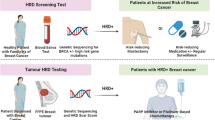
Abstract
Background
Pregnancy-associated breast cancer (PABC) defined as breast cancer diagnosed during gestation, lactation or within 1 year after delivery, represents a truly challenging situation with significantly increasing incidence rate. The genomic background of PABC has only recently been addressed while the underlying mechanisms of the disease still remain unknown. This analysis aims to further elucidate the frequency of PABC cases attributable to genetic predisposition and identify specific cancer susceptibility genes characterizing PABC.
Methods
A comprehensive 94-cancer gene panel was implemented in a cohort of 20 PABC patients treated in our clinic and descriptive correlation was performed among the results and the patients’ clinicopathological data.
Results
In the present study, 35% of PABC patients tested carried pathogenic mutations in two known cancer predisposition genes (BRCA1 and CHEK2). In total, 30% of the patients carried BRCA1 pathogenic variants. An additional 5% carried pathogenic variants in the CHEK2 gene. Variants of unknown/uncertain significance (VUS) in breast cancer susceptibility genes BRCA2, CHEK2 and BRIP1 were also identified in three different PABC patients (15%). Not all patients carrying germline mutations reported known family history of cancer.
Conclusions
Genetic testing should be considered as an option for PABC patients since the disease is highly associated with genetic susceptibility among other predisposing factors. Germline mutation identification may further modify PABC management approach and improve the prognostic outcome.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most frequent malignancy diagnosed in women; this observation generally applies to both the pregnant and the non-pregnant population [1, 2]. Pregnancy-associated breast cancer (PABC) is a complex situation that is commonly defined as breast cancer diagnosed during the period of pregnancy, lactation or within 12 months following delivery [3]. Up until today, PABC has been regarded as a rare entity since it accounts for only 0.2–3.8% of all breast cancer cases [4]. However, it has been well established that BC incidence increases with age, until the seventh decade [5]. Consequently, due to the social phenomenon of delayed childbearing, the incidence of PABC is currently on the rise in developing countries [6]. As far as developed countries are concerned, the application of non-invasive prenatal testing (NIPT) in all pregnant women today aiming to identify chromosomal abnormalities in the fetus, has significantly increased the detection of asymptomatic PABC patients [7, 8]. Additionally, the proportion of premenopausal women diagnosed with BC has increased substantially, with one in forty women diagnosed being under the age of 35 [9]. Taking all the above into account, PABC is expected to gain more scientific interest in the following years, in order to ensure that this rare and heterogenic population is offered the best individualized management for both maternal and fetal well-being.
Regarding the heredity of the disease, hallmark studies on familial BC have resulted in the identification of clinically relevant cancer susceptibility genes [10, 11]. Notably, most pregnancies occur under the age of 40, which is the age group in which BC has been more commonly associated with a positive family history and a higher occurrence of germline mutations [12]. In a study utilizing multiple-gene panel testing, 23% of breast malignancies in young women were related to germline mutations in known cancer predisposition genes such as BRCA1/2, CHEK2, ATM and PALB2 [13]. In this setting, the latest international guidelines strongly recommend that genetic testing for high and moderate risk cancer susceptibility genes should be offered to every woman under the age of 50 with BC, thus encompassing PABC patients [14, 15]. Additionally, tumors in PABC patients bear distinct biological characteristics that deem them more aggressive, including higher grade and proliferation rates, advanced T stage at diagnosis, nodal involvement, greater prevalence of the triple-negative subtype (TNBC), and hormone receptor negativity [16]. Consequently, the genetic risk evaluation in this cohort of patients can have a substantial impact on PABC management and follow-up, offering a significant benefit not only to the affected mother to be, but also to family members [17].
Still, the genetic background of PABC remains an understudied field, despite the fact than in recent years the incorporation of multi-gene panel testing into clinical practice has enabled researchers to more accurately and cost-effectively estimate the proportion of cancers attributable to genetic predisposition and hereditary cancer syndromes [18]. Notably, numerous genes with non-silent mutations have been found to be differentially expressed between PABC and non-PABCpatient-derived tissues, as it was demonstrated in a recent systematic review published by members of our research group [19], implying a differential genomic background between pregnancy and non-pregnancy associated BC. In the current study, we applied multiple-gene panel testing to 94 cancer susceptibility genes aiming to identify the proportion of PABC cases attributable to genetic predisposition and to assess the prevalence of germline (i.e. inherited) mutations in PABC patients treated in our clinic.
Materials and methods
In this cohort study, 20 women diagnosed with breast cancer during pregnancy or in the first year after delivery were enrolled. All participants were required to have completed the 18th year of age and to have attended the Breast Unit of the Obstetrical/Gynecological Clinic or the Department of Clinical Therapeutics of the National and Kapodistrian University of Athens, at the Alexandra Hospital in Athens, Greece. The sample was pooled from the hospital’s patient database according to the abovementioned inclusion criteria. The study was approved by the Institutional Review Board (IRB) of the participating hospital.
Participation was voluntary and once informed consent was granted by each participant, medical files of the patients were reviewed and researchers interviewed enrollees in person to collect demographic and clinical data, including age at diagnosis, family cancer history, prior genetic testing results, histopathologic evaluation (tumor stage, size, grade, lymph node status, hormone receptor, and HER2 status). Breast cancer diagnosis was established based on a combination of standard clinical, radiological and histological criteria [20]. Finally, a blood sample was collected from each PABC patient.
Subsequently, genomic DNA was isolated from whole blood using the QIAsymphony DSP DNA Mini Kit (Qiagen, Germantown, USA) and used to prepare indexed libraries to target the sequence of 94 cancer predisposing genes using the Trusight Cancer Panel – Νextera DNA Flex Pre-Enrichment Library Prep (Illumina, San Diego, USA). Libraries were qualitatively and quantitatively evaluated using a Fragment Analyzer (Advanced Analytical Technologies, Heidelberg, Germany) and sequenced on a MiSeq genetic analyzer (Illumina, Inc., San Diego, CA), according to the manufacturer’s protocols. Annotation was performed against the human reference genome GRCh38 using VariantStudio V.3 (Illumina, Inc., San Diego, CA). Based on the data generated from this software, alterations were identified as pathogenic when classified as disease causing or as variants of unknown significance (VUS) when evidence regarding their pathogenicity was either conflicting or limited [21]. The minimum base and amplicon coverage were 50×, and 100×, respectively, while the mean read depth was 182×. All sequenced variants were interpreted according to the recommendations of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [22].
Results
We have screened 20 PABC patients, unselected for age or family history, for the presence of germline mutations. The detection rate of pathogenic mutations among the cases tested was 35% (7/20). Of those, six patients of our cohort had a pathogenic BRCA1 mutation and one patient a CHEK2 mutation. Of note, three PABC patients carried variants of unknown/uncertain significance (VUS) in three breast cancer susceptibility genes.
Demographic characteristics
Detailed data concerning the major demographic variables and the pregnancy characteristics of the PABC subjects enrolled in this study are presented in Table 1, including age, ethnicity, BMI, time of diagnosis, and number of pregnancy. The age of diagnosis ranged from 26 to 45 years, with a mean age of 34 years. The majority of participants was Greek (18/20 = 90%), while one was of Albanian (5%) and one of Romani (5%) ancestry. According to the body mass index (BMI) chart, 35% of participants was classified as overweight and 25% as obese.
Histopathological types
Histopathological features of pregnancy-associated breast tumors are presented in Table 2. The most frequent histological type among PABC patients enrolled in our study was invasive ductal carcinoma (IDC) (90%). Interestingly, among them there were two cases of IDCs with medullary features, which fall into the basal-like molecular subtype [23]: one case concerned a 30-year-old woman diagnosed with PABC at the 28th week of pregnancy and the other was a case of bilateral disease with asynchronous onset at ages 41 (11 weeks pregnant) and 43 (non-pregnant). Concerning the latter case, the histopathological findings differed between the two diagnoses, since the pregnancy associated tumor exhibited medullary-like features, while two years later the same patient was diagnosed with metaplastic breast cancer of the other breast. Of the remaining PABC patients, there was one case of invasive lobular carcinoma (ILC) and one case of metaplastic carcinoma of the breast diagnosed simultaneously with ductal carcinoma. The majority of tumors was of high grade (80%), and pathological measurements regarding tumor size revealed that 75% of them were above 2 cm (T2, T3). As far as receptor status is concerned, 45% of PABC cases were ER negative, 60% HER2 negative and 50% of them PR negative; of note, 30% of the participants’ tumors were classified as triple receptor-negative breast cancer (TNBC). Axillary node infiltration was identified in 40% of cases.
Germline mutation analysis
For those with a positive mutation result from genetic testing, Table 3 shows the identified germline mutations in cancer predisposition genes and the exact location of each mutation in the affected gene, in comparison with the family history of hereditary breast and ovarian cancer syndromes as well as other common cancers. Specifically, participant #553 has a germline mutation (BRCA1: c.5328delC) along with a family history of vulvar cancer, #750 has a known pathogenic mutation (BRCA1: c.5212G > A) with a family history of breast and ovarian cancer, #2754 is a carrier of a pathogenic mutation (BRCA1: c.5251C > T) with a family history of breast cancer, prostate cancer and basal cell carcinoma (BCC) of the skin, and finally PABC patient #2740 carries a germline mutation (BRCA1 g.169527_180579del11052) and has a history of endometrial and lung cancer. Interestingly, PABC subjects #964 and #749 were identified to have the same pathogenic mutation (BRCA1: c.3700_3704delGTAAA), however the former has no reported family history, while the latter has a family history of breast and endometrial cancer. Furthermore, one of our patients carried a known pathogenic CHEK2 c.1100delC mutation, despite having no family history of cancer.
Concerning the other CHEK2 variant (c.1175C > T) that was identified in a 36-year-old patient with Luminal B- HER-2 negative PABC, it is classified as a variant of unknown/uncertain significance (VUS). Furthermore, another 37yo patient (#3227) carried a VUS in BRCA2 (c.8386C > T), while having Luminal A-HER-2 negative BC and no cancer family history. Lastly, patient #2122 is a carrier of a VUS in the BRIP1 gene; this PABC patient was diagnosed five months post-partum with Luminal B-HER-2 negative breast cancer at 32 years of age and had a positive family history of cancer, including a first- and a second- degree relative with prostate and breast cancer respectively.
Discussion
In this descriptive study we have established the prevalence of BRCA1 germline mutations in a group of 20 pregnancy associated breast cancer patients, which were selected regardless of family history. All in all, we identified seven PABC patients carrying known pathogenic germline mutations (35%), six of them in the BRCA1 gene and one in the CHEK2 gene. Additionally, variants of unknown/uncertain significance (VUS) in breast cancer susceptibility genes CHEK2, BRCA2 and BRIP1 were determined to be present in three different PABC patients (15%). To our knowledge, this is the first recent study aiming to investigate the germline mutation frequency and spectra in a clinic-based series of PABC patients.
The notably high frequency of BRCA1 mutations found in the present PABC group (30%) places pregnant breast cancer patients in a high-risk setting. Previous studies, however heterogenous they are, report high BRCA1 mutation prevalence in PABC patients, ranging from 11.4–19.6% [24, 25]. In this setting, PABC patients with germline mutations in BC susceptibility genes should be placed under close clinical monitoring, in accordance to the evidence-based clinical practice guidelines that have been developed to ensure the appropriate management for carriers of a BRCA pathogenic variant [15, 26]. This suggestion is consistent with the findings of the only similar study to ours published more than two decades ago by Johansson et al. (1998), who investigated the influence of pregnancy on the risk of developing breast cancer in germline BRCA1/2 mutation carriers [27]. According to their results, more women with BRCA1 mutations developed PABC and in the same context they proposed close monitoring of women with BRCA1 familial mutations during and after pregnancy, thus substantiating our observations.
Nowadays, reports state that BRCA1/2 mutations account for only approximately 50% of the identifiable germline cancer predisposition variants in BC patients [28]. In parallel, overwhelming evidence suggest that germline pathogenic variants in genes of intermediate penetrance, such as ATM and CHEK2, confer an increased risk of BC, and their analysis is encompassed in gene panels alongside the BRCA1/2 genes [15, 29]. Notably, the checkpoint kinase 2 (CHEK2) gene is a tumor suppressor gene involved in cell cycle checkpoint regulation, DNA damage repair activation and apoptosis [30]. Given its essential role, CHEK2 inherited pathogenic variants have been implicated in BC predisposition [31]. Of these, the founder mutation CHEK2 c.1100delC is one of the most frequently identified among Northern Europeans [32], while it is considered to be less common in the Mediterranean region, including BC patients of Greek decent (0.16%) [33]. This observation does not coincide with our results, since we identified one carrier of the pathogenic CHEK2 c.1100delC and another carrier of a CHEK2 VUS among our PABC patients, highlighting the importance of further investigations of other mutations in order to unravel their contribution to PABC susceptibility.
Notably, multigene panel testing (MGPT) allows the sequencing of multiple genes simultaneously and has offered a cost effective and efficient way to assess cancer genetics in a phenotypically directed clinical setting [28, 34]. Multigene panel tests in general depict more clearly the proportion of breast cancers attributable to genetic predisposition, since they allow detection of even moderate or low penetrance genetic variants [35]. However, there are several issues to take into consideration regarding their use. Specifically, as gene panel testing options for breast cancer risk assessment continue to grow in variety, the specific multigene test that will be used should be chosen carefully. From a clinical perspective, choosing a gene panel that analyses a wide array of moderate or low penetrance genes may lead to the identification of genetic variants which are not medically actionable and thus do not exhibit the attendant cancer prevention benefits [36]. Additionally, multigene tests increase the likelihood of detecting a variant of unknown/uncertain significance (VUS). These VUSs add complexity that may cause difficulty for clinicians in making management recommendations and advising patients. In our study, a PABC patient carried a VUS in BRIP1 (BRCA1 interacting protein C-terminal helicase 1) which is a gene that contributes to the DNA repair function of BRCA1; the impact of this variant on molecular function and subsequent roles in cancer risk is uncertain. This is not a rare finding, since according to data available in ClinVar, 933 variants in BRIP1 clinically classified as a VUS have been reported to date [37]. However, it is a noteworthy observation, since classification of VUSs identified in PABC patients can contribute to earlier detection and screening of breast cancer and, as data from multigene panel testing accumulates, eventually improve treatment options in PABC.
Limitations of the study include that large genomic rearrangements (LGRs), such as copy number variants (CNVs) are usually missed by multigene panel testing; hence they were not reported in our study. Furthermore, it is important to point out that our analysis focused on a small number of patients due to the rarity of the disease, deeming our results merely indicative and in need of further confirmation in a larger cohort of patients, in order to draw safe conclusions on whether a pathogenic variant of a gene can be associated with PABC. Lastly, since this is a descriptive study aiming to assess the prevalence of germline mutations in PABC, the limitations naturally include the absence of a comparison group. The approach utilized does not allow for causal statements; however, our results imply a noteworthy correlation and support the recommendation of at least BRCA1 testing for all PABC patients, regardless of family history and age of diagnosis.
Conclusions
In conclusion, this study highlights the high frequency of PABC cases attributable to genetic predisposition, indicating that genetic testing at an appropriate time is of great importance for this sub-group, since it might ensure that PABC patients receive the most appropriate treatment in respect to their specific needs and their sensitive situation. Additionally, our results imply a potential use of multigene panel testing that is not limited to young women at high risk but extends to patients with no family history. Interestingly, some of the PABC patients included in this study had family history of breast, ovarian, prostate, colorectal or endometrial cancer, while others reported no family history of malignancies, even though they had a positive result of genetic testing for pathogenic variants in high-risk genes. Taken together, these observations indicate that family history of cancer remains an important variable during decision-making about genetic testing among PABC patients, but it should not be the only criteria for patient selection if we want to assure that carriers will not be missed. Lastly, the use of multi-gene panel testing for hereditary breast cancer risk, not limited to BRCA1/2 pathogenic mutations, is essential to increase the likelihood of detecting an underlying germline genetic component and achieve better PABC patient outcomes. Unfortunately, as already mentioned the incidence of pregnancy-associated breast cancer is expected to increase considerably in the years to come while the introduction of non-invasive prenatal testing has led to higher cancer detection rates in pregnant women [38]; therefore, further research in a larger cohort of patients is deemed necessary.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Parazzini F, Franchi M, Tavani A, Negri E, Peccatori FA. Frequency of pregnancy related Cancer: a population based linkage study in Lombardy, Italy. Int J Gynecol Cancer. 2017;27(3):613–9. https://doi.org/10.1097/IGC.0000000000000904.
de Haan J, Verheecke M, Van Calsteren K, Van Calster B, Shmakov RG, Mhallem Gziri M, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–46. https://doi.org/10.1016/S1470-2045(18)30059-7.
Wang B, Yang Y, Jiang Z, Zhao J, Mao Y, Liu J, et al. Clinicopathological characteristics, diagnosis, and prognosis of pregnancy-associated breast cancer. Thorac Cancer. 2019;10(5):1060–8. https://doi.org/10.1111/1759-7714.13045.
Vinatier E, Merlot B, Poncelet E, Collinet P, Vinatier D. Breast cancer during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):9–14. https://doi.org/10.1016/j.ejogrb.2009.06.030.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. https://doi.org/10.1002/ijc.29210.
Lee GE, Mayer EL, Partridge A. Prognosis of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2017;163(3):417–21. https://doi.org/10.1007/s10549-017-4224-6.
Amant F, Verheecke M, Wlodarska I, Dehaspe L, Brady P, Brison N, et al. Presymptomatic identification of cancers in pregnant women during noninvasive prenatal testing. JAMA Oncol. 2015;1(6):814–9. https://doi.org/10.1001/jamaoncol.2015.1883.
Lenaerts L, Jatsenko T, Amant F, Vermeesch JR. Noninvasive prenatal testing and detection of occult maternal malignancies. Clin Chem. 2019;65(12):1484–6. https://doi.org/10.1373/clinchem.2019.306548.
Cardoso F, Loibl S, Pagani O, Graziottin A, Panizza P, Martincich L, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48(18):3355–77. https://doi.org/10.1016/j.ejca.2012.10.004.
Margaritte P, Bonaiti-Pellie C, King MC, Clerget-Darpoux F. Linkage of familial breast cancer to chromosome 17q21 may not be restricted to earlyonset disease. Am J Hum Genet. 1992;50(6):1231-4. Erratum in: Am J Hum Genet 1993;52(3):654.
Rahman N. Realizing the promise of cancer predisposition genes. Nature. 2014;505(7483):302–8. https://doi.org/10.1038/nature12981.
Lee HB, Han W. Unique features of young age breast cancer and its management. J Breast Cancer. 2014;17(4):301–7. https://doi.org/10.4048/jbc.2014.17.4.301.
Rummel SK, Lovejoy L, Shriver CD, Ellsworth RE. Contribution of germline mutations in cancer predisposition genes to tumor etiology in young women diagnosed with invasive breast cancer. Breast Cancer Res Treat. 2017;164(3):593–601. https://doi.org/10.1007/s10549-017-4291-8.
Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. 2017;35:203–17. https://doi.org/10.1016/j.breast.2017.07.017.
Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic,version 1.2020 featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw. 2020;18:380–91. https://doi.org/10.6004/jnccn.2020.0017.
Murphy CG, Mallam D, Stein S, Patil S, Howard J, Sklarin N, et al. Current or recent pregnancy is associated with adverse pathologic features but not impaired survival in early breast cancer. Cancer. 2012;118(13):3254–9. https://doi.org/10.1002/cncr.26654.
Partridge AH, Pagani O, Abulkhair O, Aebi S, Amant F, Azim HA, et al. First international consensus guidelines for breast cancer in young women (BCY1). Breast. 2014;23(3):209–20. https://doi.org/10.1016/j.breast.2014.03.011.
Kurian AW, Ward KC, Hamilton AS, Deapen DM, Abrahamse P, Bondarenko I, et al. Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol. 2018;4(8):1066–72. https://doi.org/10.1001/jamaoncol.2018.0644.
Korakiti A-M, Moutafi M, Zografos E, Dimopoulos M-A, Zagouri F. The genomic profile of pregnancy-associated breast Cancer: a systematic review. Front Oncol. 2020;10:1773. https://doi.org/10.3389/fonc.2020.01773.
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. ERRATUM: Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(10):1674. https://doi.org/10.1093/annonc/mdz189.
Pesaran T, Karam R, Huether R, Li S, Farber-Katz S, Chamberlin A, et al. Beyond DNA: an integrated and functional approach for classifying Germline variants in breast Cancer genes. Int J Breast Cancer. 2016;2016:1–10. https://doi.org/10.1155/2016/2469523.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Kleer CG. Carcinoma of the breast with medullary-like features: Diagnostic challenges and relationship with brca1 and ezh2 functions. Arch Pathol Lab Med. 2009;133(11):1822–5. https://doi.org/10.1043/1543-2165-133.11.1822.
Blanquisett AH, Vicent CH, Gregori JG, Zotano ÁG, Porta VG, Simón AR. Breast cancer in pregnancy: an institutional experience. Ecancermedicalscience. 2015;9. https://doi.org/10.3332/ecancer.2015.551.
Gooch JC, Chun J, Kaplowitz E, Guth A, Axelrod D, Shapiro R, et al. Pregnancy-associated breast cancer in a contemporary cohort of newly diagnosed women. Breast J. 2020;26(4):668–71. https://doi.org/10.1111/tbj.13510.
Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR, et al. Management of hereditary breast cancer: American society of clinical oncology, American society for radiation oncology, and society of surgical oncology guideline. J Clin Oncol. 2020;38(18):2080–106. https://doi.org/10.1200/JCO.20.00299.
Johannsson O, Loman N, Borg Å, Olsson H. Pregnancy-associated breast cancer in BRCA1 and BRCA2 germline mutation carriers. Lancet. 1998;352(9137):1359–60. https://doi.org/10.1016/S0140-6736(05)60750-7.
Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123(10):1721–30. https://doi.org/10.1002/cncr.30498.
Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26(7):1291–9. https://doi.org/10.1093/annonc/mdv022.
Cai Z, Chehab NH, Pavletich NP. Structure and activation mechanism of the CHK2 DNA damage checkpoint kinase. Mol Cell. 2009;35(6):818–29. https://doi.org/10.1016/j.molcel.2009.09.007.
Stolarova L, Kleiblova P, Janatova M, Soukupova J, Zemankova P, Macurek L, et al. CHEK2 Germline variants in Cancer predisposition: stalemate rather than checkmate. Cells. 2020;9(12). https://doi.org/10.3390/cells9122675.
Muranen TA, Greco D, Blomqvist C, Aittomäki K, Khan S, Hogervorst F, et al. Genetic modifiers of CHEK2∗1100delC-associated breast cancer risk. Genet Med. 2017;19(5):599–603. https://doi.org/10.1038/gim.2016.147.
Apostolou P, Fostira F, Papamentzelopoulou M, Michelli M, Panopoulos C, Fountzilas G, et al. CHEK2 c.1100delC allele is rarely identified in Greek breast cancer cases. Cancer Genet. 2015;208:129–34. https://doi.org/10.1016/j.cancergen.2015.02.006.
Manchanda R, Patel S, Gordeev VS, Antoniou AC, Smith S, Lee A, et al. Cost-effectiveness of population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. J Natl Cancer Inst. 2018;110(7):714–25. https://doi.org/10.1093/jnci/djx265.
Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–9. https://doi.org/10.1200/JCO.2013.53.6607.
Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN. Gene panel testing for inherited cancer risk. JNCCN J Natl Compr Cancer Netw. 2014;12(9):1339–46. https://doi.org/10.6004/jnccn.2014.0128.
Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44(D1):D862–8. https://doi.org/10.1093/nar/gkv1222.
Korakiti AM, Zografos E, van Gerwen M, Amant F, Dimopoulos MA, Zagouri F. Long-term neurodevelopmental outcome of children after in utero exposure to chemotherapy. Cancers. 2020;12(12):1–16. https://doi.org/10.3390/cancers12123623.
Acknowledgements
Not applicable.
Funding
There was no specific funding available for this research study.
Author information
Authors and Affiliations
Contributions
FZ conceptualized the project and the methodology. EZ and AMK, with the support of AA, analyzed the data, generated Tables 1, 2 and 3, and prepared the original draft under the supervision of FZ and MAD. IR, CD, SM, AG, AK, and NB have been involved in the collection of samples and performed data curation. MAD was actively involved in the interpretation of the results, providing important intellectual content. All authors provided critical feedback, contributed to the manuscript, and approved the final version in accordance with criteria established by the International Committee of Medical Journal Editors (ICMJE).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was performed in accordance to the ethical standards of the institution and followed the tenets of the Helsinki Declaration; ethics approval was granted by the Institutional Review Board of the Alexandra Hospital in Athens, Greece, and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: FZ has received honoraria for lectures and has served in an advisory role for Astra-Zeneca, Daiichi, Eli-Lilly, Merck, Novartis, Pfizer, and Roche. MAD has received honoraria from participation in advisory boards from Amgen, Bristol-Myers-Squibb, Celgene, Janssen, Takeda. The remaining authors (EZ, AMK, AA, IR, CD, SM, AG, AK, and NB) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zografos, E., Korakiti, AM., Andrikopoulou, A. et al. Germline mutations in a clinic-based series of pregnancy associated breast cancer patients. BMC Cancer 21, 572 (2021). https://doi.org/10.1186/s12885-021-08310-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08310-9