Abstract
Due to the prominent role of the liver in the body and detoxification, its functionality can be affected in an irreversible manner by diseases. This phenomenon renders the liver to stop working, leading to morbidity and mortality. Therefore, liver transplantation is the only way to tackle this issue.
In order to compensate for the lack of adequate healthy liver tissue for transplantation, therapeutic approaches such as hepatocyte transplantation have been proposed as an alternative. Recognizing the fact that mesenchymal stem cells are adult stem cells with the capacity to differentiate into several cell types, different methods have been invented to produce hepatocyte-like cells from mesenchymal stem cells. They can be divided into three main categories, such as addition of cytokines and growth factors, genetic modifications, and adjustment of microenvironment as well as physical parameters.
In this review, we attempted to introduce diverse efficient methods for differentiating mesenchymal stem cells and their capability for transformation into hepatocyte-like cells.
Similar content being viewed by others
Introduction
The liver is designed to accomplish many tissue-dependent tasks such as detoxification of drugs and endogenous substrates, as well as synthesis of plasma proteins and bile. Parenchymal hepatocytes, consisting of 80% total hepatic volume, are the major cells that carry out the aforementioned functions [1, 2]. For end-stage liver-related suffering patients, transplantation is the only possible way. Many of these patients in the waiting lists die before transplantation; hence, other therapeutic approaches like hepatocyte transplantation and bioartificial liver (BAL) devices can be proposed as alternative treatments [3].
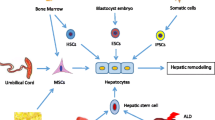
In order to take advantage of fundamental researches in the clinic to treat various degenerative and autoimmune liver diseases, a reliable source of functional cells is warranted [4]. Having enough knowledge about liver formation and embryogenesis can contribute to the development of differentiation and reprogramming protocols. Hepatocytes can be differentiated from embryonic stem cells, induced pluripotent stem cells (iPSC), and mesenchymal stem cells (MSCs), providing a potential source of functional cells for transplantation.
Stem cells (SCs) are said to have three principal characteristics: the ability for self-renewal, differentiation into multiple cell lineages, and functional activity in a given tissue in vivo [5]. According to their origin, they are classified into two categories. The cells that are risen form blastocyst stage, which are called embryonic stem cells (ESCs), while the ones that compose niches of mature adult tissues and bone marrow are known as adult stem cells [5]. MSCs are stem cells that are well-known for their proliferation and differentiation abilities in vitro [6]. Friedenstein et al. were the first to isolate MSCs in 1968 from the bone marrow and introduced them to the scientific community [7].
There are a variety of sources that MSCs can be collected from, which makes them an outstanding supply in order to apply them for cell therapy in liver diseases. There are various approaches for differentiation of MSCs into hepatocyte-like cells (HLCs) [8,9,10]. Therefore, in this review, different assessment methods for differentiation of MSCs into HLCs are categorized, which might elucidate the best strategy for researches and further clinical scale-up in the future.
MSC characteristics and sources
The International Society for Cellular Therapy (ISCT) has introduced criteria for the definition of MSCs including fibroblast-like morphology; plastic adherence; differentiation to adipocytes, osteoblasts, and chondroblasts; and positive expression of CD44, CD105, CD73, and CD90, with negative expression of CD45, CD34, and HLA-DR surface molecules [11]. It is worth mentioning that in vitro properties and surface molecular expressions might differ in MSCs from various origins [12].
Due to some significant characteristics of MSCs, the research in this field is growing exponentially. One of these significant characteristics is somehow easy isolation methods that can be utilized for standard culturing of MSCs [10]. Another important criterion of MSCs is their immunomodulatory properties. They can produce many cytokines while not having immunogenic properties. MSCs do not express or merely express low quantities of MHC class I and II antigens. Additionally, they lack B7 family co-stimulatory molecules that are essential for initiating immune responses [12]. According to these features, MSCs can be considered as a universal stem cell source for transplantation without immunological rejections and need for immunosuppression drugs. Lastly, the other significant character of MSCs is their differentiation capacity. MSCs can be differentiated into other mesodermal cell types like chondrocytes, adipocytes, and osteocytes in response to specific stimuli. Even, they can be transdifferentiated into tissues of all three embryonic layers [13,14,15] (Fig. 1). This capacity proposes a great clinical potential in regenerative medicine. In Table 1 and Additional file 1: Table S1, a comparison of different MSC sources and their differentiation potentials is summarized. It was reported that MSCs derived from specific sources exhibit preference in their differentiation pattern and scientists are investigating how the origin of MSCs might affect their final differentiation program [26]. Therefore, the capacity of MSCs in tissue regeneration might be related to the tissue sources, which they were collected from [4].
Bone marrow MSCs (BM-MSCs) are typically derived from bone marrow biopsy of the iliac crest, and also other BM cavities [27], but this procedure is painful. It is important to mention that BM progenitor cells lose their proliferative capacity during the aging process, which contributes to a significant decrease in their differentiation potency after age 20 [14].
MSCs can be isolated from adipose tissue (AT-MSCs) from different parts of the body, and the most common source is the discarded fatty tissues after liposuction or plastic surgeries [28]. Dental pulp-MSCs (DP-MSCs) have the capacity to be differentiated more easily toward neuronal lineage and are used in surveys correlated to Parkinson’s disease as well as neurodegenerative diseases, spinal cord injuries, Alzheimer’s diseases, and stroke [14]. Heart-derived MSCs (H-MSCs) exhibit higher levels of cardiovascular-related markers such as myosin light chain-2a [29].
MSCs can be isolated from different birth-associated tissues such as the placenta, amnion, chorion, umbilical cord (UC), and cord blood (CB). Also, it is suggested that MSCs derived from neonatal sources have more differentiating capabilities in comparison to adult sources [30]. Some researchers have elucidated that human placenta-derived MSCs have higher expansion and engraftment capacity than BM-MSCs [31, 32]. Placenta- and amnion-derived MSCs express pluripotency-related genes like Oct-4 and Nanog. They can be duplicated over 250 times and have long telomers [13, 33]. Also, it is important to consider that OCT4 upstream region is important in pluripotency in early embryo development and the establishment of embryonic stem cells [34].
Unlike peripheral blood that contains low number of MSCs if any, 10–30% of umbilical cord blood cells are composed of MSCs [14, 35]. These cells have high proliferative activity, and after several passages, they do not show any signs of senescence [36]. A jelly-like matrix material exists inside the cord that guards the cord arteries and the veins, which was initially described by Thomas Wharton in 1656 (“Wharton’s jelly”). Wharton’s jelly (WJ)-derived MSCs (Fig. 2) migrate through the developing cord, during embryogenesis, and some of these cells are trapped that can contribute to the cellular composition of the cord [13].
The structure of the human umbilical cord with a three-dimensional exploded diagram. The diagram is made by direct tracing the outlines of various features in the histological section, then shifting them along the tilted longitudinal axis. Scale bar = 5 mm [15]
The molecular embryogenesis of the liver
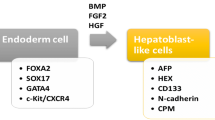
In the production of the liver parenchymal cells, the anterior part of the definitive endoderm (DE) is accountable, during gastrulation of the embryo. When the foregut closes, the progenitor cells which are trapped inside lie near the cells that create the heart and also regions of lateral plate mesoderm (LPM) that finally generate the proepicardium and septum transversum (STM). Fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) are different temporally regulated signals from cardiac mesoderm and STM respectively, that are responsible for this induction [37]. Hepatic induction starts following an FGF signal, that activates the RAS/MAPK pathway. Several FGFs (FGF1, 2, 8, and 10) are detected in the cardiac mesoderm during the beginning steps of hepatogenesis [38].
SMAD4 is the mediator of BMP signaling, which leads to histone, and subsequent transcriptional activation of liver-specific genes [39]. Studies in mouse embryos have shown that the hepatocyte nuclear factor beta (HNF1b) is one of the critical molecules during hepatic specification [40] (Fig. 3).
Molecular pathways in embryogenesis of the liver. Production of the liver parenchymal cells starts from the anterior part of the primary liver bud. FGF from cardiac mesoderm and BMP, which is mediated by SMAD4, interfere in hepatic induction through RAS/MAP kinase pathway and BMP signaling. GATA4 regulates expression of the secreted BMP4, which is highly expressed in the STM mesenchymal cells at the 8-somite stage. At early somite stages WNT signaling acts around 7–11 somites to repress the expression of Hhex. At around 21 somites, the matrix surrounding the basal surface of the epithelium is degraded, and E-cadherin expression is downregulated in the hepatic cells by the action of MMPs. GATA4 and/or GATA6 cause hepatoblast development by transactivating the Hhex promoter. Around 25 somites, Onecut-1 and Onecut-2 are redundantly essential for hepatoblast migration. Prox1 also promotes hepatoblast proliferation and migration from the primary liver bud. Tbx3 normally promotes a hepatocyte fate and represses a cholangiocyte fate through the expression of Hnf4a and c/EBPa. FGF fibroblast growth factor, HNF hepatocyte nuclear factor, BMP bone morphogenetic protein, Fox A Fork-head box protein A, Hhex hematopoietically expressed homeobox, STM septum transversum, MMPs matrix metalloproteinases, c/EBPa CCAAT-enhancer-binding proteins, Tbx3 T-Box 3, Prox1 prospero-related homeobox transcription factor
Liver bud is surround by STM mesenchymal cells that GATA4, a zinc finger transcription factor, is abundantly expressed inside them [41]. GATA4 regulates the expression of secreted BMP4 and is highly expressed like GATA4 in the STM mesenchymal cells at the 8-somite stage of mouse development [37]. Analyses in 2–4 somite stages revealed that Fork-head box protein A (FoxA) and GATA4 are expressed in the anterior endoderm and can attach to the albumin (ALB) enhancer before the onset of ALB expression [42], which following this attachment, the repositioning of nucleosomes happens [43].
In addition, the WNT signaling pathway gets involved during hepatic development through a very complex intervention. Studies have shown that canonical WNT signaling has different impacts, due to the developmental stage. In the primary stages of somite (4-6 somites) and in the posterior endoderm, expression of Hhex (Hematopoietically-expressed homeobox; a critical transcriptional regulator during hepatic development) is repressed as a result of WNT signaling action [44] (Fig. 3).
Hepatic differentiation is continued by active transcription of albumin regarding as the liver-specific gene in the primary stages of embryogenesis (day 8.5; E8.5) in the mouse ventral foregut endoderm, serving as the first line of evidence for hepatic differentiation [45]. And after that, the hepatic endoderm cells transform into a columnar morphology and start expressing some hepatic genes that are markers of early hepatic cell fate AFP (alpha fetoprotein), Ttr (transthyretin), Rbp (retinol binding protein), and Hnf4a, all of which are reliable indicators of early hepatic cell fate [46].
At around 21 somites in the mouse, following the onset of downregulation of E-cadherin expression, the degradation of the matrix covering the epithelium basal surface starts and these happenings result in the hepatic progenitor cells migration into the stroma (by the action of matrix metalloproteinases (MMPs)) occurs [47]. As previously mentioned, Hhex and WNT signaling participate in regulating the onset of hepatogenesis in different ways. At this stage, proliferation and positioning of the ventral endoderm which is placed in the cardiogenic field is regulated by Hhex. It seems that mentioned events regulate the induction of hepatic cell fate. In addition to mesodermal functions, which was already discussed, the transactivation of the Hhex promoter by GATA4 and/or GATA6 might contribute to hepatoblast development by transactivating the Hhex promoter [41].
Further transcriptional regulators have been considered due to their role in later events (around 25 somites and later). For instance, Onecut-1 (homeodomain factors HNF6) and Onecut-2 are redundantly essential for hepatoblast migration [47]. The prospero-related homeobox transcription factor (Prox1) also is responsible for hepatoblast proliferation and migration from the primary liver bud [48]. T-Box 3 (Tbx3) generally endorses a hepatocyte fate and suppresses cholangiocyte fate through the expression of Hnf4a and c/EBPa (CCAAT-enhancer-binding proteins) [49]. HNF4a controls apolipoprotein B and glucose metabolism through glucose-6-phosphatase and glycogen synthase, which both are essential for lipid metabolism [50].
During hepatogenesis, the hepatoblasts that migrate into the STM have the ability to differentiate into either cholangiocytes or hepatocytes. Cells that follow a hepatocyte cell fate progressively mature and make the liver bulk and the cholangiocytes make the biliary epithelial cells [51] (Fig. 3).
Around E10 of mouse development, migration of hematopoietic stem cells into the liver starts and hepatocyte maturation happens through signals from these hematopoietic cells, such as Oncostatin M (OSM) [52].
Therefore, according to different stages of hepatocyte maturation during embryogenesis, stepwise differentiation (ES cells → DE → hepatoblasts → immature hepatocytes → mature hepatocytes) adopted from liver developmental process was applied to produce in vitro HLCs (Fig. 4) (for review, visit ref. [16,17,18,19,20,21,22,23,24,25, 53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]).
In vitro hepatic differentiation pattern. Hepatocytes can be differentiated from ES cells in vitro by mimicking the developmental processes of liver formation. Stepwise differentiation (ES cells → DE → hepatoblasts → immature hepatocytes → mature hepatocytes) adopted from liver developmental processes was applied to produce in vitro HLCs. EGF epidermal growth factor, EGFR epidermal growth factor receptor, ERK1/2 extracellular-signal-regulated kinase 1/2, FGF fibroblast growth factor, HGF hepatocyte growth factor, ITS insulin-transferrin-selenium, MAPK mitogen-activated protein kinase, OSM oncostatin M, PI3K phosphoinositide 3-kinase, NTA nicotinamide, ActA Activin A, BMP bone morphogenetic protein
Using serum and other undefined culture medium components might be responsible for varying efficiency of hepatocyte differentiation from ES cells. Therefore, Si-Tayeb et al. introduced a hepatocyte differentiation protocol via reducing serum and application a well-defined culture medium components that can elicit the efficient and reproducible generation of hepatocytes from human ES [10] (for more examples ref. [16,17,18,19,20,21,22,23,24,25, 53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]). They claim that using their protocol can help researchers to obtain more than 80% definitive endodermal cells, subsequently more than 80% hepatoblasts, and finally about 80% albumin-expressing hepatocytes (based on flow cytometry), indicating 80% efficiency of hepatocyte differentiation from human ES.
Various in vitro techniques for the differentiation of MSCs into HLCs
According to studies, it is clarified that numerous signals through the extracellular matrix, growth factors, and even juxtacrine signals, which are produced by adjacent cells, regulate the cells’ behavior cooperatively [71]. The timing and distribution patterns of the mentioned signals are specific for each organ and developmental phase and are completely controlled. Consequently, it is vital to produce environments similar to the local environment for in vitro cultures to obtain more accurate results [72]. Previous reports revealed that MSCs derived from different sources can be differentiated into hepatocytes both in mice [73] and humans, using different protocols and techniques in vitro [9, 74].
Addition of cytokines and growth factors
Knowing that the liver is derived from the DE and nodal, a member of the transforming growth factor β (TGFβ) superfamily signaling is essential for endoderm specification. During development, the ventral foregut endoderm receives BMP and FGF from its surrounding tissues and gives rise to hepatoblasts. Therefore, Activin A treatment is followed by BMP2/4 and FGF2/4 treatment [75]. For further maturation, hepatocyte growth factor (HGF), oncostatin M, dexamethasone, and/or EGF were added into the culture medium (reviewed in reference [76, 77]) (Fig. 4).
Understanding the fact that growth factors can selectively alter cell function and are capable of promoting MSC proliferation, migration, and differentiation formed the foundation for many researches in tissue engineering [53, 54]. Accordingly, investigators have attempted different protocols to differentiate these cells into other cell types such as hepatocytes. It is noteworthy to say that some researchers prefer to use sequential differentiation step by step (firstly induction and then maturation), using different growth factors, cytokines, and biochemicals that have roles in every stage-like development and regeneration [25, 55].
Hepatic differentiation protocol with Iscove’s modified Dulbecco’s medium (IMDM) and sequential cytokine supplements (Fig. 4) is the most popular hepatic differentiation procedure. In the primary induction step, the induction of MSCs into endodermal cells is carried out by EGF and FGF. EGF stimulates the proliferation of MSCs by binding to EGF receptor (EGFR) [56]. FGF belongs to a family that is composed of at least seven closely related polypeptides and, has a critical role during the onset of endodermal patterning. Amongst these, FGF-4 and basic FGF (bFGF) are used regularly. Similar to EGF, FGF also has impact on the proliferation rate of MSCs [57].
FGF, HGF, nicotinamide (NTA), and insulin-transferrin selenium (ITS) are normally added to cultures in order to induce cell differentiation. HGF is a mesenchymal origin pleiotropic cytokine, involved in the regulation of proliferation, differentiation, and chemotactic migration of MSCs [58]. Forte et al. [59] presented that when MSCs are exposed to HGF in a short-term manner c-met receptor and it’s downstream effectors such as ERK1/2, p38, MAPK, and PI3K/Akt are activated. However, the long-term exposure can cause cytoskeletal rearrangement, cell migration, and marked inhibition of proliferation. Furthermore, ITS and NTA promote the proliferation and survival of primary hepatocytes [60].
OSM and dexamethasone (Dexa) are required to induce further maturation, together with the addition of FGF, ITS, and HGF. OSM plays an important role in progression of liver maturation from developing hepatocyte [61]. A study specified that the hepatic induction effects of OSM might be related to downregulation of sox, which enforces the proliferation by maintaining the pluripotency of stem/progenitor cells [62]. Dexa can induce the expression of both HNF4 and CCAAT/EBPα. These two mentioned transcription factors are crucial for differentiation of hepatocytes [63]. Also, trichostatin A and sodium butyrate that are histone deacetylase inhibitors contribute to stem cell hepatic differentiation. As a matter of fact, the expression of hepatocyte-specific genes and functions might be enhanced through histone deacetylase inhibitors [25, 64].
In this regard, there are multistep procedures that treat cells by growth factors in different cell sources. Some protocols prefer to separately add growth factor and cytokines. For instance, they used BMP and FGF family, followed by utilization of Dexa and OSM (sequentially) [16, 63]. Others have used single-step procedures that simultaneously expose cells to growth factors like HGF and EGF [65, 66]. Also, there are protocols that use a combination of both proliferation (such as EGF and HGF) and maturation factors (such as OSM, Dexa, and ITS) in two separate steps [9]. Table 1 summarizes some of these studies.
Some researchers chose AT-MSCs as the HLC differentiation source. They deduct that this source is multipotent fraction of adherent cells that attach to culture wares and remain there as a heterogenous population of fibroblast-like cells. They are similar to BM-MSCs in differentiation potential and, while being heterogenous, reveal a surface antigen marker profile [17,18,19,20, 66].
Banas et al. collected AT-MSCs from different age group patients and used growth factors like HGF, FGF1, and FGF-4 to differentiate them into hepatocytes. Their results showed that ACD105+ AT-MSCs has high hepatic differentiation ability and the differentiated HLCs could produce ALB, with the ability to uptake low-density lipoprotein (LDL) and ammonia detoxification. Also, these cells could be incorporated in the parenchyma of the liver after transplantation into mice [16]. In another research, a method was established to generate functional HLC clusters, using the floating culture method, which induces functional HLC clusters that function efficiently both in-vitro and in-vivo. The characteristics of produced HLC clusters were analyzed by gene expression analysis, functional assays, and transplantation into mouse afflicted with chronic liver injury. The result showed that cell clusters can express various genes that are commonly found on mature hepatocytes, and also displayed functional characteristics of hepatocytes such as ALB expression, urea secretion, cytochrome P450 activity, take up LDL, and stored glycogen. Also, they stated that after transplanting of these cell clusters into chronic liver injured mouse the serum levels of ALB and total bilirubin improved significantly [17]. The objective of Yin et al. was to observe the hepatic differentiation ability of AT-MSCs in vivo and in vitro. The differentiated cells showed HLC morphology and hepatocyte-specific markers (ALB and AFP) and bioactivity assays (LDL uptake and glycogen storage). ALB was detected in the mouse livers 1-month post-transplantation. Furthermore, their study revealed that trichostatin A (TSA) enhanced ALB production and LDL uptake by the HLCs, which was compared with the functions of human liver cells [18].
Another source of MSCs is UC-MSCs, capable of differentiating into other cell types. Moreover, in vivo UC-MSC transplantation experiments introduced these cells as a promising candidate for restoration functional defects in some injuries and diseases [21,22,23,24].
For the first time, Campard et al. isolated UC-MSCs and differentiated them into HLCs, by sequential exposure to EGF, bFGF, bFGF-HGF, and finally OSM. They analyzed HLCs by reverse-transcription polymerase chain reaction, flow cytometry, and immunocytochemical assays, and then compared with undifferentiated UC-MSCs as well as freshly isolated liver cells. They concluded that UC-MSCs, with an endodermic differentiation capacity, might be an alternative source for liver-directed cell therapies. They showed that in vitro undifferentiated UC-MSCs constitutively expressed hepatic markers, including ALB, AFP, ck-19, connexin-32 (Cnx-32), and dipeptidyl peptidase IV (DPPIV). Also, the newly differentiated cells showed hepatocyte-like morphology, up-regulated several hepatic markers, stored glycogen, produced urea, and exhibited an inducible CYP 3A4 activity. Still, some hepatic markers were not found such as HepPar1 or HNF-4, which this means that their differentiation level was not reached to that of mature hepatocytes. They also, observed that some of the MSC markers were partially preserved in the differentiated UCMSCs. Finally, the differentiated UCMSCs had the capacity of engrafting, and expressing of human ALB and AFP after 2, 4, and 6 weeks post transplantation in mouse livers, while cytokeratin 19 was completely downregulated [19]. Also, Yoon et al. examined the four-step sequential exposure of UC-MSCs with OSM plus TSA or OSM, plus dimethyl sulfoxide (DMSO) for in vitro differentiation into HLCs. Consequently, the morphology and protein expression had successively changed in a step-dependent manner. The urea synthesis and ammonia concentration rates of (OSM + TSA)- and (OSM + DMSO)-treated cells were measured [20]. In another study, UC-MSCs were differentiated into HLCs in a one-step protocol, using HGF and FGF-4. The analysis showed that these cells produced hepatocyte-specific markers ALB, AFP, and CK-18; stored glycogen; and uptook LDL [21]. Zheng et al. conducted a research, which showed the in vitro ability of UC-MSCs to differentiate into HLCs by emphasizing on the critical role of OSM by a modified differentiation protocol. The function of differentiated cells was examined via Periodic acid-Schiff (PAS) staining and LDL uptake. Also, the protein expressions of total protein (TP), ALB, globulin (GLB), urea (BUN), and AFP were also measured [22].
Hasan et al. differentiated UCB-MSCs into functional HLCs under hepatogenic conditions (containing HGF and FGF-4). The function of differentiated HLCs was detected by estimating urea production and protein secretion. The cells started production of AFP from day 7, and ALB gene was measured on the 14th day. This study exhibited that UCB-derived MSCs had the ability to differentiate into functioning HLCs starting on the 7th day after culturing under hepatogenic conditions, which became well-functioning on the 21st and 28th day [2].
In a study by Raufi et al., hepatic differentiation of umbilical cord vein MSCs was performed, using HGF and OSM in a two-step protocol by a 4-week induction. Immunological analysis indicated that derived HLCs expressed liver-specific protein markers such as ALB and CK-18 and also exhibited several characteristics of hepatocytes, including expression of transthyretin, glucose 6-phosphatase, CK-18, AFP, HNF-3β, and ALB. Also, the ability of indocyanine green cell uptake and glycogen storage was surveyed [23]. Furthermore, there are more studies that have used UCB-MSCs to differentiate into HLCs, using various combinations of cytokines and growth factors such as FGF, HGF, OSM, EGF, LIF, and ITS premix [67, 68].
Wei et al. used human fetal mesenchymal stem cells (F-MSCs) which were derived from the fetal bone marrow in order to differentiate them into HLCs. For this reason, they used HGF, bFGF, and OSM, and after 21 days of differentiation, the expression of hepatocyte-specific markers such as AFP and CK-18 by immunofluorescence staining were measured [24].
A study by Snykers et al. described that BM-MSCs cannot be differentiated into HLCs without TSA after sequential exposure to growth factors, especially in the final step, and they supposed that adding FGF-4, HGF, and combination of HGF-ITS-Dexa sequentially can reflect the extracellular signaling pattern during hepatogenesis. The differentiated cells induced both glycogen storage and CK-18 expression.
When the cells are additionally exposed to TSA, endodermal differentiation was improved by changes in epithelial morphology; chronological expression of hepatic proteins, including HNF-3β, AFP, CK-18, ALB, HNF1α, multidrug resistance-associated protein (MRP)2 and C/EBPα; and functional maturation, such as ALB secretion, urea production, and cytochrome P450 (CYP)-dependent activity [25].
All in all, it seems that different sources of MSCs can be used for transformation into HLCs. The need for a comprehensive study that compares the capacity of all the sources in differentiating into HLCs with each other is warranted. However, the existing data cannot elucidate as to which source is more suitable for developing functional HLCs to satisfy the need of a promising source for transplantation.
Genetic modifications
MicroRNAs (miRs)
miRs are highly conserved, non-coding RNA molecules that are about 20 nucleotides in lengths that can repress or stimulate the translation or degradation of its target mRNAs. miRNAs can act as key regulators to determine cell fate. A combination of different miRs is responsible for the regulation of MSC differentiation [69].
Davoodian et al. studied the elucidating role of miR-122 during hepatic differentiation and showed that this process could be improved by the overexpression of miR-122. Their study confirmed that this miR can act as a prominent factor in differentiation of human AT-MSCs into HLCs [70].
Cui et al. studied the dynamic microRNA profiles of human UC-MSCs into hepatocytes. Hepatocyte formation was induced in-vitro After isolating hUC- MSCs, using growth factors. They induced hUC-MSCs for 26 days and differentiated cells could express hepatocyte-specific genes, synthesize glycogen and urea, and uptake LDL. Cellular total RNA from hUC-MSCs and hepatic differentiated hUC-MSCs was collected at seven time points, including days 2, 6, 10, 14, 22, and 26, for microRNA microarray analysis. The dynamic microRNA profiles that were detected did not have any overlap or only had partial overlap with microRNAs, which were reported to be involved in hepatocyte regeneration or hepatic differentiation of liver-derived progenitor cells. Sixty-one microRNAs out of 1205 human microRNAs exhibited steady changes and were altered at least by 2-fold between hUC-MSCs and differentiated cells microRNAs, and 25 of them showed over-expression. Also, 36 microRNAs were under-expressed and had a similar expression pattern to that of the overexpressed microRNAs. They elucidated that the microRNAs related to hepatic differentiation were not enriched in hepatocyte or hepatocellular carcinoma cells and might possibly have targets among liver-enriched transcription factors and genes [78]. In continuation, they clarified that six overexpressed miRNAs (miR-1246, miR-1290, miR-148a, miR-30a, miR-424 and miR-542-5p) have instructive role during hepatic differentiation of hUC-MSCs. Before hepatic differentiation, lentivirus holding a miRNA inhibitor sequence were used for infecting derived human MSCs. They found that downregulating of any one of the six-hepatic differentiation-specific miRNAs that mentioned could prevent HGF-induced hepatic differentiation including albumin expression and LDL uptake. While, it was detected that overexpression of any one of the six miRNAs alone or liver-enriched miR-122 cannot onset hepatic differentiation. Then, it was observed that ectopic overexpression of seven miRNAs (miR-1246, miR-1290, miR-148a, miR-30a, miR-424, miR-542-5p together with miR-122) simultaneously can stimulate human MSC conversion into functionally mature induced hepatocytes (iHep) [79]. Moreover, the induced iHep cells were transplanted into CCL4 liver injured mice and it was observed that iHeps not only could improve liver function but also restored the injured livers. Finally, they showed that the differentiated cells can improve liver injury in an animal model. In their studies, hMSCs were directly converted into hepatocytes using a seven-miRNA combination and they claimed that in comparison with conventional methods, the seven-miRNA combination promoted hepatic differentiation of hMSCs more quickly and efficiently [79, 80]. In another study, Khosravi et al. used miR-106a, miR-574-3p, and miR-451 for hepatic differentiation for in vitro UC-MSCs and confirmed the production of ALB [81]. The findings from these studies specify that miRNAs have the ability to directly convert human MSCs to a hepatocyte phenotype in vitro.
Adjustment of microenvironment and physical parameters
In vivo, cells create communities in a three-dimensional (3D) microenvironment, where factors like interaction with neighboring cells, the extracellular matrix (ECM), and systemic factors are involved in determining their phenotype [82]. In addition, there are some common qualities within most mammalian cell microenvironments, such as continuous nutrient supply, short distances between cells, waste removal, and a definite temperature.
3D scaffolds
The in-vivo environment cannot be represented correctly by traditional culturing methods on plastic culture wares; hence a paradigm shift from 2D to 3D experimental techniques is essential for fulfilling this need, different equivalents of natural, synthetic and semisynthetic ECM have been established to produce a suitable cellular microenvironment [83]. Standard 2D culture conditions which soluble growth factors are at uncommon high concentrations, make poor mimics of the cellular environment, because 3D signals are generally absent, oxygen tension is too high and adjacent cell interactions are not organized properly [84]. Most studies conducted on hepatic trans-differentiation of adult stem cells were accomplished in monolayer 2D culture, but it is identified that hepatocytes sustain their differentiated functions better in 3D multicellular aggregates or spheroids than in monolayer culture [85].
The aim of Ong et al. study was to study the hepatic differentiation capacity of MSCs in two types of 3D constructs: a cell pellet and a cell pellet accompanied by ECM from the small intestinal submucosa (SIS). SIS is a biomaterial derived from the porcine small intestine that contains collagen I, small quantities of collagen IV, fibronectin, and laminin. It seems that because SIS is composed of ECM molecules in its native state and concentration, it might have the potential to produce an appropriate biological signal to guide hepatic differentiation in-vitro. Moreover, SIS could provide angiogenesis in- vivo, which is vital for the survival of transplanted cells. In their study, the MSCs were cultured in two different constructs with growth factors, which confirmed successful hepatic differentiation. Finally, due to possible confounding factors of diffusion limitation, they showed that the SIS biomaterial is not advantageous enough for hepatic differentiation [86]. Pulavendran et al. incorporated HGF into biocompatible chitosan nanoparticles (CNP) and hepatic differentiation of MSC in the presence of HGF and CNP was explored in vitro [87].
In another study, Wang et al. produced a perfusion system for 3D cell culture and the porous polylactic glycolic acid (PLGA) polymer was used as the biodegradable scaffold. Human MSCs were seeded and proliferated in PLGA scaffolds, and a medium containing HGF, FGF, and Dex was used to induce hepatogenesis. The comparison between perfused and statically induced differentiated human MSC survival and hepatogenic ability showed that perfusion is the reason of increased survival and the uniform distribution pattern of induced cells in the scaffolds, which resulted in a higher efficiency of hepatogenesis in the PLGA construct with hMSCs. Finally, they concluded that the oscillatory perfusion induction system combined with the hepatogenic medium can be a valuable and convenient tool for in vitro hepatic tissue engineering using human MSCs [88].
Khodabandeh et al. study aimed to evaluate the ability of human UC-MSCs as an accessible and achievable source for differentiation toward HLCs by a 3D culture system in the presence of hepatogenic media containing IGF-1 with or without FGF4. Following the pre-exposer of the cells into FGF4 before being treated with IGF-1 and HGF in 3D collagen scaffold, liver-specific marker expression had increased. Long-term culturing of UC-MSCs in 3D environment induced the cells to express higher level of hepatogenic markers at protein level, and also the induced cells became functional, as well [89].
Another study investigated the potential of UB-MSCs to turn into HLCs based on a sequential 2D and scaffold-based 3D differentiation protocol. For hepatic differentiation, a sequential 4-step protocol in 3D and 2D culture systems were used for induction of the cells. Urea concentration and ALB secretion were measured, and also, gene expression levels of AFP, ALB, and CK18 were determined. Morphological changes from elongated fibroblast-like cells to round epithelial cells were observed in 2D culture, using growth factors. Finally, their results showed that sequential exposures of UC-MSCs to growth factors in the 3D culture can improve hepatic differentiation [90].
Microfluidic devices
Microfluidic devices have been utilized for cell culture, cell differentiation [91], dynamic gene expression [92], and test of cellular response to chemical gradients [93]. According to reports, similar induction protocols for hepatic differentiation of MSCs have been used in many researches [94, 95]. However, these protocols require at least 4–6 weeks to complete the hepatic differentiation process; in addition, in these studies, large numbers of hepatocytes, which are necessary for cell therapy, are not fully addressed [96]. Therefore, it seems valuable to investigate hepatic differentiation of MSCs under continuous fluid flow, using a microfluidic device which mimics the shear flow in the microenvironment. Fluid shear stress, like blood pressure effect, is important in liver tissue development [97]. Therefore, microfluidic systems are used to simulate the condition of in vitro systems much more like the in vivo systems, using the diffusive mixing possibilities of the laminar flow and concentration gradients [84].
Therefore, to create a more in vivo-like 3D systems, further innovative approaches are essential that can be achieved through micro technology. Using microfluidic cell culture systems enables researchers to control the surrounding environment of individual cells, which is not possible via traditional culturing conditions (Table 2) [98].
There are shortcomings when using conventional microfluidic devices for hepatic differentiation such as an uneven flow in a small culture chamber, which might affect the yield and quality of differentiated cells [91]. An uneven cell distribution which requires cell injection through seeding microchannels can affect cellular interaction [99]. To remove these problems, Yen et al. invented a novel biomicrofluidic device that could minimize the mentioned problems. They construct a five-layered microfluidic device which was composed of one layer of PS, patterned PDMS, patterned glass, and PMMA adaptors was designed and fabricated. The constructed device in cooperation with the other components of the biomicrofluidic system, applied for the maintenance and hepatic differentiation of MSCs effectively. They tried to maintain a constant flow rate and homogeneous medium flow of media in the cell culture chamber. They simulated field of velocity distribution in order to present a uniform flow profile in the culture region of the microfluidic device. In addition, they demonstrated that the flow field near the center line and the margin was the same. The Reynolds number of the microfluidic device for a 100-μl/h flow rate was 3.6 × 10−5. The flow is considered laminar flow when Re is smaller than 2100 and turbulent flow when larger than 4000. The 100-μl/h flow rate in our device was therefore a laminar flow. By using a two-step protocol and adding cytokines and growth factors, MSCs were differentiated to HLCs on a fabricated microfluidic device. The device consisted of a large culture chamber with an ability to create a stable and uniform flow to produce homogeneous induction of hepatic differentiation. Since the device was able to make HLCs efficiently, they are convinced that their structure can be used for scale-up production of HLCs from MSCs for cellular therapy [96].
Bioreactors
The liver continuously suffers from being exposed to a wide range of exogenous substances. Therefore, it is vital to produce cellular models that can mimic hepatic function that resembles in vivo conditions [100]. The progress made in the field of tissue engineering and micro technology has improved in the form of new tools entitled “microfluidic bioreactors,” which can be applied in many different fields [101].
In their study, Rebelo et al. manufactured a stirred-tank bioreactor with perfusion operation mode. They cultivated human BM-MSCs with hepatocytes isolated from human liver tissue as spheroids in an automated, computer-controlled bioreactor. Their study resulted in an inner core of parenchymal liver tissue with an outer layer of stromal cells. Hepatocyte polarization and morphology as well as the mesenchymal phenotype of BM-MSCs were maintained throughout the culture period, and the crosstalk between the two cell types was depicted. The viability, compact morphology, and phenotypic stability of hepatocytes were enhanced in co-cultures in comparison to monocultures. Gene expression and CYP3A4 and CYP1A2 activity was inducible until week 2 of culturing [102].
In another study, the first stage of hepatic differentiation of hn-MSCs (human neonatal mesenchymal stem cells) was performed in 2D monolayer cultures for 17 days. Then, cells were cultured in 3D as self-assembled spheroids or in multicompartment membrane bioreactor system as the second stage. The system enabled hn-MSC differentiation into HLCs which was certified by positive immune staining of hepatic markers, the hepatic transporters, and drug-metabolizing enzymes. In addition, all models displayed relevant glucose metabolism, ALB production, and urea secretion. These data suggest that the tested 3D models could improve HLC maturation, showing a relevant biotransformation capacity, by providing more reliable models for mechanistic studies and more predictive systems for in vitro toxicology applications [103].
Conclusion
Mesenchymal stem cells have the capacity to multiply and differentiate into numerous types of cell lineages including HLCs. Access to an abundant, high-quality supply of hepatocytes with therapeutic potential for cell transplantation and extracorporeal support of patients in liver failure is an important issue. Although many preclinical and clinical trials were performed to elicit their role in the hepatic regeneration, the best and most effective protocol for hepatocyte differentiation is still unknown. In this review, we suggested that MSC from different origins might be considered as a suitable source for differentiation into HLCs by different protocols. It seems that in the early stages of differentiation, 2D culture is sufficient, but during the development stage, 3D culture system with HGF and FGF cytokines will be more effective.
Although the number of HLCs is critical for transplantation, the functionality of these cells has an important clinical point of view. Different protein secretion (like urea production, ALB, and AFP) and enzymatic functions (like LDL uptake, glycogen storage, and cytochrome P450) can be tested for evaluation of HLCs in order to estimate their ability in renewing the damaged liver tissue. By the way, after using the HLCs in clinical trials, the routine method tests the liver function as well as considers the MELD score. The MELD score is composed of bilirubin level, which shows how well the liver clears the bile; INR (international normalized ratio), which reflects how well the liver makes coagulative factors needed for blood clots; and also serum sodium level.
Nonetheless, it is worth mentioning that MSCs might provide a reliable source of HLCs by reducing the gap between the availability and the demand for liver donors in the future.
Availability of data and materials
Not applicable
Abbreviations
- BAL:
-
Bioartificial liver
- iPSC:
-
Induced pluripotent stem cells
- MSCs:
-
Mesenchymal stem cells
- SCs:
-
Stem cells
- ESCs:
-
Embryonic stem cells
- HLCs:
-
Hepatocyte-like cells
- ISCT:
-
The International Society for Cellular Therapy
- BM-MSCs:
-
Bone marrow MSCs
- AT-MSCs:
-
Adipose tissue MSCs
- DP-MSCs:
-
Dental pulp-MSCs
- H-MSCs:
-
Heart-derived MSCs
- UC:
-
Umbilical cord
- CB:
-
Cord blood
- WJ:
-
Wharton’s jelly
- DE:
-
Definitive endoderm
- LPM:
-
Lateral plate mesoderm
- STM:
-
Septum transversum
- FGF:
-
Fibroblast growth factor
- BMP:
-
Bone morphogenetic protein
- TGFβ:
-
Transforming growth factor β
- HNF1b:
-
Hepatocyte nuclear factor beta
- FoxA:
-
Fork-head box protein A
- ALB:
-
Albumin
- AFP:
-
Alpha fetoprotein
- Ttr:
-
Transthyretin
- Rbp:
-
Retinol binding protein
- MMPs:
-
Matrix metalloproteinases
- Prox1:
-
Prospero-related homeobox transcription factor
- Tbx3:
-
T-Box 3
- c/EBPa:
-
CCAAT-enhancer-binding proteins
- OSM:
-
Oncostatin M
- LIF:
-
Leukemia inhibitory factor
- HGF:
-
Hepatocyte growth factor
- IMDM:
-
Iscove’s modified Dulbecco’s medium
- EGFR:
-
EGF receptor
- bFGF:
-
Basic FGF
- NTA:
-
Nicotinamide
- ITS:
-
Insulin-transferrin selenium
- Hhex:
-
Hematopoietically expressed homeobox
- Dexa:
-
Dexamethasone
- LDL:
-
Low-density lipoprotein
- TSA:
-
Trichostatin A
- Cnx-32:
-
Connexin-32
- DPPIV:
-
Dipeptidyl peptidase IV
- DMSO:
-
Dimethyl sulfoxide
- PAS:
-
Periodic acid-Schiff
- TP:
-
Total protein
- GLB:
-
Globulin
- F-MSCs:
-
Fetal mesenchymal stem cells
- MRP:
-
Multidrug resistance-associated protein
- CYP:
-
Cytochrome P450
- miRs:
-
MicroRNAs
- iHep:
-
Induced hepatocytes
- 3D:
-
Three-dimensional
- ECM:
-
Extracellular matrix
- SIS:
-
Small intestinal submucosa
- CNP:
-
Chitosan nanoparticles
- hn-MSCs:
-
Human neonatal mesenchymal stem cells
References
Neuberger J. Liver transplantation. J Hepatol. 2000;32:198–207.
Hasan MH, Botros KG, El-Shahat MA, Abdallah HA, Sobh MA. In vitro differentiation of human umbilical cord blood mesenchymal stem cells into functioning hepatocytes. Alexandria J Med. 2017;53(2):167–73.
Yu Y, Liu H, Ikeda Y, Amiot BP, Rinaldo P, Duncan SA, et al. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: relevance to cellular therapies. Stem Cell Res. 2012;9(3):196–207.
Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71(2):181–97.
Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000 Jan 7;100(1):157–68.
Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;28:35(2).
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–47.
Soto-Gutiérrez A, Navarro-Álvarez N, Zhao D, Rivas-Carrillo JD, Lebkowski J, Tanaka N, et al. Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines. Nat Protoc. 2007;2(2):347–56.
Stock P, Brückner S, Ebensing S, Hempel M, Dollinger MM, Christ B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc. 2010;5(4):617–27.
Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int. 2012;2012:1–9.
Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13(12):1477–86.
Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells - sources and clinical applications. Transfus Med Hemother. 2008;35(4):272–7.
Davies JE, Walker JT, Keating A. Concise review: Wharton’s jelly: the rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl Med. 2017;6(7):1620–30.
Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219–28.
Okura H, Komoda H, Saga A, Kakuta-Yamamoto A, Hamada Y, Fumimoto Y, et al. Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2010;16(4):761–70.
Yin L, Zhu Y, Yang J, Ni Y, Zhou Z, Chen Y, et al. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol Med Rep. 2015;11(3):1722–32.
Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134(3):833–48.
Yoon H-H, Jung B-Y, Seo Y-K, Song K-Y, Park J-K. In vitro hepatic differentiation of umbilical cord-derived mesenchymal stem cell. Process Biochem. 2010;45(12):1857–64.
Zhang Y-N, Lie P-C, Wei X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy. 2009;11(5):548–58.
Zheng G, Liu Y, Jing Q, Zhang L. Differentiation of human umbilical cord-derived mesenchymal stem cells into hepatocytes in vitro. Biomed Mater Eng. 2015;25(1 Suppl):145–57.
Raoufil A, Aminil A, Azadbakht M, Farhadifar F, Rahram Nikhn Frrfin Fthi NF. Production of hepatocyte-like cells from human umbilical vein mesenchymal stem cells. Ital J Anat Embryol 2015;120(3):150–161.
Wei X, Wang C, Liu Q, Li J, Li D, Zhao F, et al. In vitro hepatic differentiation of mesenchymal stem cells from human fetal bone marrow. J Int Med Res. 2008;36(4):721–7.
Snykers S, Vanhaecke T, De Becker A, Papeleu P, Vinken M, Van Riet I, et al. Chromatin remodeling agent trichostatin a: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Dev Biol. 2007;7(1):24.
Yun CH. The impact of mesenchymal stem cell source on proliferation, differentiation, immunomodulation and therapeutic efficacy. J Stem Cell Res Ther. 2014;04(10):1–8.
Ahrens N, Tormin A, Paulus M, Roosterman D, Salama A, Krenn V, et al. Mesenchymal stem cell content of human vertebral bone marrow. Transplantation. 2004;78(6):925–9.
Jurgens WJFM, Oedayrajsingh-Varma MJ, Helder MN, ZandiehDoulabi B, Schouten TE, Kuik DJ, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332(3):415–26.
Rossini A, Frati C, Lagrasta C, Graiani G, Scopece A, Cavalli S, et al. Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res. 2011;89(3):650–60.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9(1):12.
Brooke G, Tong H, Levesque J-P, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17(5):929–40.
Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, et al. Comparison of human placenta- and bone marrow–derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17(6):1095–108.
Nadri S, Soleimani M. Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy. 2007;9(8):729–37.
Kim S-H, Choi K-H, Lee D-K, Lee M, Hwang JY, Lee C-K. Identification and characterization of the OCT4 upstream regulatory region in Sus scrofa. Stem Cells Int. 2019;2019:1–11.
Secco M, Zucconi E, Vieira NM, Fogaça LLQ, Cerqueira A, Carvalho MDF, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26(1):146–50.
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131(2):267–82.
Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15(15):1998–2009.
Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20(6):2260–8.
Xu C-R, Cole PA, Meyers DJ, Kormish J, Dent S, Zaret KS. Chromatin “prepattern” and histone modifiers in a fate choice for liver and pancreas. Science. 2011;332(6032):963–6.
Lokmane L, Haumaitre C, Garcia-Villalba P, Anselme I, Schneider-Maunoury S, Cereghini S. Crucial role of vHNF1 in vertebrate hepatic specification. Development. 2008;135(16):2777–86.
Watt AJ, Zhao R, Li J, Duncan SA. Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol. 2007;7(1):37.
Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10(13):1670–82.
Kaestner KH. The making of the liver: competence in the foregut endoderm and induction of liver-specific genes. Cell Cycle. 2005;4(9):1146–8.
McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134(12):2207–17.
Zong Y, Stanger BZ. Molecular mechanisms of liver and bile duct development. Wiley Interdiscip Rev Dev Biol. 2012;1(5):643–55.
Shiojiri N, Sugiyama Y. Immunolocalization of extracellular matrix components and integrins during mouse liver development. Hepatology. 2004;40(2):346–55.
Margagliotti S, Clotman F, Pierreux CE, Lemoine P, Rousseau GG, Henriet P, et al. Role of metalloproteinases at the onset of liver development. Develop Growth Differ. 2008;50(5):331–8.
Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25(3):254–5.
Lüdtke TH-W, Christoffels VM, Petry M, Kispert A. Tbx3 promotes liver bud expansion during mouse development by suppression of cholangiocyte differentiation. Hepatology. 2009;49(3):969–78.
Parviz F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, et al. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet. 2003;34(3):292–6.
Ge X, Yamamoto S, Tsutsumi S, Midorikawa Y, Ihara S, Wang SM, et al. Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics. 2005;86(2):127–41.
Sadler TW (Thomas W. Langman’s medical embryology. - NLM Catalog - NCBI [Internet]. [cited 2019 Jun 1]. Available from: https://www.ncbi.nlm.nih.gov/nlmcatalog/101562744.
Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003 Jul 31;102(10):3483–93.
Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–92.
Snykers S, Vanhaecke T, Papeleu P, Luttun A, Jiang Y, Vander Heyden Y, et al. Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci. 2006;94(2):330–41.
Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(3):686–95.
Salehinejad P, Alitheen NB, Mandegary A, Nematollahi-Mahani SN, Janzamin E. Effect of EGF and FGF on the expansion properties of human umbilical cord mesenchymal cells. In Vitro Cell Dev Biol Anim. 2013;49(7):515–23.
Neuss S, Becher E, Wöltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22(3):405–14.
Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24(1):23–33.
Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, et al. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330(4):1153–61.
Miyajima A, Kinoshita T, Tanaka M, Kamiya A, Mukouyama Y, Hara T. Role of Oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000;11(3):177–83.
Paganelli M, Nyabi O, Sid B, Evraerts J, El Malmi I, Heremans Y, et al. Downregulation of Sox9 expression associates with hepatogenic differentiation of human liver mesenchymal stem/progenitor cells. Stem Cells Dev. 2014;23(12):1377–91.
Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24(1):70–7.
Alizadeh E, Eslaminejad MB, Akbarzadeh A, Sadeghi Z, Abasi M, Herizchi R, et al. Upregulation of MiR-122 via trichostatin a treatments in hepatocyte-like cells derived from mesenchymal stem cells. Chem Biol Drug Des. 2016;87(2):296–305.
Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56(3):405–15.
Sgodda M, Aurich H, Kleist S, Aurich I, König S, Dollinger MM, et al. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313(13):2875–86.
MOON Y, LEE M, YOON H, YANG M, JANG I, LEE J, et al. Hepatic differentiation of cord blood-derived multipotent progenitor cells (MPCs) in vitro. Cell Biol Int. 2008;32(10):1293–301.
Sellamuthu S, Manikandan R, Thiagarajan R, Babu G, Dinesh D, Prabhu D, et al. In vitro trans-differentiation of human umbilical cord derived hematopoietic stem cells into hepatocyte like cells using combination of growth factors for cell based therapy. Cytotechnology. 2011;63(3):259–68.
Kim N, Kim H, Jung I, Kim Y, Kim D, Han Y-M. Expression profiles of miRNAs in human embryonic stem cells during hepatocyte differentiation. Hepatol Res. 2011;41(2):170–83.
Davoodian N, Lotfi AS, Soleimani M, Mowla SJ. MicroRNA-122 overexpression promotes hepatic differentiation of human adipose tissue-derived stem cells. J Cell Biochem. 2014;115(9):1582–93.
Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–9.
Gómez-Sjöberg R, Leyrat AA, Pirone DM, Chen CS, Quake SR. Versatile, fully automated, microfluidic cell culture system. Anal Chem. 2007;79(22):8557–63.
Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, et al. Hepatocytic gene expression in cultured rat mesenchymal stem cells. Transplant Proc. 2005;37(1):276–9.
Taléns-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell J-V, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;12(36):5834–45.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45(5):1229–39.
Behbahan IS, Duan Y, Lam A, Khoobyari S, Ma X, Ahuja TP, et al. New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Rev. 2011;7(3):748–59.
Shin D, Monga SPS. Cellular and molecular basis of liver development. In: Comprehensive physiology. Hoboken: Wiley.; 2013. p. 799–815.
Cui L, Zhou X, Li J, Wang L, Wang J, Li Q, et al. Dynamic microRNA profiles of hepatic differentiated human umbilical cord lining-derived mesenchymal stem cells. PLoS One. 2012;7(9):e44737 Rameshwar P, editor.
Cui L, Shi Y, Zhou X, Wang X, Wang J, Lan Y, et al. A set of microRNAs mediate direct conversion of human umbilical cord lining-derived mesenchymal stem cells into hepatocytes. Cell Death Dis. 2013;4(11):e918.
Zhou X, Cui L, Zhou X, Yang Q, Wang L, Guo G, et al. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J Cell Mol Med. 2017;21(5):881–93.
Khosravi M, Azarpira N, Shamdani S, Hojjat-Assari S, Naserian S, Karimi MH. Differentiation of umbilical cord derived mesenchymal stem cells to hepatocyte cells by transfection of miR-106a, miR-574-3p, and miR-451. Gene. 2018;667:1–9.
Meyvantsson I, Beebe DJ. Cell culture models in microfluidic systems. Annu Rev Anal Chem. 2008;1(1):423–49.
Serban MA, Liu Y, Prestwich GD. Effects of extracellular matrix analogues on primary human fibroblast behavior. Acta Biomater. 2008;4(1):67–75.
El-Ali J, Sorger PK, Jensen KF. Cells on chips 2006;442(7101):403–411.
Glicklis R, Shapiro L, Agbaria R, Merchuk JC, Cohen S. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol Bioeng. 2000;67(3):344–53.
Ong S-Y, Dai H, Leong KW. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials. 2006;27(22):4087–97.
Pulavendran S, Rajam M, Rose C, Mandal AB. Hepatocyte growth factor incorporated chitosan nanoparticles differentiate murine bone marrow mesenchymal stem cell into hepatocytes in vitro. IET Nanobiotechnology. 2010;4(3):51.
Wang J, Zong C, Shi D, Wang W, Shen D, Liu L, et al. Hepatogenic engineering from human bone marrow mesenchymal stem cells in porous polylactic glycolic acid scaffolds under perfusion culture. J Tissue Eng Regen Med. 2012;6(1):29–39.
Khodabandeh Z. Z V, M J, a H, S B, T T-K. Improved differentiation of mesenchymal stem cells into hepatocyte-like cells using FGF4 and IGF-1 in 3D culture. Int J Stem Cell Res Transplant. 2015;03(03):109–17.
Azandeh S, Mohammad Gharravi A, Orazizadeh M, Khodadi A, Hashemi TM. Improvement of mesenchymal stem cell differentiation into the endoderm lineage by four step sequential method in biocompatible biomaterial. Bioimpacts. 2016;6(1):9–13.
Ju X, Li D, Gao N, Shi Q, Hou H. Hepatogenic differentiation of mesenchymal stem cells using microfluidic chips. Biotechnol J. 2008;3(3):383–91.
Kim YT, Jung JH, Choi YK, Seo TS. A packaged paper fluidic-based microdevice for detecting gene expression of influenza A virus. Biosens Bioelectron. 2014;61:485–90.
Jeong H-H, Lee S-H, Kim J-M, Kim H-E, Kim Y-G, Yoo JY, et al. Microfluidic monitoring of Pseudomonas aeruginosa chemotaxis under the continuous chemical gradient. Biosens Bioelectron. 2010;26(2):351–6.
Ayatollahi M, Soleimani M, Tabei SZ, Kabir SM. Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I. World J Stem Cells. 2011;3(12):113–21.
Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev Reports. 2011;7(1):103–18.
Yen M-H, Wu Y-Y, Liu Y-S, Rimando M, Ho JH-C, Lee OK-S. Efficient generation of hepatic cells from mesenchymal stromal cells by an innovative bio-microfluidic cell culture device. Stem Cell Res Ther. 2016;7(1):120.
Huang M, Fan S, Xing W, Liu C. Microfluidic cell culture system studies and computational fluid dynamics. Math Comput Model. 2010;52(11–12):2036–42.
Hong J, Edel JB, deMello AJ. Micro- and nanofluidic systems for high-throughput biological screening. Drug Discov Today 2009;14(3–4):134–146.
Ji R, Zhang N, You N, Li Q, Liu W, Jiang N, et al. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials. 2012;33(35):8995–9008.
Gebhardt R, Hengstler JG, Müller D, Glöckner R, Buenning P, Laube B, et al. New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab Rev. 2003;35(2–3):145–213.
Moon S-J, Park J-S, Heo Y-J, Kang C-M, Kim E-K, Lim M-A, et al. In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Exp Mol Med. 2013;45(10):e46.
Rebelo SP, Costa R, Silva MM, Marcelino P, Brito C, Alves PM. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: improved functionality in long-term bioreactor cultures. J Tissue Eng Regen Med. 2017;11(7):2034–45.
Cipriano M, Freyer N, Knöspel F, Oliveira NG, Barcia R, Cruz PE, et al. Self-assembled 3D spheroids and hollow-fibre bioreactors improve MSC-derived hepatocyte-like cell maturation in vitro. Arch Toxicol. 2017;91(4):1815–32.
Acknowledgements
The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
AA and NA contributed to the conceptualization. AA, NA, and SN contributed to the methodology. AA and NA contributed to the investigation and wrote the original draft of the manuscript. AA, NA, SS, and SN wrote, reviewed, and edited the manuscript. NA contributed to the resources. NA and GU supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Table S1. Comparison of different MSCs sources in differentiating potentials.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Afshari, A., Shamdani, S., Uzan, G. et al. Different approaches for transformation of mesenchymal stem cells into hepatocyte-like cells. Stem Cell Res Ther 11, 54 (2020). https://doi.org/10.1186/s13287-020-1555-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-020-1555-8