Abstract
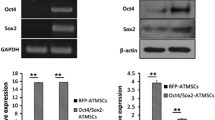
Adipose tissue is a source of multipotent stem cells and it has the ability to differentiate into several types of cell lineages such as neuron cells, osteogenic and adipogenic cells. Most studies on human adipose-derived stem cells (ASCs) have been carried out at the early passages. For clinical usage, ASCs need to be expanded in vitro for a period of time to get sufficient cells for transplantation into patients. However, the impact of long-term culture on ASCs molecular characteristics has not been established yet. Several studies have also shown that osteogenic and adipogenic cells have the ability to switch pathways during in vitro culture as they share the same progenitor cells. This data is important to ensure their functionality and efficacy before being used clinically in the treatment of bone diseases. Therefore, we aim to investigate the effect of long-term culture on the adipogenic, stemness and osteogenic genes expression during osteogenic induction of ASCs. In this study, the molecular characteristics of ASCs during osteogenic induction in long-term culture was analysed by observing their morphological changes during induction, analysis of cell mineralization using Alizarin Red staining and gene expression changes using quantitative RT-PCR. Morphologically, cell mineralization at P20 was less compared to P5, P10 and P15. Adipogenesis was not observed as negative lipid droplets formation was recorded during induction. The quantitative PCR data showed that adipogenic genes expression e.g. LPL and AP2 decreased but PPAR-γ was increased after osteogenic induction in long-term culture. Most stemness genes decreased at P5 and P10 but showed no significant changes at P15 and P20. While most osteogenic genes increased after osteogenic induction at all passages. When compared among passages after induction, Runx showed a significant increased at P20 while BSP, OSP and ALP decreased at later passage (P15 and P20). During long-term culture, ASCs were only able to differentiate into immature osteogenic cells.






Similar content being viewed by others
References
Akune T, Ohba S, Kamekura S et al (2004) PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855
Avilion AA, Nicolis SK, Pevny LH et al (2003) Multipotent cell lineages in early mouse development depend on Sox2 function. Genes Dev 17:126–140
Caetano-Lopes J, Canhao H, Fonseca JE (2007) Osteoblasts and bone formation. Acta Reum Port 32:103–110
Casteilla L, Dani C (2006) Adipose tissue-derived cells: from physiology to regenerative medicine. Diabetes Metab 32:393–401
Chambers I, Colby D, Robertson M et al (2003) Functional expression cloning of nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643–655
DiGirolamo CM, Stokes D, Colter D et al (1999) Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol 107:275
Ding J, Ghali O, Lencel P et al (2009) TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci 84(15–16):499–504
Hasegawa T, Oizumi K, Yoshiko Y et al (2008) The PPAR gamma-selective ligand BRL-49653 differentially regulates the fate choices of rat calvaria versus rat bone marrow stromal cell populations. BMC Dev Biol 8:71
Hayashi O, Katsube Y, Hirose M et al (2008) Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum and adipose tissue. Calcif Tissue Int 82:238–247
Hyslop L, Stojkovic M, Armstrong L et al (2005) Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells 23:1035–1043
Kulterer B, Friedl G, Jandrositz A et al (2007) Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomic 8:70
Liu TM, Martina M, Hutmacher DW et al (2006) Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25:750–760
Mackie EJ (2003) Osteoblasts: novel roles in orchestration of skeletal architecture. Int J Biochem Cell Biol 35:1301–1305
Michalik L, Auwerx J, Berger JP et al (2006) International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58:726–741
Mimeault M, Hauke R, Batra SK et al (2007) Stem cells: a revolution in therapeutics—recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Nature 82(3):252–264
Mitsui K, Tokuzawa Y, Itoh H et al (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642
Monfoulet L, Malaval L, Aubin JE et al (2009) Bone sialoprotein, but not osteopontin, deficiency impairs the mineralization of regenerating bone during cortical defect healing. Bone 46(2):447–452
Oedayrajsingh-Varma MJ, Van Ham SM, Kippenberg M et al (2006) Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8(2):166–177
Ogawa S, Urano T, Hosoi T et al (1999) Association of bone mineral density with a polymorphism of the peroxisome proliferator-activated receptor γ gene: PPARγ expression in osteoblasts. Biochem Biophys Res Commun 260:122–126
Parker AM, Katz AJ (2006) Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin Biol Ther 6(6):567–578
Strem BM, Hicok KC, Zhu M et al (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54(3):132–141
Wall ME, Bernacki SH, Loboa EG (2007) Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng 13(6):1291–1298
Wan Kamarul Zaman WS, Makpol S, Santhapan S Chua KH (2008) Stemness gene expression profile of human adipose derived stem cells in long term culture. Med J Malays 63(Supplement A):61–62
Weinzierl K, Hemprich A, Frerich B (2006) Bone engineering with adipose tissue derived stromal cells. J Cranio-Max Surg 34:466–471
Wilschut KJ, Jaksani S, Van Den Dolder J et al (2008) Isolation and characterization of procine adult muscle-derived progenitor cells. J Cell Biochem 105:1228–1239
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Acknowledgments
We thank the Ministry of Science, Technology & Innovation of Malaysia (MOSTI) for financial support. Grant No: 02-01-02 SF0290.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safwani, W.K.Z.W., Makpol, S., Sathapan, S. et al. Alteration of gene expression levels during osteogenic induction of human adipose derived stem cells in long-term culture. Cell Tissue Bank 14, 289–301 (2013). https://doi.org/10.1007/s10561-012-9309-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-012-9309-1