Abstract
The breast experiences substantial changes in morphology and function during pregnancy and lactation which affects its imaging properties and may reduce the visibility of a concurrent pathological process. The high incidence of benign gestational-related entities may further add complexity to the clinical and radiological evaluation of the breast during the period. Consequently, pregnancy-associated breast cancer (PABC) is often a delayed diagnosis and carries a poor prognosis. This state-of-the-art pictorial review illustrates how despite currently being underutilized, technical advances and new clinical evidence support the use of unenhanced breast MRI during pregnancy and both unenhanced and dynamic-contrast enhanced (DCE) during lactation, to serve as effective supplementary modalities in the diagnostic work-up of PABC.
Similar content being viewed by others
Key points
-
Diffusion MRI may serve as a standalone modality during pregnancy.
-
DCE MRI of the breast remains of significant value during lactation.
-
Unenhanced DTI may increase PABC lesion conspicuity as compared with DCE.
-
Non-fat suppressed T2 images can improve the delineating of non-mass DCIS lesions.
-
Increased utilization of MRI may facilitate an earlier PABC diagnosis.
Background
Pregnancy-associated breast cancer (PABC) is traditionally defined as breast cancer diagnosed during pregnancy, in the first year postpartum, or anytime during lactation [1], and typically represents a high-grade luminal b-like invasive ductal carcinoma [2]. Although it is a rare circumstance, occurring in 0.3 in 1000 pregnancies [3], breast cancer stands among the most common types of malignancies occurring during pregnancy and its incidence is on the rise in developed countries as more women delay childbearing [4, 5]. The diagnosis of PABC could be challenging because of the unique physiological changes that the breast undergoes [6], which may mask a concurrent malignant transformation both clinically and radiologically, while also dictating restrictions on the imaging work-up [7]. Ultimately, PABC is more likely to be diagnosed with an advanced disease than non-pregnant patients [8], and consequently, is associated with a poorer prognosis [9], being the most common cause of cancer-related mortality in gestational women and associated with a mortality rate that is 50% higher when compared to non-PABC [10].
Magnetic resonance imaging (MRI) and in particular, it’s workhorse sequence, dynamic contrast-enhanced (DCE) MRI, continues to serve in the mainstay of breast cancer diagnostic workup [11,12,13], and to expand in potential indications [14,15,16], owing to its high sensitivity for breast cancer detection and its unparalleled negative predictive value compared with conventional imaging [17]. In the general population, only the high costs and low availability are perhaps the main reasons to hold breast MRI from becoming widely used in screening. [18].
Nevertheless, despite its evident diagnostic superiority, inherent limitations of breast MRI surface during pregnancy and lactation, and as a result, restrictions are imposed on its utility [4, 19,20,21]. During pregnancy, DCE-MRI does not play a role in the diagnostic workup of the breast due to fetal safety concerns associated with gadolinium-based contrast agents [22], which are known to cross the placenta [23]. During lactation, gadolinium-based contrast is considered safe for administration [24]. Yet, breast DCE-MRI is considered controversial during lactation due to probable limited sensitivity caused by the increased characteristic background parenchymal enhancement (BPE), which may hinder suspicious finding [25,26,27].
In recent years, the advent of promising preliminary investigations and emergence of advanced MRI protocols, such as the increased clinical employment of unenhanced diffusion-based MRI techniques [28], has driven groups of radiologists and researchers to attempt to expand the role of breast MRI during pregnancy and lactation, with the hope to facilitate an earlier diagnosis of PABC. The aim of this pictorial article is to discuss and illustrate the latest developments of breast MRI during pregnancy and lactation. Variations in breast MRI manifestation due to the periodic physiological modifications are reviewed, as well as the MR imaging spectrum of common benign entities and PABC.
Physiological changes of the breast
Throughout pregnancy, the breast undergoes a series of unique structural and functional alterations in preparation for its eventual biological role in lactation. Within this process, called lactogenesis, the mammary gland grows with developed glandular tissue at the expense of shrunken adipose and connective tissues [29]. Regulated by key hormones, lactogenesis is composed from two stages, which is necessary for the breast in order to synthesize and secrete milk [30]. Secretory initiation takes place in the second trimester of pregnancy. In the postpartum period, secretory activation, the second stage of lactogenesis, begins and is followed with milk secretion which is triggered by the fall of progesterone blood levels [31]. The colostrum is temporarily enriched with protein and electrolytes and following several days of breastfeeding, turns into a mature, lipid-rich, and stable mother’s milk [32].
Both clinical and radiological evaluation of the breast are influenced by the physiological changes during pregnancy and lactation. Clinically, breast examination can be challenging due to the enlarged size of the breasts, their tenderness, and especially their harder, more nodular consistency [33]. Imaging-wise, each of the various radiological modalities is hampered by the various changes in the breast properties. Owing to its harmless nature and excellent utility in focal evaluation of palpable findings [34], there is consensus that US represents the most appropriate and thus, the first-line imaging modality for breast evaluation during pregnancy and lactation [35]. The role of mammography is relatively diminished during pregnancy and lactation due to the increased mammographic density of the breast parenchyma and concerns related to radiation exposure for the fetus [19]. It should be mentioned though, that in many centers, mammography is considered generally safe during pregnancy and lactation, since the radiation dose from a bilateral two view mammogram is < 3 mGy per view, equivalent to 7 weeks of background radiation [36]. The increased mammographic density (Fig. 1) may well reduce the sensitivity of screen-detected tumors [37], although mammography may still be useful in the detection of suspicious micro-calcifications. Therefore, mammography serves as an adjunct to US [27]. During lactation, patients are advised to nurse or pump immediately before undergoing mammography in order to decrease parenchymal density related to retained milk [6]. Rarely, mammography can also exhibit a unique form of scattered micro-calcifications, secondary to gestational (pregnancy) or secretory (lactation) hyperplasia, which may add further complexity for mammographic evaluation (Fig. 1) [38, 39].
Changes in mammographic density and lactation-associated microcalcifications. Left breast medio-lateral-oblique view mammograms of the same patients, performed 2 years apart, prior to pregnancy (a) and during lactation (b) are presented, demonstrating the marked increase in breast volume and mammographic density associated with pregnancy and lactation. Additionally, new onset of lactation-associated microcalcifications was depicted globally on both breasts (b and zoomed image)
These physiological changes of the breast are also reflected on the various MRI pulse sequences. Since contrast enhanced breast MRI scans are not performed during pregnancy, reports on breast MRI of pregnant patients have been limited to examinations performed prior to elected abortion [40] or using unenhanced protocol [41]. Breast MRI studies of the lactating breast are more common and include both DCE, as well as unenhanced sequences [40, 42,43,44,45,46,47,48,49,50,51,52]. The main MRI features of the breast during pregnancy and lactation are increased fibroglandular tissue [47] and increased vascularity, which is manifested by marked BPE [40, 42, 43, 49,50,51,52] and results in false positive coloring on DCE computer aided diagnosis (CAD) mapping (Fig. 2).
Changes of breast MRI properties during lactation. MR images of a BRCA carrier scanned twice at the age of 37, while lactating for 4 months (upper panel), and 2 years afterwards, post-weaning (lower panel) are presented. The sequential examinations demonstrate the marked changes in the breast composition and vascular properties between the two periods. A rich fibroglandular tissue, which is exhibited during lactation, reduces dramatically post weaning. Respectively, the breast perfusion reduces considerably, from BPE 3 to BPE 1, as shown on DCE subtraction MIP images, as well as on DCE CAD maps. Add DCE MIP
Further characterization of the lactating breast has been afforded by additional MRI sequences. On MR spectroscopy, a total choline peak, an established breast cancer biomarker [53], was evident in exams of most healthy lactating volunteers, thus limiting its clinical role in this population [44]. On diffusion-weighted imaging (DWI), the measured apparent diffusion coefficient (ADC) of the lactating breast was found to be decreased relative to normal values among premenopausal, non-lactating, and healthy volunteers. This phenomenon is most likely due to the increased viscosity of the lipid-rich milk [45], although the ADC is still higher than the malignant spectrum of values [44, 45, 47, 48].
Advanced diffusion MRI models were also used to investigate the unique features of the breast during pregnancy and lactation. Intra-voxel incoherent motion (IVIM), a bi-exponential diffusion model, is based on acquiring multiple diffusion weightings in the fast and slow regimes in order to separate the fast perfusion-based “pseudo-diffusion” component from the slow diffusion process [54]. Using IVIM analysis, the lactating breast, as anticipated, has shown to exhibit increased perfusion fraction [48], due to the pronounced vascularity of the breast parenchyma [42] and the high metabolic demand during breastfeeding [55]. Another approach could be found in diffusion tensor imaging (DTI), which is based on applying diffusion gradients to characterize tissue microstructure. These gradients go in multiple directions in order to map spatial information of the diffusion hindrance and restriction that goes beyond cellular density [56]. DTI properties among healthy, pregnant examinees resembled the measurements among non-pregnant, premenopausal examinees, with relatively high values of diffusivity, as expected for dense breasts [41]. Besides decreased diffusivity, DTI studies of the lactating breast also reported reduced anisotropy [45, 46], probably owing to the physiological transient increase in the diameter of the lactiferous ducts [57]. Furthermore, DTI has enabled the characterization of the underlying ductal-tree architecture of the lactating breast, as demonstrated by the diffusion Eigen-vectors mapping. This is clearly illustrated by the predominance of diffusivity directed to the nipple with “duct-like,” linear, and branching vectors of the first eigenvalue [30, 46, 58] (Fig. 3).
Characterization of the lactating breast using DTI. DTI-derived direction and vector maps of a 30-year-old healthy lactating volunteer are presented. Axial images overlaid on anatomical non-fat surpassed T2-weighted images at the height of the nipple are present. The direction map represents the direction of the 1st principal eigenvector in a three color code: red: right <<>> left; green: head <<>> feet; blue: anterior <<>> posterior. The vector map presents in red sticks the direction of the 1st principal eigenvector ν1. Note: The lactating breast exhibits dominant directional diffusion along the anterior–posterior axis, reflecting the structure of milk ducts heading from the base of the breast towards the nipple, while the vector map portray linear successive “duct-like” structures towards the nipple.
Benign breast disease of pregnancy and lactation
Benign entities account for the vast majority of findings among patients presenting with a palpable breast mass during pregnancy and lactation [6]. In a study that evaluated the diagnostic workup of 164 lesions among pregnant, lactating, and postpartum women, Robbins and co-authors reported that most of the cases appeared during lactation (65%), and breast cancer accounted for only 2.4% of cases (4/164), even though cancer constituted 10% of the eventual biopsies [59]. Benign conditions, however, are more common and are either the same as those observed in non-pregnant women [60] or breast abnormalities distinctive for pregnancy and lactation [61]. Examples of these mimickers include, though not exclusively, galactocele, lactating adenoma, fibroadenoma, duct ectasia, mastitis, and abscess [62], along with common contemporary mimickers that affect breast imaging, such as vaccination induced lymphadenopathy [63]. Their presentation, with focus on their MRI characteristics, will be discussed below. In addition, a summary of the typical MRI features of the common breast lesions during pregnancy and lactation is provided in Table 1.
Galactocele
A galactocele, a Greek term meaning “milky pouch,” is a milk collection retained within the fibroglandular tissue because of duct obstruction. This etiology usually regresses spontaneously on follow-up and is the most common benign breast mass among lactating patients [64]. Characterized with a cyst-like formation, a galactocele is often surrounded by a fibrous capsule with variable luminal morphology depending on the distribution of its contents: fat, protein and fluid [61]. Mostly encountered after cessation of breast-feeding, galactoceles can also be present earlier, occasionally even in the third trimester of pregnancy [6]. Similar to other pregnancy-associated breast lumps, the typical clinical manifestation is a painless, palpable mass, arising upon breastfeeding cessation [60]. From the imaging perspective, galactocele is mostly described according to its sonographic appearance [65]; usually as round or oval in shape, with variable echogenicity which most likely increases as the lesion ages and a characteristic fat-fluid level [66]. MRI features of galactocele are hardly described in the literature, as US is sufficient for its diagnostic work-up. Recently, Rosas et al. provided MR images showing a cyst with a thin septa, heterogeneous content, and fat-fluid level, which is compatible with the diagnosis [19] (Fig. 4).

Reproduced with permission from Radiologia Brasileira [19]
Galactocele. Non Fat-suppressed axial T1-weighted (panel A) and sagittal fat-suppressed T2-weighted (panel B) images of lactating patient with galactocele are presented. Note: a cyst with thin septa, heterogeneous content, and fat-fluid level is exhibited and compatible with galactocele diagnosis.
Lactating adenoma
Lactating adenoma represents a benign stromal alteration with a tendency to regress upon breastfeeding cessation [67]. Lactating adenoma is the most prevalent breast lesion occurring during pregnancy, usually appearing during the third trimester or during lactation, as a painless, palpable, and mobile breast lump [68]. Typical US features of lactating adenoma favor a benign mass, including a solid, ovoid, well-defined, and wider-than-taller lesion with homogeneous and hypoechoic appearance alongside posterior acoustic enhancement [69]. On MRI, lactating adenoma has been described as fibroadenoma-like; a well circumscribed mass, containing hypointense septa, causing mass effect by displacing the adjacent normal breast parenchyma and the main galactiferous ducts of the nipple-areolar complex [70]. Herein, we present another representative MRI case of a biopsy-confirmed lactating adenoma. Our findings suggest that lactating adenoma may exhibit benign features of enhancement kinetics on DCE MRI (Fig. 5).
MRI of lactating adenoma. MR images of patient presented with palpable mass on the left breast 2 months after breastfeeding cessation are presented. T2 weighted image showed a hypo-intense mass (a), which exhibits a gradual enhancement and early and late DCE (b, c), below the signal intensity threshold for suspicious findings on CAD (d). Despite these benign features, the patient underwent vacuum-assisted biopsy which revealed lactating adenoma
Fibroadenoma
Fibroadenomas are composed of epithelium and stroma and account for the most common benign tumor detected in young females [71]. Interestingly, before pregnancy, fibroadenomas may remain latent and asymptomatic until becoming clinically apparent as a new-onset palpable mass after hormonally stimulated growth [6]. Clinically, fibroadenomas, which are often multiple and bilateral, usually present as a painless firm, mobile, and rubbery mass. Less frequently, fibroadenomas may experience a tremendous growth spurt, resulting in central infarction, and then becoming tender [72]. On mammography, fibroadenomas often appear as a well-defined round or oval mass which may also exhibit pathognomonic benign calcifications, making a further imaging work-up unnecessary [73]. On US, fibroadenomas among pregnant or lactating women is the same as among the general population, exhibiting a circumscribed, wider-than-taller oval or round mass [74]. Infarcted or complexed fibroadenomas may show suspicious features such as irregular margins and internal cystic changes that warrant biopsy [75]. On MRI, fibroadenomas usually exhibit a benign morphology on unenhanced sequences, including a sharp contour without signs of infiltration [76]. Additionally, they exhibit benign DCE patterns such as a persistent kinetic curve [77] and a high extracellular volume fraction with low to moderate microvascular permeability [78]. A representative case of a growing fibroadenoma is given in Fig. 6.
Growing fibroadenoma during lactation. DCE-MRI subtracted images of a 26 years BRCA 1 carrier scanned twice within 18 months of routine surveillance, before conception and during lactation are presented. On baseline MRI (a), a 1.3 cm well-defined enhancing oval mass is visible on top of minimal background parenchymal enhancement (BPE 0). Yet, on follow-up during lactation, a 1.8 cm is hardly visible on DCE, due to the marked physiological BPE (grade 3) (b). The lesion enlargement and a personal history of phyllodes tumor prompt a US-guided biopsy which reassured fibroadenoma histology
Duct ectasia
Duct ectasia of the breast is among the benign processes that may affect the nipple-areolar complex during pregnancy and lactation [79]. The clinical course of duct ectasia ranges from asymptomatic to symptoms such as nipple discharge, nipple retraction, a palpable mass, and mastalgia [80]. Depending on the degree of dilatation as well as the mammographic density, duct ectasia may be visible at mammography as dense tubular structures converging on the nipple-areolar complex [81]. Sonographically, it appears as anechoic, smooth-walled, and branching structures that taper peripherally [82]. On MRI, the ductal structures may be visible on fat-suppressed T1 and T2-weighted images depending on if its contents are composed of protein or fluids, respectively. Despite being regarded as benign, a unilateral duct dilatation may be an indicator of malignancy and hence, the importance of its diagnostic work-up [83]. An illustrative case of duct ectasia mimicking malignancy is shown in Fig. 7.
MRI of duct ectasia. MRI and US images of 40-year-old lactating patient with palpable mass on the left breast are presented. Initial work-up included US which revealed a 4 cm cystic mass with thick boundaries, with relative mixed cystic areas (e) and stiffness on static elastography (f). On MRI, T2-weighted image reveals a high signal cystic structure (a), without internal restriction on ADC map (b), though with enhancement of its walls on DCE and CAD (c, d). Finally, US-guided biopsy ruled out malignancy with duct ectasia diagnosis
Mastitis and abscess
Mastitis is a common infectious condition that may affect up to one-third of lactating women [84] and is among the leading medical causes of premature breastfeeding cessation [85]. Among the most common complications are mastitis that are abscesses with a purulent collection. Its pathophysiology is thought to be related to transmission of oral bacterial from the infant to the mother’s lactiferous ducts. Maternal risk factors that were identified include previous mastitis during breastfeeding, cesarean section, breast trauma, latch problems, milk overproduction, blocked duct, and more [86]. Clinically, mastitis presents with focal mastalgia, edema, and erythema which may be accompanied fever and elevated blood test inflammatory markers. Focused US is indicated to rule out abscess when the infection is refractory to antibiotics, or for therapeutic guided-aspiration of the abscess [87]. Sonographically, it typically is characterized by an area of fluid collection with thin septations or debris, thickened walls, uncircumscribed margins and posterior acoustic enhancement [60]. With that regards, another related entity worth mentioning is granulomatous mastitis (GM), a rare benign inflammatory breast disease that affects mostly women of childbearing age with a history of breastfeeding and may mimic both abscess and carcinoma [88]. Breast MRI is not indicated during acute mastitis; however, when mastitis symptoms persist despite well-managed medical treatment, MRI may be performed. The main differential diagnosis of exclusion is inflammatory breast cancer [89], notwithstanding overlapping enhancement features of the two entities [90]. Herein, we present two cases in which MRI was utilized during for mastitis evaluation (Fig. 8) and abscess monitoring (Fig. 9).
Mastitis. Unenhanced MR images of 37-year-old pregnant patient with refractory mastitis are presented. The patient presented with breast edema, erythema and pruritus and blinded subcutaneous punch biopsies revealed adenosis on pathology. Because of continuous symptoms despite treatment, we were requested to perform MRI without contrast injection to rule out underlying inflammatory carcinoma. T2 weighted image revealed thickened skin (a) (yellow line), while no focal restriction was noted on ADC map (b)
Abscess. MRI of 31-year-old patient with a history of recurrent breast mastitis and abscesses during several separate lactation periods is presented, this time in aspiration-confirmed breast abscess post weaning. Subtracted DCE (a) MIP (c) and CAD (d) reveal large rim enhancing regions with high T2 signal (b), compatible with an abscess
Pregnancy-associated breast cancer (PABC)
Pregnancy
During pregnancy, breast DCE-MRI is contraindicated due to the increased risk of a broad set of rheumatological, inflammatory, or dermal conditions, as well as stillbirth or neonatal death, associated with gadolinium-based contrast agents used during the MRI [22]. The lone report on breast DCE-MRI during pregnancy was composed of PABC patients who elected to undergo abortion [40]. Despite the lack of supportive evidence for improved maternal outcomes for pregnant breast cancer patients undergoing therapeutic abortion [91], an elective abortion remains frequent in patients diagnosed in the first trimester [92]. In these patients, DCE-MRI can aid in improved pre-operative assessment, providing additional diagnostic information regarding tumor size, extent of disease and contralateral involvement compared to mammography and US, in up to 28% of cases [40]. A representative MRI of a pregnant breast cancer patient who elected to undergo abortion is given in Fig. 10, showing the tumor extent superimposed on the notable pregnancy-associated BPE.
MRI of PABC during pregnancy prior to elected abortion. Pre-operative MR Images of a pregnant patient with newly diagnosed IDC are shown. T2 (a), subtraction DCE (b), DWI (c) and CAD (d) reveal extensive lesion on the right breast, on top of marked BPE on both breasts, as well false positive bilateral CAD coloring secondary to the increased vascularity
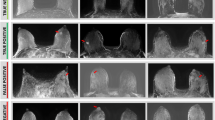
Moreover, the first attempt to utilize unenhanced diffusion MRI as a standalone modality for pregnant patients at high risk or with newly diagnosed PABC was recently reported [37]. This work demonstrated the feasibility and tolerability of breast MRI in the prone position among pregnant patients, although most cases involved pregnant women in the first and second trimesters. In order to decrease any gravitational pressure from the belly, extra pillows were placed underneath the women to assist with pelvic lifting. In terms of diagnostic performance, diffusivity maps were useful in detecting nine out of 11 lesions and excluded malignancy in 14 high-risk patients; however, the maps were unable to detect two 7 mm lesions, as anticipated under the technical limitations of this modality [93]. Representative cases of unenhanced MRI in pregnant breast cancer patients are shown in Fig. 11 [94], highlighting the potential diagnostic advantages of this approach.
Unenhanced diffusion MRI during pregnancy. T2, DWI, and DTI parametric maps of three PABC patients (A–C). T2-weighted, DWI and DTI-derived diagnostic parametric maps of λ1, MD, and λ1–λ3 of three patients with newly diagnosed IDC are presented. The lesion appears bright on DWI (b 700 s/mm2). Using the parametric threshold, the lesion could be easily depicted on l1, and MD maps, as well as on l1–l3 map, compared with the measurements in the normal tissue.
An interesting and unusual case we encountered was of a pregnant patient with newly diagnosed mucinous breast carcinoma who underwent MRI prior to elected abortion. Pure mucinous carcinoma typically appears on MRI as a circumscribed mass with extremely high signal intensity on fat-saturated T2-weighted imaging and a benign-appearing persistent enhancement curve [94, 95]. As demonstrated in Fig. 12, the palpable lesion on the left breast was not detected on DCE and CAD images because of concurrence of its benign-like kinetic features and the marked surrounding BPE. Yet, the lesions were clearly visible on fat-suppressed T2-weighted images, therefore stressing the importance of acquiring broad protocol in diagnostic breast MRI.
MRI of mucinous carcinoma during pregnancy. MR images of patient with left sided mucinous carcinoma, presented as a palpable mass during pregnancy are presented. The patient chose to undergo elected abortion. Subtraction DCE image reveals marked bilateral BPE (a) and CAD reveal bilateral diffuse benign-like coloring with progressive enhancement in time intensity curves (b) without evidence of the known underlying lesions in the left breast. However, the lesions, diagnosed on US (d), were clearly visible on sagittal T2-weighted fat-suppressed image (c)
Unenhanced diffusion MRI is also gaining recognition in the diagnostic workup of PABC for the systemic staging of pregnant patients, when the use of PET/CT is discouraged [95]. For this purpose, a whole-body MRI relying on DWI with background suppression (DWIBS) sequence has been applied [96]. This emerging MRI technique can provide non-invasive information regarding the extent of disease and distant metastasis and often provides diagnostic value that changes the patient management [97, 98].
Lactation
Breast MRI is much more common during lactation, due to the fact that injection of gadolinium-based contrast agent is considered safe for administration [20]. Past studies evaluating the gadolinium excretion into breast milk revealed that less than 0.04% of the administered dose reaches the milk [99], and, of that amount, only 0.8% is actually absorbed by the infant [100]. Accordingly, some authors openly assert that lactating patients should not be advised to suspend breastfeeding at all, given that the risks associated with interrupting breastfeeding outweigh the negligible amount of contrast media [101]. More conservative approaches suggest the option of abstaining from breastfeeding for a period of 12–24 h if this is the preference of an informed mother [102]. Since the excretion of gadolinium to breast milk has been shown to reach its peak after approximately 4 h [103], if lactating patients have concerns about breastfeeding, the authors advise to pump and dump the milk with continuation of nursing after 6 h.
The main concern regarding the use of breast MRI during lactation does not stem from safety worries, but rather reservations regarding its uncertain diagnostic performance. In light of the increased BPE, there are concerns that it may potentially obscure the presence of the underlying tumor [27]. Several publications reported that despite increased surrounding BPE, high sensitivity was observed in known PABC cases that underwent DCE-MRI [40, 49,50,51].
Herein, we present an assembly of representative cases, illustrating the spectrum of appearance and persevered diagnostic capabilities of breast MRI, even in lactating patients. The first case is a pre-operative MRI of a PABC patient who presented with a palpable mass after 3 months of lactation. IDC was diagnosed using US-guided biopsy, and MRI reassured the existence of a solitary lesion on top of the surrounding BPE (Fig. 13). Occasionally, the diagnostic workup of known, newly diagnosed PABC can get complicated by simultaneous benign lactation-related findings, as shown by Fig. 14. In this patient, a preoperative MRI performed in a lactating patient revealed the known 2.8 cm IDC, as well as another enhancing 0.9 cm lesion which warranted focused US and biopsy to reveal adenosis and lactating changes on pathology. This case demonstrated that an argument regarding reduced specificity of breast MRI during lactation could be claimed.
Preoperative MRI during lactation. Axial subtracted DCE-MRI and CAD images of a 37-year-old PABC patient, lactating for 3 months are shown. Note: DCE reveals the presence of a single small lesion in the right upper outer quadrant (12 mm IDC) with excellent conspicuity, comparing with the surrounding moderate BPE. The tumor exhibited the suspicious wash-out pattern (red) on CAD MIP image, while slight BPE exhibited persistence benign wash-in pattern (blue)
MRI of a complicated case of IDC with lactation changes. Axial subtracted DCE-MRI and CAD of lactating patient with newly diagnosed PABC, undergoing preoperative evaluation are shown. The tumor, 2.8 cm IDC in the left breast, is well visible on top of the surrounding BPE (a). Yet, additional ipsi-lateral enhancing 0.9 cm lesion is apparent (b). Upon focused US and US-guided biopsy, the second lesion came up to be adenosis and lactating changes on pathology. Interestingly, CAD depicted both lesions, as well as slight BPE in blue color, which corresponds to persistence wash-in pattern which usually represents a benign pattern (c)
One noteworthy type of cancer that deserves a specific mention is ductal carcinoma in situ (DCIS), which often displays overlapping radiological and pathological features with lesions with uncertain malignant behavior [104]. Unlike invasive carcinomas that tend to present as a mass, DCE-MRI usually depicts DCIS as non-mass enhancement (NME) with a larger median span than mammography [105]. Taking into consideration the difficulty in unravelling BPE from NME [106], this casts doubt regarding the utility of breast MRI to detect DCIS during lactation could arise. Two representative newly diagnosed DCIS cases undergoing preoperative breast MRI during lactation are presented in Figs. 15 and 16. Both patients presented with palpable mass and mammography detected suspicious linear micro-calcifications in typical segmental distribution. DCE-MRI displayed NME in the tumor region, enhancing more vividly than the surrounding lactation-induced BPE. Interestingly, additional diagnostic value was provided by non-fat suppressed T2-weighted images, allowing better depiction of lesion morphology and margins (Fig. 15).
MRI of DCIS during lactation. Images of a 40-year-old PABC patient (lactating for 4 months) with DCIS confirmed on operation are presented. The patient underwent screening mammography with adjuvant breast US (not presented) due to the extremely dense breasts on mammography (BI-RADS D) (c). Mammography revealed suspicious pleomorphic micro-calcifications in segmental distribution along 6 cm in the left upper outer quadrant (c). Pre-operative MRI showed NME on top of the surrounding prominent lactation-induced BPE, typical for DCIS, in agreement with the calcifications location (a). Furthermore, an excellent tumor delineation was afforded by non-fat surpassed T2-weighted image, showing hypo-intense region in the tumor area (b)
MRI of post-partum DCIS. Images of a 33-year-old PABC patient (lactating cessation a week prior to the MRI) diagnosed with left breast DCIS are presented. The patient palpate a lump in the left breast during the third trimester of pregnancy and underwent breast US (not presented) which depicted a benign appearing 9 mm oval mass. Upon follow-up 3 months later, post-partum, focal US depicted irregular mass with calcifications. Further diagnostic workup included US-guided biopsy, mammography which revealed extremely dense breast with segmental distribution of suspicious micro-calcifications in the inner-lower quadrant (b and zoomed image c), as well as breast MRI (a) which showed the characteristic NME of DCIS along 42 mm, in agreement with the mammographic findings, on top of the moderate background enhancement (BPE 2)
Occasionally, PABC can manifest as multi-centric carcinoma, which is difficult to fully estimate its extent using conventional imaging. A representative case of a lactating patient with newly diagnosed IDC which turned to comprise no less than seven distinct malignant ipsi-lateral lesions is presented in Fig. 17, highlighting the ability of DCE-MRI, as well as unenhanced DWI to portray the entire extent of disease. With that regards, a recent comparative study investigated tumor conspicuity in DCE-MRI and unenhanced DTI protocol among lactating patients with PABC [51]. On DCE-MRI, because of the marked BPE, tumor conspicuity was reduced by 60% as compared to non-lactating controls. On the contrary, an additional 138% increase in tumor conspicuity on DTI compared with DCE was observed, underscoring a clear advantage for unenhanced MRI to operate in the setting of lactation-induced BPE.
MRI of multi-centric IDC during lactation. MRI of a 40-year-old PABC patient (lactating for 18 months) is presented. The woman presented with a palpable left breast mass and was referred to pre-operative MRI upon IDC diagnosis. Note: Several multifocal tumor foci are shown on the subtracted DCE MRI of the left breast (red arrow heads), surrounded by a moderate BPE (grade 2) (a, b). In agreement, diffusion weighted images (b = 700) revealed restricted tumor regions (c, d), though incomplete fat saturation artifacts are also presented on the right breast (yellow arrow heads). The entire multifocal tumor distribution could be further appreciated on MIP images of DCE and CAD (e and f, respectively)
Since PABC is often a delayed diagnosis, it is associated with more advanced tumor size at the time of diagnosis compared to non-PABC [107] and eventually may lead to increased rates of mastectomy as the treatment of choice [108]. Therefore, it is not uncommon to encounter a large PABC lesion occupying a high portion of the breast size, as demonstrated in Figs. 18 and 19. These images show the complete extent of the enormous tumors which are clearly depicted on both DCE as well as on unenhanced DWI.
MRI of delayed diagnosed IDC during lactation. Axial subtraction DCE-MRI, CAD and ADC map of a 25-year-old PABC patient lactating for 8 months, with a 6.7 cm triple negative IDC on the right breast are presented. The massive lesion exhibited with an irregular rim enhancement concordant with triple negative IDC and is clearly visible on top of the surrounding mild BPE (a), with mostly persistent enhancement kinetic pattern (b) and decreased ADC values in margins of the lesions, with increased diffusivity in the central necrotic region (c)
MRI of delayed diagnosed IDC during lactation. Axial subtraction DCE-MRI, CAD and ADC map of a lactating patient with a 7 cm IDC on the right breast are presented. The huge lesion exhibited vivid enhancement as compared to the mild BPE (a), with heterogeneous kinetic features (b) and decreased ADC values as compared with the surrounding parenchymal diffusivity (c)
Post-weaning
Considering the difficulty of interpretation of DCE-MRI with marked BPE and the high likelihood of lactation-related benign entities, some authors suggest that it may be reasonable to delay the examination until several months after weaning to minimize false-positive results that may lead to unnecessary biopsies [26]. Screening MRI was once recommended in the breastfeeding period for “women who are at very high-risk for breast cancer” [7], or within the first 6 months postpartum [109]. Others suggested waiting until 3 months after cessation of breastfeeding since the imaging changes should resolve by this time-span following lactation cessation [6]. Recently, the ACR guidelines recommended resuming MRI screening for patients over 30 years old if breastfeeding is continued for more than 6 months. Otherwise, the ACR recommends resuming annual high-risk screening MRI 6–8 weeks following cessation of breastfeeding [20]. All in all, despite the variance in the literature, based on our institutional experience, the authors advocate not to postpone pre-operative MRI of newly diagnosed lactating PABC patient. Usually, from the beginning of the diagnostic work-up and until pathological confirmation of the cancer, the patients often discontinue nursing, and even this interlude period of 1–2 weeks may be sufficient to decrease the level of BPE. Among patients who are diagnosed with breast cancer post-weaning, lactation-related BPE is no longer expected and the tumor can be clearly viewed by DCE (Fig. 20).
MRI of breast cancer diagnosed post-weaning. Pre-operative MR images of 42-year-old patient with DCIS, newly diagnosed to months after cessation of breastfeeding are presented. Subtraction DCE (a) and CAD (b) reveal large nonmass enhancing lesion in the left breast, on top of minimal BPE. Non-fat surpassed T2-weighted image at the height of the nipple reveal high signal ducts in the sub-areolar region (c), representing the transformation of the breast from lactation to involution
Summary and outlook
In light of the marked physiological changes that the breast undergoes during pregnancy and lactation, clinical and radiological evaluation of the breast becomes extremely challenging. Considering the high incidence of gestational-associated benign breast entities, it is no surprise that PABC is often a delayed diagnosis [110]. The delay could be attributed to either the patient, if they postpone seeking medical evaluation, the physician, if they provide a false-negative clinical assessment of the symptomatic breast, or an imaging-related delay, via a false-negative radiological evaluation [111]. Ultimately, PABC is typically diagnosed only after clinical symptoms arise, most commonly as a large palpable mass [112]. Considering that PABC’s prognosis is not inferior from that of non-PABC when adjusted for stage and age [113], it appears that the delay in diagnosis, rather than the gestational state and associated overexpressed vascular, hormonal and growth-factor mediators [114], is responsible for its poor prognosis. This demonstrates the unmet need to adapt new screening strategies for high-risk populations during this period [20, 109], as well as to develop and utilize advanced imaging tools for achieving early diagnosis.
While there is wide agreement that US should be the first-line modality for breast imaging during pregnancy and lactation, and that mammography may have a supplementary additive role, the role of MRI remains controversial in the diagnostic work-up of PABC. In this pictorial review, we have illustrated how gestational-related physiological and benign processes are translated to MRI. Moreover, we have demonstrated the promising utility of unenhanced MRI to serve as a standalone breast imaging modality during pregnancy, and the more established utility of both contrast enhanced and unenhanced breast MRI during lactation. Specifically, it appears that since most cases of PABC reach the radiological work-up with a large palpable mass, the opportunity to facilitate an earlier diagnosis of PABC could be found among high-risk patients and BRCA mutation carriers, which account for up to 35% of PABC cases [115]. In this population, action should be taken to investigate whether screening MRI can detect PABC with asymptomatic disease.
Unenhanced breast MRI using DWI variants has shown great strides to serve as a possible cost-effective, fast, and clinically effective alternative to DCE [116]. Nevertheless, several factors are still holding it from being fully integrated into daily practice [117]. Technically, breast DWI is prone to eddy currents, geometrical and intensity distortions, and echo planar imaging ghosts artifacts [93]. Clinically, lower sensitivity of breast DWI was noted in cases of sub-centimeter lesions [118, 119], as well as in NME lesions [120]. To overcome these drawbacks, several strategies were recently attempted in order to provide robustness to artifacts and improve image quality [121,122,123,124,125]. Spatial resolution was also improved by reaching up to sub-millimeter pixel resolution [126,127,128], eventually allowing for visibility of higher lesions [129] and greater morphological concordance between DWI and DCE [130]. Thus, the authors foresee an encouraging future for breast DWI in general, and in particular with PABC.
For DCE, it is safe to assume that during pregnancy it would remain unutilized. During lactation, however, the role of DCE may expand, possibly due to the implementation of novel acquisition schemes that may allow better separation between enhancing lesions and BPE. In recent years, developments in accelerated MRI using the application of compressed sensing [131] have allowed the faster acquisition of MRI data. This relies on exploiting sparsely under-sampled k-space in peripheral regions while continuously sampling the k-space center to enable high temporal resolution with preserved spatial resolution. Several sparse methods have been integrated to MRI protocols, including time-resolved angiography with stochastic trajectory (TWIST) [132] and golden-angle radial sparse parallel (GRASP) [133]. Optimization of sparse techniques to breast MRI has promoted the novel approach of ultrafast DCE with temporal resolution of less than 10 s during the initial wash-in phase, compared with a standard temporal resolution of up to 2 min in conventional MRI [134]. Analysis of the wash-in kinetics has been found to provide valuable information for lesion characterization [134–139] and since BPE usually exhibits slow early enhancement slope and persistent delayed enhancement [140], ultrafast sequence might therefore be suitable for early visualization of malignant lesions with minimization of lactation-induced BPE [141]. The accumulation of BPE along the early phases of wash-in during ultrafast breast DCE of healthy lactating patients is demonstrated in Fig. 21. Altogether, there is a clinical necessity of further studies on larger cohort of patients to evaluate the role of breast MRI during pregnancy and lactation, and in particular as a screening tool among high-risk populations during this period.
Ultrafast DCE-MRI of the lactating breast. Subtracted ultrafast DCE-MRI of the right breast of a healthy lactating patient (34 years old, lactation duration 18 months) are presented. Using grasp-vibe, a compressed-sensing technique, acquisition of ten consecutive T1-weighted images in the first minute post injection was enabled. Note: the prominent lactation-induced BPE (grade 3) appears only in the fifth acquired T1 image, approximately after 30 s, and further enhances in the following phases
Conclusions
During pregnancy and lactation, the breast experiences substantial changes in morphology and function that affect its imaging properties and may reduce the visibility of concurrent pathological processes. Moreover, the high incidence of benign, gestational-related entities may further add complexity to the clinical and radiological evaluation of the breast during this period. Consequently, PABC is often a delayed diagnosis that carries a poor prognosis. Despite currently being underutilized, this state-of-the-art pictorial review illustrates how technical advances and new clinical evidence support the use of unenhanced breast MRI during pregnancy and both unenhanced and DCE during lactation. These modalities serve as effective supplementary options in the diagnostic work-up of PABC, especially among high risk populations, with the aim to facilitate an earlier diagnosis.
Availability of data and materials
Not applicable.
Abbreviations
- ACR:
-
American college of radiology
- ADC:
-
Apparent diffusion coefficient
- BPE:
-
Background parenchymal enhancement
- CAD:
-
Computer aided diagnosis
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- DCIS:
-
Ductal carcinoma in situ
- DWIBS:
-
DWI with background suppression
- DCE:
-
Dynamic-contrast enhanced
- GRASP:
-
Golden-angle radial sparse parallel
- GM:
-
Granulomatous mastitis
- IVIM:
-
Intra-voxel incoherent motion
- IDC:
-
Invasive ductal carcinoma
- MRI:
-
Magnetic resonance imaging
- NME:
-
Non-mass enhancement
- PABC:
-
Pregnancy associated breast cancer
- TWIST:
-
Time-resolved angiography with stochastic trajectory
References
Keinan-Boker L, Lerner-Geva L, Kaufman B, Meirow D (2008) Pregnancy-associated breast cancer. Isr Med Assoc J 10(10):722–727
Genin AS, Lesieur B, Gligorov J, Antoine M, Selleret L, Rouzier R (2012) Pregnancy-associated breast cancers: Do they differ from other breast cancers in young women? Breast. https://doi.org/10.1016/j.breast.2012.05.002
Perez F, Bragg A, Whitman G (2021) Pregnancy associated breast cancer. J Clin Imaging Sci 11:1–6. https://doi.org/10.25259/JCIS_81_2021
Kieturakis AJ, Wahab RA, Vijapura C, Mahoney MC (2021) Current recommendations for breast imaging of the pregnant and lactating patient. AJR Am J Roentgenol 216(6):1462–1475
Ruiz R, Herrero C, Strasser-Weippl K et al (2017) Epidemiology and pathophysiology of pregnancy-associated breast cancer: a review. Breast 35:136–141
Vashi R, Hooley R, Butler R, Geisel J, Philpotts L (2013) Breast imaging of the pregnant and lactating patient: physiologic changes and common benign entities. AJR Am J Roentgenol 200:329–336. https://doi.org/10.2214/AJR.12.9845
Vashi R, Hooley R, Butler R, Geisel J, Philpotts L (2013) Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. AJR Am J Roentgenol 200:321–328. https://doi.org/10.2214/AJR.12.9853
Johansson ALV, Andersson TML, Hsieh CC et al (2013) Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC). Breast Cancer Res Treat. https://doi.org/10.1007/s10549-013-2522-1
Shao C, Yu Z, Xiao J et al (2020) Prognosis of pregnancy-associated breast cancer: a meta-analysis. BMC Cancer. https://doi.org/10.1186/s12885-020-07248-8
Andrew O, Mullen LA, Harvey SC (2020) Pregnancy-associated breast cancer. Appl Radiol 12:9–17
Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology. https://doi.org/10.1148/radiol.2019182947
Scaranelo AM (2021) What’s hot in breast MRI. Can Assoc Radiol J. https://doi.org/10.1177/08465371211030944
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. https://doi.org/10.1016/j.ejca.2010.02.015
Comstock CE, Gatsonis C, Newstead GM et al (2020) Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. https://doi.org/10.1001/jama.2020.0572
Bakker MF, de Lange SV, Pijnappel RM et al (2019) Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 381:2091–2102. https://doi.org/10.1056/nejmoa1903986
Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S (2017) Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. https://doi.org/10.1148/radiol.2016161444
Marino MA, Helbich T, Baltzer P, Pinker-Domenig K (2018) Multiparametric MRI of the breast: a review. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25790
Mango VL, Goel A, Mema E, Kwak E, Ha R (2019) Breast MRI screening for average-risk women: a monte carlo simulation cost–benefit analysis. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26334
Rosas CHS, Góes ACA, Saltão LM, Tanaka AM, Marques EF, Bitencourt AG (2020) Pregnancy-lactation cycle: How to use imaging methods for breast evaluation. Radiol Bras. https://doi.org/10.1590/0100-3984.2019.0071
Carmichael H, Matsen C, Freer P et al (2017) Breast cancer screening of pregnant and breastfeeding women with BRCA mutations. Breast Cancer Res Treat 162(2):225–230
Mesurolle B, Sun S, Zhang M (2020) Utilization of breast MRI and breast MRI-guided biopsy in clinical practice: results of a survey in Québec and France. Insights Imaging. https://doi.org/10.1186/s13244-020-00886-3
Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL (2016) Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. https://doi.org/10.1001/jama.2016.12126
Sundgren PC, Leander P (2011) Is administration of gadolinium-based contrast media to pregnant women and small children justified? J Magn Reson Imaging 34(4):750-757
Webb JAW, Thomsen HS, Morcos SK et al (2005) The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. https://doi.org/10.1007/s00330-004-2583-y
Boivin G, De Korvin B, Marion J, Duvauferrier R (2012) Is a breast MRI possible and indicated in case of suspicion of breast cancer during lactation? Diagn Interv Imaging 93:823–827
Johnson HM, Lewis TC, Mitchell KB (2020) Breast cancer screening during lactation: ensuring optimal surveillance for breastfeeding women. Obstet Gynecol 135:194–198. https://doi.org/10.1097/AOG.0000000000003600
diFlorio-Alexander RM, Slanetz PJ, Moy L et al (2018) ACR appropriateness Criteria® breast imaging of pregnant and lactating women. J Am Coll Radiol. https://doi.org/10.1016/j.jacr.2018.09.013
Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE (2016) Diffusion-weighted breast MRI: clinical applications and emerging techniques. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25479
McManaman JL, Neville MC (2003) Mammary physiology and milk secretion. Adv Drug Deliv Rev 55(5):629–641
Neville MC, McFadden TB, Forsyth I (2002) Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7:49–66
Kent JC (2007) How breastfeeding works. J Midwifery Women’s Heal. https://doi.org/10.1016/j.jmwh.2007.04.007
Neville MC, Allen JC, Archer PC et al (1991) Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. https://doi.org/10.1093/ajcn/54.1.81
Langer A, Mohallem M, Berment H et al (2015) Breast lumps in pregnant women. Diagn Interv Imaging 96:1077–1087
de Holanda AAR, Gonçalves AKS, de Medeiros RD, Oliveira AM, Maranhão TM (2016) Ultrasound findings of the physiological changes and most common breast diseases during pregnancy and lactation. Radiol Bras. https://doi.org/10.1590/0100-3984.2015.0076
Ayyappan AP, Kulkarni S, Crystal P (2010) Pregnancy-associated breast cancer: spectrum of imaging appearances. Br J Radiol. https://doi.org/10.1259/bjr/17982822
Yang WT, Dryden MJ, Gwyn K, Whitman GJ, Theriault R (2006) Imaging of breast cancer diagnosed and treated with chemotherapy during pregnancy. Radiology 239(1):52–60
Wanders JOP, Holland K, Veldhuis WB et al (2017) Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-016-4090-7
Mercado CL, Koenigsberg TC, Hamele-Bena D, Smith SJ (2002) Calcifications associated with lactational changes of the breast: mammographic findings with histologic correlation. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.179.3.1790685
Stucker DT, Ikeda DM, Hartman AR et al (2000) New bilateral microcalcifications at mammography in a postlactational woman: case report. Radiology. https://doi.org/10.1148/radiology.217.1.r00oc19247
Myers KS, Green LA, Lebron L, Morris EA (2017) Imaging appearance and clinical impact of preoperative breast MRI in pregnancy-associated breast cancer. AJR Am J Roentgenol 209:W1–W7. https://doi.org/10.2214/AJR.16.17124
Nissan N, Furman-Haran E, Allweis T et al (2018) Noncontrast breast MRI during pregnancy using diffusion tensor imaging: a feasibility study. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26228
Espinosa LA, Daniel BL, Vidarsson L, Zakhour M, Ikeda DM, Herfkens RJ (2005) The lactating breast: contrast-enhanced MR imaging of normal tissue and cancer. Radiology 237:429–436. https://doi.org/10.1148/radiol.2372040837
Talele AC, Slanetz PJ, Edmister WB, Yeh ED, Kopans DB (2003) The lactating breast: MRI findings and literature review. Breast J 9:237–240
Sah RG, Agarwal K, Sharma U, Parshad R, Seenu V, Jagannathan NR (2015) Characterization of malignant breast tissue of breast cancer patients and the normal breast tissue of healthy lactating women volunteers using diffusion MRI and in vivo 1H MR spectroscopy. J Magn Reson Imaging 41:169–174. https://doi.org/10.1002/jmri.24507
Nissan N, Furman-Haran E, Shapiro-Feinberg M, Grobgeld D, Degani H (2014) Diffusion-tensor MR imaging of the breast: hormonal regulation. Radiology 271:672–680. https://doi.org/10.1148/radiol.14132084
Nissan N, Furman-Haran E, Feinberg-Shapiro M et al (2014) Tracking the mammary architectural features and detecting breast cancer with magnetic resonance diffusion tensor imaging. J Vis Exp. https://doi.org/10.3791/52048
Nissan N, Furman-Haran E, Shapiro-Feinberg M, Grobgeld D, Degani H (2017) Monitoring in-vivo the mammary gland microstructure during morphogenesis from lactation to post-weaning using diffusion tensor MRI. J Mammary Gland Biol Neoplasia. https://doi.org/10.1007/s10911-017-9383-x
Iima M, Kataoka M, Sakaguchi R et al (2018) Intravoxel incoherent motion (IVIM) and non-Gaussian diffusion MRI of the lactating breast. Eur J Radiol Open 5:24–30. https://doi.org/10.1016/j.ejro.2018.01.003
Oh SW, Lim HS, Moon SM et al (2017) MR imaging characteristics of breast cancer diagnosed during lactation. Br J Radiol. https://doi.org/10.1259/bjr.20170203
Taron J, Fleischer S, Preibsch H, Nikolaou K, Gruber I, Bahrs S (2019) Background parenchymal enhancement in pregnancy-associated breast cancer: A hindrance to diagnosis? Eur Radiol. https://doi.org/10.1007/s00330-018-5721-7
Nissan N, Allweis T, Menes T et al (2020) Breast MRI during lactation: effects on tumor conspicuity using dynamic contrast-enhanced (DCE) in comparison with diffusion tensor imaging (DTI) parametric maps. Eur Radiol. https://doi.org/10.1007/s00330-019-06435-x
Nissan N, Sorin V, Bauer E et al (2021) MRI of the lactating breast : computer-aided diagnosis false positive rates and background parenchymal enhancement kinetic features. Acad Radiol. https://doi.org/10.1016/j.acra.2021.11.003
Sharma U, Jagannathan NR (2019) In vivo MR spectroscopy for breast cancer diagnosis. BJR Open. https://doi.org/10.1259/bjro.20180040
Iima M, Le Bihan D (2016) Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 278(1):13–32
Nissan N, Sandler I, Eifer M et al (2020) Physiologic and hypermetabolic breast 18-F FDG uptake on PET/CT during lactation. Eur Radiol. https://doi.org/10.1007/s00330-020-07081-4
Le Bihan D, Mangin JF, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. https://doi.org/10.1002/jmri.1076
Geddes DT (2009) Ultrasound imaging of the lactating breast: Methodology and application. Int Breastfeed J 4:4
Solomon E, Liberman G, Nissan N, Frydman L (2017) Robust diffusion tensor imaging by spatiotemporal encoding: principles and in vivo demonstrations. Magn Reson Med. https://doi.org/10.1002/mrm.26197
Robbins J, Jeffries D, Roubidoux M, Helvie M (2011) Accuracy of diagnostic mammography and breast ultrasound during pregnancy and lactation. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.09.3662
Sabate JM, Clotet M, Torrubia S et al (2007) Radiologic evaluation of breast disorders related to pregnancy and lactation. Radiographics 27(Suppl 1):S101–S124. https://doi.org/10.1148/rg.27si075505
Parker S, Saettele M, Morgan M, Stein M, Winkler N (2017) Spectrum of pregnancy- and lactation-related benign breast findings. Curr Probl Diagn Radiol 46(6):432–440
Guirguis MS, Adrada B, Santiago L, Candelaria R, Arribas E (2021) Mimickers of breast malignancy: imaging findings, pathologic concordance and clinical management. Insights Imaging. https://doi.org/10.1186/s13244-021-00991-x
Faermann R, Nissan N, Halshtok-Neiman O et al (2021) COVID-19 vaccination induced lymphadenopathy in a specialized breast imaging clinic in Israel: analysis of 163 cases. Acad Radiol. https://doi.org/10.1016/j.acra.2021.06.003
Hogge JP, De Paredes ES, Magnant CM, Lage J (1999) Imaging and management of breast masses during pregnancy and lactation. Breast J. https://doi.org/10.1046/j.1524-4741.1999.98077.x
Ayyappan AP, Crystal P, Torabi A, Foley BJ, Fornage BD (2013) Imaging of fat-containing lesions of the breast: a pictorial essay. J Clin Ultrasound. https://doi.org/10.1002/jcu.22070
Sawhney S, Petkovska L, Ramadan S, Al‐Muhtaseb S, Jain R, Sheikh M (2002) Sonographic appearances of galactoceles. J Clin Ultrasound. https://doi.org/10.1002/jcu.10038
Sumkin JH, Perrone AM, Harris KM, Nath MAAWB (1998) Lactating adenoma: US features and literature review. Radiology. https://doi.org/10.1177/875647939801400216
Olfatbakhsh A, Gholizadeh Z, Beheshtiyan T, Hoseinpour P (2016) Five-year study of patients with lactating adenoma and review of the five-year study of patients with lactating adenoma and review of the literature. Arch Breast Cancer 2:125–128. https://doi.org/10.19187/abc.201524125-128
Ravikanth R, Kamalasekar K (2019) Imaging of lactating adenoma: differential diagnosis of solid mass lesion in a lactating woman. J Med Ultrasound. https://doi.org/10.4103/JMU.JMU_3_19
Magno S, Terribile D, Franceschini G et al (2009) Early onset lactating adenoma and the role of breast MRI: a case report. J Med Case Rep. https://doi.org/10.1186/1752-1947-3-43
Pearlman MD, Griffin JL (2010) Clinical expert series benign breast disease
Skenderi F, Krakonja F, Vranic S (2013) Infarcted fibroadenoma of the breast: report of two new cases with review of the literature. Diagn Pathol. https://doi.org/10.1186/1746-1596-8-38
Goel NB, Knight TE, Pandey S, Riddick-Young M, de Paredes ES, Trivedi A (2005) Fibrous lesions of the breast: imaging-pathologic correlation. Radiographics 25(6):1547–1559
Sperber F, Blank A, Metser U, Flusser G, Klausner JM, Lev-Chelouche D (2003) Diagnosis and treatment of breast fibroadenomas by ultrasound-guided vacuum-assisted biopsy. Arch Surg. https://doi.org/10.1001/archsurg.138.7.796
Sklair-Levy M, Sella T, Alweiss T, Craciun I, Libson E, Mally B (2008) Incidence and management of complex fibroadenomas. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.07.2330
Brinck U, Fischer U, Korabiowska M, Jutrowski M, Schauer A, Grabbe E (1997) The variability of fibroadenoma in contrast-enhanced dynamic MR mammography. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.168.5.9129437
Meeuwis C, Van De Ven SM, Stapper G et al (2010) Computer-aided detection (CAD) for breast MRI: evaluation of efficacy at 3.0 T. Eur Radiol. https://doi.org/10.1007/s00330-009-1573-5
Weinstein D, Strano S, Cohen P, Fields S, Gomori JM, Degani H (1999) Breast fibroadenoma: mapping of pathophysiologic features with three- time-point, contrast-enhanced MR imaging—pilot study. Radiology. https://doi.org/10.1148/radiology.210.1.r99ja18233
Lee SS, Hartman HJ, Kuzmiak CM, Crosby KL (2013) The management of breast symptoms in the pregnant and lactating patient. Curr Obstet Gynecol Rep. https://doi.org/10.1007/s13669-012-0037-0
Kim KW, Cho KR, Seo BK et al (2010) Sonographic findings of mammary duct ectasia: can malignancy be differentiated from benign disease. J Breast Cancer. https://doi.org/10.4048/jbc.2010.13.1.19
del Riego J, Pitarch M, Codina C et al (2020) Multimodality approach to the nipple-areolar complex: a pictorial review and diagnostic algorithm. Insights Imaging. https://doi.org/10.1186/s13244-020-00896-1
Da Costa D, Taddese A, Cure ML, Gerson D, Poppiti Jr R, Esserman LE (2007) Common and unusual diseases of the nipple-areolar complex. Radiographics 27(Suppl 1):S65-577.
Lee SJ, Sobel LD, Shamis M, Mahoney MC (2019) Asymmetric ductal ectasia: an often overlooked sign of malignancy. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.18.20651
Mediano P, Fernández L, Rodríguez JM, Marín M (2014) Case-control study of risk factors for infectious mastitis in Spanish breastfeeding women. BMC Pregnancy Childbirth. https://doi.org/10.1186/1471-2393-14-195
Scott JA, Robertson M, Fitzpatrick J, Knight C, Mulholland S (2008) Occurrence of lactational mastitis and medical management: a prospective cohort study in Glasgow. Int Breastfeed J. https://doi.org/10.1186/1746-4358-3-21
Deng Y, Huang Y, Ning P, Ma SG, He PY, Wang Y (2021) Maternal risk factors for lactation mastitis: a meta-analysis. West J Nurs Res 43(7):698–708
Spencer JP (2008) Management of mastitis in breastfeeding women. Am Fam Physician 78(6):727–731
Wolfrum A, Kümmel S, Reinisch M, Pelz E, Reinisch M (2018) Granulomatous mastitis: a therapeutic and diagnostic challenge. Breast Care. https://doi.org/10.1159/000495146
De Bazelaire C, Groheux D, Chapellier M et al (2012) Breast inflammation: indications for MRI and PET-CT. Diagn Interv Imaging. https://doi.org/10.1016/j.diii.2011.12.004
Rieber A, Tomczak RJ, Mergo PJ, Wenzel V, Zeitler H, Brambs HJ (1997) MRI of the breast in the differential diagnosis of mastitis versus inflammatory carcinoma and follow-up. J Comput Assist Tomogr. https://doi.org/10.1097/00004728-199701000-00025
Azim HA, Peccatori FA, Pavlidis N (2010) Treatment of the pregnant mother with cancer: A systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part I: Solid tumors. Cancer Treat Rev 36(2):110–121
Eibye S, Kjær SK, Mellemkjær L (2013) Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet Gynecol. https://doi.org/10.1097/AOG.0b013e3182a057a2
Furman-Haran E, Eyal E, Shapiro-Feinberg M et al (2012) Advantages and drawbacks of breast DTI. Eur J Radiol. https://doi.org/10.1016/S0720-048X(12)70017-7
Nissan N, Anaby D, Sklair-Levy M (2019) Breast MRI without contrast is feasible and appropriate during pregnancy. J Am Coll Radiol 16(4 Pt A):408–409
Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C (2012) Gynaecological cancers in pregnancy. Lancet 379(9815):558–569
Bickelhaupt S, Laun FB, Tesdorff J et al (2016) Fast and noninvasive characterization of suspicious lesions detected at breast cancer X-ray screening: capability of diffusion-weighted MR imaging with MIPs. Radiology. https://doi.org/10.1148/radiol.2015150425
Peccatori FA, Codacci-Pisanelli G, Del Grande M, Scarfone G, Zugni F, Petralia G (2017) Whole body MRI for systemic staging of breast cancer in pregnant women. Breast. https://doi.org/10.1016/j.breast.2017.07.014
Han SN, Amant F, Michielsen K et al (2018) Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: a pilot study. Eur Radiol. https://doi.org/10.1007/s00330-017-5126-z
Kubik-Huch RA, Gottstein-Aalame NM, Frenzel T et al (2000) Gadopentetate dimeglumine excretion into human breast milk during lactation. Radiology. https://doi.org/10.1148/radiology.216.2.r00au09555
Rofsky NM, Weinreb JC, Litt AW (1993) Quantitative analysis of gadopentetate dimeglumine excreted in breast milk. J Magn Reson Imaging. https://doi.org/10.1002/jmri.1880030122
Newman J (2007) Breastfeeding and radiologic procedures. Can Fam Physician 53(4):630-631
Davanzo R (2018) Controversies in breastfeeding. Front Pediatr 6:278
Schmiedl U, Maravilla KR, Gerlach R, Dowling CA (1990) Excretion of gadopentetate dimeglumine in human breast milk. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.154.6.2110745
Catanzariti F, Avendano D, Cicero G et al (2021) High-risk lesions of the breast: concurrent diagnostic tools and management recommendations. Insights Imaging 12(1):63
Chou SHS, Romanoff J, Lehman CD et al (2021) Preoperative breast MRI for newly diagnosed ductal carcinoma in situ: imaging features and performance in a multicenter setting (ECOG-ACRIN E4112 trial). Radiology. https://doi.org/10.1148/radiol.2021204743
Chikarmane SA, Michaels AY, Giess CS (2017) Revisiting nonmass enhancement in breast MRI: analysis of outcomes and follow-up using the updated BI-RADS atlas. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.17.18086
Johansson ALV, Andersson TML, Hsieh CC et al (2018) Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int J Cancer. https://doi.org/10.1002/ijc.31174
Keyser CE, Staat MB, Fausett CM, Shields LC (2012) Pregnancy-Associated Breast Cancer. Rev Obst Gynecol 5:94–99. https://doi.org/10.3909/riog0172
Zha N, Alabousi M, Abdullah P et al (2019) Breast cancer screening in high-risk patients during pregnancy and breastfeeding: a systematic review of the literature. J Breast Imaging. https://doi.org/10.1093/jbi/wby015
Taylor D, Lazberger J, Ives A, Wylie E, Saunders C (2011) Reducing delay in the diagnosis of pregnancy-associated breast cancer: how imaging can help us. J Med Imaging Radiat Oncol. https://doi.org/10.1111/j.1754-9485.2010.02227.x
Al-Amri A (2015) Clinical presentation and causes of the delayed diagnosis of breast cancer in patients with pregnancy associated breast cancer. J Fam Community Med. https://doi.org/10.4103/2230-8229.155383
Langer A, Mohallem M, Stevens D, Rouzier R, Lerebours F, Chérel P (2014) A single-institution study of 117 pregnancy-associated breast cancers (pabc): presentation, imaging, clinicopathological data and outcome. Diagn Interv Imaging. https://doi.org/10.1016/j.diii.2013.12.021
Amant F, Von Minckwitz G, Han SN et al (2013) Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. https://doi.org/10.1200/JCO.2012.45.6335
Slepicka PF, Cyrill SL, dos Santos CO (2019) Pregnancy and breast cancer: pathways to understand risk and prevention. Trends Mol Med 25(10):866–881
Zografos E, Korakiti AM, Andrikopoulou A et al (2021) Germline mutations in a clinic-based series of pregnancy associated breast cancer patients. BMC Cancer. https://doi.org/10.1186/s12885-021-08310-9
Camps-Herrero J (2019) Diffusion-weighted imaging of the breast: current status as an imaging biomarker and future role. BJR Open. https://doi.org/10.1259/bjro.20180049
van der Hoogt KJJ, Schipper RJ, Winter-Warnars GA et al (2021) Factors affecting the value of diffusion-weighted imaging for identifying breast cancer patients with pathological complete response on neoadjuvant systemic therapy: a systematic review. Insights Imaging 12:1–22. https://doi.org/10.1186/s13244-021-01123-1
Kazama T, Kuroki Y, Kikuchi M et al (2012) Diffusion-weighted MRI as an adjunct to mammography in women under 50 years of age: an initial study. J Magn Reson Imaging. https://doi.org/10.1002/jmri.23626
Pinker K, Moy L, Sutton EJ et al (2018) Diffusion-weighted imaging with apparent diffusion coefficient mapping for breast cancer detection as a stand-alone parameter: comparison with dynamic contrast-enhanced and multiparametric magnetic resonance imaging. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000465
Avendano D, Marino MA, Leithner D et al (2019) Limited role of DWI with apparent diffusion coefficient mapping in breast lesions presenting as non-mass enhancement on dynamic contrast-enhanced MRI. Breast Cancer Res. https://doi.org/10.1186/s13058-019-1208-y
Solomon E, Nissan N, Furman-Haran E et al (2015) Overcoming limitations in diffusion-weighted MRI of breast by spatio-temporal encoding. Magn Reson Med. https://doi.org/10.1002/mrm.25344
Solomon E, Nissan N, Schmidt R et al (2016) Removing silicone artifacts in diffusion-weighted breast MRI by means of shift-resolved spatiotemporally encoding. Magn Reson Med. https://doi.org/10.1002/mrm.25757
Hu Y, Ikeda DM, Pittman SM et al (2021) Multishot diffusion-weighted MRI of the breast with multiplexed sensitivity encoding (MUSE) and shot locally low-rank (shot-LLR) reconstructions. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27383
Rodríguez-Soto AE, Fang LK, Holland D et al (2021) Correction of artifacts induced by B0 inhomogeneities in breast MRI using reduced-field-of-view echo-planar imaging and enhanced reversed polarity gradient method. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27566
Hancu I, Lee SK, Hulsey K et al (2017) Distortion correction in diffusion-weighted imaging of the breast: performance assessment of prospective, retrospective, and combined (prospective + retrospective) approaches. Magn Reson Med. https://doi.org/10.1002/mrm.26328
Bogner W, Pinker K, Zaric O et al (2015) Bilateral diffusion-weighted MR imaging of breast tumors with submillimeter resolution using readout-segmented echo-planar imaging at 7 T. Radiology. https://doi.org/10.1148/radiol.14132340
McKay JA, Church AL, Rubin N et al (2020) A comparison of methods for high-spatial-resolution diffusion-weighted imaging in breast MRI. Radiology. https://doi.org/10.1148/RADIOL.2020200221
Solomon E, Liberman G, Nissan N, Furman‐Haran E, Sklair‐Levy M, Frydman L (2020) Diffusion-weighted breast MRI of malignancies with submillimeter resolution and immunity to artifacts by spatiotemporal encoding at 3T. Magn Reson Med. https://doi.org/10.1002/mrm.28213
Sanderink WBG, Teuwen J, Appelman L et al (2021) Comparison of simultaneous multi-slice single-shot DWI to readout-segmented DWI for evaluation of breast lesions at 3T MRI. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2021.109626
Otikovs M, Nissan N, Furman-Haran E et al (2021) Diffusivity in breast malignancies analyzed for b > 1000 s/mm2 at 1 mm in-plane resolutions: Insight from Gaussian and non-Gaussian behaviors. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27489
Lustig M, Donoho D, Pauly JM (2007) Sparse MRI: the application of compressed sensing for rapid MR imaging. Magn Reson Med. https://doi.org/10.1002/mrm.21391
Le Y, Kipfer H, Majidi S, Holz S, Dale B, Geppert C, Kroeker R, Lin C (2013) Application of time-resolved angiography with stochastic trajectories (TWIST)-Dixon in dynamic contrast-enhanced (DCE) breast MRI. J Magn Reson Imaging 38(5):1033–1042
Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R (2016) XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med 75(2):775–788
Mann RM, Mus RD, Van Zelst J, Geppert C, Karssemeijer N, Platel B (2014) A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000057
Yamaguchi K, Nakazono T, Egashira R et al (2021) Maximum slope of ultrafast dynamic contrast-enhanced MRI of the breast: comparisons with prognostic factors of breast cancer. Jpn J Radiol. https://doi.org/10.1007/s11604-020-01049-6
Vreemann S, Rodriguez-Ruiz A, Nickel D et al (2017) Compressed sensing for breast MRI: resolving the trade-off between spatial and temporal resolution. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000384
Pelissier M, Ambarki K, Salleron J, Henrot P (2021) Maximum slope using ultrafast breast DCE-MRI at 1.5 Tesla: a potential tool for predicting breast lesion aggressiveness. Eur Radiol. https://doi.org/10.1007/s00330-021-08089-0
Onishi N, Sadinski M, Hughes MC et al (2020) Ultrafast dynamic contrast-enhanced breast MRI may generate prognostic imaging markers of breast cancer. Breast Cancer Res. https://doi.org/10.1186/s13058-020-01292-9
Oldrini G, Fedida B, Poujol J et al (2017) Abbreviated breast magnetic resonance protocol: value of high-resolution temporal dynamic sequence to improve lesion characterization. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2017.07.025
Bauer E, Levy MS, Domachevsky L, Anaby D, Nissan N (2021) Background parenchymal enhancement and uptake as breast cancer imaging biomarkers: a state-of-the-art review. Clin Imaging 83:41–50. https://doi.org/10.1016/j.clinimag.2021.11.021
Heacock L, Lewin AA, Toth HK, Moy L, Reig B (2021) Abbreviated MR imaging for breast cancer. Radiol Clin N Am 59(1):99–111
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
NN, EB, EEMM contributed to collecting data. NN, EB, EEMM and MSL contributed to manuscript preparation/editing, literature research, and study design. NN and MSL contributed to final approval. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nissan, N., Bauer, E., Moss Massasa, E.E. et al. Breast MRI during pregnancy and lactation: clinical challenges and technical advances. Insights Imaging 13, 71 (2022). https://doi.org/10.1186/s13244-022-01214-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-022-01214-7