Abstract
Acute kidney injury (AKI) remains a worldwide public health issue due to its increasing incidence, significant mortality, and lack of specific target-orientated therapy. Developments in mesenchymal stem cell (MSC) research make MSCs a promising candidate for AKI management but relevant clinical trials show confusing results (NCT00733876, NCT01602328). One primary cause of the limited therapeutic effect may result from poor engraftment of transplanted cells. To solve this problem, investigators have developed a series of preconditioning strategies to improve MSC engraftment in animal AKI models. In this review, we summarize these previous studies, providing an integrated and updated view of different preconditioning strategies aimed at promoting the therapeutic effect of MSCs in AKI patients.
Similar content being viewed by others
Background
Acute kidney injury (AKI) is a common clinical disease defined as an abrupt decline in glomerular filtration, resulting in dysregulation of extracellular volume and electrolytes, which can subsequently induce a series of complications and failure of other organs [1]. The causes of AKI are numerous, including renal ischemia, nephrotoxins, sepsis, and so on. Although much work has been completed in this area over recent decades, the prevalence of AKI is still rapidly increasing [2, 3]. It is estimated that the worldwide occurrence of AKI has reached approximately 13 million people per year. For inpatients, 5% of all hospitalized patients and 40% of critically ill patients may develop AKI during their hospitalization [4, 5]. Except for the high morbidity rate, the prognosis of AKI is also not very good. The mortality rates in intensive care unit patients with AKI can reach 50–70% [6] and those who survive the acute phase also bear a high risk of developing chronic kidney disease (CKD). A recent meta-analysis found an 8.8-fold increase in risk for CKD and a 3.3-fold increased risk for end-stage renal disease (ESRD) in patients surviving AKI after hospital discharge [7]. Both the high morbidity rate and poor prognosis place a heavy burden on the public health care system, as it is estimated that the annual medical expenses for AKI treatment have exceeded $10 billion in the US and £400–600 million in the UK [8].
Currently, therapeutic choices are still confined to supportive care and preventive strategies, since kidneys have a remarkable self-repair capacity [9]. However, none of these appear to have decreased the mortality rate [10]. Some drugs have also been explored for preventing or treating AKI, such as diuretics, dopamine, fenoldopam, atrial natriuretic peptide, recombinant human insulin-like growth factor-1 (IGF-1), and erythropoietin (EPO) [11,12,13,14,15]. Despite good results in animal experiments, there is still limited evidence for their use in humans according to KDIGO-AKI guidelines [16]. During the past decade, we have witnessed an explosion of cell-based therapy in clinical use. While pharmacologic interventions often target only a single aspect of the highly complex pathophysiology following AKI, cell-based therapies may have the advantage of acting through multiple mechanisms to promote tubular epithelial cell repair [17]. Thus far, according to ClinicalTrials.gov, more than 4000 clinical trials employing stem cells from different origins have been conducted, and 46 of those consist of mesenchymal stem cell (MSC) therapy for AKI and CKD [18,19,20,21,22]. Application of MSC therapy may become a potentially effective supplemental regimen for current situations.
MSC therapy and its application in AKI
MSCs are fibroblast-like multi-potent cells first identified by Friedenstein in the 1960s and 1970s [23]. They are characterized by the robust ability for self-renewal, regeneration, proliferation, and multi-lineage differentiation [24]. By exposure to appropriate conditions, MSCs can differentiate into adipocytes, chondrocytes, and osteocytes [25]. MSCs were initially isolated from bone marrow, but now various sources, including placenta, amniotic fluid, umbilical cord blood, Wharton’s jelly, and adipose tissue, are available for preclinical and clinical use [26]. Due to very high variability in MSC preparations [27], in 2006 the International Society of Cell Therapy (ISCT) proposed criteria for the definition of MSCs: adherence to plastic with the capacity to differentiate into chondrocytes, osteoblasts, and adipocytes in in vitro culture conditions; positive for CD105, CD73, and CD90; negative for the hematopoietic markers CD45, CD34, CD19, CD79 and HLA-DR expression on the cell surface [28].
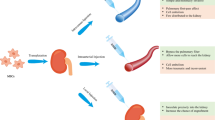
MSCs secrete a number of factors, including transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF), vascular endothelial growth factor (vEGF), and IGF-1, which can exert anti-apoptotic [29, 30], immunomodulation [31, 32], anti-oxidative [33, 34], and pro-angiogenic factors [35, 36] and target almost all pathophysiological components of AKI. MSCs can also release plenty of microvesicles (MVs), which are particularly enriched in specific molecules, especially functional mRNAs and microRNAs. Their role in vivo may be related to cell-to-cell communication and to protein and RNA exchange among cells both locally and at a distance [37]. In addition to their paracrine/endocrine activity, some studies have also demonstrated MSCs may have the ability to directly differentiate into target cells [38, 39]. This point has not been accepted by all experts, however, because most MSCs may disappear from the kidney and other organs within 72 h after infusion, which is not enough time for differentiation [40, 41] (Fig. 1).
Renal repair function of MSCs in acute kidney injury. After infusion, MSCs temporarily adhere to glomerular and postglomerular capillaries. Through a series of mechanisms, MSCs exert anti-apoptotic/anti-oxidative, anti-inflammation, immunomodulation, and pro-angiogenic effects. a Cytokines and growth factors are delivered to the injured tubular cells through paracrine actions. b This mechanism can also proceed through endocrine actions. c MSCs can secrete plenty of microvesicles, which are particularly enriched in functional mRNAs and microRNAs. Crosstalk between microvesicles and injured tubular cells causes beneficial changes in the respective gene expression profiles. d Some studies have also demonstrated MSCs may have the ability to directly differentiate into target cells
Adequate evidence demonstrates that MSCs are effective in improving outcome after AKI in different animal models. The majority of animal models included are cisplatin-induced AKI, sepsis-associated AKI, ischemia-reperfusion injury, and glycerol-induced AKI, covering most causes of human AKI [41,42,43,44,45,46].
Besides the excellent outcomes in animal models, two clinical trials have been explored on the use of MSCs in the AKI setting, but results remain contradictory. In a 2008 phase I clinical trial (NCT00733876), the safety and efficacy of MSCs were demonstrated in patients who were at high risk (underlying CKD, advanced age, diabetes mellitus, congestive heart failure, chronic obstructive lung disease, and prolonged pump times) of developing AKI after undergoing on-pump cardiac surgery. After analyzing data from 18 included patients, they concluded MSCs were useful for protecting against AKI development (0% AKI incidence in MSCs group versus 20% in control group) [47]. Based on this positive result, another phase II, randomized, double-blind, multicenter trial used MSCs to treat patients for postcardiac surgical AKI (NCT01602328) in 2017 [48]. They randomized 156 adult subjects and, at the end of the study, time to renal function recovery, the need for dialysis, and 30-day all-cause mortality were compatible between MSCs group and control group. These contradictory results are confusing to physicians when treating AKI patients because of the high price and potential tumorigenicity of MSC therapy. We need more clinical trials to address these issues, and another ongoing trial (NCT03015623) may give us clear insight into the role of MSC therapy for AKI patients (Table 1).
Concerns about the clinical application of MSCs in AKI
Some concerns remain regarding the clinical application of MSCs in AKI. First, considering their antigenicity, previous studies were performed using autologous MSCs to avoid immune rejection of donor cells [49]. However, the high expense, complex process, and timing constraints of the harvesting period from individual patients restricts their application clinically. What is more, autologous MSCs obtained from elderly donors and those with multiple medical comorbidities have significantly reduced capacity for proliferation and differentiation, with increased apoptosis signals hampering their use in the patients who will get the most benefit from such therapy [50, 51]. In fact, the absence of major histocompatibility class II antigens (MHC II) makes MSCs immunoprivileged in vivo, and increasing experimental findings have suggested autologous MSC therapy has comparable safety and effectiveness in both the short and long term after AKI [44, 52]. According to a mapping and multiscale analysis in 2016, the number of registered trials using allogeneic MSCs exceeded those with autologous MSCs (53% versus 47%) [53].
The second concern is the precise definition of MSCs. The criteria proposed by ISCT in 2006 is a minimum standard for identifying MSCs. MSCs from various sources, however, may have different biological characteristics [54,55,56,57]. Recent studies on pericytes even challenge the widely accepted view of endogenous pericytes as MSCs and suggest their progenitor potential is induced by artificial conditions and high concentrations of mitogens ex vivo [58]. This evidence raises the concern that the current definition of MSCs, which is based on surface markers and/or differentiation parameters, may not be the optimum criteria for MSCs. However, using specific DNA methylation patterns has bright prospects with regard to MSC classification [59]. In 2017, a concise review suggested using multiple methods, such as genomic, epigenomic, transcriptomic, proteomic, and metabolomic, to measure colony-forming ability, CD marker expression, telomere length, and cellular morphology, which may be useful to establish a next-generation definition for MSCs [60].
The required dose of MSCs for clinical therapy and its relevance to injury repair is a topic of active research. Although there is still no related clinical data for AKI, a preclinical study suggests medium-dose and high-dose MSC therapy (2 × 106 and 5 × 106 MSCs per kilogram bodyweight) result in better renoprotective effects after AKI compared with low-dose therapy [44]. Data from another phase I/II multicenter randomized controlled clinical study for the treatment of knee osteoarthritis also confirmed this dose-related effect [61]. This relationship may be more complicated in the field of cardiac regeneration as some are demonstrating a direct and others an inverse dose response [62,63,64,65]. It seems that different tissues need different doses of MSC therapy for repair, and more large population and appropriate control studies in the future may help us to obtain a more definitive answer to this question.
Finally, why do relevant clinical trials in AKI show confusing results? One explanation for the limited effect of MSC therapy in human AKI may be the relatively low number of transplanted MSCs in kidneys. MSCs either die due to the harsh microenvironment in vivo or cannot find their way to the injured kidneys [66, 67]. Only 1% of the delivered cells reach the target site, while most are trapped in the liver, lungs, and spleen [68,69,70,71,72]. Investigators have attempted to increase the number of injected cells but this may be risky as disturbances in blood flow may cause embolism problems [73]. Others have attempted to inject cells into the damaged tissue directly, but the invasive procedures include a high risk of hemorrhage and the number of injected MSCs is also not accurate because most of the cells may escape from the injected site [74, 75]. To strengthen the therapeutic potential of transplanted MSCs, many innovative preconditioning methods have been explored and shown excellent results in recent years [76, 77]. Below, we will discuss these novel strategies.
Preconditioning can enhance the migratory ability of MSCs
Based on the way MSCs work, these strategies are designed to either increase the effective quantity of MSCs in injured tissues (e.g., increase the survival rate of MSCs or promote their homing ability) or enhance their paracrine/endocrine ability (Fig. 2). Of these, improvement of MSC homing is of great importance because there is evidence that culture-expanded MSCs may lose a few surface molecules and be unable to migrate [67, 78, 79]. Understanding the MSC homing mechanisms may help us solve this problem.
After an injury, chemokines are released by the injured organ. By chemotaxis, MSCs can follow the gradient of soluble chemoattractants to the injured site. Then, with the help of sequences of molecular and cellular events, MSCs experience rolling and adhesion and finally transmigrate across the endothelium [80,81,82,83,84,85,86]. Based on this theory, some novel preconditioning strategies being explored either increase the affinity of MSCs to migratory stimuli or modulate the target sites to secrete more chemokines.
Increasing the affinity bility of MSCs to migratory stimuli (MSC-based strategies)
MSCs express many chemokine receptors on their surface which play an essential role in their migration by interaction with appropriate ligands. Currently, over 50 chemokines and 20 chemokine receptors have been discovered [87]. According to their cysteine residues, they have been divided into four categories: CXC family, CC family, XC family, and CX3C family [88]. Different isolation techniques and in vitro culture conditions may change the MSC expression of chemokine receptors [89, 90], but it has been widely acknowledged that CCR1, CCR2, CCR7, CXCR4, CX3CR1, CXCR6, c-met, and CD44 are primarily linked to the in vivo migratory abilities of MSCs [91,92,93].
The stromal derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis plays an important role in governing stem cell homing and engraftment in the bone marrow following transplantation [94,95,96]. SDF-1 is a member of the chemokine family and is widely expressed by both bone marrow-derived mesenchymal stromal cells (BMSCs) and endothelial cells [94] and is predominantly promoted under ischemic conditions, including AKI [97,98,99]. CXCR4, which can act as its unique receptor, is expressed on human stem and progenitor cells [100]. Unlike the abundant CXCR4 expression on hematopoietic stem cells, only approximately 1% of all human BMSCs present CXCR4, and most of the CXCR4 mRNA and antigen (83–98%) is expressed inside cells [101, 102]. Furthermore, after four to five passages of ex vivo expansion, its expression becomes barely detectable, which largely decreases the homing ability of the cells [103, 104]. Multiple strategies, such as incubation with cytokines or chemical compounds, co-injection, hypoxia stimulation, and genetic modifications, have been explored as novel preconditioning methods to enhance the interaction of SDF-1 with CXCR4 (Table 2).
Incubation with cytokines or chemical compounds
Incubation with cytokines or chemical compounds is a simple and fast preconditioning strategy for MSCs. Some cytokines or chemical compounds can trigger signaling pathways, leading to the overexpression of CXCR4. After preconditioning with 20 ng/ml IGF-1 for 24 h, MSCs were transplanted into a cisplatin-induced AKI mice model [105]. This method successfully resulted in increased cell survival rate and robust migration of MSCs towards the ischemic site followed by structural and functional recovery. This phenomenon was caused by a twofold increase in CXCR4 expression on the MSC surface [105]. Pretreatment with S-nitroso N-acetyl penicillamine, which serves as a nitric oxide donor, caused transplanted MSCs to significantly up-regulate the level of SDF-1, which resulted in better survival and migratory and secretory ability [10].
Co-injection
A synergistic effect is common in pharmacology and co-injection with drugs can enhance the migratory ability of MSCs. Muscone is the main active ingredient of musk with a supposed function as a refreshing agent, promoting blood flow and detumescence [106, 107]. Preconditioning with muscone significantly improved BMSC engraftment in injured kidney as well as other bioactivities, including cell proliferation and secretion, with increased expression of both CXCR4 and CXCR7 in gentamicin-induced AKI rats [108]. Pretreatment with EPO significantly increased this chemotactic effect of transplanted MSCs in an ischemia/reperfusion-AKI (IR-AKI) model, because EPO can increase SDF-1 levels in the AKI microenvironment and activate the PI3K/AKT and MAPK signaling pathways in MSCs [109].
Hypoxia
The low oxygen tension in injured organs may become another obstacle for the application of MSCs which are cultured under normal oxygen tension. Once localized to the ischemic tissue, MSCs encounter more severe hypoxic conditions, ranging from 0.4 to 2.3% O2, which often results in apoptosis [110]. Pre-exposure of MSCs to hypoxia may activate the expression of some genes, e.g., those encoding CXCR4, CXCR7, CX3CR1, SDF-1α, and hypoxia-inducible factor (HIF), all of which take part in cell migration [111,112,113]. In addition, cobalt is a kind of hypoxia mimetic preconditioning agent which significantly enhances MSC migratory ability by activating HIF-1α and up-regulating CXCR4, promoting improved morphology and function following AKI [114].
Genetic modifications
Genetic modifications which make MSCs overexpress migratory genes is a more accurate way to enhance MSCs’ migratory ability compared with other preconditioning strategies. For example, overexpression of CXCR4 in BMSCs effectively promoted the migration of BMSCs to the injured site of the kidney by their paracrine actions, resulting in greater improvement in renal function [115]. However, the effect was entirely abolished by pre-incubation with AMD3100, a CXCR4-specific antagonist, further confirming the important role of the SDF-1/CXCR4 axis in MSC migration [115].
Next-generation preconditioning strategies
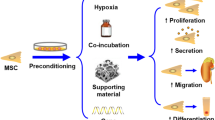
With the development of bioengineering, some novel preconditioning approaches are being explored, such as the use of hydrogels or microgels. Using MSC engineering with biomaterials could mimic cellular microenvironments more consistent with those encountered in vivo and deliver more cells to injured tissues. Although the mechanism has not been clarified, improvement of cell engraftment was observed while using an IGF-1C domain-modified chitosan hydrogel in IR-AKI model rats, which suggests enhanced MSC migratory ability through hydrogel delivery [116]. Similarly, a spherical, naturally derived extracellular matrix-based, type-I collagen microgel led to MSCs expressing significantly increased levels of SDF-1 and offered a significant functional improvement in a hind-limb ischemia mouse model [117].
Modulating target site secretion of chemokines (site-based strategies)
Apart from various methods focusing on improving the migratory function of MSCs, stimulation of the host tissue to promote recruitment is another feasible strategy. Magnification of the naturally occurring electric fields at sites of bone fracture may become a powerful cue in directing migration of human MSCs [118]. Pulse-focused ultrasound (pFUS) has been shown to enhance the homing of human MSCs through mechanotransduction, eliciting transient local increases of chemoattractants in healthy murine skeletal muscle and kidneys [119, 120]. Recently, Burks et al. [121] targeted pFUS to kidneys of mice suffering from cisplatin-induced AKI in order to evaluate whether this method could enhance MSC homing and relieve renal injury. Following pFUS treatment, tissue levels of many known chemoattractants were significantly altered, and the pFUS+MSC group had more MSCs home to the kidney and better renal function compared with the MSC group. They demonstrated pFUS could be a neoadjuvant approach to improve MSC homing to diseased organs.
Conclusions
Despite encouraging results in animal models, a large gap between scientific observation and clinical application of MSCs still exists. Improvement of MSC homing is a major challenge in clinical applications. Several preconditioning strategies have been established for enhancing the migratory ability of MSCs in AKI models. In our review, we summarize these studies and conclude that the improvement of migratory effects are mostly through the SDF-1/CXCR4 axis.
Some concerns should also be considered when applying these preconditioning strategies. Genetic modification methods are thought to have potential tumor progression risk, but so far no tumorigenic transformational changes have been observed. For pFUS, tissue damage following stimulation needs to be carefully monitored, especially using pFUS with microbubble ultrasound contrast agents. As well as the strategies discussed, investigators have also explored many other preconditioning agents, such as equipped scaffolds, melatonin, MSC-derived MV, and so on. In our opinion, combination of MSC-based and target site-based strategies may further improve the therapeutic effects and lead to better subsequent outcomes.
Another important issue for MSC application in AKI is the need for potency assays. Due to the heterogeneous population of cells and multiple mechanisms of action, no consensus on a potency assay for MSCs has been achieved. Considering their complex biological qualities, an assay matrix using biologic assays, biologic and analytical assays, or analytical assays alone to assay anti-apoptotic, immunomodulation, anti-oxidative, pro-angiogenic, and migratory functionalities of MSCs may be a proper tool to verify a specific intended effect between different MSC products and expand their application clinically.
To this end, we look forward to an optimistic future of cell-based therapy in kidney disease, while understanding the pathophysiology of AKI and clarifying the renoprotective mechanism of MSCs may further expand the success of regenerative medicine using MSCs for treating AKI.
Abbreviations
- AKI:
-
Acute kidney injury
- BMSCs:
-
Bone marrow-derived mesenchymal stromal cells
- CKD:
-
Chronic kidney disease
- CXCR4:
-
Chemokine (C-X-C motif) receptor 4
- EPO:
-
Erythropoietin
- ESRD:
-
End-stage renal disease
- HGF:
-
Hepatocyte growth factor
- HIF:
-
Hypoxia-inducible factor
- IGF-1:
-
Insulin-like growth factor-1
- IR-AKI:
-
Ischemia/reperfusion-AKI
- ISCT:
-
International Society of Cell Therapy
- MHC II:
-
Major histocompatibility class II
- MSCs:
-
Mesenchymal stem cells
- MV:
-
Microvesicles
- pFUS:
-
Pulse-focused ultrasound
- SDF-1:
-
Stromal derived factor-1
- TGF-β:
-
Transforming growth factor-β
- vEGF:
-
Vascular endothelial growth factor
References
Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8.
Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42.
Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170–9.
Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51.
Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–66.
Duann P, Lianos EA, Ma J, Lin PH. Autophagy, Innate immunity and tissue repair in acute kidney injury. Int J Mol Sci. 2016;17(5):662.
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8.
Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207.
Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67–75.
Masoud MS, Anwar SS, Afzal MZ, Mehmood A, Khan SN, Riazuddin S. Pre-conditioned mesenchymal stem cells ameliorate renal ischemic injury in rats by augmented survival and engraftment. J Transl Med. 2012;10:243.
Ludens JH, Hook JB, Brody MJ, Williamson HE. Enhancement of renal blood flow by furosemide. J Pharmacol Exp Ther. 1968;163(2):456–60.
Ludens JH, Williamson HE. Effect of furosemide on renal blood flow in the conscious dog. Proc Soc Exp Biol Med. 1970;133(2):513–5.
Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature. 1986;324(6096):473–6.
Hammerman MR. Potential role of growth factors in the prophylaxis and treatment of acute renal failure. Kidney Int Suppl. 1998;64:S19–22.
Bernhardt WM, Eckardt KU. Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care. 2008;14(6):621–6.
KDIGO workgroup. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36(4 Suppl):S187–92.
Hickson LJ, Eirin A, Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. 2016;89(4):767–78.
Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10(6):740–9.
Peired AJ, Sisti A, Romagnani P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016;2016:4798639.
Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl. 2011;1(3):103–6.
Aghajani NA, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8(1):273.
Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of Guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403.
Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21(9):1045–56.
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50.
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301.
Bieback K, Wuchter P, Besser D, et al. Mesenchymal stromal cells (MSCs): science and f(r)iction. J Mol Med (Berl). 2012;90(7):773–82.
Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
He A, Jiang Y, Gui C, Sun Y, Li J, Wang JA. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can J Cardiol. 2009;25(6):353–8.
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57(7):1759–67.
Hoogduijn MJ, Popp F, Verbeek R, et al. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10(12):1496–500.
Li JH, Zhang N, Wang JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Investig. 2008;31(2):103–10.
Tsubokawa T, Yagi K, Nakanishi C, et al. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298(5):H1320–9.
Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19(12):1885–93.
Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7(4):e35579.
Garg A, Newsome PN. Bone marrow mesenchymal stem cells and liver regeneration: believe the hypoxia. Stem Cell Res Ther. 2013;4(5):108.
Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41(1):283–7.
Liu N, Han G, Cheng J, Huang J, Tian J. Erythropoietin promotes the repair effect of acute kidney injury by bone-marrow mesenchymal stem cells transplantation. Exp Biol Med (Maywood). 2013;238(6):678–86.
Gao J, Liu R, Wu J, et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials. 2012;33(14):3673–81.
Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26(8):2075–82.
Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–804.
Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–42.
Lange C, Tögel F, Ittrich H, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68(4):1613–7.
Tögel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–85.
Duffield JS, Park KM, Hsiao LL, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115(7):1743–55.
Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–67.
Tögel FE, Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis. 2012;60(6):1012–22.
Swaminathan M, Stafford-Smith M, Chertow GM, et al. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2018;29(1):260–7.
Atoui R, Chiu RC. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med. 2012;1:200–5.
Zhuo Y, Li SH, Chen MS, Wu J, Kinkaid HY, Fazel S, et al. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg. 2010;139:1286–94. 1294.e1–2
Nayan M, Paul A, Chen G, Chiu RC, Prakash S, Shum-Tim D. Superior therapeutic potential of young bone marrow mesenchymal stem cells by direct intramyocardial delivery in aged recipients with acute myocardial infarction: in vitro and in vivo investigation. J Tissue Eng. 2011;2011:741213.
Le BK, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6.
Monsarrat P, Vergnes JN, Planat-Bénard V, Ravaud P, Kémoun P, Sensebé L, et al. An innovative, comprehensive mapping and multiscale analysis of registered trials for stem cell-based regenerative medicine. Stem Cells Transl Med. 2016;5:826–35.
Chen JY, Mou XZ, Du XC, Xiang C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac J Trop Med. 2015;8:739–46.
Davies JE, Walker JT, Keating A. Concise review: Wharton's jelly: the rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl Med. 2017;6:1620–30.
Kwon A, Kim Y, Kim M, Kim J, Choi H, Jekarl DW, et al. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep. 2016;6:23544.
Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, Aicher WK. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. 2016;2016:5646384.
Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–59. e5
de Almeida DC, Ferreira MR, Franzen J, Weidner CI, Frobel J, Zenke M, et al. Epigenetic classification of human mesenchymal stromal cells. Stem Cell Rep. 2016;6:168–75.
Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6:2173–85.
Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14:246.
Losordo DW, Henry TD, Davidson C, Sup LJ, Costa MA, Bass T, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–36.
Hare JM, Fishman JE, Gerstenblith G, DiFede VDL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79.
Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105.
Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT study). Circ Res. 2017;121:1279–90.
Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45(4):567–81.
Karp JM, Leng TGS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–16.
LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111(4):589–601.
Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–8.
Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21(12):3197–207.
Mäkinen S, Kekarainen T, Nystedt J, et al. Human umbilical cord blood cells do not improve sensorimotor or cognitive outcome following transient middle cerebral artery occlusion in rats. Brain Res. 2006;1123(1):207–15.
Haddad-Mashadrizeh A, Bahrami AR, Matin MM, et al. Evidence for crossing the blood barrier of adult rat brain by human adipose-derived mesenchymal stromal cells during a 6-month period of post-transplantation. Cytotherapy. 2013;15(8):951–60.
Walczak P, Zhang J, Gilad AA, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39(5):1569–74.
Dell'Accio F, De Bari C, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44(7):1608–19.
Huang AH, Yeger-McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthr Cartil. 2008;16(9):1074–82.
Naderi-Meshkin H, Bahrami AR, Bidkhori HR, Mirahmadi M, Ahmadiankia N. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol Int. 2015;39(1):23–34.
Ezquer FE, Ezquer ME, Vicencio JM, Calligaris SD. Two complementary strategies to improve cell engraftment in mesenchymal stem cell-based therapy: increasing transplanted cell resistance and increasing tissue receptivity. Cell Adhes Migr. 2017;11(1):110–9.
Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160–70.
Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–96.
McEver RP. Rolling back neutrophil adhesion. Nat Immunol. 2010;11(4):282–4.
Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4(6):432–44.
Kamei M, Carman CV. New observations on the trafficking and diapedesis of monocytes. Curr Opin Hematol. 2010;17(1):43–52.
Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102(6):2060–7.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87.
Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105(3):223–30.
Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–23.
Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9(9):970–80.
Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–44.
Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013;13(5):856–67.
Smith H, Whittall C, Weksler B, Middleton J. Chemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cells. Stem Cells Dev. 2012;21(3):476–86.
Wu Y, Zhao RC. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev. 2012;8(1):243–50.
Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24(5):1254–64.
Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–27.
Lapidot T, Dar A, Kollet O. How do stem cells find their way home. Blood. 2005;106(6):1901–10.
Cottler-Fox MH, Lapidot T, Petit I, et al. Stem cell mobilization. Hematol Am Soc Hematol Educ Program. 2003;1:419–37.
Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35(21):5627–35.
Lai P, Li T, Yang J, et al. Upregulation of stromal cell-derived factor 1 (SDF-1) expression in microvasculature endothelial cells in retinal ischemia-reperfusion injury. Graefes Arch Clin Exp Ophthalmol. 2008;246(12):1707–13.
Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27(1):6–13.
Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67(5):1772–84.
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–24.
Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643–5.
Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009;69(9):991–1000.
Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–41.
Ahmadbeigi N, Seyedjafari E, Gheisari Y, Atashi A, Omidkhoda A, Soleimani M. Surface expression of CXCR4 in unrestricted somatic stem cells and its regulation by growth factors. Cell Biol Int. 2010;34(7):687–92.
Xinaris C, Morigi M, Benedetti V, et al. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22(3):423–36.
Lin DL, Chang HC, Huang SH. Characterization of allegedly musk-containing medicinal products in Taiwan. J Forensic Sci. 2004;49(6):1187–93.
Wu Q, Li H, Wu Y, et al. Protective effects of muscone on ischemia-reperfusion injury in cardiac myocytes. J Ethnopharmacol. 2011;138(1):34–9.
Liu P, Feng Y, Dong C, Yang D, Li B, Chen X, et al. Administration of BMSCs with muscone in rats with gentamicin-induced AKI improves their therapeutic efficacy. PLoS One. 2014;9:e97123.
Liu N, Tian J, Cheng J, Zhang J. Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment. Exp Cell Res. 2013;319(13):2019–27.
Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16(2):159–68.
Hung SC, Pochampally RR, Hsu SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2(5):e416.
Liu H, Liu S, Li Y, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7(4):e34608.
Liu H, Xue W, Ge G, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem Biophys Res Commun. 2010;401(4):509–15.
Yu X, Lu C, Liu H, et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013;8(5):e62703.
Liu N, Patzak A, Zhang J. CXCR4-overexpressing bone marrow-derived mesenchymal stem cells improve repair of acute kidney injury. Am J Physiol Renal Physiol. 2013;305(7):F1064–73.
Feng G, Zhang J, Li Y, Nie Y, Zhu D, Wang R, et al. IGF-1 C domain-modified hydrogel enhances cell therapy for AKI. J Am Soc Nephrol. 2016;27:2357–69.
Thomas D, Fontana G, Chen X, Sanz-Nogués C, Zeugolis DI, Dockery P, et al. A shape-controlled tuneable microgel platform to modulate angiogenic paracrine responses in stem cells. Biomaterials. 2014;35:8757–66.
Zhao Z, Watt C, Karystinou A, et al. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. Eur Cell Mater. 2011;22:344–58.
Ziadloo A, Burks SR, Gold EM, et al. Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells. 2012;30(6):1216–27.
Burks SR, Ziadloo A, Hancock HA, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One. 2011;6(9):e24730.
Burks SR, Nguyen BA, Tebebi PA, et al. Pulsed focused ultrasound pretreatment improves mesenchymal stromal cell efficacy in preventing and rescuing established acute kidney injury in mice. Stem Cells. 2015;33(4):1241–53.
Acknowledgements
The authors would like to thank the laboratory members for their contributions and funding support from the sources indicated.
Funding
This work is supported by the National Natural Science Foundation of China (number 81700553) and Public Welfare Industry Research Project of China (number 201502010).
Author information
Authors and Affiliations
Contributions
LZ and JC contributed to the conception of this manuscript. LZ and CH were responsible for the literature review. LZ, PZ, and HJ drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication of the present manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, L., Hu, C., Zhang, P. et al. Novel preconditioning strategies for enhancing the migratory ability of mesenchymal stem cells in acute kidney injury. Stem Cell Res Ther 9, 225 (2018). https://doi.org/10.1186/s13287-018-0973-3
Published:
DOI: https://doi.org/10.1186/s13287-018-0973-3