Abstract
Veno-venous extracorporeal membrane oxygenation (ECMO) is a helpful intervention in patients with severe refractory hypoxemia either because mechanical ventilation cannot ensure adequate oxygenation or because lung protective ventilation is not feasible. Since ECMO is a highly invasive procedure with several, potentially devastating complications and its implementation is complex and expensive, simpler and less invasive therapeutic options should be first exploited. Low tidal volume and driving pressure ventilation, prone position, neuromuscular blocking agents and individualized ventilation based on transpulmonary pressure measurements have been demonstrated to successfully treat the vast majority of mechanically ventilated patients with severe hypoxemia. Veno-venous ECMO has a place in the small portion of severely hypoxemic patients in whom these strategies fail. A combined analysis of recent ARDS trials revealed that ECMO was used in only 2.15% of patients (n = 145/6736). Nevertheless, ECMO use has sharply increased in the last decade, raising questions regarding its thoughtful use. Such a policy could be harmful both for patients as well as for the ECMO technique itself. This narrative review attempts to describe together the practical approaches that can be offered to the sickest patients before going to ECMO, as well as the rationale and the limitations of ECMO. The benefit and the drawbacks associated with ECMO use along with a direct comparison with less invasive therapeutic strategies will be analyzed.
Similar content being viewed by others
Introduction
Mechanical ventilation (MV) constitutes one of the most researched and evolving areas of intensive care. In no other era has MV been better understood and safer applied than in the current decade. Despite the substantial progress, the harmful effects of MV cannot be erased and in the most difficult to ventilate patients, veno-venous extracorporeal membrane oxygenation (ECMO) is a promising intervention.
ECMO provides gas exchange via an extracorporeal circuit. The idea is not new; from 1966 to 1979, ECMO was used in several hundreds of patients with acute respiratory failure [1, 2]. The technology was rather crude at that time and the first randomized trial by Zapol et al. demonstrated no benefit at all, along with major complications, mostly bleeding [3]. These results sidelined the use of ECMO for respiratory failure for several years. It continued to be applied mostly in severe hypoxemia in neonates since the mid-1970s [4]. A renewed impetus in ECMO use for acute respiratory failure arose with the H1N1 flu epidemic in 2008–2009 followed by two randomized clinical trials. Since then, interest in ECMO has risen exponentially.
There are various configurations of ECMO, depending on whether the aim is to support the lungs, the heart, or both. In this narrative review, we will focus on the use of veno-venous ECMO (v-v ECMO) for acute hypoxemic respiratory failure. This review attempts to describe together the practical approaches that can be offered to the sickest patients before going to ECMO, as well as the rationale and the limitations of ECMO. This corresponds to the needs of clinicians taking care of these patients.
Veno-venous ECMO: technique and physiology of gas exchange
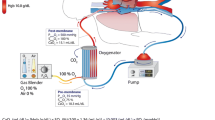
Figure 1 illustrates the working principle of v-v ECMO. ECMO draws blood from the venous system, enriches it with oxygen, removes carbon dioxide (CO2), and returns the final product again to the venous circulation. Blood is withdrawn via a central venous catheter and is subsequently propelled to a membrane oxygenator [5]. Gas with a certain amount of inspired fraction of oxygen (FiO2) is pumped into the membrane oxygenator, where gas exchange takes place: CO2 diffuses from the venous blood to the gas, while oxygen leaves the gas to saturate the hemoglobin (Hb) of the venous blood. CO2 is continuously removed from the membrane oxygenator through an exit port [5]. Haldane and Bohr effects play a significant role in this process.
Illustration of the ECMO technique and physiology of gas exchange (see main text for a detailed description). Venous blood is drawn via a central venous catheter inserted in the femoral vein, which is propelled at a set extracorporeal blood flow (ECBF) rate to a membrane oxygenator where gas exchange takes place. Gas with a user-adjusted fraction of O2 enters the oxygenator in order to saturate the hemoglobin of the venous blood. CO2 diffuses from the venous blood to the gas and leaves the oxygenator via an exit port. Heated and humidified oxygenated blood is then returned to the venous circulation via the jugular vein. V’O2 ECMO reflects the amount of oxygen delivered by the ECMO system and is depending on the ECBF and the oxygen content in the blood before (CvO2INLET) and after (CvO2OUTLET) the membrane oxygenator, respectively. White arrows in the subclavian veins and vena cava indicate native venous return; the oxygen content of the native system (CvO2NATIVE) is mixed with the oxygenated blood from the ECMO system. The arterial oxygen content of the mixed blood (CvO2) is described by Equation 2 and depends on the ratio of ECBF to cardiac output (CO2). CO, cardiac output; CO2, carbon dioxide; CvO2, oxygen content in venous blood; ECBF, extracorporeal blood flow; O2, oxygen
Oxygenation
While arterio-venous ECMO improves both arterial oxygen content (CaO2) and cardiac output (CO), v-v ECMO affects only CaO2. The amount of oxygen delivered by ECMO to the extracorporeal blood (V′O2ECMO, ml/min) is described by the following equation:
where ECBF is extracorporeal blood flow in dl/min and CvO2inlet and CvO2outlet are the oxygen content in the venous blood before and after the membrane oxygenator, respectively, (ml O2/dl of whole blood) [5].
The ECBF, externally regulated on the ECMO module interface, is the most significant factor affecting oxygenation in ECMO. Resistance to blood flow can diminish the maximum ECBF that can be achieved and is related to catheter characteristics (length, diameter, site of the drainage), cardiac output, and membrane oxygenator function. The CaO2outlet can be increased by increasing FiO2 in the sweep gas or by raising the Hb.
When two systems, ECMO and native venous return, provide blood of different concentrations of O2 at different flows rates, the arterial oxygen content in the mixed blood (CvO2) is the average of the amount of oxygen in each of the two systems [6]:
where CO indicates cardiac output and CvO2native the oxygen content of the native venous return. Equation (2) dictates that the ECBF/CO ratio is crucial: the higher the ratio, the higher the C\(v\)O2 and the oxygen saturation (SvO2) and partial pressure of O2 in the mixed venous blood entering the pulmonary artery. For ECBF to be sufficiently high to correct severe hypoxemia, large cannulas are required. Two factors could limit the efficiency of gas exchange correction. Firstly, high C\(v\)O2 and SvO2 in the pulmonary circulation offered by ECMO globally tend to increase oxygenation, but paradoxically reduce or eliminate hypoxic pulmonary vasoconstriction, which increases pulmonary shunt [7]. The vasoconstriction of pulmonary vessels in the presence of hypoxia, constitutes an important protective physiological reflex, that improves ventilation–perfusion and shunt by redirecting blood flow away from hypoxic areas of the lungs to better ventilated and oxygenated regions [8]. This is why there is a kind of competition between the oxygenated blood flow brought by ECMO and the native blood flow. This explains why ECMO blood flow needs to be very high compared to native blood flow. Secondly, a fraction of oxygenated blood from ECMO re-enters the membrane oxygenator without prior mixing with systemic circulation (recirculation) and may contribute to hypoxemia [9]. Both factors explain why increasing oxygen delivery from ECMO has less than predicted impact on PaO2 and SaO2.
CO2 removal during ECMO
CO2 removal during ECMO depends on the sweep gas flow rate and the ECMO blood flow rate. Increases in sweep gas flow rate results in faster CO2 clearance from the oxygenator and thus higher CO2 removal from the blood. As CO2 is more soluble and diffusible in blood than O2, decarboxylation is achieved faster than oxygenation for the same blood flow rate. Hence, oxygenation in ECMO mostly depends on the blood flow rate while decarboxylation is primarily regulated by the sweep gas flow rate. Notably, oxygenation and CO2 removal through ECMO are interrelated: CO2 removal and consequent alkalinization of blood enhances the oxygen uptake (Bohr effect), while oxygenation promotes CO2 removal from blood as oxygenated hemoglobin has decreased capacity to carry CO2 (Haldane effect) [10].
Indications and contraindications
The principal indications for v-v ECMO are acute, refractory hypoxemia, usually in the context of acute respiratory distress syndrome (ARDS) [9, 11,12,13,14,15,16]. In this scenario, v-v ECMO is applied whenever MV fails to ensure adequate oxygenation and/or when lung protective ventilation, with reasonable plateau and driving pressures is not feasible. Prone position should be offered first before considering ECMO. v-v ECMO is also used as a bridge to lung transplantation or after primary graft dysfunction following lung transplantation [17, 18]. It is contraindicated in patients with irreversible underlying lung disease who are not candidates for lung transplantation. Moreover, its use is discouraged in conditions precluding recovery or unacceptable functional outcome if the patient recovers (i.e., moribund state, metastatic malignancy, hypoxic brain injury, etc.).
Evidence of benefit from veno-venous ECMO
The modern ECMO machines have little to do with those used in late 1970s. Improvements in the efficacy, ease of use, and, more importantly, safety, accelerated the widespread use of ECMO during the H1N1-associated ARDS in 2009. In that same year, the results of the first major randomized controlled trial employing v-v ECMO for severe ARDS were published [15]. In the CESAR trial, 180 adults with severe ARDS were randomized to be either transferred to one single center with ECMO capability or to remain at any of the 68 hospitals in the UK where they were already receiving conventional management [15]. The primary composite endpoint, death or severe disability at six months, was significantly lower for patients randomized to receiving ECMO (37% vs 53%, p = 0.03), with an absolute risk reduction of 16%. In reality, the CESAR trial did not directly compare ECMO to no ECMO; 24% of patients in the ECMO group never received ECMO. Almost all of them (93%), however, underwent protocolized lung protective MV. Contrariwise, a lung protective ventilation strategy was not mandated for patients in the control group and only 70% did, at some point during their course, receive low-volume, low-pressure MV. Prone positioning was employed in only 42% of control and 37% of ECMO patients [19]. Hence, the CESAR trial did not conclusively prove that ECMO was effective, but did show the importance of lung protective specialized management in severe ARDS.
Recently, an international randomized controlled trial [ECMO to rescue Lung Injury in severe ARDS (EOLIA trial)] compared early v-v ECMO with standard lung protective ventilation in patients with severe ARDS [12]. Patients with severe ARDS receiving MV for less than 7 days were randomized to standard of care, including protocolized MV (n = 125) or to early ECMO (n = 124). The entry criteria were severe hypoxemia (PaO2/FiO2 < 50 or 80 mmHg for > 3 or > 6 h, respectively) or hypercapnia (PaCO2 ≥ 60 mmHg with a pH < 7.25 for > 6 h with the respiratory rate increased to 35 breaths/minute and ventilation settings adjusted to keep a plateau pressure of ≤ 32 cmH2O) despite ventilator optimization. The later included an FiO2 of ≥ 0.80, a tidal volume of 6 ml per kilogram of predicted body weight (PBW), and a positive end-expiratory pressure (PEEP) of ≥ 10 cmH2O. Neuromuscular blocking agents and prone position were strongly encouraged. Crossover from control to ECMO for refractory hypoxemia was allowed if the patients had refractory hypoxemia despite the use of available and feasible adjunctive therapies. Ultra-protective ventilation was applied to patients on ECMO with further tidal volume and respiratory rate reduction. The study failed to achieve the goal of 20% reduction in 60-day mortality and was terminated early for futility. Although not statistically significant, the 11% absolute reduction in mortality with ECMO was considered by many physicians as clinically important. Therefore, the results of the EOLIA trial were re-examined through the prism of Bayesian analysis, which combines a prior probability function (calculated from prior data and beliefs), with a likelihood function (calculated from new data) to create a posterior probability function [20, 21]. The latter is an updated summary of knowledge and the remaining uncertainty. Bayesian analysis of the EOLIA trial demonstrated that the posterior probability of any reduction in mortality with ECMO was very high (88–99%) and the probability of an absolute risk reduction ≥ 2% ranged from 78 to 98% [20].
Pooled mortality data from the CESAR and EOLIA trials and from three observational studies on v-v ECMO for severe ARDS (total of 773 patients), in which matching techniques were used, disclosed that ECMO was associated with a significant reduction in 60-day mortality compared with conventional ventilation (relative risk, 0.69; 95% CI 0.50–0.95) [22]. Nevertheless, there was a moderate risk of major 7hemorrhage and a low risk of cannula- and circuit-related complications.
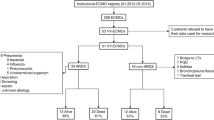
The reported use of ECMO in recent ARDS trials is presented in Table 1.
ECMO use in pandemics
During the global pandemic of influenza H1N1 in 2009–2010, collaborative networks from Australia and New Zealand (ANZICS), France (the REVA Network), Italy and the UK reported survival rates of 70% in patients with severe ARDS treated with ECMO [11, 13, 14, 16]. At the same time, however, other centers published equally auspicious outcomes without the use of ECMO [23]. Matched score analysis from the UK and REVA network presented inconsistent results in terms of mortality [13, 16].
The new pandemic of coronavirus disease 2019 (COVID-19) is complicated by ARDS at rates ranging from 5 to 42% [24, 25]. In the first retrospective studies of patients with severe COVID-19 who received ECMO, mortality was sometimes as high as 80–94% [24,25,26,27,28,29,30,31,32,33,34,35]. Mortality was noticeably lower (31–54%) in subsequent multicenter studies, conducted in younger patients [36,37,38,39,40]. In critically ill patients with COVID-19, it has been recently shown that presenting ICU physiology, but also hospital socioeconomic status, hospital capacity and strain contribute to significant interhospital variation in observed mortality [41]. This finding may also explain why studies report different outcomes for ECMO in COVID-19, especially patient factors. The first nationwide study, coming from Chile, demonstrated that, during the 1st wave of the pandemic, ECMO was employed in 14.89:100,000 patients with COVID-19 representing 0.42:100,000 population, and 1.2% of COVID-19 intubated patients) [42]. The 90-day mortality of ECMO supported COVID-19 patients was 38.8%, comparable to that reported for other indications of ECMO. Lower respiratory system compliance and higher driving pressure before ECMO were associated with higher mortality. Outcomes of studies investigating v-v ECMO in severe COVID-19 are presented in Table 2.
Recently, protection of the right heart with single-access, dual stage (right atrium-to-pulmonary artery cannulation) v-v ECMO or ECMO with right ventricular assist device yielded promising results in severe COVID-19 disease, but further studies are required to assess the value of this approach [30, 43].
Complications
Even with modern ECMO circuits, complications associated with the technique are manifold, can be devastating, and their incidence is not precisely known and probably underestimated, since they are not consistently reported across studies. These complications are broadly divided into mechanical (i.e., associated with device insertion or function) and medical (i.e., associated with either anticoagulation or the effect of ECMO on human organism) complications (Table 3).
Device-related complications
The commonest adverse events reported are bleeding at the site of cannulation (7.8%), vascular injury, insertion site infection and deep venous thrombosis (DVT). Cannulation of improper vessels, retroperitoneal injury, limb ischemia, air embolism, and, rarely, cardiac perforation during guidewire advancement may occur.
Circuit failure occurs in 13–25% of cases, most often due to clot formation within the circuit or oxygenator. Other reasons include oxygenator failure (5.9%), gas in the circuit and pump failure. The most dreadful circuit-related complications are massive gas embolism, resulting from extremely negative pressures between the drainage cannula and ECMO pump, and massive blood loss secondary to tubing disconnection or breach. ECMO necessitated exchange in 31% of 265 ARDS patients and in 45% of these cases the exchange was required on an urgent basis [44].
Medical complications
Bleeding and thrombosis
The exposure of blood to non-endothelial surfaces and high shear stress activates the adhesion of platelets, thrombin generation and clot formation in the cannula or circuit. Furthermore, the coagulation cascade is triggered by endothelial injury from intravascular catheters and blood flow disturbances. The true incidence of thrombosis is unknown. When systematically investigated, cannula associated DVT develops in 62–85% of patients undergoing v-v ECMO and with a 16% incidence of pulmonary embolism [45,46,47]. Systemic anticoagulation does not abolish the risk of thromboembolism.
Hemorrhage complicates the course of 16–50% of patients receiving v-v ECMO [48,49,50] with the commonest sites being the cannula site (13.2%), gastrointestinal tract (5.5%), lungs (6.1%) and central nervous system (3.9%) [48]. Hemorrhage is one of the leading causes of morbidity and mortality in patients undergoing ECMO [48, 51]. Ried et al. demonstrated a bleeding rate of 23.2% in v-v ECMO, with a mortality rate of 48.5% among these patients [51]. Very high rates of major bleeding requiring transfusion (43%) complicated the application of ECMO in a recently published large multicenter study of COVID-19 patients [39]. The etiology of ECMO-related bleeding is multifactorial and it is related to the circuit, the systemic anticoagulation and the patient. Thrombocytopenia has a considerable prevalence (up to 21%), regardless of the type of ECMO mode, and is commonly complicated with bleeding [50].
Hemolysis
Intravascular hemolysis occurs commonly under ECMO (5–18%) [52]. It is caused by shear stress and erythrocyte breakdown resulting from turbulent flow either within the circuit, due to partial obstruction from the thrombus, fibrin deposition in the pump and/or negative pressure in the access line or, less frequently, in the systemic circulation due to intravascular thrombosis. Released extracellular Hb causes vasoconstriction, endothelial dysfunction and platelet aggregation. Hemolysis is especially frequent at lower blood flow rates, such as those used for extracorporeal CO2 removal [53].
Neurologic complications
Approximately 7–9% of patients suffer from neurologic injury during v-v ECMO [54, 55].The clinical spectrum ranges from neurocognitive disturbances to seizures (1.1%), ischemic stroke (1.9%), intracranial hemorrhage (3.5%), and brain death (1.6%) [54]. The pathophysiology includes hypotension (cardiac arrest, shock, etc.), loss of cerebral autoregulation, large arterial pressure variations during ECMO, reperfusion injury, anticoagulation, and embolism. Brain hemorrhage and ischemic stroke are the most devastating neurologic complications associated with a mortality rate of 60–96% and 50–84%, respectively, and significant long-term functional impairment [56]. Intracerebral hemorrhage was reported as the commonest cause of death in H1N1 influenza patients treated with v-v ECMO [11, 57]. Intracerebral hemorrhage occurred in 12–13% of patients with COVID-19 treated with ECMO [39, 42], and the incidence was higher than that reported for ECMO for ARDS caused by other viruses [58]. It is worth mentioning that the EOLIA study was the only one that did not find an increase in intracranial bleeding with ECMO, probably reflecting that experience in ECMO use is associated with safer use of anticoagulation [12]. Importantly, sudden changes in PaCO2 have been independently associated with neurological complications [54, 57, 59]. In a large international study, a PaCO2 decrease greater than 50% in the first 24 h following ECMO onset significantly increased all neurologic complications [54].
Sepsis and systemic inflammatory syndrome
In all extracorporeal therapies, the exposure of blood to non-biological surfaces, the blood shear stress and the air–blood interface initiate and amplify a systemic inflammatory response. The inflammatory response to ECMO is intricate and involves the triggering of a variety of coagulation and inflammatory cascades. Widespread endothelial injury, capillary leaks and multiple organ dysfunction is possible [60, 61]. More than 50% of patients develop an infection while on ECMO therapy, predominantly ventilator-associated pneumonia (35%), and 26% develop sepsis with significant increase in mortality [52, 62].
Renal failure
More than two-thirds of patients develop acute kidney injury during ECMO treatment and approximately 50–65% of them eventually need renal replacement therapy [52, 63]. Beyond patient-related factors, ECMO may harm the kidneys through multiple mechanisms: (1) renal hypoperfusion in case of bleeding, hemolysis, catheter obstruction and vascular thromboembolism; (2) fluid overload; (3) ischemia–reperfusion injury promoted by hemodynamic fluctuations and renin–angiotensin–aldosterone dysregulation; (4) systemic inflammatory response syndrome, and (5) coagulopathy and catheter-related infections.
ECMO versus conventional management
Prior to employing a complex, costly and potentially dangerous treatment such as ECMO, the clinician should exhaust any available simpler, less resource intensive and safer therapeutic options (Fig. 2). To date, there are two conventional interventions with proven survival benefit in ARDS patients, namely lung protective ventilation with low tidal volumes (6 ml/kg PBW) and prone position [64,65,66,67,68,69,70]. Resolution of patient–ventilator dyssynchronies, application of sufficient PEEP, administration of neuromuscular blocking agents, and transpulmonary pressure measurements constitute further strategies that may benefit patients.
Illustration of therapeutic options in ARDS patients that should be considered prior to employing ECMO treatment. Equal weight is given to the management of asynchrony, transpulmonary pressure (PL) targets, and administration of neuromuscular blocking agents, in order to illustrate that specific patients may benefit from specific strategies (e.g. PL measurements in obese patients, and paralysis in patients with high respiratory drive or reverse triggering (entrainment) despite sedation). VT, tidal volume; Pplat, plateau pressure; VV ECMO, veno-venous extracorporeal membrane oxygenation
Lung protective ventilation
The application of “gentle” mechanical ventilation with low tidal volumes and plateau and driving pressures, low respiratory rates and low mechanical power has been shown to reduce the risk and magnitude of VILI [66, 67, 70,71,72]. Before proceeding to ECMO for lung protection, the abilities of conventional ventilation should be fully exploited. Remarkably, the LUNG SAFE study demonstrated that the principles of lung protective ventilation were not rigorously respected in clinical practice: plateau pressure was measured in only 40% of patients with ARDS, whereas only two-thirds of patients in whom respiratory system mechanics were calculated actually received lung protective ventilation [73]. Furthermore, the inverse relationship between FiO2 and PaO2 and the absence of correlation between PEEP and PaO2/FiO2 indicated that physicians primarily used FiO2 to treat hypoxemia. Thus, even following ECMO initiation, many patients continue to have injurious ventilator settings.
The employment of ultra-protective ventilation is appealing in patients with severe ARDS. The LIFEGARDS international study showed that ECMO permitted ultra-lung protective ventilation: tidal volume and plateau pressures were both significantly reduced from 6.4 ± 2.0 to 3.7 ± 2.0 ml/kg PBW and from 32 ± 7 to 24 ± 7 cmH2O, respectively [74]. Notwithstanding, ultra-low tidal volume ventilation can also be applied in approximately two-thirds of moderately severe to severe ARDS patients without extracorporeal treatment [75], while it is not without risks: it can lead to lung collapse with associated hypoxemia and compliance deterioration and is frequently accompanied by severe acidosis [75, 76]. Finally, whether very-low tidal volume ventilation with ECMO is superior to conventional low ventilation is largely unknown.
Recruitment and positive end-expiratory pressure
Applying appropriately sufficiently high end-expiratory pressure (PEEP) is an essential component of ARDS ventilation. Adequate PEEP recruits atelectatic regions, improves regional lung compliance, decreases shunt, and reduces cyclic closing and reopening of alveoli without increasing stress and hemodynamic compromise [77]. Although, higher versus moderate PEEP for the same degree of hypoxemia had not shown survival benefit in the general population with ARDS [78,79,80], subsequent individual patient-data meta-analysis, indicated that higher PEEP was associated with lower mortality in hypoxemic patients with PaO2/FiO2 < 200 mmHg and with no benefit in mild ARDS [81].
Prone position
Prone position improves oxygenation, reduces the risk of VILI and may improve right heart function in patients with hypoxemic respiratory failure. The redistribution of lung densities with a recruitment of dorsal regions associated with increase in chest wall elastance, follow a more homogenous ventilation distribution, while alveolar shunt reduction results from improvement in ventilation/perfusion matching [82]. Gattinoni et al. demonstrated that prone position significantly improved arterial oxygenation in ARDS patients and might have a survival advantage in those with very severe hypoxemia[69]. The landmark PROSEVA trial by Guerin et al. proved that long-term prone positioning significantly reduced 28-day mortality (16% vs 32.8%, p < 0.001) in ARDS patients with PaO2/FiO2 < 150 mmHg [68]. Meta-analyses confirmed that prone position carries a substantial survival benefit, provided that it is implemented in severely hypoxemic patients and for longer than 12-h sessions [83,84,85]. Complications are infrequent and include tube dislodgement or obstruction, pressure ulceration and, rarely, cardiac arrest. For sustained oxygenation improvement, prone position sessions should last and should be repeated several times. In the PROSEVA study, patients underwent four prone position sessions on average, which lasted 17 consecutive hours each, even if oxygenation did not improve following proning [68]. This recommendation emphasizes that the beneficial effect of prone position in patients with severe hypoxemia is expected mainly through the increase in lung homogeneity and the associated reduction of regional stress and strain [86] and, secondarily, through improvement in oxygenation [87]. Therefore, beyond PaO2/FiO2 changes following proning, reduction of plateau airway pressure, driving pressure and transpulmonary pressure (if available) should be evaluated as well. The optimal duration of prone position is not known. In patients with COVID-19 related lung injury, proning sessions of 36 h could be safely performed and were associated with more sustained oxygenation improvement compared to standard 16 h of proning [88].
Given the evidence, one would expect that clinicians would enthusiastically embrace a simple, relatively safe intervention, with proven survival benefit and no additional cost. Nevertheless, data from subsequent international studies indicate a radically different clinical attitude. In the LUNG SAFE study, proning was used in only 16.3% of patients with severe ARDS [73]. Similarly, Li X et al. disclosed that not more than 31% out of 672 ARDS patients managed with v-v ECMO had previously underwent a trial of prone positioning [89]. Remarkably, the positive results of Guerin et al. [68] failed to change routine clinical practice at least before the COVID-19 pandemic. The proportion of patients in whom prone positioning was used before ECMO was lower in the more recent studies (19%) versus those published before 2013 [89]. Even in experienced ECMO centers, prone position was offered to only 26% of patients [74]. In a recent multinational survey, only 21% of experienced v-v ECMO physicians would be reluctant to initiate ECMO without beforehand turning patients prone [90]. In a prospective international 1-day prevalence study, the rate of proning was higher in non-European than in European countries (28.6 vs 13%; p = 0.019) [91]. Notably, prone position is part of pre-ECMO support in no more than 60% of patients with COVID-19 (https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx).
Neuromuscular blocking agents
Spontaneous breathing activity, under the influence of high respiratory drive with or without MV, may harm the lungs due to patient–ventilator dyssynchrony, excessive transpulmonary and transmural vascular pressures and pendelluft phenomenon [92]. The higher the respiratory drive, the more injurious are the inspiratory efforts for the lung. Neuromuscular blocking agents abolish spontaneous breathing activity and they may have anti-inflammatory properties. The ACURASYS study and two meta-analyses demonstrated a reduction in mortality and days on MV and improved oxygenation in the most hypoxemic ARDS patients treated with continuous neuromuscular blockade [93,94,95]. The incidence of ICU acquired weakness did not increase as a result of cisatracurium. Subsequent studies did not find a survival benefit from early administration of neuromuscular blocking agents in ARDS [65, 96], but it still seems prudent to consider their use prior to ECMO in the sickest patients. This is not often the case in clinical practice, though: around 40% of patients received ECMO without any trial of neuromuscular blockade as reported in the LIFEGARDS study [74].
Transpulmonary pressure targeted ventilation
Recruitment and ventilation settings adjusted to reach targets of transpulmonary pressures (PL), measured via an esophageal catheter, allow individualization of MV and have been shown to improve oxygenation while, concomitantly, reduce the risk of lung injury [97,98,99,100]. Transpulmonary pressures calculation allows the partitioning of respiratory mechanics between the lung and chest wall. In influenza A (H1N1)-associated ARDS patients referred to an ECMO center, setting PEEP to approach a maximum end-inspiratory PL of 25cmH2O improved oxygenation and obviated the use of ECMO for the group of patients with high chest wall elastance (50% in this study) [101]. Similarly, an end-expiratory PL guided open lung approach significantly increased oxygenation and obviated the use of ECMO in all 8 patients with severe ARDS (PaO2/FiO2 62 ± 7 mmHg) not responding to any conventional ventilation management, including neuromuscular blockade and prone positioning [102]. Individual titration of mechanical ventilation based, among others, on esophageal manometry might be particularly valuable in obese patients and could improve their outcome compared to standard practice [103]. In this group, the high prevalence of complete airway closure (> 41%), can make driving pressures unreliable [104]. Furthermore, the hemodynamic tolerance to high PEEP was found remarkably good in class III obese patients (mean BMI = 57 kg/m2) [105]. The technique is largely neglected in daily practice, likely due to technical challenges.
Ethical considerations
From an ethical perspective, the decision to apply ECMO can be complex [106]. There are clinical scenarios in which the technique is clearly futile [90], but, in most cases it is noticeably difficult to predict outcome. Survival predicting tools have been proposed [107, 108], but the personal perception of “futility” and patient´s wishes and values, often expressed by family, play an important role [90]. Judgement may be clouded by emotional and physical stress, the hope offered by sophisticated therapies, or the inputs from friends or media. Additional limitations further challenge the decision to initiate ECMO during pandemics [109]. Even more intricate is to determine when to withdraw the treatment. All these aspects cannot be addressed by algorithms or guidelines. A thorough and honest discussion between the physician and the patient or his relatives regarding the benefits, risks and expectations of the treatment is essential. The goal should be the bridge to recovery [106, 110, 111].
Summary and critical appraisal
Extracorporeal membrane oxygenation is, undoubtedly, a powerful aid against refractory hypoxemia and the inability to deliver lung protective ventilation with MV alone. It is also a highly invasive technique with many serious complications. Moreover, resource implications associated with ECMO are substantial: according to guidelines, ECMO should be employed in centers with experienced and organized multidisciplinary ECMO teams, available 24 h per day, with high nurse-to-patient ratio and where blood bank, radiology and vascular, thoracic and abdominal surgical departments are directly disposable to address emergencies [112]. Furthermore, it is definitely an expensive modality: financial analyses estimated that ECMO raised the costs per patient by approximately 57.000 to 70.000 USD [15, 113] or at 30.000 USD/quality-adjusted life year [114]. Concurrently, existing evidence suggests that v-v ECMO might have a mortality benefit in a very small portion of patients: those with very severe hypoxemia (PaO2/FiO2 < 80 mmHg), who receive injurious mechanical ventilation settings and in whom all conservative strategies such as lung protective ventilation, repeated prone positioning and neuromuscular blocking agents have failed [115]. Table 2 details the reported use of ECMO as adjunctive therapy in recent large randomized clinical trials in a total of 6736 ARDS patients; only 2.15% of patients required ECMO, which ranged from 0 to 6.14% for individual studies. Finally, there is still no confirmatory data that ECMO, even in severely hypoxemic patients, prevails over optimal conventional management techniques.
Despite the above considerations, ECMO use exploded in the last decade and continues to rapidly expand worldwide, even in small hospitals. Internationally, between 2010 and 2019, the number of centers that provide ECMO at any age and for any cause increased from 183 to 4637 and the number of ECMO runs per year increased from 3445 to 12,850 according to the Extracorporeal Life Support Organization registry database [116]. Analyzing data from the Federal Statistical Office of Germany, Karagiannidis et al. found that v-v ECMO use almost tripled between 2007 and 2014, whilst mortality among patients who had received ECMO for less than 48 h was 70% [117]. This mortality rate was higher than that reported in epidemiologic studies on severe ARDS [73], indicating that either some patients were too sick to benefit from ECMO anyway and/or that dreadful complications affected the outcome [118].There is no definite answer why doctors resort so easily to a complicated treatment such as ECMO. Some physicians may perceive that ECMO implementation is more straightforward and, hence, less time consuming and less prone to errors as compared to the individualization of mechanical ventilation settings based on lung mechanics measurements. Nonetheless, proper use prerequisites a thorough understanding of the patient’s physiology. Other reasons include the belief that the more advanced and sophisticated the treatment, the higher the benefit for the patient, along with the excitement of dealing with advanced technology. Finally, financial interests should not be belittled: reimbursement from ECMO programs can be substantial for institutions and may constitute a strong incentive for its implementation [119].
Conclusion
Lung protective ventilation, prone position, neuromuscular blockade and individualized ventilation driven by transpulmonary pressures, are efficient in the majority of hypoxemic patients, even in those with severe hypoxemia. v-v ECMO is a valuable tool in highly selected patients, in whom the aforementioned strategies fail to correct life threatening hypoxemia. This modality should be reserved after implementing simpler and less invasive strategies. Failure to do so may be harmful both for the patient as well as for the ECMO technique itself.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ACURASYS:
-
ARDS et Curarisation Systematique
- CaO2 :
-
Arterial oxygen content
- CvO2 :
-
Arterial oxygen content in the mixed blood
- SaO2 :
-
Arterial oxygen saturation
- CO2 :
-
Carbon dioxide
- CO:
-
Cardiac output
- CESAR (study):
-
Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure [15]
- EOLIA (study):
-
ECMO to rescue Lung Injury in severe ARDS [12]
- ECBF:
-
Extracorporeal blood flow
- ECMO:
-
Extracorporeal membrane oxygenation
- FiO2 :
-
Inspired fraction of oxygen
- Hb:
-
Hemoglobin
- LUNG SAFE (study):
-
Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE [73]
- O2 :
-
Oxygen
- CvO2native :
-
Oxygen content of the native venous return
- CvO2outlet :
-
Oxygen content in the venous blood after the membrane oxygenator
- CvO2inlet :
-
Oxygen content in the venous blood before the membrane oxygenator
- V’O2ECMO :
-
Oxygen delivery by extracorporeal membrane oxygenation
- SvO2 :
-
Oxygen saturation in the pulmonary artery
- MV:
-
Mechanical ventilation
- PO2 :
-
Partial pressure of oxygen
- PEEP:
-
Positive end-expiratory pressure
- PROSEVA (study):
-
Prone positioning in severe acute respiratory distress syndrome[68]
- PaO2/FiO2 :
-
Ratio of partial pressure of oxygen to inspired fraction of oxygen
- P L :
-
Transpulmonary pressures
- v-v ECMO:
-
Veno-venous extracorporeal membrane oxygenation
- VILI:
-
Ventilator-induced lung injury
- LIFEGARDS (study):
-
VentiLatIon management oF patients with Extracorporeal membrane oxyGenation for Acute Respiratory Distress Syndrome [74]
References
Gille JP, Bagniewski AM. Ten years of use of extracorporeal membrane oxygenation (ECMO) in the treatment of acute respiratory insufficiency (ARI). Trans Am Soc Artif Intern Organs. 1976;22:102–9.
Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome): use of the Bramson membrane lung. N Engl J Med. 1972;286:629–34.
Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–6.
Engle WA, West KW, Hocutt GA, Pallotto EK, Haney B, Keith RJ, et al. Adult outcomes after newborn respiratory failure treated with extracorporeal membrane oxygenation*. Pediatr Crit Care Med. 2017;18:73–9.
Quintel M, Bartlett RH, Grocott MPW, Combes A, Ranieri MV, Baiocchi M, et al. Extracorporeal membrane oxygenation for respiratory failure. Anesthesiology. 2020;132:1257–76.
Bartlett RH. Physiology of gas exchange during ECMO for respiratory failure. J Intensive Care Med. 2017;32:243–8.
Marshall BE, Marshall C. A model for hypoxic constriction of the pulmonary circulation. J Appl Physiol. 1988;64:68–77.
Dawson CA. Role of pulmonary vasomotion in physiology of the lung. Physiol Rev. 1984;64:544–616.
Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J. 2015;61:115–21.
Christiansen J, Douglas CG, Haldane JS. The absorption and dissociation of carbon dioxide by human blood. J Physiol. 1914;48:244–71.
Davies A, Jones D, Bailey M, Beca J, Bellomo R, Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95.
Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75.
Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306:1659.
Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–63.
Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)–induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–85.
Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–8.
Tipograf Y, Salna M, Minko E, Grogan EL, Agerstrand C, Sonett J, et al. Outcomes of extracorporeal membrane oxygenation as a bridge to lung transplantation. Ann Thorac Surg. 2019;107:1456–63.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–63.
Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251.
Lewis RJ, Angus DC. Time for clinicians to embrace their inner bayesian?: reanalysis of results of a clinical trial of extracorporeal membrane oxygenation. JAMA. 2018;320:2208.
Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–72.
Miller RR, Markewitz BA, Rolfs RT, Brown SM, Dascomb KK, Grissom CK, et al. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A(H1N1) infection. Chest. 2010;137:752–8.
Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–8.
Ñamendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348–9.
Falcoz P-E, Monnier A, Puyraveau M, Perrier S, Ludes P-O, Olland A, et al. Extracorporeal Membrane oxygenation for critically ill patients with COVID-19-related acute respiratory distress syndrome: worth the effort? Am J Respir Crit Care Med. 2020;202:460–3.
Jacobs JP, Stammers AH, St. Louis J, Hayanga JWA, Firstenberg MS, Mongero LB, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: experience with 32 patients. ASAIO J. 2020;66:722–30.
Kon ZN, Smith DE, Chang SH, Goldenberg RM, Angel LF, Carillo JA, et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. 2021;111:537–43.
Li X, Guo Z, Li B, Zhang X, Tian R, Wu W, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J. 2020;66:475–81.
Mustafa AK, Alexander PJ, Joshi DJ, Tabachnick DR, Cross CA, Pappas PS, et al. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg. 2020;155:990.
Osho AA, Moonsamy P, Hibbert KA, Shelton KT, Trahanas JM, Attia RQ, et al. Veno-venous extracorporeal membrane oxygenation for respiratory failure in COVID-19 patients: early experience from a major academic medical center in North America. Ann Surg. 2020;272:e75–8.
Sultan I, Habertheuer A, Usman AA, Kilic A, Gnall E, Friscia ME, et al. The role of extracorporeal life support for patients with COVID-19: Preliminary results from a statewide experience. J Card Surg. 2020;35:1410–3.
Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–4.
Yang X, Hu M, Yu Y, Zhang X, Fang M, Lian Y, et al. Extracorporeal membrane oxygenation for SARS-CoV-2 acute respiratory distress syndrome: a retrospective study from Hubei, China. Front Med (Lausanne). 2020;7:61460.
Zeng Y, Cai Z, Xianyu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care. 2020;24:148.
Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. The Lancet. 2020;396:1071–8.
Jacobs JP, Stammers AH, Louis JST, Hayanga JWA, Firstenberg MS, Mongero LB, et al. Multi-institutional analysis of 100 consecutive patients with COVID-19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J. 2021;67:496–502.
Lorusso R, Mueller T. COVID-19 and ECMO: a call for close cooperation and more investigation. Lancet Respir Med. 2021. https://doi.org/10.1016/S2213-2600(21)00128-4.
Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respi Med. 2021. https://doi.org/10.1016/S2213-2600(21)00096-5.
Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–31.
Churpek MM, Gupta S, Spicer AB, Parker WF, Fahrenbach J, Brenner SK, et al. Hospital-level variation in death for critically ill patients with COVID-19. Am J Respir Crit Care Med. 2021. https://doi.org/10.1164/rccm.202012-4547OC.
Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, et al. ECMO for COVID-19-associated severe ARDS in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021. https://doi.org/10.1164/rccm.202011-4166OC.
Cain MT, Smith NJ, Barash M, Simpson P, Durham LA, Makker H, et al. Extracorporeal membrane oxygenation with right ventricular assist device for COVID-19 ARDS. J Surg Res. 2021;264:81–9.
Lubnow M, Philipp A, Foltan M, Bull Enger T, Lunz D, Bein T, et al. Technical complications during veno-venous extracorporeal membrane oxygenation and their relevance predicting a system-exchange – retrospective analysis of 265 cases. PLoS ONE. 2014;9:e112316.
Fisser C, Reichenbächer C, Müller T, Schneckenpointner R, Malfertheiner MV, Philipp A, et al. Incidence and risk factors for cannula-related venous thrombosis after venovenous extracorporeal membrane oxygenation in adult patients with acute respiratory failure. Crit Care Med. 2019;47:e332–9.
Menaker J, Tabatabai A, Rector R, Dolly K, Kufera J, Lee E, et al. Incidence of cannula-associated deep vein thrombosis after veno-venous extracorporeal membrane oxygenation. ASAIO J. 2017;63:588–91.
Parzy G, Daviet F, Persico N, Rambaud R, Scemama U, Adda M, et al. Prevalence and risk factors for thrombotic complications following venovenous extracorporeal membrane oxygenation: a CT scan study. Crit Care Med. 2020;48:192–9.
Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63:60–7.
Mazzeffi M, Greenwood J, Tanaka K, Menaker J, Rector R, Herr D, et al. Bleeding, transfusion, and mortality on extracorporeal life support: ECLS working group on thrombosis and hemostasis. Ann Thorac Surg. 2016;101:682–9.
Jiritano F, Serraino GF, ten Cate H, Fina D, Matteucci M, Mastroroberto P, et al. Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med. 2020;46:1154–69.
Ried M, Sommerauer L, Lubnow M, Müller T, Philipp A, Lunz D, et al. Thoracic bleeding complications in patients with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2018;106:1668–74.
Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–8.
Gross-Hardt S, Hesselmann F, Arens J, Steinseifer U, Vercaemst L, Windisch W, et al. Low-flow assessment of current ECMO/ECCO2R rotary blood pumps and the potential effect on hemocompatibility. Crit Care. 2019;23:348.
Cavayas YA, Munshi L, Del Sorbo L, Fan E. The early change in PaCO2 after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am J Respir Crit Care Med. 2020;201:1525–35.
Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med. 2017;45:1389–97.
Sutter R, Tisljar K, Marsch S. Acute neurologic complications during extracorporeal membrane oxygenation: a systematic review. Crit Care Med. 2018;46:1506–13.
Luyt C-E, Bréchot N, Demondion P, Jovanovic T, Hékimian G, Lebreton G, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2016;42:897–907.
Bermea RS, Raz Y, Sertic F, Rubin J, Wolf M, Olia S, et al. Increased intracranial hemorrhage amid elevated inflammatory markers in those with COVID-19 supported with extracorporeal membrane oxygenation. Shock. 2021. https://doi.org/10.1097/SHK.0000000000001730 (Publish Ahead of Print).
Muellenbach RM, Kilgenstein C, Kranke P, Küstermann J, Kredel M, Roewer N, et al. Effects of venovenous extracorporeal membrane oxygenation on cerebral oxygenation in hypercapnic ARDS. Perfusion. 2014;29:139–41.
Burrell AJC, Lubnow M, Enger TB, Nanjayya VB, Philipp A, Malfertheiner MV, et al. The impact of venovenous extracorporeal membrane oxygenation on cytokine levels in patients with severe acute respiratory distress syndrome: a prospective, observational study. Crit Care Resusc. 2017;19:37–44.
Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:387.
Grasselli G, Scaravilli V, Di Bella S, Biffi S, Bombino M, Patroniti N, et al. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients’ outcome. Crit Care Med. 2017;45:1726–33.
Kielstein JT, Heiden AM, Beutel G, Gottlieb J, Wiesner O, Hafer C, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28:86–90.
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8.
Aoyama H, Uchida K, Aoyama K, Pechlivanoglou P, Englesakis M, Yamada Y, et al. Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: a systematic review and network meta-analysis. JAMA Netw Open. 2019;2:e198116.
Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55.
Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323.
Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68.
Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–73.
Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42:1567–75.
for the PROVE Network Investigators, Serpa Neto A, Deliberato RO, Johnson AEW, Bos LD, Amorim P, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018;44:1914–22.
Urner M, Jüni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8:905–13.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.
Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome. An international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200:1002–12.
Richard JC, Marque S, Gros A, Muller M, Prat G, Beduneau G, et al. Feasibility and safety of ultra-low tidal volume ventilation without extracorporeal circulation in moderately severe and severe ARDS patients. Intensive Care Med. 2019;45:1590–8.
Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–8.
Suter PM, Fairley HB, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975;292:284–9.
National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36.
Mercat A, Richard JCM, Vielle B, Jaber S, Osman D, Diehl J-L, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646.
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637.
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865.
Richard J-C, Bregeon F, Costes N, Bars DLE, Tourvieille C, Lavenne F, et al. Effects of prone position and positive end-expiratory pressure on lung perfusion and ventilation. Crit Care Med. 2008;36:2373–80.
Lee JM, Bae W, Lee YJ, Cho Y-J. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med. 2014;42:1252–62.
Munshi L, Del Sorbo L, Adhikari NKJ, Hodgson CL, Wunsch H, Meade MO, et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:S280–8.
Sud S, Friedrich JO, Adhikari NKJ, Taccone P, Mancebo J, Polli F, et al. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2014;186:E381-390.
Katira BH, Osada K, Engelberts D, Bastia L, Damiani LF, Li X, et al. Positive end-expiratory pressure, pleural pressure, and regional compliance during pronation: an experimental study. Am J Respir Crit Care Med. 2021. https://doi.org/10.1164/rccm.202007-2957OC.
Albert RK, Keniston A, Baboi L, Ayzac L, Guérin C, Proseva Investigators. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:494–6.
Carsetti A, Damia Paciarini A, Marini B, Pantanetti S, Adrario E, Donati A. Prolonged prone position ventilation for SARS-CoV-2 patients is feasible and effective. Crit Care. 2020;24:225.
Li X, Scales DC, Kavanagh BP. Unproven and expensive before proven and cheap: extracorporeal membrane oxygenation versus prone position in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:991–3.
Abrams D, Pham T, Burns KEA, Combes A, Curtis JR, Mueller T, et al. Practice patterns and ethical considerations in the management of venovenous extracorporeal membrane oxygenation patients: an international survey*. Crit Care Med. 2019;47:1346–55.
Guérin C, Beuret P, Constantin JM, Bellani G, Garcia-Olivares P, Roca O, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44:22–37.
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury*. Crit Care Med. 2012;40:1578–85.
Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17:R43.
Neto AS, Pereira VGM, Espósito DC, Damasceno MCT, Schultz MJ. Neuromuscular blocking agents in patients with acute respiratory distress syndrome: a summary of the current evidence from three randomized controlled trials. Ann Intensive Care. 2012;2:33.
Papazian L, Forel J-M, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16.
The National Heart, Lung, and Blood Institute PETAL Clinical Trials Network . Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008.
Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–31.
Baedorf Kassis E, Loring SH, Talmor D. Recruitment maneuvers: using transpulmonary pressure to help Goldilocks. Intensive Care Med. 2017;43:1162–3.
Chen L, Chen G-Q, Shore K, Shklar O, Martins C, Devenyi B, et al. Implementing a bedside assessment of respiratory mechanics in patients with acute respiratory distress syndrome. Crit Care. 2017;21:84.
Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–104.
Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38:395–403.
van der Zee P, Dos Reis MD, Meeder H, Endeman H, Gommers D. vvECMO can be avoided by a transpulmonary pressure guided open lung concept in patients with severe ARDS. Crit Care. 2019;23:133.
Florio G, Ferrari M, Bittner EA, De Santis SR, Pirrone M, Fumagalli J, et al. A lung rescue team improves survival in obesity with acute respiratory distress syndrome. Crit Care. 2020;24:4.
Coudroy R, Vimpere D, Aissaoui N, Younan R, Bailleul C, Couteau-Chardon A, et al. Prevalence of complete airway closure according to body mass index in acute respiratory distress syndrome: pooled cohort analysis. Anesthesiology. 2020. https://doi.org/10.1097/ALN.0000000000003444.
De Santis Santiago R, Teggia Droghi M, Fumagalli J, Marrazzo F, Florio G, Grassi LG, et al. High pleural pressure prevents alveolar overdistension and hemodynamic collapse in ARDS with class III obesity. Am J Respir Crit Care Med. 2020. https://doi.org/10.1164/rccm.201909-1687OC.
Bein T, Brodie D. Understanding ethical decisions for patients on extracorporeal life support. Intensive Care Med. 2017;43:1510–1.
Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The respiratory extracorporeal membrane oxygenation survival prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–82.
Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt C-E, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–13.
Murugappan KR, Walsh DP, Mittel A, Sontag D, Shaefi S. Veno-venous extracorporeal membrane oxygenation allocation in the COVID-19 pandemic. J Crit Care. 2021;61:221–6.
Bein T, Weber-Carstens S, Herridge M. Extracorporeal life support, ethics, and questions at the bedside: how does the end of the pathway look? Intensive Care Med. 2015;41:1714–5.
Ramanathan K. Ethical challenges of adult ECMO. Indian J Thorac Cardiovasc Surg. 2021;37:303–8.
Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190:488–96.
Mishra V, Svennevig JL, Bugge JF, Andresen S, Mathisen A, Karlsen H, et al. Cost of extracorporeal membrane oxygenation: evidence from the Rikshospitalet University Hospital, Oslo. Norway Eur J Cardiothorac Surg. 2010;37:339–42.
Barrett KA, Hawkins N, Fan E. Economic evaluation of venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome*. Crit Care Med. 2019;47:186–93.
Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69.
Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–96.
Quintel M, Gattinoni L, Weber-Carstens S. The German ECMO inflation: when things other than health and care begin to rule medicine. Intensive Care Med. 2016;42:1264–6.
Blum JM, Lynch WR, Coopersmith CM. Clinical and billing review of extracorporeal membrane oxygenation. Chest. 2015;147:1697–703.
Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, Laranjeira LN, de Paisani DM, Damiani LP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low peep on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335.
Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-F io 2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321:846.
The ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382:989–98.
Constantin J-M, Jabaudon M, Lefrant J-Y, Jaber S, Quenot J-P, Langeron O, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7:870–80.
Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008.
Hodgson CL, Cooper DJ, Arabi Y, King V, Bersten A, Bihari S, et al. Maximal recruitment open lung ventilation in acute respiratory Distress Syndrome (PHARLAP). A phase II, multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200:1363–72.
Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364.
Le Breton C, Besset S, Freita-Ramos S, Amouretti M, Billiet PA, Dao M, et al. Extracorporeal membrane oxygenation for refractory COVID-19 acute respiratory distress syndrome. J Crit Care. 2020;60:10–2.
Funding
Laurent Brochard is supported by the Keenan Chair.
Author information
Authors and Affiliations
Contributions
DG, EA and LB conceived the idea; EA, DG and LB performed the literature search and drafted the manuscript, AJ and MCS drafted the figures. The article was critically reviewed and revised by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
There are no conflicts of interest in relation to the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akoumianaki, E., Jonkman, A., Sklar, M.C. et al. A rational approach on the use of extracorporeal membrane oxygenation in severe hypoxemia: advanced technology is not a panacea. Ann. Intensive Care 11, 107 (2021). https://doi.org/10.1186/s13613-021-00897-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-021-00897-3