Abstract
Background
The thyroid hormone (T3)-induced formation of adult intestine during amphibian metamorphosis resembles the maturation of the mammalian intestine during postembryonic development, the period around birth when plasma T3 level peaks. This process involves de novo formation of adult intestinal stem cells as well as the removal of the larval epithelial cells through apoptosis. Earlier studies have revealed a number of cytological and molecular markers for the epithelial cells undergoing different changes during metamorphosis. However, the lack of established double labeling has made it difficult to ascertain the identities of the metamorphosing epithelial cells.
Results
Here, we carried out different double-staining with a number of cytological and molecular markers during T3-induced and natural metamorphosis in Xenopus laevis. Our studies demonstrated conclusively that the clusters of proliferating cells in the epithelium at the climax of metamorphosis are undifferentiated epithelial cells and express the well-known adult intestinal stem cell marker gene Lgr5. We further show that the adult stem cells and apoptotic larval epithelial cells are distinct epithelial cells during metamorphosis.
Conclusions
Our findings suggest that morphologically identical larval epithelial cells choose two alternative paths: programmed cell death or dedifferentiation to form adult stem cells, in response to T3 during metamorphosis with apoptosis occurring prior to the formation of the proliferating adult stem cell clusters (islets).
Similar content being viewed by others
Background
Intestinal remodeling during Xenopus metamorphosis serves as an excellent model to study the development of vertebrate adult organ-specific adult stem cells, which are essential for physiological tissue renewal and regeneration. This transformation of the larval intestine to the adult form during amphibian metamorphosis involves the removal of larval epithelium and de novo formation of the adult epithelium with concurrent maturation of the other intestinal tissues in a process similar to the maturation of the mammalian intestine around birth [1–5]. The tadpole intestine consists of largely a monolayer of larval epithelial cells surrounded by thin layers of connective tissue and muscles. During metamorphosis, the larval epithelial cells undergo apoptosis and clusters of proliferating adult epithelial cells are formed de novo, which subsequently proliferate and differentiation to form a multiply folded adult epithelium surrounded by thick layers of connective tissue and muscles [1, 6–12]. Gene expression analyses of known adult stem cell markers of mammalian intestine, such as Lgr5 [13], suggest that the clusters of proliferating cells are adult stem cells.
Like all other processes during amphibian metamorphosis, intestinal remodeling is under the control of thyroid hormone (T3) [14, 15]. This process can be easily induced by adding physiological concentrations of T3 to premetamorphic tadpole rearing water or prevented by blocking the synthesis of endogenous T3. In addition, it is organ autonomous and can be induced with T3 even in intestinal organ cultures of premetamorphic tadpoles. Such properties makes intestinal remodeling a superior model to study the development of adult organ-specific stem cells as compared to the mammalian models, where it is difficult to manipulate the uterus-enclosed late stage embryos for such studies.
Earlier work in Xenopus laevis has shown that T3 induces the vast majority of the larval epithelial cells to undergo programmed cell death or apoptosis and that the proliferating adult epithelial cells are formed de novo, apparently from the dedifferentiation of a small number of larval epithelial cells, via a yet-unknown mechanism [1, 7–12, 16–19]. These proliferating adult epithelial cells can be easily identified as clusters of cells or islets that can be labeled with DNA synthesis markers, such as 3H and 5-bromo-2′-deoxyuridine, or strongly stained with methyl green-pyronin Y (MPGY) at the climax of metamorphosis [16–18, 20]. In addition, in situ hybridization analyses have shown that well-known markers of the adult mammalian intestinal stem cells, such as leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) and Musashi-1 (Msi-1), are expressed in clusters of intestinal epithelial cells at the climax of metamorphosis, suggesting that the clusters or islets are proliferating adult stem cells. However, there has been no report of using double labeling to ascertain the identities and property of these cell clusters. Here by using a combination of different staining methods, we successfully carried out different double labeling that allowed us to conclusively demonstrate that the clusters of epithelial cells induced by T3 at the climax of intestinal metamorphosis are proliferating, Lgr5+ adult stem cells. We further show that these cells can be strongly stained with MPGY and lack intestinal fatty acid binding protein (IFABP), which is expressed in the differentiated epithelial cells. Finally, we demonstrated that apoptotic and the proliferating cells are distinct populations of epithelial cells at the climax of metamorphosis.
Results and discussions
Proliferating adult intestinal epithelial cells exist as cell clusters and lack the differentiation marker IFABP
The remodeling of the intestine leads to distinct changes in the morphology of the intestinal cross-section. This can be easily detected by staining the tissue sections with a mixture (MPGY) of methyl green, which stains DNA, and pyronin Y, which stains RNA [20–22]. Earlier studies have shown that MGPY stains strongly clusters of epithelial cells formed at the climax of metamorphosis or after T3 treatment, while the surrounding cells that are poor stained. As the epithelial cell clusters or islets at the climax of metamorphosis can be labeled with DNA synthesis markers [16, 20], it has been assumed that the clusters with active DNA synthesis are the same as those stained strongly with MGPY. To demonstrate this directly, we treated premetamorphic tadpoles at stage 54 with T3 for 0–6 days to induce metamorphosis. One hour prior to being sacrificed, the tadpoles were injected with 5-ethynyl-2′-deoxyuridine (EdU) to label the newly synthesized cellular DNA. The intestinal cross-sections from the resulting tadpoles were double-stained with MGPY and for EdU. As shown in Fig. 1, in premetamorphic intestine, the epithelium was uniformly stained with MGPY and some of the epithelial cells were EdU positive (Fig. 1A, a″). T3 treatment for 3 days had little effect on either intestinal morphology or staining (Fig. 1B, b″). However, after 6 days of T3 treatment, clusters of cells in the epithelium appeared and were more strongly stained by MGPY than the surrounding cells (Fig. 1C). Furthermore, these clusters were labeled by EdU (Fig. 1C, c″), indicating that the cell clusters strongly stained by MGPY are indeed the proliferating cell clusters.
MGPY stains strongly the clusters (islets) of proliferating adult intestinal epithelial cells during T3-induced intestinal metamorphosis. Premetamorphic stage 54 tadpoles treated with 10 nM T3 for 0 (A), 3 (B), or 6 days (C) and were sacrificed 1 h after injection with EdU. Cross-sections of the intestine from the resulting tadpoles were double-stained for EdU and with MGPY. Higher magnifications of boxed areas in (A–C) are shown in (a′–c′) and (a″–c″). The approximate epithelium-mesenchyme boundary was drawn based on morphological differences between epithelial cells and mesenchyme cells in the pictures of the double-stained tissues, under enhanced contrast and/or brightness by using Photoshop, if needed (dotted lines). Note that the clusters (islets) of EdU labeled cells in the epithelium after 6 days of T3 treatment were strongly stained by MGPY (C, c″). Arrowheads indicate the clusters of proliferating cells or islets (c′)
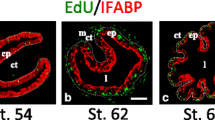
To investigate this during natural metamorphosis, we carried out similar double labeling on intestinal cross-sections from tadpoles at premetamorphosis (stage 54), climax (stage 62), and end of metamorphosis (stage 66). The results showed that before or after metamorphosis, the epithelium were uniformly labeled with MGPY and some cells were also positive for EdU (Fig. 2A, C, a″, c″). Interestingly, the EdU label were preferentially at the bottom of the newly formed epithelial folds at the end of metamorphosis, suggesting that the proliferating adult cells become restricted to the bottom of the fold, which resembles the crypt in adult mammalian intestine where stem cells reside [23–25]. In contrast, at the climax of metamorphosis, clusters of epithelial cells were much more strongly stained by MGPY than the surrounding cells and these clusters were also labeled by EdU (Fig. 2B, b″), just like that during T3 induced metamorphosis. Thus, the newly formed epithelial cell clusters during metamorphosis are proliferating cells that are strongly stained by MGPY.
MGPY and EdU co-stain the clusters (islets) of proliferating adult intestinal epithelial cells at the climax of natural metamorphosis. Tadpoles at premetamorphic stage 54 (A), climax (B stage 62), and end of metamorphosis (C stage 66) were injected with EdU 1 h before being sacrificed. Cross-sections of the intestine from the resulting tadpoles were double-stained for EdU and with MGPY. Higher magnifications of boxed areas in (A–C) are shown in (a′–c′) and (a″–c″). The dotted lines depict the epithelium-mesenchyme boundary (see Fig. 1). Arrowhead indicates the clusters of proliferating cells or islets (b′)
Since differentiated larval epithelial cells are capable of proliferating, e.g., those at stage 54 (Figs. 1A, 2A), it is possible that the cells in the clusters formed at the climax of metamorphosis are differentiated cells. To investigate this, we carried out double-labeling of intestinal cross-sections during T3 (Fig. 3) or natural (Fig. 4) metamorphosis by using EdU for cell proliferation and immunohistochemistry for IFABP, a marker for differentiated intestinal epithelial cells [22, 26]. As shown in Fig. 3, after 0–3 days of T3 treatment, there were only low levels of EdU positive cells in the epithelium but the entire epithelium was uniformly labeled with anti-IFABP antibody (Fig. 3A, B). Furthermore, the EdU labeled epithelial cells were positive for IFABP (Fig. 3a″, b″). These findings are consistent with earlier observations showing that larval epithelial cells are mitotically active and express IFABP uniformly [16, 20, 22, 26]. After 6 days of T3 treatment, however, the number of EdU positive cells in the epithelium increased dramatically and existed as clusters in regions adjacent to the connective tissue and lacked IFABP signal (Fig. 3C, c″).
The newly formed proliferating adult intestinal epithelial cells during T3-induced metamorphosis have little or no expression of IFABP. Premetamorphic stage 54 tadpoles treated with 10 nM T3 for 0 (A), 3 (B), or 6 days (C) and were sacrificed 1 h after injection with EdU. Cross-sections of the intestine from the resulting tadpoles were double-stained for IFABP by immunohistochemistry and for EdU. Higher magnifications of boxed areas in (A–C) are shown in (a′–c′) and (a″–c″). The dotted lines depict the epithelium-mesenchyme boundary (see Fig. 1). Note that the EdU labeling revealed profound cell proliferation after T3 treatment. The proliferating cells in the epithelium after 6 days of T3 treatment were present mainly in clusters where IFABP staining was weak or absent (C, c″)
Clusters of proliferating adult intestinal epithelial cells at the climax of natural metamorphosis lack IFABP. Tadpoles at premetamorphic stage 54 (A), climax (B stage 62), and end of metamorphosis (C, stage 66) were injected with EdU 1 h before being sacrificed. Cross-sections of the intestine from the resulting tadpoles were double-stained for EdU and IFABP by immunohistochemistry. Higher magnifications of boxed areas in (A–C) are shown in (a′–c′) and (a″–c″). The dotted lines depict the epithelium-mesenchyme boundary (see Fig. 1). Note that the EdU-labeled proliferating cells in the epithelium were few and expressed IFABP at premetamorphosis (A) and increased in form of clustered cells that lacked IFABP at the climax of metamorphosis (B, b″). At the end of metamorphosis, EdU-labeled proliferating cells were localized mainly in the troughs of the epithelial folds where IFABP expression was low (C, c″)
When we carried out similar analyses on intestinal cross-sections during natural metamorphosis, we also observed that at the climax of metamorphosis (stage 62), the EdU labeled cells were present as clusters between the luminal, larval epithelial cells that were positive for IFABP and the connective tissue (Fig. 4B, b″), while before (stage 54) or after (stage 66) metamorphosis (Fig. 4A, C, respectively), the EdU positive cells had IFABP, although at the troughs of the epithelial folds of post-metamorphic intestine (stage 66), the EdU positive cells had little or lower levels of IFABP (Fig. 4c″). These findings suggest that T3 induces the formation of clusters of proliferating cells that are dedifferentiated or undifferentiated. The same conclusion was reached when double-labeling was carried out with immunohistochemistry against IFABP and PCNA (proliferating cell nuclear antigen) (data not shown).
The proliferating epithelial cell clusters express adult intestinal stem cell marker Lgr5
We have previously shown that the well-known adult mammalian intestinal stem cell marker Lgr5 is upregulated during metamorphosis and is expressed preferentially or specifically in the epithelial cell clusters formed during metamorphosis [13]. To determine if Lgr5+ clusters are expressed in the proliferating cell clusters of the epithelium, we carried out double-labeling with EdU and Lgr5 in situ hybridization for Lgr5 mRNA on intestinal cross-sections during natural or T3-induced metamorphosis. As shown in Fig. 5, after 0–3 day T3 treatment of premetamorphic tadpoles, there were few Lgr5+ cells and no cells were found to co-stain with Lgr5 and EdU (Fig. 5A, B, a′, b′). However, after 6 days of treatment, Lgr5 positive cell clusters were numerous in the epithelium and these clusters were labeled with EdU (Fig. 5C, c′). Similarly, we found that at the climax (stage 62) during natural metamorphosis, the epithelial cell clusters were co-labeled with Lgr5 and EdU (Fig. 6b, b′). However, no such clusters were present before (stage 54) (Fig. 6A) or after (stage 66) (Fig. 6C) metamorphosis. Thus, the proliferating epithelial cells in the clusters formed during metamorphosis are adult stem cells.
The EdU-labeled clusters (islets) of proliferating adult intestinal epithelial cells during T3-induced intestinal metamorphosis express the adult intestinal stem cell marker Lgr5. Premetamorphic stage 54 tadpoles treated with 10 nM T3 for 0 (A), 3 (B), or 6 days (C) and were sacrificed 1 h after injection with EdU. Cross-sections of the intestine from the resulting tadpoles were double-stained for Lgr5 by in situ hybridization and for EdU. Higher magnifications of boxed areas in (A–C) are shown in (a′–c′). The dotted lines depict the epithelium-mesenchyme boundary (see Fig. 1). Note that the clusters (islets) of EdU labeled cells in the epithelium after 6 days of T3 treatment had high levels of Lgr5 mRNA (c, c′)
Lgr5 and EdU co-stain the clusters (islets) of proliferating adult intestinal epithelial cells at the climax of natural metamorphosis. Tadpoles at premetamorphic stage 54 (A), climax (B stage 62), and end of metamorphosis (C stage 66) were injected with EdU 1 h before being sacrificed. Cross-sections of the intestine from the resulting tadpoles were double-stained for EdU and Lgr5. Higher magnifications of boxed areas in (A–C) are shown in (a′–c′). The dotted lines depict the epithelium-mesenchyme boundary (see Fig. 1)
Apoptotic and proliferating cells represent distinct populations of epithelial cells at the climax of metamorphosis
T3 induces both larval epithelial cell death and adult epithelial development. We next used double-labeling to simultaneously detect apoptotic cells with TUNEL and proliferating cells with EdU. Consistent with earlier reports [27], after 3 days of T3 treatment of premetamorphic tadpoles, larval epithelial cell death could be detected by TUNEL (Fig. 7B) and few apoptotic cells were detected after 6 days, although many EdU positive cells were present (Fig. 7C). No co-staining of any cells by TUNEL and EdU was detected throughout the T3 treatment. Similarly, during natural metamorphosis, there were many TUNEL and EdU positive cells at stage 60 (climax of metamorphosis) but no co-stained cells were detected (Fig. 8B, b′). Like what was observed during T3 treatment, apoptotic labeling peaked before cell proliferation, with little apoptotic signal detected by stage 62 when EdU labeling was the strongest (Fig. 8C, c′). These findings indicate that T3-induced apoptosis occurs earlier than the massive proliferation of the adult stem cells during metamorphosis and that the proliferating cells and apoptotic cells are distinct cell populations in the intestinal epithelium at the climax of metamorphosis.
EdU and TUNEL-labeling reveals that apoptotic and proliferating cells are non-overlapping epithelial cells during T3-induced intestinal metamorphosis. Premetamorphic stage 54 tadpoles treated with 10 nM T3 for 0 (A), 3 (B), or 6 days (C) and were sacrificed 1 h after injection with EdU. Cross-sections of the intestine from the resulting tadpoles were double-stained for apoptosis by TUNEL and for EdU. Higher magnifications of boxed areas in (A–C) are shown in (a′–c′). The dotted lines depict the epithelium-mesenchyme boundary (see Fig. 1). Note that apoptosis in the epithelium occurred prior to the appearance of the clusters (islets) of EdU labeled cells and in distinct epithelial cells during T3 treatment (C, c′)
Epithelial apoptosis takes place prior to the appearance of clusters (islets) of proliferating adult intestinal stem cells during natural metamorphosis. Tadpoles at premetamorphic stage 54 (A), climax (B stage 60, C stage 62), and end of metamorphosis (D stage 66) were injected with EdU 1 h before being sacrificed. Cross-sections of the intestine from the resulting tadpoles were double-stained for apoptosis by TUNEL and for EdU. Higher magnifications of boxed areas in (A–D) are shown in (a′–d′). The dotted lines depict the epithelium-mesenchyme boundary (see Fig. 1)
Conclusion
Adult organ-specific stem cells are critical for organ homeostasis, repair, and regeneration and mis-regulation of such stem cells often leads to diseases such as cancer. Thus, extensive studies have been carried out to understand the regulation of organ-specific stem cells as well as cancer stem cells [28–36]. Intestinal remodeling during amphibian metamorphosis resembles the maturation of mammalian intestine around birth and thus has served as a model to study the development of adult organ-specific stem cells in vertebrates [2, 4, 5, 9, 37–43]. The advancements in genetic approaches for gene function studies in vivo, such as the knockout and knockin in Xenopus [44–46], undoubtedly further enhance the value of this unique model system for studying adult organ-specific stem cells. While earlier single labeling studies have provided valuable information for analyzing cell transformations in the epithelium, the lack of double-labeling has hindered analyses and/or interpretations regarding adult stem cells. Here, we have adapted different protocols that allowed us to double label different epithelial cells with several combinations of different labeling methods, including chemical labeling with EdU, staining with MGPY, in situ hybridization, and immunohistochemistry. These have allowed us to directly demonstrate experimentally for the first time that adult intestinal stem cells formed during metamorphosis are the proliferating cell clusters formed at the climax of metamorphosis. Considering our earlier findings that the adult stem cells are derived from larval epithelium [12, 19], our double-labeling studies of proliferating and apoptotic cells indicate that in response to T3, the epithelial cells take two mutually exclusive pathways, apoptosis or dedifferentiation followed by proliferation, which leads to the formation of the adult intestinal epithelium. Finally, our findings here pave the way to use any one of the labeling methods in this study to analyze stem cell development during metamorphosis.
Methods
Animals and treatments
Wild-type X. laevis tadpoles were reared in the laboratory or purchased from Nasco or Xenopus 1. The tadpoles were staged based on Nieuwkoop and Faber [47]. Premetamorphic X. laevis tadpoles at stage 54 were treated with 10 nM T3 for 0–6 days at 18 °C. At least 3 tadpoles were analyzed for each stage or day of T3 treatment. All animal studies were done in accordance with the guidelines established by the National Institute of Child Health and Human Development Animal Use and Care Committee.
In situ hybridization
The in situ probe for Lgr5 was made as described previously [13]. Intestinal fragments were isolated from the anterior part of the small intestine of tadpoles at indicated stages, fixed in 4 % MOPS/EGTA/magnesium sulfate/formaldehyde buffer (MEMFA), followed by cryosectioning. Tissue sections cut at 7 µm were subjected to in situ hybridization by using the antisense probe as previously described [48]. For double staining with EdU staining, the sections were first processed for digoxygenin in situ hybridization, and then the slides were washed in 1× phosphate buffered saline plus 0.05 % Tween-20 for 5 min, followed by EdU staining.
5-Ethynyl-2′-deoxyuridine (EdU) labeling
EdU staining was performed as described [49]. Briefly, 6.7, 40 and 40 µL of 2.5-mg/mL EdU were injected into stage 54, 62, and 66 tadpoles, respectively. One hour after injection, the tadpoles were sacrificed, and the intestine was fixed in 4 % MEMFA and processed for cryosectioning. EdU was detected by using the Click-iT Plus EdU Alexa Fluor 594 Imaging kit (Life Technologies) according to supplier’s instructions.
Immunohistochemistry
To identify differentiated intestinal absorptive cells, the sections were incubated with the rabbit anti-IFABP (intestinal fatty acid binding protein) antibody (diluted 1:500; [22]) overnight at 4 °C. Samples were washed several times with 1× phosphate buffered saline and primary antibodies were detected by using Alexa Fluor 568 Goat Anti-Rabbit IgG (H+L) Antibody (diluted 1:100; molecular probes). For double labeling with EdU staining, the sections were first processed for immunostaining, and then the slides were washed in 1× phosphate buffered saline plus 0.05 % Tween-20 for 5 min, followed by EdU staining.
Methyl green-pyronin Y (MPGY) staining
Sections were stained with MPGY (Muto), a mixture of methyl green, which binds strongly to DNA, and pyronin Y, which binds strongly to RNA, for 5 min at room temperature [22]. Adult epithelial stem/progenitor cells were intensely stained red because of their RNA-rich cytoplasm [3]. For double staining with EdU labeling, the sections were first processed for EdU staining. After photographing the EdU labeling, the slides were washed in 1X Phosphate Buffered Saline plus 0.05 % Tween-20 for 5 min, followed by MPGY staining. The image of the MPGY staining was taken. The images from MPGY and EdU staining from the same slide were merged by using Adobe Photoshop CS5.1 to determine whether MPGY and EdU labeled the same cells.
TUNEL assays
TUNEL (terminal deoxyribonucleotidyl transferase-mediated dUTP-biotin nick end labeling) assays were performed by using DeadEnd™ Colorimetric TUNEL System (Promega) as described [50]. For double staining, EdU staining was performed after the TUNEL assays.
Abbreviations
- T3:
-
Thyroid hormone
- TR:
-
Thyroid hormone receptor
- IFABP:
-
Intestinal fatty acid binding protein
- TUNEL:
-
Terminal deoxyribonucleotidyl transferase-mediated dUTP-biotin nick end labeling
- MPGY:
-
Methyl green pyronin Y
- EdU:
-
5-Ethynyl-2′-deoxyuridine
References
Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. The development of the adult intestinal stem cells: insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci. 2011;1(1):30.
Ishizuya-Oka A, Shi YB. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci. 2011;1(1):37.
Ishizuya-Oka A, Shi YB. Regulation of adult intestinal epithelial stem cell development by thyroid hormone during Xenopus laevis metamorphosis. Dev Dyn. 2007.
Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I, van den Bergh Weerman MA, O’Carroll D, Hardwick JC, Hommes DW, van den Brink GR. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun. 2011;2:452.
Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci USA. 2011;108(26):10585–90.
Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: embryogensis and remodeling during metamorphosis. Curr Topics Dev Biol. 1996;32:205–35.
Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux’s Arch Dev Biol. 1992;201:322–9.
Ishizuya-Oka A, Shimozawa A. Inductive action of epithelium on differentiation of intestinal connective tissue of Xenopus laevis tadpoles during metamorphosis in vitro. Cell Tissue Res. 1994;277(3):427–36.
Hasebe T, Fu L, Miller TC, Zhang Y, Shi YB, Ishizuya-Oka A. Thyroid hormone-induced cell-cell interactions are required for the development of adult intestinal stem cells. Cell Biosci. 2013;3(1):18.
Schreiber AM, Mukhi S, Brown DD. Cell–cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331(1):89–98.
Schreiber AM, Cai L, Brown DD. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci USA. 2005;102(10):3720–5.
Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi YB. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. Faseb J. 2009;23:2568–75.
Sun G, Hasebe T, Fujimoto K, Lu R, Fu L, Matsuda H, Kajita M, Ishizuya-Oka A, Shi YB. Spatio-temporal expression profile of stem cell-associated gene LGR5 in the intestine during thyroid hormone-dependent metamorphosis in Xenopus laevis. PLoS One. 2010;5(10):e13605.
Shi Y-B. Amphibian metamorphosis: from morphology to molecular biology. New York: John Wiley & Sons, Inc.; 1999.
Dodd MHI, Dodd JM. The biology of metamorphosis. In: Lofts B, editor. Physiology of the amphibia. New York: Academic Press; 1976. p. 467–599.
McAvoy JW, Dixon KE. Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool. 1977;202:129–38.
Marshall JA, Dixon KE. Cell proliferation in the intestinal epithelium of Xenopus laevis tadpoles. J Exp Zool. 1978;203:31–40.
Marshall JA, Dixon KE. Cell specialization in the epithelium of the small intestine of feeding Xenopus laevis tadpoles. J Anat. 1978;126(1):133–44.
Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells. 2011;29(1):154–61.
Ishizuya-Oka A, Ueda S. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 1996;286(3):467–76.
Ishizuya-Oka A, Shimizu K, Sakakibara S, Okano H, Ueda S. Thyroid hormone-upregulated expression of Musashi-1 is specific for progenitor cells of the adult epithelium during amphibian gastrointestinal remodeling. J Cell Sci. 2003;116(Pt 15):3157–64.
Ishizuya-Oka A, Ueda S, Damjanovski S, Li Q, Liang VC, Shi Y-B. Anteroposterior gradient of epithelial transformation during amphibian intestinal remodeling: immunohistochemical detection of intestinal fatty acid-binding protein. Dev Biol. 1997;192(1):149–61.
Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974;141(4):503–19.
Al-Nafussi AI, Wright NA. Cell kinetics in the mouse small intestine during immediate postnatal life. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;40(1):51–62.
Schmidt GH, Winton DJ, Ponder BA. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988;103(4):785–90.
Shi Y-B, Hayes WP. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol. 1994;161:48–58.
Buchholz DR, Tomita A, Fu L, Paul BD, Shi Y-B. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol. 2004;24:9026–37.
de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–91.
Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–59.
Sun G, Fu L, Shi Y-B. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci. 2014;4:73.
Tarayrah L, Chen X. Epigenetic regulation in adult stem cells and cancers. Cell Biosci. 2013;3(1):41.
Huang J. Thematic series: epigenetics in stem cells and cancer. Cell Biosci. 2013;3(1):38.
Gu B, Lee MG. Histone H3 lysine 4 methyltransferases and demethylases in self-renewal and differentiation of stem cells. Cell Biosci. 2013;3(1):39.
Chen J, Li Q. Enhancing myogenic differentiation of pluripotent stem cells with small molecule inducers. Cell Biosci. 2013;3(1):40.
Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–84.
van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60.
Wen L, Hasebe T, Miller TC, Ishizuya-Oka A, Shi YB. A requirement for hedgehog signaling in thyroid hormone-induced postembryonic intestinal remodeling. Cell Biosci. 2015;5:13.
Luu N, Wen L, Fu L, Fujimoto K, Shi YB, Sun G. Differential regulation of two histidine ammonia-lyase genes during Xenopus development implicates distinct functions during thyroid hormone-induced formation of adult stem cells. Cell Biosci. 2013;3(1):43.
Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol Cell Biol. 2001;21(14):4761–72.
Flamant F, Poguet AL, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRalpha gene. Mol Endocrinol. 2002;16(1):24–32.
Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem. 2009;284(2):1234–41.
Plateroti M, Chassande O, Fraichard A, Gauthier K, Freund JN, Samarut J, Kedinger M. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology. 1999;116(6):1367–78.
Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi YB. Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol. 2010;11(5):R55.
Wang F, Shi Z, Cui Y, Guo X, Shi YB, Chen Y. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 2015;5:15.
Lei Y, Guo X, Deng Y, Chen Y, Zhao H. Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci. 2013;3(1):21.
Shi Z, Wang F, Cui Y, Liu Z, Guo X, Zhang Y, Deng Y, Zhao H, Chen Y. Heritable CRISPR/Cas9-mediated targeted integration in Xenopus tropicalis. FASEB J. 2015 (in press).
Nieuwkoop PD, Faber J. Normal table of Xenopus laevis, 1st edn. Amsterdam: North Holland Publishing; 1956.
Hasebe T, Hartman R, Matsuda H, Shi YB. Spatial and temporal expression profiles suggest the involvement of gelatinase A and membrane type 1 matrix metalloproteinase in amphibian metamorphosis. Cell Tissue Res. 2006;324:105–16.
Okada M, Miller TC, Fu L, Shi YB. Direct activation of amidohydrolase domain-containing 1 gene by thyroid hormone implicates a role in the formation of adult intestinal stem cells during Xenopus metamorphosis. Endocrinology. 2015;156:3381–93.
Hasebe T, Kajita M, Fujimoto K, Yaoita Y, Ishizuya-Oka A. Expression profiles of the duplicated matrix metalloproteinase-9 genes suggest their different roles in apoptosis of larval intestinal epithelial cells during Xenopus laevis metamorphosis. Dev Dyn. 2007;236(8):2338–45.
Authors’ contributions
MO, LW, TCM, and DS designed and carried out experiments and interpreted the findings. MO and YBS prepared the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the intramural Research Program of NICHD, NIH. MO was supported in part by Japan Society for the Promotion of Science (NIH) Fellowship.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Morihiro Okada and Luan Wen contributed equally
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Okada, M., Wen, L., Miller, T.C. et al. Molecular and cytological analyses reveal distinct transformations of intestinal epithelial cells during Xenopus metamorphosis. Cell Biosci 5, 74 (2015). https://doi.org/10.1186/s13578-015-0065-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-015-0065-3