Abstract
Background
Vibrio cholerae O1/O139 were the predominant circulating serogroups exhibiting multi-drug resistance (MDR) during the cholera outbreak which led to cholera treatment failures.
Objective
This meta-analysis aimed to evaluate the weighted pooled resistance (WPR) rates in V. cholerae O1/O139 isolates obtained from environmental samples.
Methods
We systematically searched the articles in PubMed, Scopus, and Embase (until January 2020). Subgroup analyses were then employed by publication year, geographic areas, and the quality of studies. Statistical analyses were conducted using STATA software (ver. 14.0).
Results
A total of 20 studies investigating 648 environmental V. cholerae O1/O139 isolates were analysed. The majority of the studies were originated from Asia (n = 9). In addition, a large number of studies (n = 15 i.e. 71.4%) included in the meta-analysis revealed the resistance to cotrimoxazole and ciprofloxacin. The WPR rates were as follows: cotrimoxazole 59%, erythromycin 28%, tetracycline 14%, doxycycline 5%, and ciprofloxacin 0%. There was increased resistance to nalidixic acid, cotrimoxazole, furazolidone, and tetracycline while a decreased resistance to amoxicillin, ciprofloxacin, erythromycin, chloramphenicol, ampicillin, streptomycin, and ceftriaxone was observed during the years 2000–2020. A significant decrease in the doxycycline and ciprofloxacin-resistance rates in V. cholerae O1/O139 isolates was reported over the years 2011–2020 which represents a decrease in 2001–2010 (p < 0.05).
Conclusions
Fluoroquinolones, gentamicin, ceftriaxone, doxycycline, kanamycin, and cefotaxime showed the highest effectiveness and the lowest resistance rate. However, the main interest is the rise of antimicrobial resistance in V. cholerae strains especially in low-income countries or endemic areas, and therefore, continuous surveillance, careful appropriate AST, and limitation on improper antibiotic usage are crucial.
Similar content being viewed by others
Introduction
Globally, WHO reported about 2.9 million new cases of cholera in 69 cholera-endemic countries and 21,000–143,000 cholera-associated deaths to occur every year worldwide [1]. Also, more than one million new cases and 5654 deaths were reported from 34 countries [2]. Two serogroups, O1 and O139 were related to the cholera outbreak and also were the predominant circulating serogroups exhibiting multi-drug resistance during the cholera outbreak [2, 3].
Over the years, some antimicrobials such as tetracyclines and fluoroquinolones have been excellently active against cholera-associated strains [4,5,6,7]. However, recently, cholera treatment failures are frequently observed with the recurrent emergence of resistant strains [4]. So, there has been a rising concern about the development of antimicrobial resistance in V. cholerae strains especially in low-income countries [4]. So, a comprehensive and elucidated resistance rate data is essential. Since, V. cholerae is a primarily/natural inhabitant of aquatic environment ecosystems worldwide, aquatic environment as the reservoir of toxigenic V. cholerae contribute significantly to variation and transmission in cholera epidemics [8, 9]. Nevertheless, the epidemiological impact of environmental V. cholerae strains is not clearly understood. To answer this vexing question, we conducted this systematic review and meta-analysis to provide extensive and elaborated data on the antimicrobial resistance patterns of environmental V. cholerae isolates against commonly used antimicrobials, across various regions over different periods.
Methods
Guidelines
This review is reported accordant with the Preferred Reporting Items for Systematic Reviews and Meta Analyses guidelines (PRISMA) [10].
Search strategy
We systematically searched for relevant studies in PubMed, Scopus, and Embase (Until January 2020) by using the related keywords; (“Vibrio cholerae” OR “V. cholerae”) AND (“Antibiotic resistance” OR “Antimicrobial resistance”) in the Title/Abstract/Keywords fields. No limitation was used while searching databases, but the inclusion of the study in our full analysis required at least the abstract to be available in English. The search strategy was designed and conducted by study investigators. The records found through database searching were merged and the duplicates were removed using EndNote X8 (Thomson Reuters, New York, NY, USA).
Study selection
One of the research teams (N.O.) randomly evaluated the search results and confirmed that no relevant study had been ignored. All these steps were done by the three authors (H.K, N.O., A.M.), and any disagreements about article selection were resolved through discussion, and a fourth author (E.K.) acted as arbiter. Three reviewers (H.K., N.O., A.M.) screened all titles and abstracts separately and excluded irrelevant or duplicate articles first. Three reviewers then independently evaluated the remaining articles for inclusion. Discrepancies were resolved by discussion.
Eligibility criteria
The following items were extracted from each included study: the first author, year published, continent, country, number of environmental V. cholerae O1/O139 isolates, number of resistant environmental V. cholerae O1/O139 isolates, antibiotic susceptibility testing methods (AST; disk diffusion, agar dilution, microbroth dilution, E-test), and interpretation of resistance. The exclusion criteria were as follows: (1) studies that contained redundant data or were overlapping articles; (2) those which presented clinical or non O1/O139 V. cholerae isolates, animal research, reviews, meta-analysis and/or systematic review, and conference abstracts; (3) those in which resistance rates were not presented or reported; and (4) articles that included fewer than 5 V. cholerae O1/O139 isolates.
Quality assessment process
The quality of the included studies was assessed by two reviewers (H.K., N.O.) separately using an adapted version of the tool proposed by the Newcastle–Ottawa assessment scale adapted for cross-sectional studies [11]. A score ranging from 0 to 7 points was attributed to each study (≥ 6 points: high quality, ≤ 5 points: low quality). A higher score indicated a higher study quality. A third reviewer (E.K) adjudicated in any cases where there was disagreement.
Publication bias
Publication bias was analysed using Egger’s linear regression test.
Statistical analysis
Those studies presenting raw data on antibiotic susceptibility in environmental V. cholerae O1/O139 isolates were included in the meta-analysis that have performed pool computing pool using a random-effects model with Stata/SE software, v.14.1 (StataCorp, College Station, TX). The inconsistency across studies was examined by the forest plot as well as the I2 statistic. Values of I2 (25%, 50%, and 75%) were interpreted as the presence of low, medium, or high heterogeneity, respectively. So, the DerSimonian and Laird random-effects models were used [11]. Subgroup analyses were then employed by publication year, geographic areas, and the quality of studies. Publication bias was assessed using Egger’s test. All statistical interpretations were reported on a 95% confidence interval (CI) basis.
Study outcomes
The main outcome of interest was the weighted pooled resistance rate (WPR) of environmental V. cholerae O1/O139 to antibiotics. A subgroup analysis was performed; The subgroup analysis was then employed by (1) publication date (2001–2010, and 2011–2020), (2) continents, and (3) the quality of studies.
Results
Systematic literature search
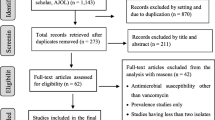
A total of 1650 records were identified in the initial search. From these, 1550 articles were excluded after an initial screening of the title and abstract due to their irrelevancy and redundancy. The full texts of the remaining 85 articles were reviewed (Fig. 1). Of the 85 articles, 64 were excluded for the following reasons: being meta-analysis, review, conference abstract, or not containing relevant, clinical, resistance data, or O1/O139 V cholerae clinical isolates. Finally, 20 cross-sectional studies [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] were included in this meta-analysis (Additional file 1). The studies included in this meta-analysis evaluated antibiotic resistance to ciprofloxacin, erythromycin, furazolidone, tetracycline, doxycycline, chloramphenicol, nalidixic acid, cotrimoxazole, ampicillin, streptomycin, gentamicin, ceftriaxone, and norfloxacin.
Characteristics of included studies
All 21 studies included in the analysis were performed in 11 countries and had investigated 648 O1/O139 V. cholerae environmental isolates obtained from the drinking water, streams, storage tanks, wells, and seafoods. The majority of the studies were originated from Asia (n = 9) and Africa (n = 8), followed by America (n = 3). Disk diffusion agar test was the most common antimicrobial susceptibility testing method (n = 19), followed by a combination of two methods (agar dilution & disk diffusion agar) (n = 1). All 21 studies had a cross-sectional design. The majority of the studies (n = 15 i.e. 71.4%) included in the meta-analysis revealed the resistance to cotrimoxazole and ciprofloxacin. The WPR rates of each antibiotic and the subgroup analyses by year, continent, and the quality are shown in the Supplementary Table. The WPR rates of environmental V. cholerae O1/O139 isolates to antibiotics shown in Fig. 2.
Tetracyclines-resistance
Tetracycline
The susceptibility to tetracycline was determined in 12 studies that included 285 environmental O1/O139 V. cholerae isolates; the WPR was 14% (95% CI 3–29) with substantial heterogeneity (I2 = 91.39%) (Table 1). We performed a subgroup analysis for two periods (2001–2010, and 2011–2020), to analyze the trends for changes in the prevalence of tetracycline resistance in more recent years (Table 1). The subgroup analysis that compared the data during 2001–2010 (WPR 13%; 95% CI 3–26) and 2011–2020 (WPR 14%; 95% CI 1–36) indicated an increase in the resistance rate. The highest resistance rate was reported in Africa, (4%, 95% CI 0–21) (Table 1).
Doxycycline
The susceptibility to doxycycline was determined in 7 studies that included 173 O1/O139 V. cholerae isolates; the WPR was 5% (95% CI 0–14) with substantial heterogeneity (I2 = 73.83%) (Table 1). As shown in Table 1, the prevalence of doxycycline-resistance significantly decreased from 17% (95% CI 7–30) of 48 strains between 2001 and 2010 to 3% (95% CI 0–10) of 125 strains in 2011–2020, thus, the frequency of O1/O139 V. cholerae during the years 2011–2020 represents a > fivefold decrease over the years 2001–2010 (p = 0.02). The prevalence of doxycycline resistance was 7% (95% CI 0–21) among 129 isolates in Africa, and 0% (95% CI 0–7) among 44 isolates in America.
Sulfonamides-resistance
Cotrimoxazole
The susceptibility to cotrimoxazole was determined in 15 studies that included 1624 O1/O139 V. cholerae isolates; the WPR was 59% (95% CI 30–85) with substantial heterogeneity (I2 = 95.43%) (Table 1). The prevalence of cotrimoxazole-resistance increased from 51% (95% CI 11–89) of 764 strains during 2001–2011 to 66% (95% CI 30–95) of 860 strains in 2012–2020. The prevalence of cotrimoxazole resistance was 66% (95% CI 29–95) among 663 isolates in Africa, 63% (95% CI 21–97) among 629 isolates in Asia, and 37% (95% CI 0–100) among 332 isolates in America.
Fluoroquinolones
Ciprofloxacin
The susceptibility to ciprofloxacin was determined in 15 studies that included 376 O1/O139 V. cholerae isolates; the WPR was 0% (95% CI 0–0) (Table 1). The subgroup analysis that compared the data over the years 2001–2010 (WPR 2%; 95% CI 0–7) and 2011–2020 (WPR 0%; 95% CI 0–0) indicated a decrease in the resistance rate and, this difference was statistically significant (P = 0.03; Table 1).
Norfloxacin
The susceptibility to norfloxacin was determined in 4 studies that included 64 O1/O139 V. cholerae isolates; the WPR was 0% (95% CI 0–3) (Table 1).
Ofloxacin
The susceptibility to ofloxacin was determined in 4 studies that included 123 O1/O139 V. cholerae isolates; the WPR was 0% (95% CI 0–1) (Table 1).
Aminoglycosides-resistance
Erythromycin
The susceptibility to erythromycin was determined in 5 studies that included 71 O1/O139 V. cholerae isolates; the WPR was 28% (95% CI 4–60) with substantial heterogeneity (I2 = 83.77%) (Table 1). The prevalence of erythromycin-resistance gradually increased from 50% (95% CI 32–68) of 16 strains during 2001–2010 to 20% (95% CI 0–64) of 55 strains in 2011–2020 (Table 1). Thus, the frequency of erythromycin-resistant strains during the years 2011–2020 represents a 2.5-fold decrease over the years 2001–2010. The prevalence of erythromycin resistance was 44% (95% CI 7–84) among 43 isolates in Africa, and 12% (95% CI 1–28) among 28 isolates in Asia (Table 1).
Streptomycin
The susceptibility to streptomycin was determined in 11 studies that included 201 O1/O139 V. cholerae isolates; the WPR was 60% (95% CI 28–89) with substantial heterogeneity (I2 = 95.2%) (Table 1). The prevalence of streptomycin-resistant strains gradually decreased from 64% (95% CI 68–92) of 91 strains during 1980–2000 to 57% (95% CI 62–88) of 110 strains in 2001–2010 (Table 1). The prevalence of streptomycin resistance was 42% (95% CI 0–100) among 58 isolates in Africa, 62% (95% CI 48–75) among 38 isolates in America, and 74% (95% CI 35–100) among 106 isolates in Asia (Table 1).
Gentamicin
The susceptibility to gentamicin was determined in 8 studies that included 247 V. cholerae O1/O139 isolates; the WPR was 0% (95% CI 0–0) (Table 1).
Furazolidone- resistance
The susceptibility to furazolidone was determined in 7 studies that included 185 V. cholerae O1/O139 isolates; the WPR was 62% (95% CI 20–96) with substantial heterogeneity (I2 = 96.81%) (Table 1). The subgroup analysis that compared the data during 2001–2010 (WPR 6%; 95% CI 13–98) and 2011–2020 (WPR 69%; 95% CI 59–79) indicated an increase in the resistance rate (Table 1). The highest prevalence of furazolidone resistance was 97% (95% CI 78–100) among 50 isolates in Asia (Table 1).
Chloramphenicol-resistance
The susceptibility to chloramphenicol was determined in 10 studies that included 244 O1/O139 V. cholerae isolates; the WPR was 12% (95% CI 1–29) with substantial heterogeneity (I2 = 91.25%) (Table 1). The subgroup analysis compared the data during 2001–2010 (WPR 27%; 95% CI 0–71) and 2011–2020 (WPR 3%; 95% CI 0–11) (Table 1). Thus, the frequency of chloramphenicol-resistance during the years 2011–2020 represents a ninefold decrease over the years 2001–2010. The highest resistance rate was reported in Asia, followed by Africa (100%, 95% CI 74–100; 11%, 95% CI 1–25) (Table 1).
Nalidixic acid-resistance
The susceptibility to nalidixic acid was determined in 12 studies that included 249 O1/O139 V. cholerae isolates; the WPR was 37% (95% CI 9–70) with substantial heterogeneity (I2 = 95.72%) (Table 1). The subgroup analysis compared the data during 2001–2010 (WPR 19%; 95% CI 2–45) and 2011–2020 (WPR 49%; 95% CI 5–94) (Table 1). Thus, the frequency of nalidixic acid- resistance during the years 2011–2020 represents a ~ 2.5-fold increase over the years 2001–2010. The highest resistance rate was reported in America (37%, 95% CI 0–100).
β-Lactams-resistance
Ampicillin
The susceptibility to ampicillin was determined in 13 studies that included 379 O1/O139 V. cholerae isolates; the WPR was 43% (95% CI 19–70) with substantial heterogeneity (I2 = 96.05%) (Table 1). The subgroup analysis compared the data during 2001–2010 (WPR 50%; 95% CI 20–79) and 2011–2020 (WPR 39%; 95% CI 6–77) indicated a decrease in the resistance rate. The highest resistance rate was reported in Europe followed by Asia, Africa, and America (51%, 95% CI 3–98; 52%, 95% CI 24–80; 17%, 95% CI 0–58).
Ceftriaxone
The susceptibility to ceftriaxone was determined in 15 studies that included 1851 O1/O139 V. cholerae isolates; the WPR was 12% (95% CI 2%-27%) with substantial heterogeneity (I2 = 97.99%) (Table 1). The subgroup analysis compared the data during 2001–2010 (WPR 24%; 95% CI 1–60), and 2011–2020 (WPR 5%; 95% CI 0–18) (Table 1). Thus, the frequency of ceftriaxone-resistance during the years 2011–2020 represents a 4.8-fold decrease over the years 2001–2010. The highest resistance rate was reported in Africa, followed by Europe (43%, 95% CI 0–100; 8%, 95% CI 2–20). A significant difference was found in the methods used for AST (p = 0.02).
Publication bias
Begg’s and Egger’s regression tests were performed to assess publication bias. The shapes of the funnel plots do not show obvious evidence of asymmetry. However, the p. value of Egger’s test confirmed the existence of publication bias for all the WPRs evaluated [(A) ciprofloxacin, p = 0.465; (B) erythromycin, p = 0.546; (C) furazolidone, p = 0.894; (D) tetracycline, p = 0.467; (E) doxycycline, p = 0.577; (F) chloramphenicol, p = 0.401; (G) nalidixic acid, p = 0.527; (H) cotrimoxazole, p = 0.74; (I) amoxicillin, p = 0.622; (J) streptomycin, p = 0.995; (K) gentamicin, p = 0.645; (L) ceftriaxone, p = 0.489; (M) ampicillin, p = 0.096; (N) norfloxacin, p = 0.795; (O) kanamycin, p = 0.967; (P) cefotaxime, p = 0.549; and (Q) amikacin, p = 0.81] (Table 1).
Discussion
This systematic review and meta-analysis was conducted to consider the global prevalence of antibiotic resistance in environmental V. cholerae O1/O139 isolates. It is significant to obtain more data about the resistance profiles of circulating environmental V. cholerae O1/O139 strains. Cholera as an ancient and acute infectious disease is considered a major public health issue primarily in developing countries [32]. The antimicrobial resistance is increasing in V. cholerae isolates over recent years and has become a major risk to cholera treatment strategies [4]. Thus, a comprehensive and potent policy is required to control and treat cholera.
Tetracyclines has long been the most effective antibiotic class for cholera treatment. Nevertheless, previously published studies reported an increase in tetracycline-resistant strains of V. cholerae worldwide [33, 34]. This meta-analysis revealed that resistance status to tetracycline and doxycycline in environmental V. cholerae O1/O139 isolates were 14% and 5%, respectively. We found that the tetracycline-resistance rate of V. cholerae isolates was considerably varying in different geographical areas. The regional divergences in tetracycline-resistance rate of V. cholerae isolates may result from infection control policies, antibiotic stewardship, the lack of a uniform consumption pattern, exposure to the same antibiotics in different regions, and various AST methods. Our meta-analysis presented that trend of tetracycline-resistance had a minor increase from 2001–2010 to 2011–2020 (13% and 14%). Also, the frequency of doxycycline resistant O1/O139 V. cholerae isolates shows, fivefold decrease over the years 2001–2010. Additionally, the frequency of tetracycline and doxycycline-resistant isolates in Africa is more to compare with Asia and America. The tetracycline-resistance determinants may be developed, transferred and exchanged between environmental and clinical V. cholera isolates through the horizontal gene transfer mechanisms, the active efflux of antibiotics from the bacterial cell, the production of ribosomal protection proteins (encoded by tet genes), target site mutation, decreased drug permeability, and enzymatic degradation of the antibiotics [35, 36]. Recently, a systematic review and meta-analysis [37] of tetracyclines resistance in clinical V. cholera O1 isolates showed higher rates than observed in our study; 20% (95% CI, 10–30) for tetracycline and 7% (95% CI, 3–10) for doxycycline. The analyses differed in the origin of the isolates since, in our analyses, the data on V. cholera isolates of clinical origin were not included. Three representatives of fluoroquinolones were analysed in our study; ciprofloxacin, norfloxacin, and ofloxacin. In our study, the resistance rate of environmental O1/O139 V. cholerae isolates against fluoroquinolones was0%. The prevalence of ciprofloxacin-resistant O1/O139 V. cholerae isolates shows, 2% decrement over the years 2001–2010. Our data in line with some studies [38, 39] showed that fluoroquinolones have excellent activity against V. cholerae species. However, fluoroquinolones-resistance in V. cholerae strains started rising from July 1996 [40]. Mohammed and colleagues [41] conducted a systematic review and meta-analysis to review prior data on antimicrobial resistance of V. cholerae from sub-Saharan Africa. They reported a huge high resistance rate (44.0%) to fluoroquinolones. However, no resistance to fluoroquinolones was reported in Miwanda study [42]. The variable levels of resistance to fluoroquinolones may have been resulted from surveillance programs, widespread consumption of these antibiotics, and different AST methods.
Although previously published study proposed erythromycin, a macrolide as a substitute to tetracyclines in young children and pregnant women [43, 44]. Our findings showed that the highest resistance rate was towards erythromycin (28%; 4%-60%). Previous studies conducted in Asia and Africa reported high rates of erythromycin-resistance in V. cholerae [34, 41, 43].
The use of chloramphenicol has been restricted in some areas such as India in the past due to the availability of more effective antibiotics with fewer side effects [45]. Our study revealed an absolute chloramphenicol-resistance rate (100%) in Asia. The prevalence of chloramphenicol-resistance in V. cholerae isolates shows, sevenfold decrease over the years 2001–2010.
Hitherto furazolidone and nalidixic acid have been commonly used for cholera therapy. But currently, because of the high resistance rate in V. cholerae isolates these antibiotics were less effective [46]. In the present study, a high resistance rate of V. cholera against furazolidone (88%) and nalidixic acid (37%) were found. Yousefi et al., in another meta-analysis on Iranian isolates, claimed similar findings [33]. A significant increase in furazolidone-resistance might be related to the increased consumption of this antibiotic in low-income countries [46].
Trimethoprim/Sulfamethoxazole (cotrimoxazole) was frequently referred as antibiotic for gastroenteritis therapy. Nevertheless, in our study, a high resistance rate against trimethoprim/sulfamethoxazole was found (59%) among environmental V. cholerae strains, and this result is in line with the other studies [47,48,49]. Therefore, the rapid and reliable diagnosis between V. cholerae and other causative agents of gastroenteritis helps us to apply appropriate options in cholera therapy [49].
The main cause of shedding V. cholera to environments is the consumption of wastewater and human excreta for farming or in the aquaculture systems [50, 51]. While, antibiotic excretion from urine and faeces of humans or farm animals, and/or disposal of antibiotics may lead to the development of resistant strains or treatment failures [46, 50, 51].
Conclusions
This meta-analysis has provided the major insights into the epidemiologically antibiotic resistance pattern of environmental V. cholera in three periods (1980–2000, 2001–2010 and 2011–2020) and has emphasized the distribution of antibiotic-resistant strains in continents. Our meta-analysis showed a low resistance rate against some antibiotics, including fluoroquinolones, gentamicin, ceftriaxone, doxycycline, kanamycin, and cefotaxime. However, antimicrobial resistance is on the increasing slope, and the main interest is the rise of antimicrobial resistance in V. cholerae strains especially in low-income countries or endemic areas. Finally, to control the development and the increase of resistant strains, continuous surveillance, careful and appropriate AST and limitation on improper antibiotic usage are crucial.
Availability of data and material
All the data in this review are included in the manuscript.
Abbreviations
- MDR:
-
Multidrug-resistant
- WPR:
-
Weighted pooled resistance
- AST:
-
Antibiotic susceptibility testing
- PRISMA:
-
Preferred reporting items for systematic reviews and meta analyses guidelines
- CI:
-
Confidence interval
References
Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9(6):e0003832.
Ramamurthy T, Mutreja A, Weill FX, Das B, Ghosh A, Nair GB. Corrigendum: revisiting the global epidemiology of cholera in conjunction with the genomics of Vibrio cholerae. Front Public Health. 2019;7:237.
Verma J, Bag S, Saha B, Kumar P, Ghosh TS, Dayal M, et al. Genomic plasticity associated with antimicrobial resistance in Vibrio cholerae. Proc Natl Acad Sci USA. 2019;116(13):6226–31.
Das B, Verma J, Kumar P, Ghosh A, Ramamurthy T. Antibiotic resistance in Vibrio cholerae: understanding the ecology of resistance genes and mechanisms. Vaccine. 2020;38(Suppl 1):A83–92.
Ghosh A, Ramamurthy TJTI. Antimicrobials and cholera: are we stranded? Indian J Med Res. 2011;133(2):225.
Saha D, Karim MM, Khan WA, Ahmed S, Salam MA, Bennish ML. Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med. 2006;354(23):2452–62.
Yaghoubi S, Zekiy AO, Krutova M, Gholami M, Kouhsari E, Sholeh M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. 2021:1–20.
Dalsgaard A, Serichantalergs O, Forslund A, Lin W, Mekalanos J, Mintz E, et al. Clinical and environmental isolates of Vibrio cholerae serogroup O141 carry the CTX phage and the genes encoding the toxin-coregulated pili. J Clin Microbiol. 2001;39(11):4086–92.
Singh D, Matte MH, Matte G, Jiang S, Sabeena F, Shukla B, et al. Molecular analysis of Vibrio cholerae O1, O139, non-O1, and non-O139 strains: clonal relationships between clinical and environmental isolates. Appl Environ Microbiol. 2001;67(2):910–21.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Ceccarelli D, Alam M, Huq A, Colwell RR. Reduced susceptibility to extended-spectrum beta-lactams in Vibrio cholerae isolated in Bangladesh. Front Public Health. 2016;4:231.
Zachariah R, Harries AD, Arendt V, Nchingula D, Chimtulo F, Courteille O, et al. Characteristics of a cholera outbreak, patterns of Vibrio cholerae and antibiotic susceptibility testing in rural Malawi. Trans R Soc Trop Med Hyg. 2002;96(1):39–40.
Islam MS, Jahid MIK, Rahman MM, Rahman MZ, Shafiqu M, Islam MSK, et al. Biofilm acts as a microenvironment for plankton-associated Vibrio cholerae in the aquatic environment of Bangladesh. Microbiology and Immunology. 2007;51:369–79.
Campos LC, Zahner V, Avelar KES, Alves RM, Pereira DSG, Vital Brazil JM, et al. Genetic diversity and antibiotic resistance of clinical and environmental Vibrio cholerae suggests that many serogroups are reservoirs of resistance. Epidemiol Infect. 2004;132:985–92.
Gu W, Yin J, Yang J, Li C, Chen Y, Yin J, et al. Characterization of Vibrio cholerae from 1986 to 2012 in Yunnan Province, southwest China bordering Myanmar. Infect Genet Evol. 2014;21:1–7.
Abana D, Gyamfi E, Dogbe M, Opoku G, Opare D, Boateng G, et al. Investigating the virulence genes and antibiotic susceptibility patterns of Vibrio cholerae O1 in environmental and clinical isolates in Accra, Ghana. BMC Infect Dis. 2019;19(1):76.
Alam MT, Weppelmann TA, Longini I, De Rochars VM, Morris JG Jr, Ali A. Increased isolation frequency of toxigenic Vibrio cholerae O1 from environmental monitoring sites in Haiti. PLOS ONE. 2015;10(4):e0124098.
Li BS, Tan HL, Wang DC, Deng XL, Chen JD, Zhong HJ, et al. Phenotypic and genotypic characterization Vibrio cholerae O139 of clinical and aquatic isolates in China. Curr Microbiol. 2011;62(3):950–5.
Bhanumathi R, Sabeena F, Isac SR, Shukla BN, Singh DV. Molecular characterization of Vibrio cholerae O139 bengal isolated from water and the aquatic plant Eichhornia crassipes in the River Ganga, Varanasi, India. Appl Environ Microbiol. 2003;69(4):2389–94.
Kumar R, Lalitha KV. Prevalence and molecular characterization of Vibrio cholerae O1, non-O1 and non-O139 in tropical seafood in Cochin, India. Foodborne Pathog Dis. 2013;10(3):278–83.
Waturangi DE, Wennars M, Suhartono MX, Wijaya YF. Edible ice in Jakarta, Indonesia, is contaminated with multidrug-resistant Vibrio cholerae with virulence potential. J Med Microbiol. 2013;62:352–9.
Al-Hilu SA, Al-Mohana AM, Jaber Z. Conventional and molecular detection of vibrio cholerae isolated from environmental water with the prevalence of antibiotic resistance mechanisms. Int J Res Pharm Sci 2019;10(3)
Kiiru J, Mutreja A, Mohamed AA, Kimani RW, Mwituria J, Sanaya RO, et al. A study on the geophylogeny of clinical and environmental Vibrio cholerae in Kenya. PLOS ONE. 2013;8(9):e74829.
Dixit SM, Johura FT, Manandhar S, Sadique A, Rajbhandari RM, Mannan SB, et al. Cholera outbreaks (2012) in three districts of Nepal reveal clonal transmission of multi-drug resistant Vibrio cholerae O1. BMC Infect Dis. 2014;14:392.
Ibarra JO, Alvarado DE. Antimicrobial resistance of clinical and environmental strains of Vibrio cholerae isolated in Lima-Peru during epidemics of 1991 and 1998. Braz J Infect Dis. 2007;11:100–5.
Mercy N, Mohamed AA, Zipporah N, Chowdhury G, Pazhani GP, Ramamurthy T, et al. Phenotypic and genetic characterization of vibrio cholera o1 isolated from various regions of Kenya between 2007 and 2010. Pan Afr Med J. 2014.
Akoachere JFTK, Masalla TN, Njom HA. Multi-drug resistant toxigenic Vibrio cholerae O1 is persistent in water sources in New Bell-Douala, Cameroon. BMC Infect Dis. 2013;13(1):1–12.
Akoachere JFTK, Mbuntcha CKP. Water sources as reservoirs of Vibrio cholerae O1 and non-O1 strains in Bepanda, Douala (Cameroon): Relationship between isolation and physico-chemical factors. BMC Infect Dis. 2014;14(1):1–10.
Onyuka JHO, Kakai R, Onyango DM, Arama PF, Gichuki J, Ofulla AVO. Prevalence and antimicrobial susceptibility patterns of enteric bacteria isolated from water and fish in lake victoria basin of western kenya. 2011.
Eja ME, Etok CA, Asikong BE, Mboto CI, Arikpo GE. Incidence of enteric bacterial pathogens in water found at the bottom of commercial freezers in Calabar, Southeastern Nigeria. 2006.
Mukandavire Z, Morris JG. Modeling the Epidemiology of Cholera to Prevent Disease Transmission in Developing Countries. Microbiol Spectr. 2015;3(3):3–21.
Yousefi A, Vaez H, Sahebkar A, Khademi F. A systematic review and meta-analysis on the epidemiology of antibiotic resistance of Vibrio cholerae in Iran. Ann Ig. 2019;31(3):279–90.
Dengo-Baloi LC, Sema-Baltazar CA, Manhique LV, Chitio JE, Inguane DL, Langa JP. Antibiotics resistance in El Tor Vibrio cholerae 01 isolated during cholera outbreaks in Mozambique from 2012 to 2015. PLOS ONE. 2017;12(8):e0181496.
Thaker M, Spanogiannopoulos P, Wright GD. The tetracycline resistome. Cell Mol Life Sci. 2010;67(3):419–31.
Loo KY, Letchumanan V, Law JWF, Pusparajah P, Goh BH, Ab Mutalib NS, et al. Incidence of antibiotic resistance in Vibrio spp. Rev Aquac. 2020;12(4):2590–608.
Ahmadi MH. Global status of tetracycline resistance among clinical isolates of Vibrio cholerae: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2021;10(1):1–12.
Bhattacharya SK, Bhattacharya MK, Dutta P, Dutta D, De SP, Sikdar SN, et al. Double-blind, randomized, controlled clinical trial of norfloxacin for cholera. Antimicrob Agents Chemother. 1990;34(5):939–40.
Dutta D, Bhattacharya S, Bhattacharya M, Deb A, Deb M, Manna B, et al. Efficacy of norfloxacin and doxycycline for treatment of Vibrio cholerae O139 infection. J Antimicrob Chemother. 1996;37(3):575–81.
Mukhopadhyay AK, Basu I, Bhattacharya SK, Bhattacharya MK, Nair GB. Emergence of fluoroquinolone resistance in strains of Vibrio cholerae isolated from hospitalized patients with acute diarrhea in Calcutta, India. Antimicrob Agents Chemother. 1998;42(1):206–7.
Mohammed Y, Aboderin AO, Okeke IN, Olayinka AT. Antimicrobial resistance of Vibrio cholerae from sub-Saharan Africa: a systematic review. Afr J Lab Med. 2018;7(2):778.
Miwanda B, Moore S, Muyembe JJ, Nguefack-Tsague G, Kabangwa IK, Ndjakani DY, et al. Antimicrobial drug resistance of Vibrio cholerae, democratic Republic of the Congo. Emerg Infect Dis. 2015;21(5):847–51.
Awuor SO, Omwenga EO, Daud II. Geographical distribution and antibiotics susceptibility patterns of toxigenic Vibrio cholerae isolates from Kisumu County, Kenya. Afr J Prim Health Care Fam Med. 2020;12(1):e1–6.
Ngandjio A, Tejiokem M, Wouafo M, Ndome I, Yonga M, Guenole A, et al. Antimicrobial resistance and molecular characterization of Vibrio cholerae O1 during the 2004 and 2005 outbreak of cholera in Cameroon. Foodborne Pathog Dis. 2009;6(1):49–56.
Chatterjee P, Kanungo S, Bhattacharya SK, Dutta S. Mapping cholera outbreaks and antibiotic resistant Vibrio cholerae in India: An assessment of existing data and a scoping review of the literature. Vaccine. 2020;38(Suppl 1):A93–104.
Bagheri-Josheghani S, Bakhshi B, Mousavi M. Prevalence of antibiotic resistance in Vibrio cholerae: a meta-analysis. 2021.
Adabi M, Bakhshi B, Goudarzi H, Zahraei SM, Pourshafie MR. Distribution of class I integron and sulfamethoxazole trimethoprim constin in Vibrio cholerae isolated from patients in Iran. Microb Drug Resist. 2009;15(3):179–84.
Masoumi-Asl H, Gouya MM, Rahbar M, Sabourian R. The epidemiology and antimicrobial resistance of cholera cases in Iran during 2013. Iran J Microbiol. 2016;8(4):232–7.
Aminshahidi M, Arastehfar A, Pouladfar G, Arman E, Fani F. Diarrheagenic Escherichia coli and Shigella with high rate of extended-spectrum beta-lactamase production: two predominant etiological agents of acute Diarrhea in Shiraz, Iran. Microbial Drug Resist. 2017;23:1037–44.
Kirpich A, Weppelmann TA, Yang Y, Ali A, Morris JG Jr, Longini IM. Cholera transmission in Ouest Department of Haiti: dynamic modeling and the future of the epidemic. PLOS Negl Trop Dis. 2015;9(10):e0004153.
Lupica A, Gumel AB, Palumbo A. The computation of reproduction numbers for the environment-host-environment cholera transmission dynamics. J Biol Syst. 2020;28(02):183–231.
Acknowledgements
This study Supported by the National Natural Science Foundation of China (81672020).
Author information
Authors and Affiliations
Contributions
XHY, AZV, YML, VHK, contributed to the conception, design, drafting of the work. YJ, YJ, NO participated in the design of the study and performed the statistical analysis. EK, AM, YJ conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable in this section.
Consent for publication
Not applicable in this section.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
The characteristics of cross-sectional studies included in this meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, Xh., Li, Ym., Vaziri, A.Z. et al. Global status of antimicrobial resistance among environmental isolates of Vibrio cholerae O1/O139: a systematic review and meta-analysis. Antimicrob Resist Infect Control 11, 62 (2022). https://doi.org/10.1186/s13756-022-01100-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-022-01100-3