Abstract
Fermentation of chemicals from lignocellulose hydrolysate is an effective way to alleviate environmental and energy problems. However, fermentation inhibitors in hydrolysate and weak inhibitor tolerance of microorganisms limit its development. In this study, atmospheric and room temperature plasma mutation technology was utilized to generate mutant strains of Enterobacter cloacae and screen for mutants with high inhibitor tolerance to acid hydrolysate of corncobs. A highly inhibitor-tolerant strain, Enterobacter cloacae M22, was obtained after fermentation with non-detoxified hydrolysate, and this strain produced 24.32 g/L 2,3-butanediol and 14.93 g/L organic acids. Compared with that of the wild-type strain, inhibitor tolerance was enhanced twofold with M22, resulting in improvement of 2,3-butanediol and organic acid production by 114% and 90%, respectively. This work presents an efficient method to screen for highly inhibitor-tolerant strains and evidence of a novel strain that can produce 2,3-butanediol and organic acids using non-detoxified acid hydrolysate of corncobs.
Similar content being viewed by others
Introduction
2,3-Butanediol (2,3-BDO) is a staple chemical material and liquid fuel that is used extensively in the medical, food, and aerospace industries. 2,3-BDO can be used to manufacture flavour agents, edible spices, and solvents (Garg and Jain 1995; Tran and Chambers 1987; Zeng et al. 2016). The traditional chemical method used tetracarbonic hydrocarbons produced by petroleum pyrolysis as raw material and then obtained 2,3-butanediol by hydrolysis at high temperature and pressure. Dependence on petroleum resources and high production costs have limited the development of chemical method. Biological fermentation is not dependent on oil resources and therefore is considered a green route for 2,3-BDO production, with carbohydrates converted to 2,3-BDO by microorganisms under mild conditions. Fermentation is accompanied by the formation of organic acids such as formic acid, acetic acid, and succinic acid, which also have many applications. In recent years, succinic acid has been extensively used in the synthesis of biodegradable polymers such as polybutylene succinate (PBS) and polyamide (Debuissy et al. 2017; Jiang et al. 2014).
The feedstock for 2,3-BDO and organic acid fermentation has been a focus of study worldwide. Typical 2,3-BDO fermentation used sugars such as glucose and xylose as substrates. Because of the high cost of sugars, some cheap feedstock such as starch, molasses, glycerol, and lignocellulosic materials have been utilized as substrates for 2,3-BDO fermentation (Bialkowska and Aneta 2016). Lignocellulosic materials are considered the most plentiful feedstock and an alternative for 2,3-BDO and organic acid production. The hemicellulose fraction accounts for 20–35% of lignocellulose materials, and hemicellulose is prone to being hydrolysed into monosaccharides with diluted acid pretreatment. However, the formation of by-products (e.g. formic acid, acetic acid, furfural, and 5-hydroxymethyl furfural) always accompanies dilute acid pretreatment of lignocellulose, which inhibits microbial growth and fermentation (Lloyd and Wyman 2005). Therefore, these inhibitors need to be removed when using dilute acid hydrolysate of lignocellulose as a substrate for 2,3-BDO fermentation. Many processes such as activated carbon adsorption, calcium neutralization, and organic solvent extraction have been applied to detoxification of the dilute acid hydrolysate of lignocellulose (Cheng et al. 2010; Jiang et al. 2012). However, the process of detoxification often leads to the loss of sugar, which reduces production yield. Another disadvantage of this process is the consumption of additional reagents that cannot be recycled.
The high raw material and operating costs of hydrolysate detoxification make fermentation uneconomical. Thus, using non-detoxified acid hydrolysate as a raw material for fermentation with inhibitor-tolerant strains seems attractive (Schirmer-Michel et al. 2009; Varga et al. 2004). Most wild-type microbes have low inhibitor tolerance. Mutation breeding methods are effective in improving strain characteristics. Many physical and chemical mutation methods involving ultraviolet radiation, X-ray, sodium azide, and diethyl sulphate have been reported in the literature (Bhagwat and Duncan 1998; Wang et al. 2007). However, traditional mutation methods are detrimental to health and have low mutation efficiency.
Atmospheric and room temperature plasma (ARTP) mutation is a novel technique that can be applied to the breeding of bacteria, fungi, yeast, actinomycetes, and microalgae (Zhang et al. 2014a), is easy and safe to perform, and has high mutation efficiency. Wang et al. used ARTP to mutate Streptomyces avermitilis and obtained a mutant with a higher abamectin yield (Wang et al. 2010). ARTP mutation was also applied to the breeding of Crypthecodinium cohnii for extracellular polysaccharide production (Liu et al. 2015). Cao et al. used the oleaginous microalgae Chlorella pyrenoidosa as the parent strain and, by ARTP, obtained a mutant with high lipid productivity. The dry weight and lipid productivity of the mutant strain were increased by 22.07% and 16.85%, respectively (Cao et al. 2017). These results confirm that ARTP is an efficient mutagenesis tool for microbial breeding.
Many microorganisms such as Enterobacter, Klebsiella, Bacillus, Aeromonas, Pseudomonas, Serratia have been employed for 2,3-BDO production (Garg and Jain 1995; Bialkowska 2016). Enterobacter cloacae is considered to be one of the most promising strain for industrial application because of its stable performance under a wide range of environmental conditions and prominent tolerance to inhibitors. In addition, Enterobacter cloacae can use an extensive range of substrates to produce 2,3-BDO. The ability of Enterobacter cloacae to produce 2,3-BDO and succinic acid have been investigated by our group. Enterobacter cloacae can produce 77.1 g/L 2,3-BDO and 28.1 g/L succinic acid from glucose. When using xylose as substrate, a maximum of 40.67 g/L 2,3-BDO and 21.79 g/L succinic acid were obtained (Cheng et al. 2013; Wu et al. 2018). So, Enterobacter cloacae was used as the original strain in this study. Enterobacter cloacae cannot grow at the original pH of hydrolysate which is about 2. Appropriate pH of cell growth and 2,3-BDO formation is 6.5. So the tolerance of Enterobacter cloacae to hydrolysate in this study was conducted at pH 6.5.
In this study, the ARTP mutation technique was used to create a library of Enterobacter cloacae mutants to screen for strains with high tolerance to inhibitors in corncob acid hydrolysate. The mutant strains were then used to produce 2,3-BDO and organic acids with non-detoxified corncob acid hydrolysate as the substrate. The selected mutant strain may be applied to biological detoxification of dilute acid hydrolysate of lignocellulose in other fermentation processes.
Materials and methods
Strain and media
The original strain used in this study was E. cloacae CICC 10011, which was purchased from the China Center of Industrial Culture Collection (CICC). The E. cloacae M22 obtained in this study has been deposited in China General Microbiological Culture Collection Center (CGMCC) and the preservation number is CGMCC NO. 17265.
The seed medium consisted of 30 g/L xylose, 2 g/L (NH4)2SO4, 4.4 g/L K2HPO4, 1.3 g/L KH2PO4, 1 g/L yeast extract, 0.2 g/L MgSO4·7H2O, 1 mL/L trace element solution, and 2 mL/L Fe solution. The trace element solution contained 70 mg/L ZnCl2, 0.1 g/L MnCl2·4H2O, 60 mg/L H3BO3, 0.2 g/L CoCl2·2H2O, 20 mg/L CuCl2·2H2O, 25 mg/L NiCl2·6H2O, 35 mg/L Na2MoO4·2H2O, 0.9 mL/L HCl, and Fe solution containing 5 g/L FeSO4·7H2O, and 4 mL/L HCl. The pH of seed medium was 6.5.
Acid hydrolysate of corncobs was diluted to various concentrations and used as fermentation medium containing 1.5 g/L yeast extract.
Corncob pretreatment
The corncobs used as raw material for hydrolysis of dilute acids was obtained from Shandong province in China. The particle size of the corncobs was 20–40 mesh, and the main components of corncob are 2.67 ± 0.04% benzene-ethanol extractives, 40.46 ± 0.45% cellulose, 32.87 ± 0.48% hemicellulose, 17.12 ± 0.34% lignin.
Corncob particles were pre-treated with a mixed acid solution (1.5% sulfuric acid + 0.5% phosphoric acid), loaded into 500-mL flasks with a loading capacity of 30 g and 90 g, respectively, and then heated to 130 °C for 1 h in an autoclave. The hydrolysate and solid residues were separated by filtration, and the filter cake was washed with 60 mL distilled water to increase the amount of sugars collected. The pH of the hydrolysate was adjusted to 5.5 with NaOH and 6.5 with ammonia.
Tolerance of Enterobacter cloacae wild-type strains to hydrolysate
Seed culture and fermentation experiments were carried out in a 500-mL flask. The strains were inoculated into 50 mL seed culture medium, and the flasks were cultivated on a shaker at 30 °C and 150 rpm for 14–16 h. Then, the seeds were inoculated into fermentation medium containing 25%, 50%, 75%, and 100% corncob acid hydrolysate at an inoculation rate of 10% and cultivated on a shaker at 35 °C and 150 rpm for fermentation. CaCO3 (5 g/L) was used as a pH buffer during fermentation.
Atmospheric and room temperature plasma mutation of Enterobacter cloacae
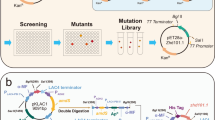
The ARTP mutation breeding system was supplied by Professor Xinhui Xing of Tsinghua University and consisted of a coaxial type plasma generator, gas supply control subsystem, radiofrequency power supply, and sample plate made of stainless steel (Zhang et al. 2014b). The experimental process is shown in Fig. 1. Bacteria reached logarithmic growth (OD ≈ 1) before mutation. Ten microliters of the bacterial liquid was pipetted onto a sterilized sample plate and then treated for 0, 60, 120, 180, 240, 300, or 360 s. The helium gas flow was 10 standard litres per minute, and plasma was induced at 120 W.
After ARTP mutation, the cells on the stainless-steel plates were washed with fresh seed culture medium and then cultivated on a shaker at 30 °C and 150 rpm for 1 h. The activated bacteria were properly diluted, and 100 μL bacterial suspension was coated on each plate with 75% diluted acid hydrolysate. The plates were placed in an incubator for 24 h at 30 °C, and then the numbers of surviving clones were counted.
Fermentation experiments
The seed cells were inoculated into 500-mL flasks containing 100 mL fermentation medium at 10% (v/v), then cultivated on a shaker at 35 °C and 150 rpm for fermentation. The pH was buffered with 5 g/L GaCO3. Each data point is the average of two replicates.
Fermentation was performed in a 1-L fermenter at 35 °C and 500 rpm with ventilation of 0.2 vvm. The seed culture was transferred to 500 mL fermentation medium with an inoculation volume of 10%, and the pH was maintained at 6.5 throughout fermentation by adding 4 mol/L sodium hydroxide solution with an automatic controller.
Analytical methods
Cell concentration was monitored by measuring the optical density at 600 nm (OD600) with a UV spectrophotometer (JingHua 7600CRT, China). Then, components of the hydrolysate and fermentation products were analysed using a high-performance liquid chromatograph (HPLC, Shimadzu LC-20AT, Japan). The components were separated in an Aminex HPX-87H ion exclusion column (Bio Rad, USA) at 65 °C, and 5 mM H2SO4 was used as the mobile phase with a flow rate of 0.8 mL/min. Concentrations of glucose, xylose, formic acid, acetic acid, succinic acid, and 2,3-BDO were determined using an RI detector. HMF and furfural were analysed with a UV detector at 210 nm.
Results
Corncob pretreatment
Table 1 shows the main compounds of the dilute sulphuric acid hydrolysate from corncobs. The concentration of total sugars was 67.57 g/L, and the mass ratio of glucose, xylose, and arabinose was 1:8:1.2. Xylose was the main carbon source and was attributable to the degradation of hemicellulose during dilute acid pretreatment. The amount of acetic acid was greater than that of the other inhibitors.
Tolerance of Enterobacter cloacae wild-type strains to hydrolysate
Culture medium containing 25%, 50%, 75%, or 100% acid hydrolysate from corncobs was used as a substrate to investigate the tolerance of E. cloacae to hydrolysate. In addition, culture medium containing same sugar concentration as hydrolysate were used to study the effects of different sugar concentrations on cell growth. The results showed that sugar concentration had no significant effect on cell growth (Fig. 2), which proved that the sugars in hydrolysate have no inhibitory effect on cell growth within the range of concentration investigated. Tables 2 and 3 show the concentration of each component before and after fermentation, respectively. Use of 25% hydrolysate resulted in no obvious inhibition of E. cloacae. The sugar consumption achieved 99.01% at 18 h with a sugar consumption rate of 0.83 g/L/h. The final concentration of 2,3-BDO was 6.27 g/L. The 2,3-BDO productivity and yield were 0.35 g/L/h and 0.42 g/g sugar, respectively. The initial concentrations of formic acid and acetic acid were 0.17 g/L and 1.68 g/L, respectively, in 25% hydrolysate. The production of organic acids during fermentation were 0.82 g/L succinic acid, 0.04 g/L formic acid and 3.05 g/L acetic acid. When 50% hydrolysate was used as the substrate, the utilization of sugar decreased to 85% and the fermentation time was extended to 40 h due to relatively high inhibition. The sugar consumption rate was only 0.69 g/L/h. The production of 2,3-BDO increased to 10.84 g/L but the the 2,3-BDO productivity and yield decreased to 0.27 g/L/h and 0.39 g/g sugar, respectively. The values of organic acids in Table 3 were the final concentrations in the fermentation broth. The actual amount of organic acids formed during fermentation were 1.43 g/L succinic acid, 0.02 g/L formic acid and 5.47 g/L acetic acid. Use of 75% hydrolysate almost completely inhibited the growth of E. cloacae. Only 2% of sugar was utilized, and cell growth only reached 0.53. Therefore, 75% hydrolysate was used as the preliminary screening medium.
Mutation and primary screening of the mutants
The mutants were screened by culture and fermentation, and, in general, a lethality rate of 90–99% was considered appropriate. In this study, an efficient and rapid screening method of directional screening on plates containing 75% diluted acid hydrolysate was developed. The number of colonies on plates after different treatment times is shown in Fig. 3. E. cloacae was subjected to ARTP mutation for times ranging from 60 to 360 s. Untreated E. cloacae, used as a control, resulted in 30 surviving clones. The corresponding numbers of surviving colonies were 0, 4, 193, 18, 10, and 1 when the treatment times were 60, 120, 180, 240, 300, and 360 s, respectively. When the treatment time was less than 180 s, the positive mutation rate was low and few colonies grow on the plates due to the presence of inhibitors. The positive mutation rate reached the maximum at 180 s of treatment time and the number of colonies on the plate increased sharply. When the treatment time was greater than 180 s, most of the cells died because of the high lethal rate, and few colonies grew on the plate. Therefore, an ARTP mutation time of 180 s was considered optimal. One hundred and ninety-three colonies were selected from the solid medium and inoculated into test tubes containing 5 mL seed culture medium for 14 h incubation at 30 °C. Then, the bacterial fluid was streak-inoculated onto plates containing 100% hydrolysate medium. There were only 23 strains that grew on the 100% hydrolysate solid medium.
Treatment time is a key parameter in ARTP mutation. The optimum treatment time is determined by the original strain and screening conditions. For example, 60 s of treatment time is suitable for the mutation of Spirulina platensis to improve carbohydrate productivity (Fang et al. 2013), whereas Hua used LB agar plates containing 9.0% NaCl to screen for mutants of E. cloacae. At a treatment time of 2 min, 90% cell lethality was observed (Hua et al. 2010). As for the oleaginous microalgae Chlorella pyrenoidosa, when the treatment time was 40 s, 50 s, or 60 s, mortality rates reached 95.69%, 98.05%, and 99.88%, respectively (Cao et al. 2017).
Fermentation of 2,3-butanediol and organic acids by mutants with hydrolysate
The fermentation performance of 23 mutants was investigated with 100% hydrolysate as the substrate. Figure 4 shows the cell growth of mutants at 24 h. Only 10 mutants were able to grow steadily (OD600 > 6.0) and produce 2,3-BDO and organic acids. Another 13 mutants grew to varying degrees, but there were no fermentation products. Table 4 shows the fermentation results for the mutants. The sugar utilization of 10 mutants was higher than 80%, and their ability to ferment 2,3-BDO and organic acids with non-detoxified hydrolysate was much greater than that of the wild-type strain. Good genetic stability is essential for a mutant to be optimal for fermentation. Therefore, the stable fermentation performance of the mutants with non-detoxified hydrolysate was verified. The mutants were cultivated in seed medium for five generations and then inoculated into non-detoxified hydrolysate for fermentation. The inhibitor tolerance of most mutants decreased, with only mutant M22 maintaining greater fermentation ability (Table 5). The sugar utilization of M22 was 88.69%, and the production of 2,3-BDO and organic acids was 24.32 g/L and 14.93 g/L, respectively. Long-term stability of M22 is shown in Table 6. The 43 generations of M22 transmission still maintained good fermentation performance.
M22 was used for the co-production of 2,3-BDO and organic acids with non-detoxified hydrolysate in a 1-L fermenter. Sodium bicarbonate (5 g/L) was added at fermentation times of 12 and 36 h, respectively, to enhance the production of succinic acid. Previously, E. cloacae has been reported to show high co-production of 2,3-BDO and succinic acid from sugar (Cheng et al. 2013; Wu et al. 2018), and addition of bicarbonate salts to the fermentation medium can increase the amount of dissolved CO2, which is an indispensable substrate for the formation of succinic acid. The final concentration of succinic acid reached 7.33 g/L in this study. After fermentation, 1.02 g/L formic acid and 4.68 g/L acetic acid were produced. The final concentrations of 2,3-BDO and organic acids were 23.2 g/L and 19.93 g/L, respectively, the yield of 2,3-BDO was 0.44 g/g sugar. (Figure 5).
Discussion
The inhibition of fermentation inhibitors in acid hydrolysate on microorganisms limit the utilization of lignocellulose material. The mechanisms underlying inhibition of organic acids and furans have been reported. Undissociated acetic acid is the key factor for inhibition of other microorganisms (Cheng et al. 2005). Molecules of organic acids in a free state can freely shuttle through cell membranes, leading to acidification of the intracellular environment and inhibiting the synthesis of DNA and RNA (Parawira and Tekere 2011). In 2,3-BDO fermentation, a small amount of acetic acid has no obvious inhibitory effect; rather, it is beneficial (Wu et al. 2013a). However, the simultaneous presence of acetic acid and furfural strongly inhibits interactions (Wu et al. 2013b). Furan derivatives, mainly furfural and HMF, are dehydrated from pentose and hexose, respectively, in acidic environments. Their inhibitory effects on microorganisms are as follows: (i) they destroy the integrity of protoplasmic membranes and increase the permeability of cell membranes; (ii) they have direct inhibitory effects on various enzymes in the glycolysis pathway; (iii) they inhibit the synthesis or accelerate the degradation of NAD(P)H in cells; and (iv) dipole moments of large aldehyde groups result in a sharp rise in reactive oxygen in cells, which damages the cytoskeleton and inhibits cell growth (Allen et al. 2010; Feron et al. 1991; Liu et al. 2013; Miller et al. 2009). According to the literature, the furfural has higher negative effect of on biomass formation. The inhibitions of HMF and acetic acid were about 1/2 and 1/10, respectively, of that of furfural (Wu et al. 2013a). In this study, the furfural and HMF were converted to their less toxic alcohols, furfuryl alcohol and furan-2,5-diyldimethanol, respectively, during the fermentation. Phenolic compounds in hydrolysate are derived from lignin as it degrades. These compounds can destroy or decompose cytomembranes. In general, the smaller the substituents on aromatic rings, the greater the inhibition (Almeida 2010).
Fermentation of 2,3-BDO from non-detoxified biomass hydrolysate by Enterobacter has been reported previously (Table 7). Hazeena et al. used non-detoxified oil palm frond hydrolysate as the substrate for fermentation of 2,3-BDO by E. cloacae SG-1. The lag period of bacterial growth was longer than that of glucose because of inhibitors present in the hydrolysate, and the concentration of 2,3-BDO was 7.67 g/L (Hazeena et al. 2016). Joo et al. investigated the effects of inhibitory compounds on E. aerogenes. Three hydrolysates, yellow poplar, larix, and rice hull hydrolysates, were used as substrates to produce 2,3-BDO, and 14.27 g/L, 12.44 g/L, and 10.24 g/L, respectively, were produced (Joo et al. 2016). E. aerogenes ATCC 29007 also has the ability to resist inhibitors. With a Miscanthus hydrolysate containing 34.62 g/L of reducing sugar as a substrate, 11.00 g/L of 2,3-BDO was obtained (Lee et al. 2015).
In this study, ARTP mutation technology was used to generate mutants of E. cloacae to screen for a strain with high inhibitor tolerance to corncob acid hydrolysate. Fermentation performance and genetic stability with non-detoxified hydrolysate of one mutant, E. cloacae M22, was examined. Compared with that of the wild-type strain, inhibitor tolerance was enhanced twofold in M22. The wild-type strains can tolerate only 50% hydrolysate and produce 10.84 g/L 2,3-BOD and 6.92 g/L organic acids. The mutant strain M4 can tolerate 100% hydrolysate and produce 23.2 g/L 2,3-BOD and 13.03 g/L organic acids which increased by 114% and 88%, respectively. E. cloacae M22 showed super inhibitor tolerance, and 23.2 g/L 2,3-BDO was produced from non-detoxified corncob acid hydrolysate. This production yield was superior to that reported elsewhere (Hazeena et al. 2016; Joo et al. 2016; Lee et al. 2015).
References
Allen SA, Clark W, Mccaffery JM, Cai Z, Lanctot A, Slininger PJ, Liu ZL, Gorsich SW (2010) Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels 3(1):2
Almeida JRM (2010) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol. 82(4):340–349
Bhagwat B, Duncan EJ (1998) Mutation breeding of banana cv Highgate (Musa spp., AAA Group) for tolerance to Fusarium oxysporum f. sp. cubense using chemical mutagens. Sci Horticult 73(1):11–22
Bialkowska AM (2016) Strategies for efficient and economical 2,3-butanediol production: new trends in this field. World J Microbiol Biotechnol 32:200
Cao S, Zhou X, Jin WB, Wang F, Tu RJ, Han SF, Chen HY, Chen C, Xie GJ, Ma F (2017) Improving of lipid productivity of the oleaginous microalgae Chlorella pyrenoidosa via atmospheric and room temperature plasma (ARTP). Bioresour Technol 244:1400–1406
Cheng KK, Liu HJ, Liu DH (2005) Multiple growth inhibition of Klebsiella pneumoniae in 1, 3-propanediol fermentation. Biotechnol Lett 27(1):19–22
Cheng KK, Liu Q, Zhang JA, Li JP, Xu JM, Wang GH (2010) Improved 2,3-butanediol production from corn cob acid hydrolysate by fed-batch fermentation using Klebsiella oxytoca. Process Biochem 45(4):613–616
Cheng KK, Wu J, Wang GY, Li WY, Feng J, Zhang JA (2013) Effects of pH and dissolved CO2 level on simultaneous production of 2,3-butanediol and succinic acid using Klebsiella pneumoniae. Bioresour Technol. 135:500–503
Debuissy T, Sangwan P, Pollet E, Averous L (2017) Study on the structure-properties relationship of biodegradable and biobased aliphatic copolyesters based on 1,3-propanediol, 1,4-butanediol, succinic and adipic acids. Polymer 122:105–116
Fang MY, Jin LH, Zhang C, Tan YY, Jiang PX, Ge N, Li HP, Xing XH (2013) Rapid mutation of Spirulina platensis by a new mutagenesis system of atmospheric and room temperature plasmas (ARTP) and generation of a mutant library with diverse phenotypes. PLoS ONE 8(10):e77046
Feron VJ, Til HP, Vrijer FD, Woutersen RA, Cassee FR, Bladeren JV (1991) Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res 259:363–385
Garg SK, Jain A (1995) Fermentative production of 2,3-butanediol: a review. Bioresour Technol 51(3):103–109
Hazeena SH, Pandey A, Binod P (2016) Evaluation of oil palm front hydrolysate as a novel substrate for 2,3-butanediol production using a novel isolate Enterobacter cloacae SG1. Renew Energy 98:216–220
Hua XF, Wang J, Wu ZJ, Zhang HX, Li HP, Xing XH, Liu Z (2010) A salt tolerant Enterobacter cloacae mutant for bioaugmentation of petroleum- and salt-contaminated soil. Biochem Eng J 49(2):201–206
Jiang LQ, Fang Z, Guo F, Yang LB (2012) Production of 2,3-butanediol from acid hydrolysates of Jatropha hulls with Klebsiella oxytoca. Bioresour Technol 107:405–410
Jiang M, Chen GS, Lu P, Jian Dong (2014) Preparation of aqueous soluble polyamides from renewable succinic acid and citric acid as a new approach to design bio-inspired polymers. J Appl Polym Sci. https://doi.org/10.1002/APP.39807
Joo J, Lee SJ, Yoo HY, Kim YH, Jang M, Lee J, Han SO, Kim SW, Park C (2016) Improved fermentation of lignocellulosic hydrolysates to 2,3-butanediol through investigation of effects of inhibitory compounds by Enterobacter aerogenes. Chem Eng J 306:916–924
Lee SJ, Lee JH, Yang X, Kim SB, Lee JH, Yoo HY, Park C, Kim SW (2015) Phenolic compounds: strong inhibitors derived from lignocellulosic hydrolysate for 2,3-butanediol production by Enterobacter aerogenes. Biotechnol J 10(12):1920–1928
Liu CG, Xue C, Lin YH, Feng WB (2013) Redox potential control and applications in microaerobic and anaerobic fermentations. Biotechnol Adv 31(2):257–265
Liu B, Sun Z, Ma X, Yang B, Jiang Y, Wei D, Chen F (2015) Mutation breeding of extracellular polysaccharide-producing microalga Crypthecodinium cohnii by a novel mutagenesis with atmospheric and room temperature plasma. Int J Mol Sci 16(6):8201–8212
Lloyd TA, Wyman CE (2005) Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol 96(18):1967–1977
Miller EN, Jarboe LR, Turner PC, Pharkya P, Yomano LP, York SW, Numm D, Shanmugam KT, Ingram LO (2009) Furfural inhibits growth by limiting sulfur assimilation in ethanologenic Escherichia coli strain LY180. Appl Environ Microbiol 75(19):6132–6141
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31(1):20–31
Schirmer-Michel AC, Flores SH, Hertz PF, Ayub ZMA (2009) Effect of oxygen transfer rates on alcohols production by Candida guilliermondii cultivated on soybean hull hydrolysate. J Chem Technol Biotechnol 84(2):223–228
Tran AV, Chambers RP (1987) The dehydration of fermentative 2,3-butanediol into methyl ethyl ketone. Biotechnol Bioeng 29(3):343–351
Varga E, Klinke HB, Reczey K, Thomsen AB (2004) High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol. Biotechnol Bioeng 88(5):567–574
Wang XL, Li ZW, Chen LH, Liu XB, Xie HB (2007) The development of physical mutation techniques in industrial microbe breeding. Biotechnol Bull 2:114–118
Wang LY, Huang ZL, Li G, Zhao HX, Xing XH, Sun WT, Li HP, Gou ZX, Bao CY (2010) Novel mutation breeding method for Streptomyces avermitilis using an atmospheric pressure glow discharge plasma. J Appl Microbiol 108(3):851–858
Wu J, Cheng KK, Li WY, Feng J, Zhang JA (2013a) Effect of acetic acid, furfural and 5-hydroxymethylfurfural on fermentation of 2,3-butanediol using Klebsiella oxytoca. Chin J Biotechnol 29(3):350–357
Wu J, Cheng KK, Wang GY, Li WY, Feng J, Zhang JA (2013b) Analysis of acetic acid, furfural and 5-hydroxymethylfurfural affecting 2,3-butanediol production using Klebsiella oxytoca. J Chem Technol Biotechnol 88(12):2239–2243
Wu J, Liu HJ, Yan X, Zhou YJ, Lin ZN, Cheng KK, Zhang JA (2018) Co-production of 2,3-BDO and succinic acid using xylose by Enterobacter cloacae. J Chem Technol Biotechnol 93:1462–1467
Zeng F, Tenn WJ, Aki SNVK, Xu JY, Liu B, Hohn KL (2016) Influence of basicity on 1,3-butadiene formation from catalytic 2,3-butanediol dehydration over gamma-alumina. J Catal 344:77–89
Zhang X, Wang L, Zhang C, Chen Y, Chang H, Li H, Xing X (2014a) Recent progress on atmospheric and room temperature plasma mutation breeding technology and its applications. Ciesc J 65:2676–2684
Zhang X, Zhang XF, Li HP, Wang LY, Zhang C, Xing XH, Bao CY (2014b) Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl Microbiol Biotechnol 98(12):5387–5396
Authors’ contributions
JW carried out the writing and editing of manuscript. J-AZ carried out the review of manuscript and funding acquisition. Y-JZ and WZ carried out the sample detection. K-KC and H-JL carried out the data analysis and data curation. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2016YFE0131300) and the NSFC-NRCT project (No. 51861145103). We appreciate Prof. Xinhui Xing and his group for their help in ARTP mutation.
Ethics approval and consent to participate
This article does not contain any studies with human participants performed by any of the authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wu, J., Zhou, YJ., Zhang, W. et al. Screening of a highly inhibitor-tolerant bacterial strain for 2,3-BDO and organic acid production from non-detoxified corncob acid hydrolysate. AMB Expr 9, 153 (2019). https://doi.org/10.1186/s13568-019-0879-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-019-0879-1