Abstract
Background
Osteoarthritis (OA) is defined by signs and symptoms of inflammation within the affected joint. The aim of this study is to determine the mRNA expression levels of selected cytokines and matrix-metalloproteinases of cells found in synovial fluid (SF) obtained from osteoarthritic knee joints compared to healthy controls.
Methods
SF was obtained from 40 patients undergoing total knee arthroplasty due to evident OA and from 10 healthy controls. Expression of TNF-α, IL-1β, MMP-1 and MMP-3 was assayed among both groups by performing qPCR. Patients were configured concerning age, gender and BMI.
Results
IL-1β, MMP-1 and MMP-3 showed significantly higher expression among the OA group compared to control (P < 0.001). Strong correlation appeared between expression of MMP-1 and MMP-3 among OA patients (r = 0.856); no correlation was found between age, gender or BMI and cytokine/proteinase expression. Expression of IL-1β, MMP-1 and MMP-3 within SF was elevated in OA-patients.
Conclusion
Consequently, cells within SF expressing cytokines and proteinases may play a relevant role in the progression of joint destruction. Considering the fact that SF in an OA joint comprises abnormal amounts of detrimental bioactive proteins, temporary clearance, dilution or suppression/modulation by means of lavage or disease-modifying medication may be promising to constitute interim relief or even postpone disease progression due to decreased inflammatory and/or degrading activity within the articular environment.
Similar content being viewed by others
Background
Osteoarthritis (OA) is characterized by slowly progressing destruction of the articular cartilage as well as an inflammatory reaction within the realms of the affected joint [1]. OA represents the most common joint disorder in the world and results in pain, deformity and gradual loss of joint function [1]-[3]. The origin includes mechanical injury, genetic factors and aging [4]. Disease progression is characterized by formation of osteophytes, subchondral bone cysts, increased thickness of the subchondral bone plate and loss of articular cartilage [5]. The exact etiology of OA, however, is not fully understood.
Synovial inflammation is commonly found in early stage OA and is characterized by joint swelling, redness, heat and pain [6],[7]. The synovium is infiltrated by an increased number of synovial lining cells and immune cells such as macrophages, mast cells, T- and B-lymphocytes and plasma cells. Activated synovial cells, immune cells and chondrocytes produce large quantities of matrix metalloproteinases (MMPs) -1, -3, -9, -13 and ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs [8]. These proteolytic enzymes eventually degrade extracellular matrix macromolecules and cause a loss of cartilage integrity and biomechanical strength [9]-[11].
Proinflammatory cytokines act as soluble mediators and favor catabolic activities in articular cartilage [12],[13]. Cytokine production is associated with certain immune cells while their specific origin in OA, however, is still unclear [14]. The proinflammatory cytokines IL-1β and TNF-α are found in elevated concentration in synovial fluid (SF) and joint tissue of OA patients. Both activate the JNK, p38 MAPK and NF-κB signaling pathways in chondrocytes [15],[16]. They are capable of suppressing proteoglycan, collagen II and aggrecan expression while stimulating MMP expression and the production of further catabolic cytokines such as IL-6, IL-8, MCP-1 and CCL-5 [17].
OA is no longer understood as a disease merely caused by wear and tear but rather as an inflammatory process [18]. Definition of baseline cytokine and proteinase levels and their mRNA concentration in SF of healthy and OA patients is crucial to evaluate the potential for diagnosing early OA using cytokines as biomarkers [19]. The objective of this study was to elucidate whether the cells in the SF are producing the detrimental proteins mentioned above. Thus, we aimed to quantify mRNA expression patterns of the main inflammatory mediators IL-1β and TNF-α and degradative enzymes MMP-1 and MMP-3 within synovial fluid of OA patients compared to healthy controls. Such information would further define the role of the mentioned cytokines and proteases within the complex cascades of incidental/progressive OA and could strengthen their potential as biomarkers and/or novel therapeutic targets.
Methods
Patients and donors
Patient and donor characteristics are presented in Table 1. A total of 40 patients (18 females, 22 males) with clinically as well as radiographically-evident OA were included and SF was obtained prior to total knee arthroplasty (TKA). Furthermore, SF was obtained from a total of 10 controls/donors (4 females, 6 males) with no history of joint surgery or any cartilage/OA-related disease, within 4 hours after death due to insult or atraumatic intracranial bleeding. SF harvesting among patients as well as controls was performed utilizing a sterile syringe with the needle injected into the knee joint using a standardized anterolateral portal, strictly avoiding hemarthrosis. Single incision arthrotomy allowed then for direct visualization of the knee joint and cartilage evaluation was performed by an experienced orthopedic surgeon in order to exclude the presence of OA.
The study was performed in accordance with the Declaration of Helsinki. Informed consent from each patient as well as approval from the local ethics committee was obtained prior to investigation (Ethics commission, Charité, Berlin, EA1/083/09).
RNA extraction
After centrifugation of SF from OA patients and controls directly after harvest, the cell-pellet was disrupted in RA-1 lysis buffer (Macherey-Nagel, Düren, Germany) and stored at −80°C until further analysis. Total RNA was then isolated from the cell-pellet yielded using the NucleoSpin RNA II® kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s protocol.
Reverse transcription
A volume of 8 μl mRNA template, 1 μl 10 mM Deoxynucleotide-Mix (dNTP-Mix, Invitrogen Life Technologies, Paisley, UK) and 1 μl random primer (Invitrogen Life Technologies, Paisley, UK) was incubated for 5 minutes at 65°C in a thermocycler (Eppendorf, Hamburg, Germany) and then cooled down to 4°C. A total of 9.5 μl reaction mix consisting of 4 μl reaction buffer (M-MLV RT 5x Reaktionspuffer, Promega GmbH, Mannheim, Deutschland), 1 μl RNase inhibitor (RNase Inhibitor 40 U/μl, Promega GmbH, Mannheim, Deutschland) and 4.5 μl H2O were added and incubated at 42°C for 2 minutes. After adding 1 μl of reverse transcriptase (M-MLV RT(H-) 200 U/μl, Promega GmbH, Mannheim, Deutschland), an incubation period of 50 minutes at 42°C was followed by 15 minutes at 70°C, producing the cDNA to be further analyzed via qPCR. After cooling to 4°C, residual RNA was eliminated using 1 μl of RNase (RNse H 250 U/μl, Promega GmbH, Mannheim, Deutschland) for 20 minutes at 37°C.
Quantitative polymerase chain reaction (qRT-PCR)
Expression profiles were assessed via qRT-PCR on an iCycler detection system (Bio-Rad, Munich, Germany) using SYBR Green (Applied Biosystems, Foster City, CA, USA) as a fluorescent reporter. Primers were designed by TIB MOLBIOL (Syntheselabor GmbH, Berlin, Germany) and tested with the Basic Local Alignment Search Tool (BLAST). The human primer sequences used were as follows:
IL-1β: 5′-CAGGGACAGGATATGGAGCAA-3′ (forward)
5′-GCAGACTCAAATTCCAGCTTGTTA-3′ (reverse)
TNF-α: 5′-CTTCTCCTTCCTGATCGTGGC-3′ (forward)
5′-GGGTTTGCTACAACATGGGC-3′ (reverse)
MMP-1: 5′-CCGGTTTTTCAAAGGGAATAAGTA-3′ (forward)
5′-CGATATGCTTCACAGTTCTAGGGAA-3′ (reverse)
MMP-3: 5′-AAACCCACCTTACATACAGGATTG-3′ (forward)
5′-AAGTCTCCATGTTCTCTAACTGCA-3′ (reverse)
GAPDH: 5′-CATGTTCGTCATGGGTGTG-3′ (forward)
5′-GGCAGTGATGGCATGGACTG-3′ (reverse)
The 25 μl reaction-mix consisted of 1.0 μl cDNA template, 12.5 μl iQ Supermix (Bio-Rad, Munich, Germany), 0.5 μl primer forward (10 μM), 0.5 μl primer reverse (10 μM), 1.0 μl SYBR-Green (Applied Biosystems, Foster City, CA, USA) and 9.5 μl DNase-free water. iCycler settings involved activation of the polymerase reaction at 95°C (15 minutes), followed by 38 cycles of denaturation at 94°C (30 seconds), primer annealing at 59°C according to primer-specific requirements (40 seconds), elongation at 72°C (30 seconds) and fluorescence detection at 80°C (15 seconds). GAPDH was used as an internal control and all reactions were performed in triplicates followed by a specific melting curve analysis of each assay at 60°C to 95°C (30 seconds). Quantification of mRNA expression was assessed by the 2- ΔΔCT method [20].
Statistics
The major determinant for outcome comparison was the expression level of the targeted cytokines and proteases according to the groups described above. Statistical analysis was performed using the software package SPSS version 17 (SPSS Inc., Chicago, IL, USA). All data were tested for normal distribution using the Kolmogorov-Smirnov test. The Mann-Whitney U-test was performed to assess differences in expression levels between groups corrected for cofounding variables (age, gender, BMI). Furthermore, Fisher’s exact test was conducted to compare the frequency of detectable expressions between the groups. To control the family-wise error rate, the Bonferroni correction was applied. Unless stated otherwise, descriptive results were demonstrated as the mean ± standard deviation (SD). Significance was set at P < 0.05 for all tests.
Results
Participant characteristics
An overview of participant characteristics is given in Table 1. All patients showed clinically and radiographically-evident knee OA. Controls showed no history of knee-related pathologies and there was no intraarticular, no ligamentous and particularly no meniscal or cartilage pathology to be found in any control knee joint and the surrounding soft tissues were also without pathological findings. There was a significant overall group difference for age and BMI (P < 0.05) between groups.
qPCR outcome
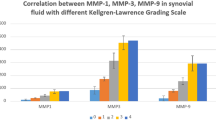
IL-1β, MMP-1 and MMP-3 showed significantly higher expression levels among the OA group compared to the control (P < 0.001) while TNF-α expression differences never reached the level of significance (P = 0.077). Median and interquartile ranges (IQR: 25th to 75th percentile) are reported to depict differences in distribution of MMP-1, MMP-3, IL-1β and TNF-α between patients and donors/controls and an overview is given in Table 2.
Frequency of detectable expression was 85.4% (OA) versus 0% (control) for MMP-1, 97.6% (OA) versus 12.5% (control) for MMP-3, 95.1% (OA) versus 25.0% (control) for IL-1β and 95.1% (OA) versus 37.5% (control) for TNF-α; the corresponding bar charts are given in Figure 1.
Furthermore, a strong correlation appeared between expression levels of MMP-1 and MMP-3 among OA patients (r = 0.856) while no such correlation was found between age, gender or BMI and the expression levels investigated.
Discussion
Inflammation plays a pivotal role in the pathogenesis of OA [4]. However, the cellular origin of inflammatory mediators and catabolic enzymes remains an area of speculation [14]. The aim of this study was to quantify and compare inflammatory and degradative markers on mRNA levels in cells floating in the SF of OA patients and healthy controls. The selection of TNF-α, IL-1β, MMP-1 and MMP-3 represents well-established inflammatory mediators and catabolic proteinases in OA [15],[18]. The present study demonstrates that there is significantly more IL-1β, MMP-1 and MMP-3 mRNA transcribed in SF cells of OA patients compared to healthy controls. The mean age of OA patients was slightly older compared to the donor group and older age could lead to a different onset of inflammatory response. Furthermore, there was a difference in mean BMI comparing OA patients and donors. Higher BMI causes higher joint load and one could imagine that this might interfere with the inflammatory response of the investigated joints. However, the immense differences between the two groups concerning frequencies of detectable expression of IL-1β, MMP-1 and MMP-3 are not likely to be caused by the slight differences of patient age or BMI.
Increased levels of active IL-1β have already been found in SF of OA patients using ELISA [21]-[24]. Similar findings were made analyzing OA cartilage and synovial tissue using immunohistochemistry [25]-[27]. To our knowledge, this is the first study to analyze mRNA levels of IL-1β in SF cells of OA patients. Our data demonstrate a high inflammatory activity of SF cells via IL-1β. However, it is not clear to what extent these cells act synergistically with cells within the synovial tissue and cartilage. Furthermore, IL-1β’s mRNA still has to be translated and IL-1β’s inflammatory potential is influenced by the presence of IL-1β-converting enzyme, allocation of suitable receptors and other inflammatory processes. Our analysis of TNF-α mRNA concentration showed a wide range of results within the group of healthy controls and no significant difference to our OA group (P = 0.077). Although TNF-α is accepted as one of the two most important inflammatory cytokines in OA today, conflicting reports are found in the literature. Early studies showed highly inconsistent levels of TNF-α expression in SF and synovial tissue of OA patients [24],[28]-[30]. Furthermore, TNF-α levels in SF significantly correlate with clinical symptoms but not with radiographic findings of OA [31]. An answer to these contrary reports might be variations in number and susceptibility of chondrocyte TNF-α receptors, the presence of soluble TNF-α receptors and activity of TNF-α-converting enzymes [24]. Alternatively, TNF-α-expressing cells such as macrophages could mainly be localized in the synovial tissue [32].
IL-1β and TNF-α stimulate the production of cartilage-degrading enzymes [33],[34]. As such, MMPs are of particular interest because they are considered as the major degradative enzymes in OA [11]. Augmented MMP levels were found in SF of OA patients and they are expressed by lining cells, neutrophils, macrophages and chondrocytes in the synovial tissue [35]-[38]. Moreover, our data indicate that a significant amount of MMPs is present within the synovial fluid. Again, it is not clear whether these MMPs are produced by cells in the surrounding tissues or by cells within SF. First and foremost, our data indicate that cells in the SF are significant contributors. Thus, this study gives valuable information about potential treatment options including replacement of SF. Arthroscopic joint irrigation has been reported to cause significant release of pain in early studies. Livesely and colleagues showed more pain relief after arthroscopic lavage and physiotherapy compared to physiotherapy alone [39]. In contrast, Moseley and co-workers showed no difference between arthroscopic irrigation and sham operations of OA knee joints, nor regarding pain or function [40]. However, patients in the latter study were predominantly male and younger than our cohort leading to the thought that only certain patients may profit from arthroscopic irrigation. Removing inflamed SF at an early OA stage might inhibit the start of the self-escalating inflammatory cycle whereas joint lavage of late-stage OA might have no or only mild/temporary effects.
Early diagnosis of OA presents another clinical challenge and is crucial for disease prevention. Conventional diagnostic tools such as radiographic and magnetic resonance imaging (MRI) showed limited sensitivity or are time-consuming and expensive [41]. Analysis of cartilage matrix molecules in body fluids is still only used as research tool and gene expression profiles in peripheral blood leukocytes showed ambivalent cytokine levels [4],[42]. Alam and colleagues successfully showed in a canine animal model that biomarkers in SF such as tartrate-resistant acid phosphatase, MMP-2, and tissue inhibitor of MMP-2 can be used for diagnosis of early OA [43]. Another canine animal model by Garner and co-workers showed high sensitivity and specificity of IL-8 and monocyte chemoattractant protein-1 in SF to surgically-induced OA. Our data show that mRNA of IL-1β, MMP-1 and -3 in SF is a sensitive indicator for symptomatic OA [44]. Further research should evaluate whether these markers are also able to detect early or non-symptomatic OA and whether they can function as a prognostic tool.
Conclusion
Our findings show that SF of OA patients contains cells that actively promote inflammation via expression of proinflammatory mediators and joint-degrading enzymes. Although origin and type of cells still have to be investigated, this may offer an explanation as to why removal of OA SF can cause OA symptom relief. Furthermore, qPCR-based analysis of SF might offer a promising diagnostic tool and the data presented here lead to speculation towards potential pharmacologic OA intervention on a biological basis.
Abbreviations
- OA:
-
osteoarthritis
- mRNA:
-
messenger ribonucleic acid
- SF:
-
synovial fluid
- TNF:
-
tumor necrosis factor
- IL:
-
interleucin
- MMP:
-
matrix metalloproteinase
- qPCR:
-
quantitative polymerase chain reaction
- BMI:
-
body mass index
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- NF-κB:
-
nuclear factor 'kappa-light-chain-enhancer' of activated B-cells
- MCP:
-
monocyte chemotactic protein
- CCL:
-
chemokine ligand
- dNTP:
-
desoxy ribonukleotide triphosphate
- RT:
-
reverse transcriptase
- cDNA:
-
complementary desoxyribonucleic acid
- BLAST:
-
Basic Local Alignment Search Tool
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- ELISA:
-
enzyme linked immunosorbent assay
- MRI:
-
magnetic resonance imaging
References
Loeser RF, Goldring SR, Scanzello CR, Goldring MB: Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012, 64: 1697–1707. 10.1002/art.34453
Arden N, Nevitt MC: Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006, 20: 3–25. 10.1016/j.berh.2005.09.007
Zhang Y, Jordan JM: Epidemiology of osteoarthritis. Clin Geriatr Med 2010, 26: 355−+. 10.1016/j.cger.2010.03.001
Goldring MB, Goldring SR: Osteoarthritis. J Cell Physiol 2007, 213: 626–634. 10.1002/jcp.21258
Burr DB: Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Research Society 2004, 12(Suppl A):S20-S30. 10.1016/j.joca.2003.09.016
Benito MJ, Veale DJ, Fitzgerald O, van den Berg WB, Bresnihan B: Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 2005, 64: 1263–1267. 10.1136/ard.2004.025270
Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M: Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis - results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil 2005, 13: 361–367. 10.1016/j.joca.2005.01.005
Sellam J, Berenbaum F: The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010, 6: 625–635. 10.1038/nrrheum.2010.159
Cawston TE, Wilson AJ: Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol 2006, 20: 983–1002. 10.1016/j.berh.2006.06.007
Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, Mikecz K, Sandy JD: Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5 - hyaluronan complexes in articular cartilages. Osteoarthr Cartil 2007, 15: 719–734. 10.1016/j.joca.2006.12.008
Rengel Y, Ospelt C, Gay S: Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther 2007, 9(5):221. 10.1186/ar2304
Goldring SR, Goldring MB: The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 2004, (427 Suppl): S27-S36. 10.1097/01.blo.0000144854.66565.8f
Goldring MB, Berenbaum F: The regulation of chondrocyte function by proinflammatory mediators - Prostaglandins and nitric oxide. Clin Orthop Relat Res 2004, (427 Suppl): S37-S46. 10.1097/01.blo.0000144484.69656.e4
de Lange-Brokaar BJE, Ioan-Facsinay A, van Osch GJVM, Zuurmond AM, Schoones J, Toes REM, Huizinga TWJ, Kloppenburg M: Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage 2012, 20: 1484–1499. 10.1016/j.joca.2012.08.027
Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y: Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis 2008, 67: 75–82. 10.1136/ard.2008.098764
Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR: Role of interleukin-1 and tumor necrosis factor a in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 2005, 52: 128–135. 10.1002/art.20776
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H: Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011, 7: 33–42. 10.1038/nrrheum.2010.196
Pelletier JP, Martel-Pelletier J, Abramson SB: Osteoarthritis, an inflammatory disease - Potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001, 44: 1237–1247. 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F
Chu CR, Williams AA, Coyle CH, Bowers ME: Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther 2012, 14: 212–212. 10.1186/ar3845
Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods 2001, 25: 402–408. 10.1006/meth.2001.1262
Kubota E, Imamura H, Kubota T, Shibata T, Murakami K: Interleukin 1 beta and stromelysin (MMP3) activity of synovial fluid as possible markers of osteoarthritis in the temporomandibular joint. J Oral Maxillofac Surg 1997, 55: 20–27. 10.1016/S0278-2391(97)90438-9
Marks PH, Donaldson MLC: Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy 2005, 21: 1342–1347. 10.1016/j.arthro.2005.08.034
Mannami K, Mitsuhashi T, Takeshita H, Okada K, Kuzuhara A, Yamashita F, Sakakida K: Concentration of interleukin-1 beta in serum and synovial fluid in patients with rheumatoid arthritis and those with osteoarthritis. Nihon Seikeigeka Gakkai zasshi 1989, 63: 1343–1352.
Westacott CI, Sharif M: Cytokines in osteoarthritis: mediators or markers of joint destruction? Semin Arthritis Rheum 1996, 25: 254–272. 10.1016/S0049-0172(96)80036-9
Tetlow LC, Adlam DJ, Woolley DE: Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage - Associations with degenerative changes. Arthritis Rheum 2001, 44: 585–594. 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C
Z-m S, Ling M, Liu M, Zhang Y-G: Expression of interleukin-1beta and tumor necrosis factor-alpha in the synovium and synovial fluid of patients with Kashin-Beck disease and osteoarthritis. Nan Fang Yi Ke Da Xue Xue Bao 2009, 29: 5–8.
Aigner T, Soeder S, Haag J: Il-1 beta and BMPS - Interactive players of cartilage matrix degradation and regeneration. Eur Cell Mater 2006, 12: 49–56.
Di Giovine FS, Nuki G, Duff GW: Tumour necrosis factor in synovial exudates. Ann Rheum Dis 1988, 47: 768–772. 10.1136/ard.47.9.768
Hopkins SJ, Meager A: Cytokines in synovial fluid ii. The presence of tumor necrosis factor and interferon. Clin Exp Immunol 1988, 73: 88–92.
Brennan FM, Chantry D, Jackson AM, Maini RN, Feldmann M: Cytokine production in culture by cells isolated from the synovial membrane. J Autoimmun 1989, 2(Suppl):177–186. 10.1016/0896-8411(89)90129-7
Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, Ishikawa T, Hanaoka E, Yamashita K, Yamashita M, Equchi Y, Toyone T, Takahashi K, Ohtori S: Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord 2011, 12: 144. 10.1186/1471-2474-12-144
Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE: The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 2006, 8(6):R187. 10.1186/ar2099
Milner JM, Cawston TE: Matrix metalloproteinase knockout studies and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Curr Drug Targets Inflamm Allergy 2005, 4: 363–375. 10.2174/1568010054022141
Aida Y, Maeno M, Suzuki N, Shiratsuchi H, Motohashi M, Matsumura H: The effect of IL-1 beta on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci 2005, 77: 3210–3221. 10.1016/j.lfs.2005.05.052
Lohmander LS, Hoerrner LA, Lark MW: Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum 1993, 36: 181–189. 10.1002/art.1780360207
Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TWJ, Tekoppele JM, Hanemaaijer R, DeGroot J: MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis 2005, 64: 694–698. 10.1136/ard.2004.022434
Meszaros E, Malemud CJ: Prospects for treating osteoarthritis: enzyme-protein interactions regulating matrix metalloproteinase activity. Ther Adv Chronic Dis 2012, 3: 219–229. 10.1177/2040622312454157
Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y: Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 2000, 59: 455–461. 10.1136/ard.59.6.455
Livesley PJ, Doherty M, Needoff M, Moulton A: Arthroscopic lavage of osteoarthritic knees. J Bone Joint Surg Br Vol 1991, 73: 922–926.
Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM, Wray NP: A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002, 347: 81–88. 10.1056/NEJMoa013259
Pelletier J-P, Martel-Pelletier J: Imaging in osteoarthritis trials: useful or just expensive? Nat Clin Pract Rheum 2009, 5: 76–77. 10.1038/ncprheum0990
Attur M, Belitskaya-Lévy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, Smiles S, Lee S, Patel J, Al-Mussawir H, McDaniel G, Kraus VB, Abramson SB: Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum 2011, 63: 1908–1917. 10.1002/art.30360
Alam MR, Ji JR, Kim MS, Kim NS: Biomarkers for identifying the early phases of osteoarthritis secondary to medial patellar luxation in dogs. J Vet Sci 2011, 12: 273–280. 10.4142/jvs.2011.12.3.273
Garner BC, Stoker AM, Kuroki K, Evans R, Cook CR, Cook JL: Using animal models in osteoarthritis biomarker research. J Knee Surg 2011, 24: 251–264. 10.1055/s-0031-1297361
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. MS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sauerschnig, M., Stolberg-Stolberg, J., Schulze, A. et al. Diverse expression of selected cytokines and proteinases in synovial fluid obtained from osteoarthritic and healthy human knee joints. Eur J Med Res 19, 65 (2014). https://doi.org/10.1186/s40001-014-0065-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-014-0065-5