Abstract
In the past decade, adipose tissue became a highly interesting source of adult stem cells for plastic surgery and regenerative medicine. The isolated stromal vascular fraction (SVF) is a heterogeneous cell population including the adipose-derived stromal/stem cells (ASC), which showed regenerative potential in several clinical studies and trials. SVF should be provided in a safe and reproducible manner in accordance with current good manufacturing practices (cGMP). To ensure highest possible safety for patients, a precisely defined procedure with a high-quality control is required. Hence, an increasing number of adipose tissue-derived cell isolation systems have been developed. These systems aim for a closed, sterile, and safe isolation process limiting donor variations, risk for contaminations, and unpredictability of the cell material. To isolate SVF from adipose tissue, enzymes such as collagenase are used. Alternatively, in order to avoid enzymes, isolation systems using physical forces are available. Here, we provide an overview of known existing enzymatic and non-enzymatic adipose tissue-derived cell isolation systems, which are patented, published, or already on the market.
Similar content being viewed by others
Introduction
Subcutaneous human adipose tissue is an abundant source of mesenchymal stromal/stem cells (MSC), which are promising for several therapeutic applications in esthetic and regenerative medicine. For a long time, adipose tissue has been considered to be an inert tissue and has usually been discarded as surgical waste after liposuction [1, 2]. Nevertheless, it is a highly vascularized tissue and an abundant source of multiple cell types such as MSC [3, 4]. MSC with similar characteristics can be found in a variety of other tissues including the bone marrow, muscle, connective tissue, skin, placenta, blood, cord blood, synovium, periosteum, and perichondrium [2, 5–19]. Bone marrow-derived mesenchymal stromal/stem cells (BM-MSC) have been extensively used since their discovery in the early 70s [20], but harvesting bone marrow is accompanied by certain drawbacks including painful procurement as well as low stem cell yield. In comparison to BM-MSC, MSC from adipose tissue, the adipose-derived stromal/stem cells (ASC), occur at a 100–1000-fold higher frequency within adipose tissue on a volume basis [21]. Harvesting adipose tissue is minimally invasive and less painful, and liposuction is one of the most commonly performed cosmetic procedures without general anesthesia [1]. Additionally, the adipose source offers two options for selection of regenerative cells: the stromal vascular fraction (SVF) and the ASC contained therein.
The stromal vascular fraction (SVF)
SVF is a heterogeneous mixture of cells isolated by enzymatic or non-enzymatic dissociation of adipose tissue followed by centrifugation in order to remove the differentiated adipocytes, which float over the aqueous layer. The heterogeneous population of SVF includes endothelial cells, erythrocytes, fibroblasts, lymphocytes, monocytes/macrophages, and pericytes among others, but most importantly ASC [22–26]. SVF may be used as a source material to isolate cells for tissue regeneration and has already been applied in clinical trials [27–32].
Adipose-derived stromal/stem cells (ASC)
ASC can be isolated from the SVF by in vitro cultivation on plastic surfaces, which results in the accumulation of spindle-shaped cells characterized by their self-renewal potency and ability to give rise to at least adipogenic, osteogenic, and chondrogenic lineages [33–35] (Fig. 1). Besides that, there is a growing body of evidence that these cells can generate a variety of other cell types including neuronal/glial-like cells [36–39], cardiomyocytes [40, 41], endothelial cells [42–44], hepatocyte-like cells [45, 46], various epithelial cell types [47–49], keratinocyte-like cells [50], and dental bud structures [51]. In addition to their extensive differentiation potential, ASC have been shown to secrete high levels of growth factors such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), keratinocyte growth factor (KGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), transforming growth factor-beta (TGF-β), insulin growth factor (IGF), and brain-derived neurotrophic factor (BDNF) [52]. The growth factors are secreted at bioactive levels and act primarily angiogenic and anti-apoptotic, and their secretion is significantly increased under hypoxic conditions [53–57]. Besides growth factors, ASC also release cytokines including fms-related tyrosine kinase 3 (Flt-3) ligand, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), macrophage-colony stimulating factor (M-CSF), interleukin (IL) such as IL-6, IL-7, IL-8, IL-11, and IL-12, leukemia inhibitory factor (LIF), and tumor necrosis factor-alpha (TNF-α) [44, 58]. Further, they are able to interact with cells of the immune system and have demonstrated to possess immunomodulatory and anti-inflammatory effects [59–62]. ASC have been successfully used in clinical studies and trials for treating soft tissue defects, bone defects, gastrointestinal lesions, immune disorders, neurological injuries, and cardiovascular diseases [32, 63–72].
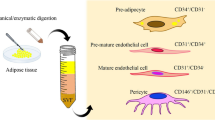
Adipose-derived cells: origin, immunophenotype, morphology, and differentiation potential. Lipoaspirate can be easily obtained from the patient and processed to obtain a heterogeneous cell population, the stromal vascular fraction (SVF). Adipose-derived stromal/stem cells (ASC) can be isolated from the SVF by in vitro cultivation on plastic surfaces. ASC are characterized mainly by mesenchymal stem cell marker (CD73, CD90, CD105) at the expense of hematopoietic stem cell marker (CD45) and their spindle-shaped morphology with the ability to differentiate into the adipogenic, osteogenic, and chondrogenic lineages. The differentiation potential can be analyzed by histological stainings, such as Oil red O for adipogenic, Alizarin red for osteogenic, and Alcian Blue for chondrogenic differentiation
Definition, standardization, and regulation of adipose-derived cells
Although SVF and ASC have been shown to possess a wide therapeutic range in vivo, there is still no standard protocol with uniform parameters for the isolation and also unclear aspects about the identity of cells isolated from adipose tissue. Zuk et al. [2] were the first who identified a cell population derived from human lipoaspirate, which partially resemble BM-MSC in their potency and functionality. These so-called processed lipoaspirate (PLA) cells have the ability to self-renew or differentiate efficiently into the adipogenic, osteogenic, myogenic, and chondrogenic lineage. Various research groups identified consistent immunophenotype surface marker whereas the expression profile changes with passaging. Pittenger et al. [16] identified mesenchymal, hematopoietic, and endothelial stem cell surface marker in BM-MSC. A similar set of markers was identified in SVF and ASC ([34], reviewed in [73]). Castro-Malaspina et al. [74] characterized human MSC within bone marrow via fibroblast colony-forming units showing cell adherence and the ratio between the number of cells plated and the number of colonies formed. Consequently, existing knowledge about SVF and ASC was identified and collected by the International Federation for Adipose Therapeutics and Science (IFATS) together with the International Society for Cellular Therapy (ISCT) in order to provide guidance for standardization between different research groups. IFATS and ISCT established a “living document” for SVF and ASC where existing literature is summarized and can be modified in response to new data and findings from ongoing clinical studies. According to this declaration, cells should have a viability of >70 % for SVF and >90 % for ASC. Frequency of stromal progenitors analyzed with a fibroblast colony forming unit assay (CFU-F) is expected to be >1 % for SVF and >5 % for ASC. Regarding SVF cell identity, immunophenotype should show the following characteristic primary marker profile for stromal cells: CD13, CD29, CD44, CD73, CD90 positive (>40 %), and CD34 positive (>20 %), but CD31 (<20 %) and CD45 negative (<50 %). In contrast, ASC should be positive for CD13, CD29, CD44, CD73, CD90, and CD105 (>80 %), but negative for CD31, CD45, and CD235a (<2 %). Moreover, ASC are expected to have the capacity to differentiate into the adipogenic, osteogenic, and chondrogenic lineage. The differentiation potential can be analyzed by histological stainings, such as Oil red O or Nile red for adipogenic differentiation, Alizarin red or von Kossa for osteogenic differentiation, and Alcian Blue or Safranin O for chondrogenic differentiation. Additionally, specific biomarkers can be investigated, such as adiponectin, CCAAT/enhancer binding protein alpha (C/EBPα), fatty acid binding protein (FABP) 4, leptin and peroxisome proliferator-activated receptor gamma (PPARγ) for adipogenic differentiation, osteocalcin, osterix, and runt-related transcription factor (runx) 2 for osteogenic differentiation and aggrecan, collagen type II, and (SRY (sex determining region Y)-box (Sox) 9 for chondrogenic differentiation [33].
Adipose tissue-derived cells, which are for clinical use, need to fulfill some additional requirements according to current good manufacturing practice (cGMP) compared to those used for research applications. These requirements are set by regulatory agencies and are intended to ensure highest possible safety for patients. A major point is to avoid contact with anything that might carry risk factors, such as pathogens or substances which might cause negative reactions in the patient. This also includes animal-derived components, which are frequently used in common cell culture procedures. Another requirement for production under cGMP is the use of certified and validated instruments and components, which might not be available for every production step. In case of ASC production by common enzymatic method, the following points have to be considered: raw material, in this case adipose tissue, has to be obtained by a validated procedure in a certified facility. This means that the physician’s rooms need to be checked for suitability in addition to applied standard surgery room requirements. Equipment needs to be certified, and personal has to receive appropriate training. To avoid contamination, the graft needs to be transported in a closed sterile container, with suitable temperature maintenance and monitoring. Besides microbiological quality control of the adipose tissue, serological tests of donor blood material have to be performed in order to ensure that no infectious diseases are transferred via the fat graft. A medical questioner’s form has to be answered, and a declaration of consent has to be signed by the donor [75–78].
Enzymatic and non-enzymatic SVF isolation
The most common isolation technique is based on enzymatic digestion of adipose tissue to obtain the SVF. In general, enzymes such as collagenase, trypsin, or dispase are used to digest adipose tissue [33]. Although the isolation techniques for adipose tissue-derived cells are rather diverse, they follow a certain standard procedure. Differences lie mainly in numbers of washing steps, enzyme concentrations, centrifugation parameters, erythrocyte lysis methods as well as in filtration, and eventually culture conditions [34, 35, 79–85]. Briefly, the adipose tissue is washed, followed by enzymatic digestion and the cells separated by centrifugation from mature adipocytes released oil and enzyme solution. Commonly used collagenases are type I and type II as well as subtypes or combinations of those [26, 82, 85–90]. GMP grade collagenases are produced by recombinant bacteria and are usually delivered in lyophilized form. Their activity varies between batches, and their purity varies between manufacturers. Nevertheless, enzyme concentrations are usually given in weight per volume percent (w/v) resulting in irregularities between different isolations even if the same protocol is used. Concentrations stated in literature range from 0.075 % (w/v) to 0.3 % (w/v) [81, 91, 92]. An erythrocyte lysis step is usually included to get rid of erythrocyte contamination and to decrease the amount of cells with hematopoietic origin. After another optional washing step, the SVF is either cryopreserved or cultured in expansion medium. The plastic-adherent cell fraction, including ASC, can be obtained after passaging or cryopreservation or further cultivated for expansion for a more homogeneous ASC population. As the use of enzymes is accompanied with high costs and might have an impact on safety [79] and efficacy [93, 94], several groups focus on non-enzymatic isolation methods using shear force, centrifugal force, radiation force, and pressure. This mechanical step replaces the enzymatic digestion to separate the cells or cell aggregates from adipose tissue. Nevertheless, similar to the enzymatic cell isolation methods, the range of protocols and methods for the non-enzymatic cell isolation shows also high variations.
Adipose tissue-derived cell isolation systems
Based on experiences in esthetic surgery, it is known that transfer of autologous fat is beneficial and therefore preferable to all short-acting fillers [95, 96]. But the use of autologous fat can also have serious disadvantages, such as partial necrosis after transplantation of larger quantities. For improving the survival rate of transplanted fat grafts, vascularization is required for nutrition and incorporation inside the surrounding tissue. The additional use of stem cells as SVF and ASC can eliminate these limitations. It has been shown that cell-assisted lipotransfer (CAL) can reduce postoperative atrophy and enhance neovascularization [97–103].
Automated closed devices could help to standardize the isolation process with the assurance of a sterile process. Therefore, a number of different companies together with research laboratories started to develop automated devices to perform parts or even the whole cell isolation process. Some of those devices were primarily designed for enrichment of adipose tissue for autologous fat grafts during plastic surgery and later upgraded for isolation of cell fractions from adipose tissue.
Table 1 summarizes a survey of currently patented, published, or commercially available enzymatic adipose tissue-derived cell isolation systems. The following systems aim for automation of preparation by collagenase-based digestion: AdiStem™ Small/Large Kit and AdiLight (AdiStem Pty Ltd., China), Sepax 2 (Biosafe Group SA, Switzerland), Cellthera Kit I and II and Method for isolation of adipose tissue-derived stromal vascular fraction (Cellthera, s.r.o., Czech Republic), A-Stromal™ kit (Cellular Biomedicine Group, Inc./Cellular Biomedicine Group HK, Ltd., USA), Celution® 800/CRS and 820/CRS (Cytori Therapeutics, Inc., USA), Sceldis® (ED Co. Ltd. & Purebiotech Co., Ltd., South Korea/Medica Group, United Arab Emirates), Automated systems and methods for isolating regenerative cells from adipose tissue (General Electric Company, USA), GID SVF-1™ (GID Group, Inc., USA), HuriCell (Hurim BioCell, Co., Ltd., South Korea), Apparatus and methods for cell isolation (Ingeneron, Inc., USA), STEM-X™ (Medikan International Inc., USA), Beauty Cell (N-Biotek, Inc., South Korea), UNISTATION™ (NeoGenesis Co., Ltd., South Korea), CHA STATION™ and Multi Station (PNC International Co., Ltd., South Korea/PNC North America Division Of Advanced Bio-Medical Equipment Co., INC), CID300 (SNJ Co., Ltd., South Korea/TOPMED CO., LTD., South Korea), Stempeutron™ (Stempeutics Research Pvt. Ltd., India), Tissue Genesis Icellator Cell Isolation System and Hand-held micro-liposuction adipose harvester, processor, and cell concentrator (Tissue Genesis, Inc., USA).
Other systems do not include enzymatic digestion but break the processed adipose tissue by mechanical forces. Table 2 summarizes a survey of currently patented, published, or commercially available non-enzymatic adipose tissue-derived cell isolation systems. The following non-enzymatic cell isolation systems generate SVF-enriched adipose tissue: Devices for harvesting and homogenizing adipose tissue containing autologous endothelial cells (Baxter International Inc., USA), Puregraft® (Bimini Technologies LLC, USA), Fastkit (Fastem) (CORIOS Soc. Coop., Italy), LipiVage™ (Genesis Biosystems, Inc., USA), Revolve™/GID 700™ (LifeCell Corporation, USA/GID Group, Inc., USA), Lipogems® (Lipogems International S.p.A., Italy), Lipo-Kit GT (Medikan International Inc., USA), StromaCell™ (MicroAire Surgical Instruments, LLC, USA), and myStem® (MyStem LLC, USA). Several other non-enzymatic isolation systems aim at the isolation of adipose tissue-derived cells and obtain pure SVF: Method for isolating stromal vascular fraction (Agency Science, Tech & Res, China), Procedure and device for separating adult stem cells from fatty tissue and Device for separating adult stem cells (Human Med AG, Germany), Ultrasonic cavitation derived stromal or mesenchymal vascular extracts and cells derived therefrom obtained from adipose tissue and use thereof and Isolation of stromal vascular fraction from vascular tissues (IntelliCell BioSciences Inc., USA), Non-enzymatic method for isolating human adipose-derived stromal/stem cells (Pennington Biomedical Research Center, USA), Isolation of stem cells from adipose tissue by ultrasonic cavitation, and methods of use (Rusty Property Holdings Pty Ltd., Australia/Amberdale Enterprises Pty Ltd., Australia/Tavid Pty, Australia), and Selective lysing of cells using ultrasound (Solta Medical, Inc., USA).
Note: Affiliations are status May–July 2015. Due to the fast growing/changing market, some of the device/company names might have already changed at time of publication.
In vitro/in vivo analyses of cells derived from enzymatic isolation devices/procedures
Several of the presented systems have already been tested in preclinical and clinical studies, and few comparative studies using cells isolated by different systems. SVF cells isolated using AdiStem™ cell isolation kit were combined with platelet-rich plasma (PRP) and transplanted into nude NOD/SCID mice resulting in joint regeneration [104]. AdiStem™ isolated cells were activated with a photobiostimulator AdiLight with a total viable cell number of 12 × 106/ml fat compared to a standard isolation method with 10 × 106 cells/ml fat [105]. AdiLight photobiostimulation-activated SVF were endobronchially infused to idiopathic pulmonary fibrosis (IPF) patients with an output of marginal improvement of walking and forced vital capacity [106]. Another safety study with this system was performed using infusions of autologous SVF in IPF patients with no deterioration in functional parameters and quality of life [107]. A major study was performed by Michalek et al. [32] where 1128 patients suffering from osteoarthritis were treated with autologous SVF cells isolated with Cellthera Kit I and II. Patients observed an improvement in pain, movement, and stiffness after SVF injection directly into the joint. Enzymatic cell isolation using the Celution® 800/CRS system exhibits a cell number of 2.95 × 105 cells/ml with a viability of 86.6 % [87]. In a comparison test, the Celution® 800/CRS system demonstrated the highest cell yield (2.41 × 105 cells/g) compared to Multi Station (1.07 × 105 cells/g), Lipo-Kit GT (0.35 × 105 cells/g), and CHA STATION™ (0.05 × 105 cells/g) [108]. Adipose-derived regenerative cell (ADRC)-enriched fat grafting using the Celution® 800/CRS device resulted in decreased fat absorption and increased neovascularization in nude mice [109]. Autologous SVF cells isolated with the same device improved hand disability and reduced pain in systemic sclerosis [110]. With the same system, autologous ADRC and adipose tissue were transurethrally injected, resulting in reduction of male stress urinary incontinence [111]. ADRC-enriched fat graft injections derived from Celution® device improved breast contour in a clinical trial for breast conservation therapy (BCT) [112]. Isolation of SVF with the GID SVF-1™ yielded an average cell number of 7.19 ± 2.11 × 105 nucleated cells/ml of dry adipose tissue. This cell number is dependent on the patient’s age and decreases with increasing age [113]. Güven et al. [86] observed a higher cell yield with the Sepax 2 isolation system compared to standard manual isolation (2.6 ± 1.2 × 105 vs. 1.6 ± 0.9 × 105 nucleated cells/ml of liposuction) and 24 % higher clonogenicity. SVF cells derived via Sceldis® were added to a mixture of platelet-rich plasma, hyaluronic acid, and CaCl2 and injected to treat knee pain due to meniscus tear [114, 115]. The invention of Khan et al. resulted in cell numbers of ~6 × 105 cells/ml (66 % viability) for donor 1 and ~1 × 106 cells/ml (51 % viability) for donor 2 [116]. In an animal model of focal cerebral ischemia, cells isolated with the HuriCell isolation device showed neuroprotective effects in ischemic brain injury [117]. Stubbers and Coleman [118] invented an apparatus and methods for cell isolation yielding 4.9 × 106–24.7 × 106 total nucleated cells/100 g lipoaspirate. SVF isolated with the Tissue Genesis Icellator Cell Isolation System were successfully used with aspirated adipose tissue for breast and face augmentation or reconstruction [119]. Domenis et al. [120] analyzed three isolation devices (Lipo-Kit, Celution®, Fastem) and showed that SVF-enriched adipose fat grafts increased thickness of the tissue and improved long-term effects in breast reconstruction compared to standard lipotransfer. However, the non-enzymatic device Fastem was less effective in stem cell enrichment compared to the enzymatic devices Lipo-Kit and Celution®.
In vitro/in vivo analyses of cells derived from non-enzymatic isolation devices/procedures
Washing and filtration of adipose tissue using Puregraft® showed higher tissue viability and lower presence of red blood cells, free lipids, and contaminants compared to other fat grafts [121, 122]. Processed adipose tissue derived from the Revolve™ system exhibited significant higher fat retention injected in nude mice than with a standard centrifugation or decantation method [123]. Washing of adipose tissue with the GID 700™ resulted in significant reduced amounts of triglycerides, lactate dehydrogenase, and hematocrit and maintained osmolarity of the adipose graft [124]. SVF cells derived from the harvesting and irrigation device LipiVage™ showed mesenchymal and endothelial progenitor cells maintaining their growth and differentiation capacity when applied through a fibrin spray system [125]. LipiVage™ yielded a higher number of viable adipocytes and sustained a higher level of intracellular enzyme (glycerol-3-phophatase dehydrogenase (G3PDH)) activity within fat grafts [126]. Bianchi et al. [127] showed that Lipogems® isolated cells exhibit a significantly higher percentage of mature pericytes and ASC and lower amount of hematopoietic cells than enzymatic isolated cells and higher percentage of exosomes [128]. Moreover, Lipogems® showed arteriogenic and paracrine properties for the rescue of ischemic limb [129] and ASC derived from Lipogems® exhibit enhanced transcription of vasculogenic genes, enhanced differentiation capacity in mouse embryonic stem cells, and efficient direct multi-lineage reprogramming in human skin fibroblasts compared to enzymatically isolated ASC when exposed to a radio electric asymmetric conveyer (REAC) [130]. Human lipoaspirated adipose tissue microfragmented with Lipogems® resulted in a better mesenchymal stem cell source compared to normal lipoaspirated tissue, while maintaining the structural composition of the original tissue [131]. Fat transplantation using Lipogems® applied in combination with orthognatic surgery reduces postoperative pain and swelling and improves final esthetic outcomes [132]. In addition, Lipogems® may improve the healing, osteointegration, and stability of the implants in newly formed bone [133] and can also improve the symptoms of fecal incontinence due to muscular and neural local trauma [134]. Another device for harvesting and homogenizing adipose tissue for endothelial cells was described by Hu et al. [135] obtaining 1.12–2.13 × 106 cells larger than 7.8 μm from 1 g adipose tissue but after enzymatic isolation of the non-enzymatic isolated cells. An increased population of microvascular endothelial cells can be collected with an elongated cannula with cutting edges to disrupt the connective tissue. Victor invented a SVF isolation method using ultrasonic cavitation and yielded 1.67–2.24 × 107 cells with a viability of 97.1–98.9 % [136]. Bright et al. [137] dissociated adipose tissue by lysing mature adipocytes using ultrasonic cavitation to obtain cell yields of about 2–4 million cells/gram adipose tissue. Clinical studies were performed using intra-articular SVF injection for patients suffering from osteoarthritis (knee, hip) and intravenous SVF injection for rheumatoid osteoarthritis showing improvement in pain, stiffness, and physical function. Patients suffering from chronic migraine experienced a decline in frequency and severity of migraines after systemic treatment with autologous SVF isolated with the ultrasonic cavitation protocol from Bright et al. [138]. Another method was claimed by Schafer [139], focusing on the separation of adipose cells using ultrasonic energy/acoustic standing wave with a yield of small (<50 μm) but more vital pre-adipocytes for successful grafting. This graft could stimulate the production of vascular structure. The idea of a simple method comprising manual shaking and washing the stem cells out from adipose tissue derives from Gimble et al. [140] with a cell yield of 2.5 × 106 cells per 100 ml adipose tissue. These cells possess equally high adipogenic and osteogenic differentiation potential, compared to a standard enzymatic isolation method.
Conclusion
In the past decade, subcutaneous adipose tissue came into the focus of plastic surgery and regenerative medicine. The isolated SVF as well as ASC have been successfully used in clinical studies and trials. But there are still drawbacks associated with current strategies to provide cellular therapeutics, which is defined in the regulations of the different countries. To fulfill the criteria of the regulatory authorities for the translation of cell-based therapies into clinics, a great deal of work remains to be done: primarily, a common standard operating protocol, toxin- and xeno-free reagents including replacement of enzymes and a quick quality control to predict donor variations in cell identity and efficiency. Therefore, several adipose tissue-derived cell isolation systems have been already developed with the main goal to provide a closed, sterile, and safe isolation process avoiding contaminations and unpredictability of the cell material. However, not all of the cell isolation systems are closed systems, which is the prerequisite for a sterile isolation unless the isolation is performed in a cleanroom facility. Each method or system has different advantages and disadvantages and is under continuous development and optimization. Differences within the systems include parameters such as operation (manual or automatic), handling (easy until cumbersome to use), and costs (e.g., expensive apparatus or high cost consumables). Publication of study outcomes, comparative studies as well as standardization of cell products will allow the field to bring further effective therapy to the clinics.
References
Coleman 3rd WP, Glogau RG, Klein JA, Moy RL, Narins RS, Chuang TY, et al. Guidelines of care for liposuction. J Am Acad Dermatol. 2001;45(3):438–47. doi:10.1067/mjd.2001.117045.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi:10.1089/107632701300062859.
Harwood Jr HJ. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012;63(1):57–75. doi:10.1016/j.neuropharm.2011.12.010.
Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci: AMS. 2013;9(2):191–200. doi:10.5114/aoms.2013.33181.
Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood, NJ). 2001;226(6):507–20.
Anker PS I ’t, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–45. doi:10.1634/stemcells.2004-0058.
Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22(5):649–58. doi:10.1634/stemcells.22-5-649.
De Bari C, Dell’Accio F, Tylzanowski P, Luyten F. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi:10.1002/1529-0131(200108)44.
Young H, Steele T, Bray R, Hudson J, Floyd J, Hawkins K, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62.
Zvaifler N, Marinova-Mutafchieva L, Adams G, Edwards C, Moss J, Burger J, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–88.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42. doi:10.1046/j.1365-2141.2000.01986.x.
Wakitani S, Goto T, Pineda S, Young R, Mansour J, Caplan A, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am Vol. 1994;76:579–92.
Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002;195(12):1549–63. doi:10.1084/jem.20011700.
Gronthos S, Franklin D, Leddy H, Robey P, Storms R, Gimble J. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi:10.1002/jcp.1138.
Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, et al. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78(10):1439–48. doi:10.1093/humrep/den356.
Pittenger M, Mackay A, Beck S, Jaiswal R, Douglas R, Mosca J, et al. Multilineage potential of adult human mesenchymal stem cells. Science (New York, NY). 1999;284:143–7. doi:10.1126/science.284.5411.143.
Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–97. doi:10.1097/01.tp.0000045055.63901.a9.
Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118–29. doi:10.1111/j.1365-2141.2005.05409.x.
Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42(7):1539–46.
Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi:10.1111/j.1365-2184.1970.tb00347.x.
Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14. doi:10.1080/14653240310004539.
Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53(2):227–46. doi:10.1194/jlr.R021089.
Cousin B, Andre M, Arnaud E, Penicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun. 2003;301(4):1016–22. doi:10.1016/S0006-291X(03)00061-5.
Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115(5):957–64. doi:10.1182/blood-2009-05-219923.
McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24(5):1246–53. doi:10.1634/stemcells.2005-0235.
Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry Part A: J Int Soc Anal Cytol. 2010;77(1):22–30. doi:10.1002/cyto.a.20813.
Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16(2):169–75. doi:10.1111/j.1524-4741.2009.00873.x.
Sterodimas A, de Faria J, Nicaretta B, Boriani F. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J. 2011;31(6):682–93. doi:10.1177/1090820x11415976.
Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32(1):48–55. doi:10.1007/s00266-007-9019-4.
Rigotti G, Marchi A, Galie M, Baroni G, Benati D, Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409–22. doi:10.1097/01.prs.0000256047.47909.71.
Riordan NH, Ichim TE, Min WP, Wang H, Solano F, Lara F, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi:10.1186/1479-5876-7-29.
Michalek J, Moster R, Lukac L, Proefrock K, Petrasovic M, Rybar J, et al. Autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transplant. 2015. doi:10.3727/096368915x686760.
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8. doi:10.1016/j.jcyt.2013.02.006.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. doi:10.1091/mbc.E02-02-0105.
Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–9. doi:10.1080/14653240310003026.
Ashjian PH, Elbarbary AS, Edmonds B, DeUgarte D, Zhu M, Zuk PA, et al. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg. 2003;111(6):1922–31. doi:10.1097/01.prs.0000055043.62589.05.
Banerjee A, Nurnberger S, Hennerbichler S, Riedl S, Schuh CM, Hacobian A, et al. In toto differentiation of human amniotic membrane towards the Schwann cell lineage. Cell Tissue Bank. 2014;15(2):227–39. doi:10.1007/s10561-013-9401-1.
Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16(3):1131–41. doi:10.1091/mbc.E04-10-0949.
Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294(2):371–9. doi:10.1016/s0006-291x(02)00469-2.
Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94(2):223–9. doi:10.1161/01.res.0000109792.43271.47.
Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75(3):775–9.
Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110(3):349–55. doi:10.1161/01.cir.0000135466.16823.d0.
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–63. doi:10.1161/01.cir.0000114522.38265.61.
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8. doi:10.1161/01.cir.0000121425.42966.f1.
Al Battah F, De Kock J, Vanhaecke T, Rogiers V. Current status of human adipose-derived stem cells: differentiation into hepatocyte-like cells. TheScientificWorldJOURNAL. 2011;11:1568–81. doi:10.1100/tsw.2011.146.
Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328(1):258–64. doi:10.1016/j.bbrc.2004.12.158.
Baer PC. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011;20(10):1805–16. doi:10.1089/scd.2011.0086.
Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330(1):142–50. doi:10.1016/j.bbrc.2005.02.141.
Vossmerbaeumer U, Ohnesorge S, Kuehl S, Haapalahti M, Kluter H, Jonas JB, et al. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 2009;11(2):177–88. doi:10.1080/14653240802714819.
Du Y, Roh DS, Funderburgh ML, Mann MM, Marra KG, Rubin JP, et al. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680–9.
Ferro F, Spelat R, Falini G, Gallelli A, D’Aurizio F, Puppato E, et al. Adipose tissue-derived stem cell in vitro differentiation in a three-dimensional dental bud structure. Am J Pathol. 2011;178(5):2299–310. doi:10.1016/j.ajpath.2011.01.055.
Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5(2):103–10. doi:10.2174/157488810791268564.
Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11(57):160–70.
Frazier TP, Gimble JM, Kheterpal I, Rowan BG. Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie. 2013;95(12):2286–96. doi:10.1016/j.biochi.2013.07.011.
Moon KM, Park YH, Lee JS, Chae YB, Kim MM, Kim DS, et al. The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int J Mol Sci. 2012;13(1):1239–57. doi:10.3390/ijms13011239.
Kutten JC, McGovern D, Hobson CM, Luffy SA, Nieponice A, Tobita K, et al. Decellularized tracheal extracellular matrix supports epithelial migration, differentiation, and function. Tissue Eng A. 2015;21(1–2):75–84. doi:10.1089/ten.TEA.2014.0089.
Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair. Society. 2014;22(3):313–25. doi:10.1111/wrr.12173.
Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212(3):702–9. doi:10.1002/jcp.21068.
Kronsteiner B, Peterbauer-Scherb A, Grillari-Voglauer R, Redl H, Gabriel C, van Griensven M, et al. Human mesenchymal stem cells and renal tubular epithelial cells differentially influence monocyte-derived dendritic cell differentiation and maturation. Cell Immunol. 2011;267(1):30–8. doi:10.1016/j.cellimm.2010.11.001.
Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13(6):1173–83. doi:10.1089/ten.2006.0313.
Wolbank S, Stadler G, Peterbauer A, Gillich A, Karbiener M, Streubel B, et al. Telomerase immortalized human amnion- and adipose-derived mesenchymal stem cells: maintenance of differentiation and immunomodulatory characteristics. Tissue Eng A. 2009;15(7):1843–54. doi:10.1089/ten.tea.2008.0205.
Kronsteiner B, Wolbank S, Peterbauer A, Hackl C, Redl H, van Griensven M, et al. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011;20(12):2115–26. doi:10.1089/scd.2011.0031.
Cho YB, Park KJ, Yoon SN, Song KH, Kim DS, Jung SH et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015. doi:10.5966/sctm.2014-0199.
Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129(6):1277–90. doi:10.1097/PRS.0b013e31824ecae6.
Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30(5):804–10. doi:10.1002/stem.1076.
Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1(2):19. doi:10.1186/scrt19.
Sergeevicheva V, Kruchkova I, Chernykh E, Shevela E, Kulagin A, Gilevich A, et al. Rapid recovery from chronic PRCA by MSC infusion in patient after major ABO-mismatched alloSCT. Case Rep Med. 2012;2012:862721. doi:10.1155/2012/862721.
Ra JC, Kang SK, Shin IS, Park HG, Joo SA, Kim JG, et al. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J Transl Med. 2011;9:181. doi:10.1186/1479-5876-9-181.
Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245–57. doi:10.1016/j.jcyt.2013.11.011.
Sandor GK, Numminen J, Wolff J, Thesleff T, Miettinen A, Tuovinen VJ, et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med. 2014;3(4):530–40. doi:10.5966/sctm.2013-0173.
Thesleff T, Lehtimaki K, Niskakangas T, Mannerstrom B, Miettinen S, Suuronen R, et al. Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68(6):1535–40. doi:10.1227/NEU.0b013e31820ee24e.
Tanikawa DY, Aguena M, Bueno DF, Passos-Bueno MR, Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;132(1):141–52. doi:10.1097/PRS.0b013e3182910a82.
Zuk P. Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells. 2013;2013:35. doi:10.1155/2013/713959.
Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56(2):289–301.
EudraLex. Clinical trial guidelines. 2010 (Volume 10).
EudraLex. Good manufacturing practice (GMP). 2015 (Volume 4).
Aarya Hari SG. Production of good manufacturing practice grade equine adiposederived mesenchymal stem cells for therapeutic use. J Stem Cell Res Ther. 2013;03(05):2157–7633. doi:10.4172/2157-7633.1000154.
Sensebe L, Gadelorge M, Fleury-Cappellesso S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: a review. Stem Cell Res Ther. 2013;4(3):66. doi:10.1186/scrt217.
Carvalho PP, Gimble JM, Dias IR, Gomes ME, Reis RL. Xenofree enzymatic products for the isolation of human adipose-derived stromal/stem cells. Tissue Eng Part C Meth. 2013;19(6):473–8. doi:10.1089/ten.TEC.2012.0465.
Patrikoski M, Juntunen M, Boucher S, Campbell A, Vemuri MC, Mannerstrom B, et al. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013;4(2):27. doi:10.1186/scrt175.
Aguena M, Fanganiello RD, Tissiani LA, Ishiy FA, Atique R, Alonso N, et al. Optimization of parameters for a more efficient use of adipose-derived stem cells in regenerative medicine therapies. Stem Cells Int. 2012;2012:303610. doi:10.1155/2012/303610.
Yang XF, He X, He J, Zhang LH, Su XJ, Dong ZY, et al. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci. 2011;18:59. doi:10.1186/1423-0127-18-59.
Markarian CF, Frey GZ, Silveira MD, Chem EM, Milani AR, Ely PB, et al. Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett. 2014;36(4):693–702. doi:10.1007/s10529-013-1425-x.
Philips BJ, Marra KG, Rubin JP. Adipose stem cell-based soft tissue regeneration. Expert Opin Biol Ther. 2012;12(2):155–63. doi:10.1517/14712598.2012.644533.
Thirumala S, Gimble JM, Devireddy RV. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med. 2010;4(3):224–32. doi:10.1002/term.232.
Guven S, Karagianni M, Schwalbe M, Schreiner S, Farhadi J, Bula S, et al. Validation of an automated procedure to isolate human adipose tissue-derived cells by using the Sepax(R) technology. Tissue Eng Part C Methods. 2012;18(8):575–82. doi:10.1089/ten.TEC.2011.0617.
Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, Tamura T, et al. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10(4):417–26. doi:10.1080/14653240801982979.
Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25(7):1823–9. doi:10.1634/stemcells.2007-0124.
Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17(6):1053–63. doi:10.1089/scd.2008.0117.
Eom YW, Lee JE, Yang MS, Jang IK, Kim HE, Lee DH, et al. Rapid isolation of adipose tissue-derived stem cells by the storage of lipoaspirates. Yonsei Med J. 2011;52(6):999–1007. doi:10.3349/ymj.2011.52.6.999.
Suga H, Shigeura T, Matsumoto D, Inoue K, Kato H, Aoi N, et al. Rapid expansion of human adipose-derived stromal cells preserving multipotency. Cytotherapy. 2007;9(8):738–45. doi:10.1080/14653240701679873.
Safwani WK, Makpol S, Sathapan S, Chua KH. Alteration of gene expression levels during osteogenic induction of human adipose derived stem cells in long-term culture. Cell Tissue Bank. 2013;14(2):289–301. doi:10.1007/s10561-012-9309-1.
Kirkpatrick CJ, Melzner I, Goller T. Comparative effects of trypsin, collagenase and mechanical harvesting on cell membrane lipids studied in monolayer-cultured endothelial cells and a green monkey kidney cell line. Biochim Biophys Acta. 1985;846(1):120–6.
Stadler G, Hennerbichler S, Lindenmair A, Peterbauer A, Hofer K, van Griensven M, et al. Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy. 2008;10(7):743–52. doi:10.1080/14653240802345804.
Kakagia D, Pallua N. Autologous fat grafting: in search of the optimal technique. Surg Innov. 2014;21(3):327–36. doi:10.1177/1553350613518846.
Garza RM, Paik KJ, Chung MT, Duscher D, Gurtner GC, Longaker MT, et al. Studies in fat grafting: part III. Fat grafting irradiated tissue—improved skin quality and decreased fat graft retention. Plast Reconstr Surg. 2014;134(2):249–57. doi:10.1097/prs.0000000000000326.
Stillaert FB, Di Bartolo C, Hunt JA, Rhodes NP, Tognana E, Monstrey S, et al. Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds. Biomaterials. 2008;29(29):3953–9. doi:10.1016/j.biomaterials.2008.06.005.
Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12(12):3375–82. doi:10.1089/ten.2006.12.3375.
Holnthoner W, Hohenegger K, Husa AM, Muehleder S, Meinl A, Peterbauer-Scherb A, et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med. 2015;9(2):127–36. doi:10.1002/term.1620.
Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Curcio CB, et al. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1(4):341–51. doi:10.5966/sctm.2011-0065.
Jiang A, Li M, Duan W, Dong Y, Wang Y. Improvement of the survival of human autologous fat transplantation by adipose-derived stem-cells-assisted lipotransfer combined with bFGF. TheScientificWorldJOURNAL. 2015;2015:968057. doi:10.1155/2015/968057.
Luo S, Hao L, Li X, Yu D, Diao Z, Ren L, et al. Adipose tissue-derived stem cells treated with estradiol enhance survival of autologous fat transplants. Tohoku J Exp Med. 2013;231(2):101–10.
Li L, Pan S, Ni B, Lin Y. Improvement in autologous human fat transplant survival with SVF plus VEGF-PLA nano-sustained release microspheres. Cell Biol Int. 2014;38(8):962–70. doi:10.1002/cbin.10284.
Van Pham P, Hong-Thien Bui K, Quoc Ngo D, Tan Khuat L, Kim PN. Transplantation of nonexpanded adipose stromal vascular fraction and platelet-rich plasma for articular cartilage injury treatment in mice model. J Med Eng. 2013;2013:7. doi:10.1155/2013/832396.
Paspaliaris B, Thornton JAF. Methods and apparatuses for isolating and preparing stem cells. 2014. US 20140093482 A1.
Tzouvelekis A, Koliakos G, Ntolios P, Baira I, Bouros E, Oikonomou A, et al. Stem cell therapy for idiopathic pulmonary fibrosis: a protocol proposal. J Transl Med. 2011;9:182. doi:10.1186/1479-5876-9-182.
Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11:171. doi:10.1186/1479-5876-11-171.
Aronowitz JA, Ellenhorn JD. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg. 2013;132(6):932e–9. doi:10.1097/PRS.0b013e3182a80652.
Kakudo N, Tanaka Y, Morimoto N, Ogawa T, Kushida S, Hara T, et al. Adipose-derived regenerative cell (ADRC)-enriched fat grafting: optimal cell concentration and effects on grafted fat characteristics. J Transl Med. 2013;11:254. doi:10.1186/1479-5876-11-254.
Granel B, Daumas A, Jouve E, Harle JR, Nguyen PS, Chabannon C, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis. 2014. doi:10.1136/annrheumdis-2014-205681.
Gotoh M, Yamamoto T, Kato M, Majima T, Toriyama K, Kamei Y, et al. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose-derived regenerative cells: 1-year outcomes in 11 patients. Int J Urol. 2014;21(3):294–300. doi:10.1111/iju.12266.
Perez-Cano R, Vranckx JJ, Lasso JM, Calabrese C, Merck B, Milstein AM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol. 2012;38(5):382–9. doi:10.1016/j.ejso.2012.02.178.
Dos-Anjos Vilaboa S, Navarro-Palou M, Llull R. Age influence on stromal vascular fraction cell yield obtained from human lipoaspirates. Cytotherapy. 2014;16(8):1092–7. doi:10.1016/j.jcyt.2014.02.007.
Pak J, Lee JH, Lee SH. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. BioMed Res Int. 2014;2014:436029. doi:10.1155/2014/436029.
Pak J, Chang JJ, Lee JH, Lee SH. Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet Disord. 2013;14:337. doi:10.1186/1471-2474-14-337.
Khan ZA, Dulgar-Tulloch AJ, Rakuff S, Shoemaker PA, Kvam EL, Chen X, et al. Automated systems and methods for isolating regenerative cells from adipose tissue. 2012. US 20120276628 A1.
Kaengkan P, Baek SE, Kim JY, Kam KY, Do BR, Lee ES, et al. Administration of mesenchymal stem cells and ziprasidone enhanced amelioration of ischemic brain damage in rats. Mol Cells. 2013;36(6):534–41. doi:10.1007/s10059-013-0235-2.
Stubbers R, Coleman ME. Apparatus and methods for cell isolation. 2015. US 20150056691 A1.
Doi K, Tanaka S, Iida H, Eto H, Kato H, Aoi N, et al. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis. J Tissue Eng Regen Med. 2013;7(11):864–70. doi:10.1002/term.1478.
Domenis R, Lazzaro L, Calabrese S, Mangoni D, Gallelli A, Bourkoula E, et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther. 2015;6(1):2. doi:10.1186/scrt536.
Schafer ME, Hicok KC, Mills DC, Cohen SR, Chao JJ. Acute adipocyte viability after third-generation ultrasound-assisted liposuction. Aesthet Surg J Am Soc Aesthet Plast Surg. 2013;33(5):698–704. doi:10.1177/1090820x13485239.
Zhu M, Cohen SR, Hicok KC, Shanahan RK, Strem BM, Yu JC, et al. Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg. 2013;131(4):873–80. doi:10.1097/PRS.0b013e31828276e9.
Ansorge H, Garza JR, McCormack MC, Leamy P, Roesch S, Barere A, et al. Autologous fat processing via the Revolve system: quality and quantity of fat retention evaluated in an animal model. Aesthet Surg J Am Soc Aesthet Plast Sur. 2014;34(3):438–47. doi:10.1177/1090820x14524416.
Dos-Anjos Vilaboa S, Llull R, Mendel TA. Returning fat grafts to physiologic conditions using washing. Plast Reconstr Surg. 2013;132(2):323e–6. doi:10.1097/PRS.0b013e3182958be1.
Zimmerlin L, Rubin JP, Pfeifer ME, Moore LR, Donnenberg VS, Donnenberg AD. Human adipose stromal vascular cell delivery in a fibrin spray. Cytotherapy. 2013;15(1):102–8. doi:10.1016/j.jcyt.2012.10.009.
Ferguson RE, Cui X, Fink BF, Vasconez HC, Pu LL. The viability of autologous fat grafts harvested with the LipiVage system: a comparative study. Ann Plast Surg. 2008;60(5):594–7. doi:10.1097/SAP.0b013e31817433c5.
Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063–77. doi:10.3727/096368912X657855.
García-Contreras M, Messaggio F, Jimenez O, Mendez A. Differences in exosome content of human adipose tissue processed by non-enzymatic and enzymatic methods. CellR4. 2015;3(1):e1423.
Bianchi F, Olivi E, Baldassarre M, Giannone FA, Laggetta M, Valente S, et al. Lipogems, a new modality of fat tissue handling to enhance tissue repair in chronic hind limb ischemia. CellR4. 2014;2(6):e1289.
Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, et al. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: a novel approach to multipotency. Cell Transplant. 2014;23(12):1489–500. doi:10.3727/096368913x672037.
Carelli S, Messaggio F, Canazza A, Hebda DM, Caremoli F, Latorre E, et al. Characteristics and properties of mesenchymal stem cells derived from micro-fragmented adipose tissue. Cell Transplant. 2014. doi:10.3727/096368914x681603.
Raffaini M, Tremolada C. Micro fractured and purified adipose tissue graft (Lipogems®) can improve the orthognathic surgery outcomes both aesthetically and in postoperative healing. CellR4. 2014;2(0):e1118.
Benzi R, Marfia G, Bosetti M, Beltrami G, Magri AS, Versari S et al. Microfractured lipoaspirate may help oral bone and soft tissue regeneration: a case report. CellR4. 2015;3(3):e1583.
Giori A, Tremolada C, Vailati R, Navone SE, Marfia G, Caplan AI. Recovery of function in anal incontinence after micro-fragmented fat graft (Lipogems®) injection: two years follow up of the first 5 cases. CellR4. 2015;3(2):e1544.
Hu CB, Myers KE, Peterson RC. Devices for harvesting and homogenizing adipose tissue containing autologous endothelial cells. 2000. US 6020196 A1.
Victor S. Isolation of stromal vascular fraction from adipose tissue obtained from postmortem source using ultrasonic cavitation. 2014. WO 2014015229 A1.
Bright R, Bright P, Hansen B, Thomas W. Isolation of stem cells from adipose tissue by ultrasonic cavitation, and methods of use. 2014. WO 2014000031 A1.
Bright R, Bright M, Bright P, Hayne S, Thomas WD. Migraine and tension-type headache treated with stromal vascular fraction: a case series. J Med Case Rep. 2014;8:237. doi:10.1186/1752-1947-8-237.
Schafer ME. Selective lysing of cells using ultrasound. 2013. US 20130012927 A1.
Gimble JM, Shah FS, Wu X. Non-enzymatic method for isolating human adipose-derived stromal stem cells. 2014. US 20140017783 A1.
Michalek J. Method for isolation of adipose tissue-derived stromal vascular fraction cells. 2014. WO 2014169885 A1.
Hicok KC, Hedrick MH. Automated isolation and processing of adipose-derived stem and regenerative cells. Methods Mol Biol (Clifton, NJ). 2011;702:87–105. doi:10.1007/978-1-61737-960-4_8.
Peterson A, Fornace L. Systems, methods and compositions for optimizing tissue and cell enriched grafts. 2010. US 20100279405 A1.
Hedrick MH, Fraser JK, Schulzki MJ, Byrnes B, Carlson G, Schreiber RE, et al. Systems and methods for isolating and using clinically safe adipose derived regenerative cells. 2012. US 20120264200 A1.
Kakudo N, Morimoto N, Ogawa T, Kusumoto K. Potential of adipose-derived stem cells for regeneration medicine: clinical application and usefulness of fat grafting. J Stem Cell Res Ther. 2014;4(204):2.
Sanchez PL, Sanz-Ruiz R, Fernandez-Santos ME, Fernandez-Aviles F. Cultured and freshly isolated adipose tissue-derived cells: fat years for cardiac stem cell therapy. Eur Heart J. 2010;31(4):394–7. doi:10.1093/eurheartj/ehp403.
Cimino WW, Llull R, Katz AJ. Tissue processing apparatus and method for processing adipose tissue. 2015. WO 2015035221 A1.
Llull R, Katz AJ, Cimino WW. Method for processing adipose tissue and processing apparatus. 2013. WO 2013106655 A1.
Do BR, Lee JK, Kim JH, Pak SH, Shin BS. Peristaltic pump, and regenerative cell extraction system using same. 2013. WO 2013089481 A1.
Raj SS, Gopal V, Priya N, Krishnegowda B, Thiruvampattil P, Majumdar AS, et al. System for isolating stromal vascular fraction (svf) cells from the adipose tissue and a method thereof. 2015. US 20150004702 A1.
Williams SK, Kosnik P, England C, Cannon TF, Vossman E, Boland E, et al. Apparatus and methods for preparing tissue grafts. 2007. WO 2007009036 A9.
Wolters R, Yang A, NELSON J, Williams SK, et al. Hand-held micro-liposuction adipose harvester, processor, and cell concentrator. 2014. WO 2014047368 A1.
Ariff GD, Cannon T, Case JL, Haller CL, Kosnik P, Luddy CP, et al. Cell separation apparatus and methods of use. 2008. WO 2008133874 A1.
SHIGEKI S, KIAT OW. Method for isolating stromal vascular fraction. 2015. WO 2015005871 A1.
Buss B. Autologous tissue harvesting and irrigation device. 2012. US 8202493 A1.
Kensy A, Winkler KW. Procedure and device for separating adult stem cells from fatty tissue. 2014. US 8628950 A1.
Winkler KW, Matthiesen ID. Device for separating adult stem cells. 2013. EP 2677024 A1.
Matthiesen I, Winkler KW. Vorrichtung zum Separieren von adulten Stammzellen. 2013. DE 102013209718 A1.
Victor S. Ultrasonic cavitation derived stromal or mesenchymal vascular extracts and cells derived therefrom obtained from adipose tissue and use thereof. 2013. US 20130189234 A1.
Victor S. Isolation of stromal vascular fraction from vascular tissues. 2014. WO 2014138383 A1.
Cimino WW, Katz AJ, Llull R. Apparatus and methods relating to collecting and processing human biological material containing adipose. 2012. WO 2012006587 A2.
Vossman E, Iwami S, Yang A, Cannon T, Paek HJ. Adipose tissue collection and pre-processing devices for use in liposuction procedure. 2014. US 20140193852 A9.
Tremolada C. Device and method for preparing tissue, particularly adipose tissue. 2013. US 20130123747 A1.
Chapman JR, Sparks R. Apparatus for centrifugation and methods therefore. 2013. WO 2013122683 A1.
Shah FS, Wu X, Dietrich M, Rood J, Gimble JM. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy. 2013;15(8):979–85. doi:10.1016/j.jcyt.2013.04.001.
Acknowledgements
This work was funded by grant from the Austrian Research Promotion Agency (FFG) (Bridge1 program, grant no. 4694564).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
HR is a cofounder of LipoRegena company. The other authors declare that they have no competing interests.
Authors’ contributions
EO and CS made substantial contributions to conception and design and drafted the manuscript. EO, CS, and CW enquired all systems listed in Tables 1 and 2. CG, HR, and SW have given final approval and revised the review critically for important intellectual content. All authors read and approved the final manuscript.
Eleni Oberbauer and Carolin Steffenhagen contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oberbauer, E., Steffenhagen, C., Wurzer, C. et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen 4, 7 (2015). https://doi.org/10.1186/s13619-015-0020-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13619-015-0020-0