [64]. This can then explain why A. muciniphila, generally thought to mediate anti-inflammatory effects, has surprisingly been shown to be over-abundant in PD feces compared to controls. Finally, although most studies focused mainly on bacteria, a few studies reported an increase in methanogens of the archaeal domain in PD stools compared to controls [42, 44, 50, 51] and Bedarf et al. reported a decrease in virus counts in PD patients’ stools [44].
Shortcomings of microbiome studies in PD research
With more fields recognizing the microbiome as a key player in diseased and healthy conditions, it should come as no surprise that the field of PD research too has focused on microbiota research in recent years. Unfortunately, it is still difficult to draw conclusions from the studies as most of them report contradictory findings. The wet lab techniques differed between the studies with most sequencing the hypervariable regions of the 16S rRNA gene but some testing for only specific bacterial taxa. Although 16S rRNA sequencing is a powerful tool in microbiome studies, it does have limitations: limited resolution, a dependence on databases chosen for species identification, reporting only on nucleic acid as compared to viable species, and relative results rather than absolute quantification of present species [65, 66]. Additionally, the time from sample collection to freezing varied greatly and storage of samples at room temperatures even for short periods of time are known to distort the relative abundances of bacterial taxa. The different studies also corrected for different confounding factors, such as medication, and were carried out in different geographical locations, factors known to affect microbiota composition. None of the studies provided information on antibiotic use in early life or previous C. difficle infections, which are known to have profound and long-lasting effects on gut microbiota. Additionally, only one study accounted for transit time as a confounding factor [50] although most of the studies reported PD patients as suffering from constipation and GI symptoms in higher frequency. Transit time has a clear effect on fecal consistency, which is known to have the highest impact on microbiota composition [36, 38]. Both Akkermansia and methanogens, here reported to be altered in PD patients’ stools, have been correlated to stool consistency [36]. A summary of the techniques and criteria employed by each study can be found in Table 2. Unfortunately, not all studies reported all findings, making it difficult to compare and perform meta-analytical studies among them. Finally, it is important to keep in mind that the majority of the studies focused on fecal composition, yet the GI tract is not homogenously colonized but rather consist of a multitude of niches [67, 68].
The microbiome in IBD
A study conducted on mouse models of colitis found Immunoglobulin A (IgA) coating to selectively identify pathology-driving bacteria. Genera from the orders Bacteroidales and Lactobacillus and family of Erysipelotrichaceae were particularly IgA-coated. Members of Clostridiales, Ruminococcaceae, Rikenellaceae, Roseburia, Faecalibacterium, and Blautia were identified from IBD patients as being selectively IgA-coated compared to healthy controls. The abundance of all these bacterial taxa have been shown to be altered in the stools of PD patients. Of interest is whether these taxa are IgA coated in PD patients too? In that same study, they also identified bacteria that were similarly IgA coated in IBD patients and healthy controls. Fecal transplantation of IgA-coated bacteria into mice did not result in pathology unless the mice were genetically susceptible to IBD [56]. This supports the interaction of genetics and environment in a dual-hit hypothesis, valid for IBD as well as PD.
Host-microbiome interactions
The interactions between the host and microbiome are manifold, complex and bidirectional. It is now well established that gut bacteria can modulate the host immune response: polysaccharides produced by B. fragilis and Bifidobacterium breve can induce regulatory T-cells (Tregs) and stimulate the secretion of the anti-inflammatory cytokine IL-10 [68, 69]. Other bacteria induce inflammation, as has been well described in IBD [56, 70, 71]. The systemic effect of gut inflammation and its potential role in neurodegeneration has recently become an area of great interest. Some bacteria in the intestine also produce SCFAs, which serve as a main energy source for colonic epithelial cells and can also induce the generation of Tregs [71]. Butyrate, a SCFA, has been shown to exert regulatory functions on dendritic cell differentiation [72]. Although some of the studies found a reduction in butyrate-producing bacteria, generally thought of as anti-inflammatory strains, the exact effect of SCFAs is still under debate with some studies even reporting exacerbation of inflammation related to SCFAs [19]. In an MPTP-induced mouse model of Parkinsonism, fecal transplant resulted in a reduction of SCFAs accompanied by an amelioration of pathology [73]. On the other hand, miRNA produced by intestinal epithelial cells can enter bacteria and regulate gene transcription and growth [74]. The fecal miRNA patterns in PD patients have been shown to differ from healthy controls [75]. Bacterial lipopolysaccharide, in turn, has been shown to alter miRNA expression in macrophages leading to a cascade of inflammatory responses [76].
Microbe-microbe interactions
Besides the effects mediated by the host on the microbiome, the constituents of the microbiome affect its composition too. Many bacteria, including commensals in our gut, possess quorum-sensing abilities: they can regulate and inhibit growth of other bacteria by secreting anti-microbials. Examples of quorum-sensing-capable bacteria are E. coli, B. subtilis, and species of Streptococcus, Streptomyces and Lactobacillus. Bacteria can also modulate the growth of other bacteria by competition for the same resources. The microbiome consists not only of bacteria though, and fungi, such as Candida albicans can also modulate the bacterial composition of the gut. C. albicans can co-aggregate and form mixed biofilms with for instance Streptococci and can compete with species such as Pseudomonas aeruginosa [77,78,79]. Additionally, although underreported, bacteriophages are among the most abundant microbes in our gut. In an experimental mouse model, infection with lytic bacteriophages had direct effects on susceptible bacterial populations, such as E. coli, and indirect effects on non-targeted bacteria through bacteria-bacteria interactions. The expansion of A. muciniphila was one of the indirect effects reported in that study [80].
Conclusion and future perspectives
Microbiome studies generate a wealth of information that is difficult to interpret. Host-microbiome interactions will surely continue to hold interest in many research fields including that of neurodegenerative diseases. Establishing a causal relationship between microbiome dysbiosis and disease initiation or progression could open the door for biomarker and identify possible intervention targets. Although all of the patients included had idiopathic PD, it would be interesting to compare microbiota from PD patients with different familial mutations: do different forms of α-syn and phenotypic presentations of PD result in specific changes to the gut environment? Additionally, do different synucleinopathies share similar changes to the microbiota? There is no doubt that the field of microbiome studies holds great promise, however, as of now, fecal microbiota cannot be used as a biomarker for PD but perhaps inspiration could be drawn from the fields of obesity and IBD studies where machine learning has successfully been employed to aid in the identification of classifiers [22, 81, 82].
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- (COMT):
-
Catechol-O-methyltransferase
- (DMNV):
-
Dorsal motor nucleus of the vagus
- (IBD):
-
Inflammatory bowel disease
- (LBs):
-
Lewy bodies
- (LNs):
-
Lewy neurites
- (MPTP):
-
1-metyl-4-fenyl-1,2,3,6-tetrahydropyridin
- (PD):
-
Parkinson’s Disease
- (SCFAs):
-
Short chain fatty acids
- (Tregs):
-
Regulatory T-cells
- (α-syn):
-
Alpha synuclein
- A (IgA):
-
Immunoglobulin
References
Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110:517–36.
Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614.
Holmqvist S, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–20.
Kim S, et al. Transneuronal propagation of pathologic alpha-Synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103:627–41.
Svensson E, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78:522–9.
Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat Rev Neurol. 2015;11:625–36.
Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol. 2015;21:10609–20.
Hui KY, et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn's disease and Parkinson's disease. Sci Transl Med. 2018;10:eaai7795 https://doi.org/10.1126/scitranslmed.aai7795.
Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of Parkinson's disease: a Danish nationwide cohort study 1977-2014. Gut. 2019;68:18–24.
Weimers P, et al. Inflammatory bowel disease and Parkinson's disease: a Nationwide Swedish cohort study. Inflamm Bowel Dis. 2019;25:111–23.
Zhu F, et al. The risk of Parkinson's disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis. 2019;51:38–42.
Matheoud D, et al. Intestinal infection triggers Parkinson's disease-like symptoms in Pink1(−/−) mice. Nature. 2019;571:565–9.
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov Disord. 2012;27:716–9.
Bhattacharyya D, et al. Lipopolysaccharide from gut microbiota modulates alpha-Synuclein aggregation and alters its biological function. ACS Chem Neurosci. 2019;10:2229–36.
Kim C, et al. Exposure to bacterial endotoxin generates a distinct strain of alpha-synuclein fibril. Sci Rep. 2016;6:30891.
Chen SG, et al. Exposure to the functional bacterial amyloid protein Curli enhances alpha-Synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep. 2016;6:34477.
Chorell E, et al. Bacterial chaperones CsgE and CsgC differentially modulate human alpha-Synuclein amyloid formation via transient contacts. PLoS One. 2015;10:e0140194.
Evans ML, et al. The bacterial curli system possesses a potent and selective inhibitor of amyloid formation. Mol Cell. 2015;57:445–55.
Sampson TR, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167:1469–1480 e1412.
Tamburrino A, et al. Cyclosporin promotes neurorestoration and cell replacement therapy in pre-clinical models of Parkinson's disease. Acta Neuropathol Commun. 2015;3:84.
Brochard V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–92.
Gilbert JA, et al. Current understanding of the human microbiome. Nat Med. 2018;24:392–400.
Dunn AB, Jordan S, Baker BJ, Carlson NS. The maternal infant microbiome: considerations for labor and birth. MCN Am J Matern Child Nurs. 2017;42:318–25.
Goedert JJ, Hua X, Yu G, Shi J. Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: analysis of the American gut project. EBioMedicine. 2014;1:167–72.
Adlerberth I, et al. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res. 2006;59:96–101.
Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25.
Dominguez-Bello MG, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5.
Goodrich JK, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–99.
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–61.
Taur Y, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018;10:eaap9489 https://doi.org/10.1126/scitranslmed.aap9489.
Ward NL, et al. Antibiotic treatment induces long-lasting changes in the fecal microbiota that protect against colitis. Inflamm Bowel Dis. 2016;22:2328–40.
Antharam VC, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51:2884–92.
An R, et al. Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut. 2018;67:2213–22.
Odamaki T, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90.
Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7.
Vandeputte D, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62.
Vandeputte D, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–11.
Roager HM, et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1:16093.
Keshavarzian A, et al. Colonic bacterial composition in Parkinson's disease. Mov Disord. 2015;30:1351–60.
Heintz-Buschart A, et al. The nasal and gut microbiome in Parkinson's disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33:88–98.
Pereira PAB, et al. Oral and nasal microbiota in Parkinson's disease. Parkinsonism Relat Disord. 2017;38:61–7.
Qian Y, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson's disease. Brain Behav Immun. 2018;70:194–202.
Lin CH, et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson's disease. J Neuroinflammation. 2019;16:129.
Bedarf JR, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson's disease patients. Genome Med. 2017;9:39.
Petrov VA, et al. Analysis of gut microbiota in patients with Parkinson's disease. Bull Exp Biol Med. 2017;162:734–7.
Scheperjans F, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30:350–8.
Hopfner F, et al. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 1667;2017:41–5.
Hasegawa S, et al. Intestinal Dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson's disease. PLoS One. 2015;10:e0142164.
Li W, et al. Structural changes of gut microbiota in Parkinson's disease and its correlation with clinical features. Sci China Life Sci. 2017;60:1223–33.
Hill-Burns EM, et al. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739–49.
Unger MM, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72.
Li F, et al. Alteration of the fecal microbiota in north-eastern Han Chinese population with sporadic Parkinson's disease. Neurosci Lett. 2019;707:134297.
Pietrucci D, et al. Dysbiosis of gut microbiota in a selected population of Parkinson's patients. Parkinsonism Relat Disord. 2019;65:124–30.
Lin A, et al. Gut microbiota in patients with Parkinson's disease in southern China. Parkinsonism Relat Disord. 2018;53:82–8.
Aho VTE, et al. Gut microbiota in Parkinson's disease: temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707.
Palm NW, et al. Immunoglobulin a coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–10.
Minato T, et al. Progression of Parkinson's disease is associated with gut dysbiosis: two-year follow-up study. PLoS One. 2017;12:e0187307.
Tan AH, et al. Altered gut microbiome and metabolome in patients with multiple system atrophy. Mov Disord. 2018;33:174–6.
Yang X, Qian Y, Xu S, Song Y, Xiao Q. Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson's disease. Front Aging Neurosci. 2017;9:441.
Mihaila D, et al. The oral microbiome of early stage Parkinson's disease and its relationship with functional measures of motor and non-motor function. PLoS One. 2019;14:e0218252.
Perez-Pardo P, et al. Gut bacterial composition in a mouse model of Parkinson's disease. Benefic Microbes. 2018;9:799–814.
Greer RL, et al. Akkermansia muciniphila mediates negative effects of IFNgamma on glucose metabolism. Nat Commun. 2016;7:13329.
Demirci M, et al. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr). 2019;47:365–71.
Ansaldo E, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364:1179–84.
Poretsky R, Rodriguez RL, Luo C, Tsementzi D, Konstantinidis KT. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS One. 2014;9:e93827.
Jo JH, Kennedy EA, Kong HH. Research techniques made simple: bacterial 16S ribosomal RNA gene sequencing in cutaneous research. J Invest Dermatol. 2016;136:e23–7.
Bauer MA, Kainz K, Carmona-Gutierrez D, Madeo F. Microbial wars: competition in ecological niches and within the microbiome. Microb Cell. 2018;5:215–9.
Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20–32.
Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017;18:851–60.
Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–77.
Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247.
Qiang Y, et al. Butyrate and retinoic acid imprint mucosal-like dendritic cell development synergistically from bone marrow cells. Clin Exp Immunol. 2017;189:290–7.
Sun MF, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun. 2018;70:48–60.
Liu S, et al. The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe. 2016;19:32–43.
Hewel C, et al. Common miRNA patterns of Alzheimer's disease and Parkinson's disease and their putative impact on commensal gut microbiota. Front Neurosci. 2019;13:113.
Raisch J, Darfeuille-Michaud A, Nguyen HT. Role of microRNAs in the immune system, inflammation and cancer. World J Gastroenterol. 2013;19:2985–96.
Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25.
Morales DK, Hogan DA. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010;6:e1000886.
Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299:1–8.
Hsu BB, et al. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe. 2019;25:803–814 e805.
Knights D, Parfrey LW, Zaneveld J, Lozupone C, Knight R. Human-associated microbial signatures: examining their predictive value. Cell Host Microbe. 2011;10:292–6.
Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–33.
Acknowledgements
We thank the members of Li’s lab for the constructive comments and inputs.
Funding
This work was supported by grants from the Swedish Research Council (K2015-61X-22297-03-4), EU-JPND (aSynProtec) and EU-JPND (REfreAME), EU H2020-MSCA-ITN-2016 (Syndegen), the Strong Research Environment MultiPark (Multidisciplinary research on Parkinson’s disease), the Swedish Parkinson Foundation (Parkinsonfonden), Torsten Söderbergs Foundation and Olle Engkvist Byggmästere Foundation. Acknowledgements are also to the supports of the National Natural Science Foundation of China (No. 81430025 and U1801681) and the Key Field Research Development Program of Guangdong Province (2018B030337001).
Author information
Authors and Affiliations
Contributions
CH and JYL conceived of the review and drafted the manuscript. QQC helped with the editing the manuscript. All the authors approve the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary information
Additional file 1: Table S1.
Compliation of the reported findings from stool microbiome studies comparing PD patients to healthy controls. + = higher in PD patients; - = higher in controls subjects; * = p < 0.05.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Haikal, C., Chen, QQ. & Li, JY. Microbiome changes: an indicator of Parkinson’s disease?. Transl Neurodegener 8, 38 (2019). https://doi.org/10.1186/s40035-019-0175-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40035-019-0175-7