Abstract
Background
ASD and ADHD are prevalent neurodevelopmental disorders that frequently co-occur and have strong evidence for a degree of shared genetic aetiology. Behavioural and neurocognitive heterogeneity in ASD and ADHD has hampered attempts to map the underlying genetics and neurobiology, predict intervention response, and improve diagnostic accuracy. Moving away from categorical conceptualisations of psychopathology to a dimensional approach is anticipated to facilitate discovery of data-driven clusters and enhance our understanding of the neurobiological and genetic aetiology of these conditions. The Monash Autism-ADHD genetics and neurodevelopment (MAGNET) project is one of the first large-scale, family-based studies to take a truly transdiagnostic approach to ASD and ADHD. Using a comprehensive phenotyping protocol capturing dimensional traits central to ASD and ADHD, the MAGNET project aims to identify data-driven clusters across ADHD-ASD spectra using deep phenotyping of symptoms and behaviours; investigate the degree of familiality for different dimensional ASD-ADHD phenotypes and clusters; and map the neurocognitive, brain imaging, and genetic correlates of these data-driven symptom-based clusters.
Methods
The MAGNET project will recruit 1,200 families with children who are either typically developing, or who display elevated ASD, ADHD, or ASD-ADHD traits, in addition to affected and unaffected biological siblings of probands, and parents. All children will be comprehensively phenotyped for behavioural symptoms, comorbidities, neurocognitive and neuroimaging traits and genetics.
Conclusion
The MAGNET project will be the first large-scale family study to take a transdiagnostic approach to ASD-ADHD, utilising deep phenotyping across behavioural, neurocognitive, brain imaging and genetic measures.
Similar content being viewed by others
Background
Overview
Autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are neurodevelopmental disorders affecting 1–2% and 5% of the population, respectively [175, 220]. ASD is defined by deficits in social communication, and restricted and repetitive patterns of behaviour and interests and altered sensory processing, whereas ADHD is defined by hyperactivity, impulsivity and inattention [16]. In ASD, 30–80% of cases exhibit ADHD symptomatology [186, 225], and 20–50% of ADHD cases display ASD symptoms [127, 234]. The introduction of the DSM-5 has allowed, for the first time, the concurrent diagnosis of ASD and ADHD, and the two disorders are now recognized to co-occur in up to 50% of cases [127, 271]. This comorbidity can be associated with a more severe ADHD phenotype and higher treatment needs overall [68, 305].
Although ASD and ADHD are diagnosed according to a symptomatic and behavioural presentation and developmental history, both conditions have a strong genetic aetiology and are highly heritable with estimates of up to 85% for ASD [304] and 70–90% for ADHD [104, 172]. Evidence of familiality comes from findings that first degree relatives of affected individuals often show subclinical behavioural or neurocognitive difficulties characteristic of ASD and ADHD [132, 205, 233, 243, 257]. Furthermore, siblings of children with ASD have a greater likelihood of having ADHD than the general population [116, 117], and siblings of children with ADHD exhibit greater ASD symptoms than healthy controls [197] suggesting shared familiality. It is becoming clear that multiple genes are implicated in ASD and ADHD and these are associated with multiple biological systems. The genetic links may also transcend diagnostic categories, with twin studies providing evidence for shared genetic liability for ASD and ADHD [116, 117]. There is also strong evidence for a degree of shared genetic aetiology [234], with population-based research suggesting that ASD and ADHD symptoms share common genetic variance throughout childhood and adolescence [270].
Copy number variations (CNV’s; [88, 290]), de novo mutations [143, 210, 230, 240], and common genetic variation from Genome-Wide Association Studies (GWAS; [113, 126]) are all implicated in the genetic aetiology of ASD. Similarly, CNVs [103], rare variants [129], and GWAS single nucleotide polymorphisms (SNPs; [83]) are implicated in the genetics of ADHD. Although there is a growing body of literature on the phenotypic, neurobiological and genetic overlap between ASD and ADHD [11, 127, 146, 234, 264], comprehensive transdiagnostic dimensional phenotyping approaches that are agnostic to diagnostic category are only beginning to emerge.
The Research Domain Criteria (RDoC) and Hierarchical Taxonomy of Psychopathology (HiTOP): complementary frameworks for research in ASD and ADHD
Traditional taxonomies, such as the DSM and ICD, inherently assume segmentation between diagnostic categories. However, high rates of comorbidity between specific disorders, for example ASD and ADHD with intellectual disability [ID; 274, 278], obsessive compulsive disorder (OCD; [166, 183]), oppositional defiant disorder (ODD) and conduct disorder (CD; [32, 185]), and depression and anxiety [90, 268], as well as significant within disorder heterogeneity [106] challenge the assumption of a clear division between diagnostic categories. As the search for biological causes and accurate ways to identify psychiatric disorders gains traction, there is a move away from categorical conceptualisations of disorders towards a more dimensional understanding of psychopathology [60, 130, 239]. The National Institute of Mental Health’s (NIMH) Research Domain Criteria (RDoC) project [141, 142] and the Hierarchical Taxonomy of Psychopathology (HiTOP) consortium [158] represent complementary approaches to addressing the limitations of traditional categorical nosologies by using dimensional models of psychiatric and neurodevelopmental disorders evaluated at multiple levels of measurement. Studying phenotypes related to ASD and ADHD as hierarchically organised dimensions resolves problems associated with comorbidity and heterogeneity [105, 157].
The principal focus of RDoC is the analysis of dimensional phenomena at multiple levels of analysis across several core functional domains. These include behaviour, cognition, neural circuits, and genes in areas such as social processes and cognitive systems. These elements are organised within the RDoC matrix, which is primarily intended as a heuristic framework to encourage and facilitate psychiatric research that is unconstrained by traditional diagnostic categories. The primary focus of HiTOP is the articulation of the structure of the symptoms of psychopathology, which are conceptualised as hierarchically organised dimensions [158, 163]. These dimensions can be studied at varying levels of generality and specificity to uncover shared and unique genetic, neurobiological, and clinical correlates [65, 169, 291]. The RDoC and HiTOP approaches are thus complementary: the hierarchically organised phenotypic dimensions furnished by HiTOP provide the structural framework for exploring the functional domains and elements of the RDoC matrix [162, 188].
Alignment of ASD and ADHD neurocognitive endophenotypes with the RDoC matrix
A number of neurocognitive traits have been identified as areas of difficulty for children with ASD and ADHD. Some neurocognitive domains are associated with similar levels of impairment across ASD and ADHD, while others appear to differentiate between them. Although atypical neurocognitive profiles are frequently observed at a group level, not all individuals within a disorder show divergence across all behavioural and neurocognitive domains. This heterogeneity has hindered clinical translation of group-level findings to individuals (e.g. [40, 59, 73, 76, 82, 102, 243, 280, 286]). Within ASD and ADHD, deficits are seen on tasks of sustained attention and arousal [20, 29, 54, 148, 148, 150, 150, 151, 289], cognitive control, for example inhibition (e.g. [21, 115, 221, 218, 300, 301]), social processes such as emotion recognition and/or theory of mind [14, 36, 128, 281], visual and verbal working memory [69, 120, 226, 227], and reward sensitivity and decision-making [147, 196, 309]. Sensorimotor abnormalities are common in ASD. In particular, oculomotor deficits are robustly associated with ASD [112, 149, 194], with emerging evidence for oculomotor impairments in ADHD [101]. These areas of neurocognitive divergence broadly align with five RDoC matrix domains: positive valence systems, cognitive systems, arousal/regulatory systems, social processes and sensorimotor systems [193, 199].
Relationship between ASD and ADHD within HiTOP model
Although dimensional models of psychopathology originated in the developmental literature [3, 139], neurodevelopmental disorders are yet to be fully integrated into the HiTOP model [163]. The HiTOP framework conceptualises psychopathology as a multi-dimensional hierarchy, with an overarching factor for general psychopathology, or ‘p’ factor, represented at the top of the hierarchy and reflecting a common liability for mental disorder [48, 66]. Below this ‘p’ factor are super spectra; internalising, externalising, and psychosis; representing shared vulnerabilities to more specific ranges of problems. Internalising symptoms encompass depression, anxiety and somatic, eating and sexual difficulties, and subsumes the narrower and distinct subspectra of fear and distress. The externalising domain subsumes substance abuse and antisocial behaviours and is further differentiated into the disinhibited externalising (e.g. impulse-control problems) and antagonistic externalising (e.g. antisocial personality traits) subspectra. The psychosis super-spectra captures phenotypic variance related to psychotic disorders, but can be further differentiated into thought disorder (i.e. positive symptoms, experiences, and traits, such as reality distortion) and detachment (negative symptoms, experience, and traits, such as social withdrawal and emotional detachment).
To date, the full HiTOP model is more clearly articulated in adult populations [158, 163] and existing work examining the placement of neurodevelopmental disorders within dimensional models of psychopathology has been characterized by low specificity in focusing exclusively on broad band psychopathology factors (i.e., p factor, externalizing and internalizing superspectra [202, 228, 265]). Despite this work, there is still insufficient evidence to indicate the placement of neurodevelopmental disorders within HiTOP [157]. An advantage of the HiTOP conceptualization beyond general dimensional approaches in developmental psychopathology is that relevant phenotypes can be examined at varying levels of generality and specificity, from broad spectra, to subspectra, empirical syndromes, symptom components and maladaptive traits [158]. If neurodevelopmental disorders are to be included in the HiTOP framework, more evidence is needed to determine whether a general neurodevelopmental disorder subfactor [265] or alternatively, more granular hyperactive, inattentive [228], and social communication [202] subfactors, might best explain the relationship between psychopathology and neurodevelopmental conditions.
MAGNET is uniquely positioned to examine the relationship of ADHD and ASD to other forms of psychopathology within a hierarchical dimensional model, because phenotypes related to these conditions are being measured at a finer level of granularity compared to previous studies [202, 228]. For example, a dimension related to stereotyped behavior has yet to be incorporated into the HiTOP model [157]. The MAGNET protocol will be able to investigate the position of this phenotype using the Childhood Routines Inventory—Revised and Restricted Interests and Repetitive Behavior subscale of the Social Responsiveness Scale, 2nd Edition. Furthermore, studies that have examined the placement of ADHD and ASD within HiTOP have not taken into consideration potential phenotypic subdimensions or subtypes of ADHD and ASD [6, 201]. Finally, MAGNET is unique in concurrent measurement of RDoC-relevant constructs (e.g., cognition, genetics) compared to previous studies (e.g., [265]), which enables us to address method variance, validate the findings with objective cognitive assessment and observer ratings, as well as address the RDoC-HiTOP interface [188].
A conceptual cross-mapping between RDoC and HiTOP has previously been outlined [162, 188]. HiTOP spectra, subspectra, empirical syndromes, symptom components, and maladaptive traits form the phenotypic targets to which biologically informed RDoC constructs can be related [162]. Thus, there is synergy between these two complementary dimensional approaches to psychopathology. Leveraging the RDoC and HiTOP approaches has the potential to pave the way for a unified nosology, which is biologically informed and has clinical application [158, 169, 188, 212]. Symptom ratings, behavioural measures and neurocognitive tasks selected to measure psychopathology, and more specifically core ASD-ADHD traits, will allow for characterisation of ASD-ADHD within an integrated RDoC-HiTOP framework. However, although the RDoC-HiTOP interface has been broadly outlined, the relationships between specific HiTOP and biologically informed constructs has yet to be fully articulated and empirically tested at a fine-grained level of analysis [188].
The MAGNET study addresses several limitations of existing research. First, by incorporating multiple measures of ADHD and ASD symptomatology within the same structural modelling study, along with measures of other HiTOP spectra and subspectra (i.e., the Child Behavior Checklist), we are in a position to investigate the placement of these neurodevelopmental conditions, their potential subdimensions and/or subtypes, within a broader structural model of child psychopathology (i.e., internalizing, externalizing, thought disorder). Second, our methodology includes an array of measures that align with the constructs and subconstructs included in the RDoC matrix and across multiple levels of analysis, including behavioural assessment, cognitive paradigms, and genes. This uniquely positions the MAGNET study to address multiple potential points of convergence at the RDoC-HiTOP interface, as well as utilize the biologically-informed constructs of RDoC to test the validity of any ADHD/ASD phenotypic subdimensions or clusters. Of particular relevance is the cross-mapping of HiTOP dimensions and the RDoC Sensorimotor Process construct, for which there is currently no evidence [188]. The MAGNET study methodology includes multiple measures of Sensorimotor subconstructs, including Sensorimotor Dynamics (e.g., sinusoidal pursuit), Initiation (e.g., Reflexive saccades), and Inhibition and Termination (e.g., stop signal and antisaccade; [199]). Furthermore, we will be able to directly assess the mapping of Social Processes subconstructs, including Reception of Facial Communication and Understanding of Mental States, onto the Disinhibition Externalizing spectrum and overlap between this and the Detachment spectrum (i.e., ADHD and comorbid ASD/ADHD), which has not yet been investigated [188]. Our comprehensive cognitive battery aligns with multiple RDoC subconstructs, including Language, Limited Capacity, Inhibition/Suppression, and Interference Control, enabling us to test specific associations with symptom dimensions, as well as determine if cognitive profiles may differentiate subtypes [99]. Finally, we extend upon previous studies by including genotyping, enabling us to investigate the RDoC-HiTOP interface at the level of genes by calculating polygenic risk scores for ADHD, ASD, and related phenotypes [83, 126].
Precision phenotyping to facilitate genetic discovery
Current studies attempting to uncover the neurobiological correlates of ASD and ADHD typically take one of two approaches. The first approach aims to recruit large sample sizes to facilitate high powered genetic analyses, with the trade-off being that only surface level phenotyping of behaviour is typically captured [239]. The second approach recruits smaller samples with deeper phenotyping using multiple modalities and informants, at the cost of reduced sample sizes, lower statistical power and greater financial expense per participant [239]. Large biobanking projects for ASD (e.g. SFARI, [1], Australian Autism CRC biobank, [8], and Norweigian Autism Birth Cohort [272]) and ADHD (e.g. International Multicentre ADHD Genetics [IMAGE] program; [10], The Lundbeck Foundation Initiative for Integrative Psychiatric Research [iPSYCH]; [83]) have achieved large sample sizes but only capture clinical symptom level data which provides minimal insight into the structure of developmental psychopathology and associated neurobiology. Other projects with more comprehensive phenotyping protocols including clinical, neurocognitive and imaging measures such as the EU-AIMS (LEAP) study [178], Biological Origins of Autism (BOA) study [269], and ENIGMA-ADHD/ASD [35, 136, 137]. However, these projects typically confine recruitment to DSM-5 categories for either ASD or ADHD, either excluding on the basis of comorbidity or not accounting for the effects.
There is increasing momentum toward transdiagnostic research efforts, as evidenced through projects such as the NIMH-funded Bipolar Schizophrenia Network on Intermediate Phenotypes (B- SNIP) project [58], which applied a dimensional framework to schizophrenia and bipolar disorder, as well as biobank initiatives like the Healthy Brain Network [7], and the Adolescent Brain Cognitive Development (ABCD) study [173]. Of these studies, the B-SNIP project is the only transdiagnostic study to our knowledge that incorporates first-degree relatives in its design. The inclusion of siblings of first degree relatives enhances the power to detect novel biomarkers with robust biological plausibility, relative to case–control designs, as relatedness is consistently known to reduce the amount of variance within outcome measures [50, 253].
Although there is consistent evidence suggesting shared genetic liability and family aggregation for ASD and ADHD, direct evidence for shared DNA variation from techniques such as GWAS or DNA has been harder to discern [12, 242]. One contributing factor to the inability of techniques such as GWAS to identify shared genetic variation between ADHD and ASD, for example, may be the coarseness of the phenotyping employed and imprecise mapping of comorbidities [42, 236]. A hierarchical approach to psychopathology and neurodevelopment, such as that proposed by HiTOP, whereby phenotypic variation is dimensionally measured in both cases and key comparison groups, and at multiple levels of measurement, provides more specific phenotypic targets for genetic discovery [291]. This circumvents the current difficulties that broad heterogeneous diagnostic categories pose for mapping the underlying genetic architecture of psychiatric illness [239, 291]. Looking outside of the confines of DSM-defined diagnostic categories for genetic associations using a hierarchical framework will allow for identification of both general and specific levels of genetic risk. Indeed, GWAS studies suggest pleiotropy is prevalent in psychopathology, with multiple genes and common genetic variation implicated across a number of disorders [275, 291]. Further, dimensional approaches confer substantially higher statistical power to detect trait-associated genetic variation [258, 259]. Case–control designs have the potential to weaken the genetic signal, with classification of subthreshold cases as controls increasing the breadth of phenotypic and genetic variability within groups [303]. To improve our mapping of the genetic architecture of the ASD-ADHD spectra, and neurodevelopment more broadly, we need to have greater precision in our studies of phenotype-genotype associations. Ways to achieve this can be through family designs, high precision phenotyping grounded in theoretically and biologically informed frameworks (e.g. HiTOP, RDoC), and comprehensive clinical review of all cases.
The Monash Autism-ADHD Genetics and Neurodevelopment (MAGNET) project
To our knowledge there is no current study taking a truly transdiagnostic approach to understand the symptomatic, neurobiological (neurocognitive and neuroimaging) and genetic overlap between ASD and ADHD. A transdiagnostic sampling strategy that combines a family study design with deep dimensional phenotyping is needed across the ASD-ADHD spectra. By drawing on both RDoC and HiTOP frameworks, the MAGNET Project will contribute to our understanding of how neurodevelopmental disorders fit into a data-driven hierarchical taxonomy. Further, by understanding how these dimensional phenotypes present in families of children with ASD and ADHD, and whether siblings share similar behavioural signatures will provide crucial evidence for familiality of different ASD-ADHD phenotypes. The MAGNET Project therefore aims to: (1) identify data-driven symptom clusters across ADHD-ASD spectra using deep phenotyping of symptoms and behaviours; (2) investigate the degree of familiality for these data-driven symptom clusters; (3) map the neurocognitive and brain imaging correlates of these data-driven symptom clusters; and (4) explore their genetic correlates.
Methods
Study design
The MAGNET Project will enrol 1200 families with children aged between 4 and 18 years of age. Children who are typically developing, as well as those with a diagnosis of ASD, ADHD, or ASD + ADHD will be recruited to ensure both ends of the ASD-ADHD spectra are appropriately sampled. Children who are under investigation for ASD and/or ADHD and are referred to the study by their paediatrician will also be recruited. In addition, unaffected and affected siblings of probands will be recruited. A dimensional enhancement approach to sampling will be taken, as it augments clinical samples with non-clinical participants and those exhibiting subthreshold symptoms [75, 161]. This sampling strategy, whereby typically developing children are transdiagnostically phenotyped alongside probands and subthreshold cases (e.g. siblings, children currently under investigation for ASD or ADHD) combines the strengths and offsets the weaknesses of categorical and dimensional approaches to psychopathology research by increasing statistical power [50, 253] whilst maintaining clinical validity and enabling direct comparisons with existing diagnostic classifications systems [75, 131]. The MAGNET protocol comprehensively phenotypes all children and siblings, irrespective of case–control status, for behavioural and neurocognitive constructs that are central to ASD and ADHD symptomatology and align with RDoC and HiTOP frameworks (e.g. internalising and externalising symptoms, attention and cognitive control, arousal, reward, working memory, perception, social processes, and sensorimotor processes). The battery uniquely captures dimensional traits across ASD-ADHD spectra using a range of symptom, parent-report, neurocognitive, and direct behavioural observation measures to capture the target domains from multiple perspectives. This approach will provide a rich source of data unconfounded by informant bias and method bias, with the opportunity to model the correspondence and complex interactions of information obtained from multiple informants [2, 176, 176, 177, 177, 213, 215, 219].
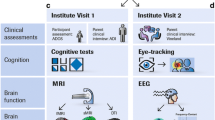
Targeted sampling through hospitals, schools, private practice clinicians, and social media across Victoria, Australia will allow for a broad and representative distribution of socioeconomic status (SES) and symptom presentation. Currently the MAGNET Project is in an open recruitment phase. The MAGNET Project will actively recruit females and children with mild-severe intellectual disability, as these children are typically under-represented, or excluded from, studies of ASD and ADHD. The study has been piloted on control and clinical children aged 4 to 18 years of age (see Table 1 for preliminary demographic and clinical data) to assist in deciding appropriate age and cognitive ranges for tasks and minimum dataset requirements. See Fig. 1 for an overview of the MAGNET Project study protocol (see Additional file 1 for the MAGNET Project Protocol).
The MAGNET Project study protocol. Participant grouping: The MAGNET Project is a single-site study recruiting children who are typically developing, as well as those with elevated ASD, ADHD, or ASD + ADHD symptoms. Affected and unaffected siblings of probands will also be recruited. All children undergo comprehensive dimensional phenotyping across behavioural constructs central to ASD and ADHD target domains. Clustering: The MAGNET Project will use both supervised and unsupervised methods for discovery of ASD-ADHD clusters using measures of symptoms and behaviours. As these methods are hypothesis free and diagnosis naïve, the number of clusters will not be determined a priori. Uniquely, the battery captures target domains from multiple perspectives (self-report, parent-report, clinician rated measures, and direct child measures [e.g. eye-tracking and neurocognitive tasks]). All control children, probands, and siblings and parents of probands provide a saliva sample for genetic analysis. Structural and functional brain measures (magnetic resonance imaging [MRI], resting state MRI, and diffusion weight imaging) are also collected. The neurocognitive, brain imaging, genetic, and functional outcomes will then be mapped to the data-driven symptom clusters
Participant eligibility
Children with a diagnosis of ASD and/or ADHD provide the clinical report from their clinician with evidence of diagnosis. Children who are under investigation, or queried for, ASD and/or ADHD are required to have a clinician (paediatrician, psychologist, and/or general practitioner) currently managing their care. Siblings of probands must share two biological parents with the proband. The healthy control children are required to have no neurodevelopmental diagnosis, no first-degree relative with a diagnosis of ASD and/or ADHD, and no history of a psychiatric (e.g. depression or anxiety) or neurological illness (e.g. head injury or tuberous sclerosis).
Probands and siblings with comorbidities such as anxiety, depression, oppositional defiant disorder (ODD), conduct disorder (CD), and obsessive–compulsive disorder (OCD) are not excluded. As a large proportion of children with ASD and ADHD experience these comorbid disorders [79, 183, 306], exclusion of these disorders may result in a sample that is not representative of the target population. Where possible, one or both biological parents complete a battery of questionnaires examining ASD and ADHD symptomatology, mental health, and quality of life. Exclusion criteria for all children include known genetic (e.g. Fragile X, Angelman’s Syndrome, tuberous sclerosis) or environmental (e.g. traumatic brain injury, foetal alcohol syndrome) causes. A peri/prenatal environment questionnaire retrospectively captures maternal alcohol and drug use, medication, illness/infection, and complications during the pregnancy and delivery. Retrospective information on the child’s development, including developmental milestones and regression is obtained via parent-report. As the questionnaire battery is extensive, at least one parent/caregiver is required to speak English. Parents complete approximately 3 hours of online questionnaires, and one (control families) or two (clinical families) 3-hour research visits at Monash University to complete the testing protocol.
All children undergo case review by a registered psychologist and paediatrician, and speech pathologist if available, to determine a ‘best clinical estimate’ of that child’s current diagnostic status. A best clinical estimate will be given for ASD, ADHD, comorbid ASD/ADHD, intellectual disability (ID), CD, and ODD (see Additional file 2). The best clinical estimate will not be used as exclusion criteria for the study. Children who do not meet thresholds for ASD and/or ADHD will still provide useful information about the dimensionality of ASD and ADHD symptoms. Children with an estimated full-scale intelligence quotient (FSIQ) in the range for ID (IQ ≤ 70) as measured using standardised psychometric assessment (e.g., Wechsler assessments) are administered a minimum dataset protocol (see Additional file 3), but will attempt additional tasks from the battery wherever possible.
Ethnicity
Single-nucleotide polymorphisms (SNPs) may vary between ethnic populations and potentially cause false positive results in genetic association studies. To avoid the potential impact of population stratification only children with four grandparents of European ancestry are invited to complete the genetic component of the protocol.
Siblings
Only full biological siblings will be eligible to take part in the study. Within simplex families, that is, families where only one child has an ASD and/or ADHD diagnosis, the child with the ASD/ADHD diagnosis is nominated as the proband. In multiplex families, families where more than one child has an ASD and/or ADHD diagnosis, the eldest child is denoted as the proband and younger children are designated as affected or unaffected siblings. Unaffected siblings of ASD/ADHD probands have no diagnosis of ASD/ADHD, are not under investigation for ASD/ADHD, and are not assigned a neurodevelopmental disorder diagnostic category during their best clinical estimate review.
Medication
The child’s current and previous medication history, medication prescriber (e.g. paediatrician, general practitioner), and reasons for any medication changes, will be recorded.
Children who are taking medication remain on their medication during Visit 2 when their relevant Wechsler and Autism Diagnostic Observation Schedule—Second Edition (ADOS-2) assessments are completed (see Additional file 4 for clinical assessment protocol). However children taking stimulant or non-stimulant medication for ADHD including methylphenidate, lisdexamfetamine, or dexamfetamine are required to withdraw from their medication 48–72 hours prior to completing the neurocognitive test battery during Visit 1 [51, 195]. Participants taking guanfacine or antipsychotics (e.g. risperidone, aripiprazole) do not withdraw for any component of the protocol as abrupt withdrawal from these medications may be associated with adverse side effects [138, 273, 307]. Children taking melatonin are not required to withdraw prior to participating.
Phenotyping overview
Each of the measures or tasks included were selected as gold standard measures that are widely used, have biological plausibility, and show robust effect sizes when differentiating controls from either ASD or ADHD (See Table 2 for the MAGNET Project symptom and environmental phenotyping measures, and Table 3 for neurocognitive phenotyping measures).
The components of the MAGNET protocol intended to measure phenotypic dimensions relevant to ASD were chosen in consultation and collaboration with the European Autism Interventions—A Multicentre Study for Developing New Medications—Longitudinal European Autism Project (EU-AIMS [LEAP]) study team [144, 178]. The EU-AIMS (LEAP) study is a European multi-centre study that aims to identify risk factors contributing to differences in brain development, social difficulties and other core ASD symptoms. Through aligning parts of the MAGNET and EU-AIMS (LEAP) protocols, the MAGNET project will also act as a replication site for the EU-AIMS (LEAP) study. The addition of measures for dimensional phenotyping of ADHD symptoms and relevant neurocognitive traits are unique to the MAGNET project and make ours the first large-scale family-based project to take a truly transdiagnostic approach to understanding ASD and ADHD (see Additional file 1 for MAGNET protocol summary).
Characterisation of ASD, ADHD and comorbid symptoms
Dimensional ASD symptomatology is measured through parent-report measures capturing social communication (Autism Quotient—Child [AQ-C], [17], Child Communication Checklist—Second Edition [CCC-2], [33], Social Responsive Scale—Second Edition [SRS-2], [64]), social competence (Child Behaviour Checklist [CBCL], [4], restricted, repetitive, and stereotyped behaviours (SRS-2, [64],The Childhood Routines Inventory—Revised [CRI-R], [96]), and autism symptomatology overall (AQ-C; [17], SRS-2; [64]). Dimensional traits central to ADHD are captured through parent-report questionnaires, and an in-house observation checklist for ADHD behaviours completed during ADOS-2 coding. Parent rated measures of attention and inattention (Strengths and Weaknesses of ADHD Symptoms and Normal Behaviour [SWAN]; [13], Conners’ Parent Rating Scale—Revised [CPRS-R]; [62], Development and Wellbeing Assessment [DAWBA], [122]), hyperactivity (Aberrant Behaviour Checklist [ABC], [9], Strengths and Difficulties Questionnaire [SDQ], [121], DAWBA, [122]), impulsivity, and overall ADHD symptomatology (CPRS-R, [62]), are comprehensively assessed, alongside an additional measure of attention appropriate for children with intellectual disability (Scale of Attention in Intellectual Disability [SAID], [110]). Teachers are invited to complete the SRS-2, SDQ, and Conners’ Teacher Rating Scale—Revised [63], although completion rates are typically lower than for parent report. Height, weight, head circumference, and joint mobility and hypomobility [26] are also recorded for every child.
Comorbidities
Comorbidities commonly observed in ASD and ADHD are captured in all children, including anxiety (Child Behaviour Checklist [CBCL], [5], Spence Children’s Anxiety Scale [SCAS], [263]) and depression (CBCL, [5], DAWBA, [122], Childhood Depression Inventory—Second Edition [CDI-2], [159, 260]). Conduct problems and oppositional defiant problems are also indexed (CBCL, [5], CPRS-R, [62], DAWBA, [122]). Level of current cognitive function is determined using age appropriate Wechsler intelligence scales [293, 294, 295, 296]. See Additional file 4 for clinical assessment protocol.
Adaptive behaviours and quality of life
Adaptive behaviour (Vineland Adaptive Behaviour Scale—Third Edition [VABS-3]; [262]) and quality of life (Child Health and Illness Profile—Child Edition [CHIP-CE], [229]) are measured in all children through parent-report questionnaires.
Language assessment
Language profiles in ASD are heterogeneous, ranging from non-verbal [114] to superior linguistic abilities [154]. Although language impairments are not a hallmark diagnostic criteria for ADHD, both linguistic and pragmatic deficits are commonly part of the symptom presentation [27]. Recent empirical records on the co-occurrence of language impairments in ASD and ADHD have identified impairments in structural and pragmatic aspects of language in both the groups [19, 164, 203, 250]. Despite the presence of language difficulties in ASD and ADHD, and indeed, in a number of other neurodevelopmental disorders and psychopathology, language constructs are not currently included in RDoC or HiTOP frameworks. Thus, the inclusion of language assessments in the MAGNET Project protocol will provide a novel and unique contribution to these nosologies.
A standardised screening measure for language difficulties (Clinical Evaluation of Language Fundamentals—Fifth Edition [CELF-5]: Screening Test; [297]) is administered to all enrolled children over 5 years of age. Children with a diagnosis of ASD and/or ADHD or those who are under investigation for these disorders, and control children who fall below criterion on the screening measure for language difficulties, are administered the Australian adaptation of the Clinical Evaluation of Language Fundamentals—Fifth Edition (CELF-5; Age group 5 to 21 years; [70], [298]) or Clinical Evaluation of Language Fundamentals—Preschool Edition (CELF-P2; Age group 3 to 6 years 11 months; [251]). This clinician-administered assessment provides a comprehensive global measure of language abilities, and characterises structural and pragmatic language in children.
The Preschool Language Scale—Fifth Edition (PLS-5; [310]) is administered to younger minimally verbal children. The PLS-5 incorporates information from clinical observation, direct measurement and parent report to assess domains of attention, play, gesture, vocal development, social communication, semantics, language structure, integrative language skills and emergent literacy skills in children from birth to 7 years 11 months. A caregiver rated questionnaire, the Children's Communication Checklist 2 (CCC-2; [33]), measures both structural (language form/content) and pragmatic traits of communication impairment in children. The CCC-2 includes an overall measure of communication skills and a Social Interaction Deviance Composite (SIDC) which indexes the strength of relationships between the social domains of communication and structural components of language, thereby aiming to attain and identify traits associated with pragmatic language difficulties. With poorer overall language performance and SIDC linked to ASD traits [33], these measures provide valuable information when differentiating comorbid presentations of language impairment in neurodevelopmental disorders. The SRS-2 also provides a parent-reported index of social communication. Recordings from the ADOS-2 provide high-resolution natural speech and language samples. See Additional file 4 for clinical assessment protocol.
Measures of neurocognition
We assess the domains of sustained attention, inhibition, cognitive control, arousal, reward, working memory, perception, social processes, and sensorimotor processes with the view to utilise neurocognitive data to discover neurobiological correlates of novel ASD-ADHD data-driven clusters. The tasks chosen are widely used, have biological plausibility and show robust effects sizes when differentiating clinical cases from controls. See Table 2 for the MAGNET Project neurocognitive phenotyping measures. Additional file 5 summarises the MAGNET Project’s neurocognitive assessment protocol.
Neurocognitive tasks
The neurocognitive tasks will be completed on a desktop computer and touchscreen laptops. Amsterdam Neuropsychological Tasks (ANT), Psytools, PsychoPy and STOP-IT software programmes were used for task administration [261, 81, 214, 282, 283].
Response inhibition, sustained attention and cognitive control
Response inhibition refers to the ability to withhold or cancel a motor response [52]. Sustained attention, or vigilance, can be defined as the ability to maintain engagement in a task over a prolonged period of time [109]. This component of attention is thought to be mediated by top down, or endogenous processes, and is controlled by internal goals [192]. These cognitive functions are measured using a Go/No-Go (ANT; [261]) and Stop Signal Task which are standard measures of top-down/endogenous sustained attention and response inhibition. Response inhibition is indexed through stop-signal reaction time (SSRT) and the percentage of failed attempts to inhibit a response on tasks. Longer stop signal reaction times and commission errors indicate poor inhibition and more omission errors and are indicative of poorer sustained attention [282]. Response inhibition and sustained attention deficits are central to the conceptualisation of ADHD [20, 28, 221, 300, 301], with some support for deficits in ASD [21, 54, 152, 246, 218]. Further, these deficits are heritable, with unaffected siblings of ADHD probands demonstrating response inhibition and sustained attention difficulties [53, 111, 243, 257]. Similarly, reduced inhibitory control has been demonstrated to be familial in ASD families [246].
Arousal
Arousal can be understood as an individual’s state of reactivity, and although arousal is intimately linked with constructs like attention, the neural correlates of these processes are largely distinct [72]. Arousal will be examined by deriving measures of intra-individual variability in response times across tasks of sustained attention and response inhibition, as suboptimal arousal is thought to underpin intra-individual variability in ADHD [28, 29, 49, 254]. Increased response time variability is a hallmark feature of neurocognitive performance in ADHD [29, 148, 150, 151, 256] and is familial [165, 200]. Variability in response time is thought to be a marker for dysfunction in the frontal areas of the brain [30, 181], which is consistent with theories of hypo-arousal and fronto-striatal dysfunction in ADHD [74, 241]. Although children with ASD show similar response time variability to typically developing children [151], variability in response time appears to index ADHD symptomatology across diagnostic boundaries as children with comorbid ASD and ADHD show similar variability to those with ADHD [279]. Thus, response time variability as a proxy measure for arousal shows promise for effectively stratifying children with ASD, ADHD and ASD-ADHD.
Reward sensitivity
Reward sensitivity refers to the tendency to respond more strongly to incentives, or rewards, and is a process implicated in decision making. ADHD is associated with divergent decision making, differing sensitivity to reward, and elevated risk-taking behaviour [80, 147, 180, 309]. Effect sizes for decision making difficulties are comparable to the attention difficulties seen in ADHD [196]. Altered reward processing in ADHD is well-studied, and posited as central to the disorder [277]. Children with ADHD show poorer decision making as they have difficulty adjusting their responses in the face of changing levels of risk [59, 125, 276]. Biological plausibility is evidenced with correlative neuroimaging in ADHD of under activation in brain regions associated with decision making (i.e. ventral and dorsolateral prefrontal cortex, and insula; [39, 94] and hypo-responsiveness in neural circuitry involved with reward anticipation (i.e. ventral striatal circuitry; [245]). Dopamine is one of the neurotransmitters implicated in decision making and reward, and indeed, dopamine deficiency is a leading hypothesis in ADHD [309]. Together, a task engaging decision making, reward sensitivity, and risk-taking behaviour is a well-positioned ADHD trait for discovery of clusters.
In ASD, there is evidence for aberrant reward processing, but to a lesser extent than that observed in ADHD [156, 277]. Children with ASD showed increased activation in the anterior cingulate cortex during reward achievement compared to controls [247]. This region is thought to be involved with self-monitoring of performance in line with reward feedback [34, 232] and risk assessment [41]. However, there is some evidence to suggest ASD and control groups perform similarly on goal-directed decision making tasks in the context of explicit reward [100] and have similar sensitivity to monetary reward [85, 266], with no difference in neural activation while processing reward [168]. The less definitive evidence in ASD may indicate that only a subgroup of these children may in fact have altered reward processing and decision making.
To assess decision making, reward sensitivity, and risk-taking, the New Cambridge Gambling Task [44] will be used. It allows for delineation of risk-taking behaviours from impulsivity, and explicitly states the probability for each trial. Unlike other gambling tasks (e.g. Iowa Gambling Task), explicit statement of probability reduces the working memory load, thus reducing confounds of additional working memory deficits.
Probabilistic reversal learning
Broadly, cognitive flexibility is a component of executive function that encompasses adaptability at a behavioural level and is studied from a variety of perspectives such as set shifting, task-switching, and reversal learning [67]. More specifically, contingency-related cognitive flexibility is the adaptation of behaviour after negative feedback, typically measured using probabilistic reversal learning paradigms. In typical development, contingency-related cognitive flexibility specifically is associated with the orbitofrontal cortex, parietal cortex, and subcortical connections [107]. Impairments in contingency-related cognitive flexibility are seen in ASD [69, 91] and ADHD probands [145, 299], with impairments also observed in unaffected first degree relatives of ASD probands [246]. In ASD, cognitive inflexibility has been associated with restricted, repetitive, and stereotyped behaviours [91, 174, 190]. Neuroimaging findings demonstrate aberrant activation of networks during cognitive flexibility tasks in children with ASD (lateral frontoparietal and midcingulo-insular networks; [280]) and fronto-striatal function, which is implicated in cognitive flexibility, is thought to be impaired in ADHD [47, 89]. In the MAGNET Project contingency-related cognitive flexibility will be measured using a probabilistic reversal learning paradigm with positive and negative feedback [178, 209]. The number of trials required to shift to a new response choice, perseverative errors, and regressive errors index cognitive inflexibility.
Working memory
Internationally, the definitions of working memory are contentious, with working memory and short-term memory sometimes still used interchangeably. Some conceptualise working memory as the process of holding information in the mind for a short period of time, which can also be thought of as short-term memory [118]. Others understand working memory, also referred to as executive memory, as the ability to maintain and manipulate information, where this manipulation may have low or high executive demands [18, 77]. Tasks are then modality specific, using verbal or visual stimuli. The MAGNET Project’s conceptualisation of working memory aligns with executive memory that has high and low executive demands. Verbal and visual working memory difficulties are seen in both ASD and ADHD [87, 153, 184, 248, 249, 252], with deficits becoming more pronounced as the cognitive load increases [226, 227, 252, 267, 284]. These difficulties on working memory tasks with higher cognitive load correspond with atypical neural processing in children with ASD [222, 284], providing biological plausibility for working memory performance as a neurocognitive marker of ASD. Further, unaffected siblings of children with ASD and ADHD showed more impaired verbal and visuospatial working memory performance than typically developing controls [31, 204, 252]. The verbal and visuospatial working memory divergence seen in unaffected siblings of children with ASD and ADHD positions working memory as a good candidate endophenotype [108, 191]. Verbal [292, 293, 297, 298] and visuospatial [45, 46] working memory tasks which increase in cognitive load across trials allows us to index working memory capacity across the broad range of cognitive abilities captured in the study.
Social processes
Emotion recognition is the ability to correctly identify another person’s emotion based on their facial expression and is crucial for effective social communication. Emotion recognition difficulties in children with ASD are a consistent and robustly replicated finding [128, 281]. Atypical processing of emotions is also thought to be familial, with unaffected relatives of individuals with ASD also showing less severe, but still significant emotion recognition difficulties [78, 206]. Although emotion recognition is not as extensively researched in ADHD, there is some evidence for emotion recognition divergence in these children [14, 36, 84, 287]. Emotion recognition in the MAGNET project is conceptualised, and measured, as the ability to recognise both simple and complex emotional states (Reading the Mind in the Eyes Task [RMET]; [22]; Karolinska Directed Emotional Faces [KDEF]; [119]).
Theory of Mind (ToM) is the ability to understand and attribute mental states to oneself and to others and understand that others can have different mental states to yourself. Profound difficulties with understanding others’ thoughts and intentions in day-to-day life are common in ASD [216]. These difficulties with ToM have been linked to genetic anomalies associated with ASD [231]. False-belief tasks are widely used for assessing ToM and individuals with ASD typically show egocentric biases when completing these tasks compared to their typically developing peers [25]. These difficulties are less definitive in high functioning individuals with ASD however, with some able to successfully complete continuous false-belief tasks [244]. The ability of such a task to separate different individuals with ASD positions it well to stratify these individuals. Conversely, the findings within ADHD are currently heterogeneous. More research is necessary to understand whether these deficits are present in only a subset of these children [217].
Oculomotor measures
Saccade and pursuit eye movement abnormalities have the potential to reliably distinguish ASD and ADHD children from controls [149]. Oculomotor abnormalities can arise as the result of abnormalities in a range of well-mapped neural circuitry throughout the brain, spanning motion sensitive visual area V5, parietal and frontal areas supporting visual attention and sensorimotor transformation, basal ganglia, brainstem and cerebellar circuitry [149]. Oculomotor control is ideal to measure in children, as it is quick, and affords sensitive, high-resolution recording, and requires minimal-to-no language comprehension for children to perform. Sensorimotor measures from ocular motor tasks include accuracy, motor dynamics (e.g. velocity profiles), initial eye acceleration in response to the onset of a visual target or target movement and integration of visual feedback in motor responses. The anti-saccade task, completed in children eight years and over, also provides a measure of how attentional processes and inhibition interface with oculomotor control [97, 140, 155, 198]. Other studies in schizophrenia and bipolar disorder have found unique relationships between genes associated with nervous system development and function and with sensorimotor processing and pursuit maintenance [171]. See Additional file 6 for oculomotor testing protocol.
Brain structure and function
Large-scale neuroimaging studies have identified robust structural differences associated with ASD and ADHD, demonstrating both common and disorder-specific brain alterations. In both ASD and ADHD, cases showed reduced subcortical volumes [134, 235] and cortical thinning in temporal regions [136, 235]. Reduced surface areas were specific to ADHD [136], whereas ASD showed increased cortical thickness in frontal regions [235]. Evidence regarding differences in diffusion weighted imaging (DWI) and resting state fMRI (rs-fMRI) are based on smaller studies demonstrating wide-spread alterations in fractional anisotropy [86, 98] and less consistent changes in rsfMRI [170, 308].
Structural and functional brain imaging (resting state fMRI) will be collected to determine if neurobiological differences exist as a function of symptom-based data-driven clusters. All scans will be performed using Siemens Skyra 3 T scanner following previously established protocols [207, 237]. Data processing pipelines will include extensive correction for in-scanner motion [207, 211] which is the most prevalent MRI artefact in paediatric populations.
Genetics
Saliva is collected from all probands, affected and unaffected biological siblings, biological parents of probands, and healthy controls for DNA extraction (see Additional file 7 for DNA collection and extraction protocol). DNA will be subjected to array-based genotyping (e.g. Illumina Global Screen Array for GWAS) and/or whole genome sequencing, as funding allows. Because our study sample size has limited power to reliably detect novel associations with DNA variants, we will capitalise on existing publicly available data and consortia science in the following ways. First, we will derive Polygenic Risk Scores (PGRS; [55, 95]) for ASD and ADHD using international datasets as the base dataset (e.g. Psychiatric Genomic Consortium [PGC] and iPSYCH; [83, 126]) and our entire sample of probands as the target dataset. We will estimate the relationships between polygenic risk scores for ADHD and/or ASD and each of our symptom-based data-driven clusters. Second, our family-based design is optimal for whole genome sequencing and will allow us to determine whether patterns of inherited versus de novo mutations differentially cluster across the data-driven clusters [50, 253]. Again, we acknowledge the limited power of our sample for whole genome sequencing, and will join collaborative efforts (e.g. PGC,iPSYCH; Autism Speaks MSSNG Project; EU-Aims; Province of Ontario Neurodevelopmental Network [POND]).
Parent phenotyping
Both biological parent’s complete self-report dimensional measures of ASD (Autism Quotient—Adults [AQ-A, [38]; SRS-2, [64], Adult Routines Inventory [ARI], [96]) and ADHD symptomatology (SWAN, [13], Conners' Adult ADHD Rating Scale, Conners et al. [61]; SDQ, [121]. Parent’s complete self-report measures of depression (Beck Depression Inventory [BDI], [24], anxiety (Beck Anxiety Inventory [BAI], [23]), and a quality of life measures (World Health Organisation Quality of Life Measure [WHOQOL-BREF], [285]) as parents of children with ASD and ADHD can experience poorer mental health and quality of life outcomes compared to parents with typically developing children [124, 167, 288].
Database access
All raw data is stored on a central database with access only granted to current members of the research team who have personalised login details. Oculomotor and neuroimaging data are downloaded to local devices from the central database for cleaning, pre-processing, and analysis. Currently, access to the MAGNET Project’s data is only granted for members of the MAGNET research team and our collaborators from the EU-AIMS (LEAP) study [178]. Genotyping information will be made available to international research consortia, such as the PGC, where participant consent for sharing has been given. Upon completion of the project, the MAGNET Project data set will be changed to open access. Consent for sharing neuroimaging data will be in line with recommendations from the Open Brain Consent working group [208].
Planned statistical analysis
A combination of supervised psychometric analyses and unsupervised clustering approaches will be used to converge on data-driven homogeneous ASD-ADHD clusters embedded within biologically-relevant dimensions based on previously derived factor score estimates [37, 106]. By using multiple measures of target constructs to create latent variable phenotypes, we can maximise our study’s statistical power and strengthen the representation of our key constructs [259]. The utility of dimensional approaches for improving statistical power in psychopathology research, particularly psychiatric genetics, is well established [160, 275, 258]. Similarly, the advantages of phenotypic precision in improving signal-to-noise ratio and thus statistical power for detecting relationships with external criterion variables, including genetic variation, has also been outlined [56, 259, 302]. Hierarchical models of psychopathology, such as HiTOP, which parse phenotypic variation into homogeneous components at varying levels of granularity and specificity are a particularly powerful approach to linking with genetics [239, 291]. Accounting for phenotypic heterogeneity through latent subtyping using hybrid models also has the potential to confer increased statistical power [105, 106]. Furthermore, combining measures of a construct across modalities removes confounding method variance [92, 219]. Obtaining information from multiple informants also controls for informant bias, whilst discrepancies between informant reports provides additional sources of information relevant to developmental psychopathology that can be the subject of further analysis [176, 176, 177, 177]. The MAGNET study leverages all of these approaches in combination, which not only improves power, but also increases consistency and efficiency and reduces bias in statistical estimation [238, 255]. Moreover, the MAGNET Project’s representative sample and measures are important prerequisites for robust clustering methods to avoid model overfitting and poor reproducibility [43, 223].
Dimension reduction strategies, such as exploratory factor analysis and exploratory structural equation modelling [15, 71, 182], or multidimensional item response theory [224], will be used on each participant’s raw scores to first identify their factor or scale score estimates representing their standing on these latent dimensions. Unbiased feature selection and optimising latent model fit in this step, prior to later clustering analyses, can reduce the interference of variance from extraneous noise. It is also acknowledged that there may be clustering and nesting within the data based on sampling (e.g. participants from the same family) and testing (e.g. testing sessions, assessors) procedures [57, 187]. Subsequent analyses will account for these effects, though the choice of correction method will depend on the characteristics of our final dataset.
Factor mixture modelling is one possible supervised clustering method that we will employ for our subtyping analyses. Factor mixture modelling can uncover homogeneous clusters within continuous and categorical data embedded within dimensional models of psychopathology by utilising probabilistic modelling techniques [37, 179, 189]. The flexibility of factor mixture modelling permits the testing and comparison of multiple models with varying numbers of a priori specified clusters. Alternatively, unsupervised machine learning techniques may be better suited for addressing specific research questions related to uncovering underlying structures in the data, and identifying clusters in a "bottom up" way. For example, HiTOP approaches to taxonomy make few assumptions regarding symptom-level data, and instead take the structure or shape of the subtypes from the data itself [133]. Community detection is one possible unsupervised approach, which combines graph theoretic analyses to detect homogeneous communities/clusters (i.e., highly connected sets of nodes). By ensuring that the algorithm achieves a connected graph, our analyses will parsimoniously account for all participants. These supervised, unsupervised, and hybrid approaches will help to empirically unify the theoretical grounding of MAGNET’s research questions with the power of cutting-edge data-driven analysis techniques to address heterogeneity. Moreover, these techniques are diagnosis-naïve, thus allowing MAGNET to fully embrace the transdiagnostic features of our biobehavioural subtypes. Finally, although MAGNET aims towards data-driven clusters using symptom and behavioural data, the potential utility of incorporating neurocognitive or genetic components in defining clusters will not be overlooked [58, 99].
Discussion
The MAGNET Project has completed initial piloting of the study protocol and entered into an open recruitment phase. It is the first large-scale study using a family design to take a truly transdiagnostic approach to ASD and ADHD that aligns with the principles of the RDoC matrix and HiTOP model of psychopathology.
Challenges in study design, recruitment, and data quality
Study design
A significant amount of time is required in the conceptualisation of an assessment battery that is appropriate for the large range of cognitive abilities and ages, while adequately capturing dimensional ASD and ADHD traits. As a number of the measures included in the protocol were not initially intended for use across broad age ranges or levels of cognitive ability, it is important to allow for extra time during piloting to determine the minimum age and cognitive level for tasks with novel applications. Although such an approach required more time initially, it will translate into a high-quality dataset upon project completion.
One of the measures used to confirm an ASD diagnosis, the ADOS-2, was chosen as it is internationally recognised as part of gold standard assessment. Uniquely, all children who participate in the MAGNET Project complete an ADOS-2. The ADOS-2 research training dictates coding of observed behaviours with no clinical interpretation to ensure research-reliable coding. Differentiating the social difficulties of children with ADHD on the ADOS-2 can be challenging, which has also been previously noted by Grzadzinski et al. [127]. ADOS data from children with ASD and ADHD also has the potential to improve clinical phenotyping across the ASD-ADHD spectra. Analysis of individual ADOS items may elucidate which items are more sensitive to ASD and which items are driven by ADHD presentations. Clinical cases are reviewed using all measures, including the ADOS-2 and the DAWBA, by the team’s paediatrician and psychologist to determine a best clinical estimate (see Additional file 2). The best clinical estimate process has been imperative in confirming diagnostic status.
Recruitment
The inclusion of children with ID will facilitate a sample that is largely representative of our target population, specifically within ASD. However, recruitment uptake for families of children with ID has been slow. These families often have children with high treatment needs which can be time consuming, in turn reducing the likelihood of these parents enrolling in a time-intensive research protocol. An alternative targeted recruitment strategy for these families will be needed moving forward, including direct communication with specialist school settings to engage teaching staff in the recruitment process for their learning community. Partnering with community grant funds and the Australian National Disability Insurance Scheme (NDIS) are further strategies the MAGNET team intends to utilise for recruitment of these children.
As ASD and ADHD are highly heritable, with evidence for shared genetic liability in families, this inherently limits the number of possible unaffected siblings. A large number of families with children with ASD and/or ADHD will therefore be required to achieve sufficient numbers of unaffected siblings for high powered statistical analysis.
Data quality
A number of the large-scale biobanking projects and multi-site studies can experience significant missingness in their data. Protecting against missing data has been a key priority in the MAGNET Project protocol development. Initial piloting highlighted that ensuring parents completed all online questionnaires before attending the in-person research visits reduced missing data, and increased attendance rates to research visits. Comprehensive data collection at the initial point of contact with families will also allow us to determine if attrition and resulting missingness is attributed to characteristics of the family or child, thus allowing us to model the missingness and avoid bias in our results. With the oversight from the project’s supervising psychologist, families are provided with a results summary after participating, including outcomes from cognitive assessments, language assessments (where applicable), ASD and ADHD symptom scales, and ADOS-2 ratings. To increase retention rates between the first and second research visit for each family, the neurocognitive tasks are completed in the first research visit and the cognitive assessment and ADOS-2 are completed in the second. Importantly, other measures used in the best clinical estimate review, such as the DAWBA, are completed prior to participants first research visit. Saliva collection from all members of the family pedigree has also been challenging, especially from fathers. Currently we have noted that mothers will primarily bring children to their research visits. Good follow-up and regular contact with the family is imperative in ensuring the least amount of missing genetic data. Minimal manual handling of data with automatic backups of all clinical, neurocognitive, and oculomotor data reduces the risk of missing data through technical or human error. Sophisticated analysis strategies to manage missingness will be utilised by the MAGNET Project that accommodates some missing data under assumptions of Missing Completely At Random, or Missing At Random, such as multiple imputation, auxiliary variables, and expectations-maximisation algorithm [93, 123].
The diversity of clinical specialists on the MAGNET Project team, including psychologists, cognitive neuroscientists, paediatricians, psychiatrists, and speech pathologists, is relatively unique. When research teams are large, this increases potential variability in administration of assessments, and thus variability in data quality. The MAGNET Project team undergo regular and ongoing staff training and clinical supervision from the project’s supervising psychologist. As a result, all members are consistently building skills to maximise participant engagement and data-capture across all tasks and assessments.
Limitations
It is possible that the MAGNET Project’s sampling strategy will not achieve a true community sample upon completion. However, a variety of recruitment avenues and methods will be utilised to achieve a sample with breadth in symptomatology and phenotype. The project will provide insight into ASD and ADHD’s place within a hierarchical taxonomy of psychopathology and neurodevelopment. Although the study primarily targets traits central to these disorders, the full breadth of neurodevelopmental difficulties and common comorbidities (e.g. anxiety) are not captured with the same degree of granularity. The MAGNET Project will therefore provide one piece of the much larger puzzle in the quest for understanding neurodevelopment in a hierarchical framework. The broad range of cognitive abilities captured by the project, which allows a more representative sample, also means a proportion of children with more severe ID may not be able to complete all neurocognitive and/or imaging protocols. Nevertheless, our minimum dataset protocol is designed to provide minimal missing data across key tasks.
Conclusion
Clinical heterogeneity and unitary conceptualisations of ASD and ADHD have hampered attempts to understand the structure of developmental psychopathology and associated neuropsychology, neurobiology and genetics. Current attempts to uncover the genetic aetiology of ASD/ADHD are limited with respect to one or more of the following: (1) recruitment is restricted to diagnostic categories that ignore the dimensional organisation of psychopathology symptoms, comorbidity, and within-group heterogeneity [163]; (2) minimal phenotyping in large samples; or (3) deep phenotyping in smaller samples [239]. Using deep phenotyping, dimension reduction techniques, factor mixture modelling, and machine learning techniques, the MAGNET Project aims to identify unique, homogeneous ASD-ADHD clusters of individuals with similar behavioural, neurocognitive, neuroimaging and, potentially, genetic profiles. The MAGNET Project will be one of the first studies to combine a dimensional conceptualisation of developmental psychopathology, in combination with deep phenotyping in a large sample to investigate the behavioural, neurocognitive, neuroimaging and genetic markers in ASD and ADHD. This study is well-positioned to uncover novel, homogeneous data-driven clusters with potential implications for ASD and ADHD diagnosis and treatment.
Availability of data and materials
Currently MAGNET Project data is stored on a central database with access currently granted to members of the research team and our collaborators from the EU-AIMS (LEAP) study [178]. Upon study completion database access will be opened to the scientific community.
References
Abrahams BS, Arking DE, Campbell DB, Mefford HC, Morrow EM, Weiss LA, Packer A. SFARI Gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Molecular Autism. 2013;4(1):2–4. https://doi.org/10.1186/2040-2392-4-36.
Achenbach TM. As others see us: clinical and research implications of cross-informant correlations for psychopathology. Curr Dir Psychol Sci. 2006;15(2):94–8. https://doi.org/10.1111/j.0963-7214.2006.00414.x.
Achenbach TM, Edelbrock CS. The classification of child psychopathology: a review and analysis of empirical efforts. Psychol Bull US Am Psychol Assoc. 1978. https://doi.org/10.1037/0033-2909.85.6.1275.
Achenbach TM, Edelbrock CS. Manual for the child behaviour checklist and revised child behaviour profile. Burlington: Department of P. Univerisity of Vermont Education; 1983.
Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles: an integrated system of mult-informant assessment. Burlington: University of Vermont, Research Center for Children, Youth & Families; 2001.
Agelink van Rentergem JA, Deserno MK, Geurts HM. Validation strategies for subtypes in psychiatry: a systematic review of research on autism spectrum disorder. Clin Psychol Rev. 2021;87: 102033. https://doi.org/10.1016/j.cpr.2021.102033.
Alexander LM, Escalera J, Ai L, Andreotti C, Febre K, Mangone A, Milham MP. An open resource for transdiagnostic research in pediatric mental health and learning disorders. BioRxiv. 2017. https://doi.org/10.1101/149369.
Alvares GA, Dawson PA, Dissanayake C, Eapen V, Gratten J, Grove R, Whitehouse AJO. Study protocol for the australian autism biobank: an international resource to advance autism discovery research. BMC Pediatr. 2018;18(1):284. https://doi.org/10.1186/s12887-018-1255-z.
Aman MG, Singh NN. Aberrant behavior checklist. East Aurora: Slosson; 1986.
Anney RJL, Hawi Z, Sheehan K, Mulligan A, Pinto C, Brookes KJ, Gill M. Parent of origin effects in attention/deficit hyperactivity disorder (ADHD): Analysis of data from the international multicenter ADHD genetics (IMAGE) program. Am J Med Genet B Neuropsychiatr Genet. 2008;147(8):1495–500. https://doi.org/10.1002/ajmg.b.30659.
Antshel KM, Zhang-James Y, Wagner KE, Ledesma A, Faraone SV. An update on the comorbidity of ADHD and ASD: a focus on clinical management. Expert Rev Neurother. 2016;16(3):279–93. https://doi.org/10.1586/14737175.2016.1146591.
Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Neale BM. Analysis of shared heritability in common disorders of the brain. Science. 2018. https://doi.org/10.1126/science.aap8757.
Arnett AB, Pennington BF, Friend A, Willcutt EG, Byrne B, Samuelsson S, Olson RK. The SWAN captures variance at the negative and positive ends of the ADHD symptom dimension. J Atten Disord. 2013;17(2):152–62. https://doi.org/10.1177/1087054711427399.
Aspan N, Bozsik C, Gadoros J, Nagy P, Inantsy-Pap J, Vida P, Halasz J. Emotion recognition pattern in adolescent boys with attention-deficit/hyperactivity disorder. Biomed Res Int. 2014;2014: 761340. https://doi.org/10.1155/2014/761340.
Asparouhov T, Muthén B. Exploratory structural equation modeling. Struct Equ Model. 2009. https://doi.org/10.1080/10705510903008204.
Association AP, Association AP. Diagnostic and statistical manual of mental disorders. 5th ed. Washington: American Psychiatric Publishing; 2013.
Auyeung B, Baron-Cohen S, Wheelwright S, Allison C. The autism spectrum quotient: children’s version (AQ-Child). J Autism Dev Disord. 2008;38(7):1230–40. https://doi.org/10.1007/s10803-007-0504-z.
Baddeley A. Working memory. Oxford: Oxford University Press; 1986.
Baixauli-Fortea I, Miranda Casas A, Berenguer-Forner C, Colomer-Diago C, Roselló-Miranda B. Pragmatic competence of children with autism spectrum disorder Impact of theory of mind, verbal working memory, ADHD symptoms, and structural language. Appl Neuropsychol Child. 2019;8(2):101–12. https://doi.org/10.1080/21622965.2017.1392861.
Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(3):65–94.
Barneveld PS, De Sonneville L, Van Rijn S, Van Engeland H, Swaab H. Impaired response inhibition in autism spectrum disorders, a marker of vulnerability to schizophrenia spectrum disorders? J Int Neuropsychol Soc. 2013;19(6):646–55. https://doi.org/10.1017/S1355617713000167.
Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psychiatry. 1997;38(7):813–22. https://doi.org/10.1111/j.1469-7610.1997.tb01599.x.
Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7.
Beck AT, Ward CH, Mendelson MM, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Begeer S, Bernstein DM, van Wijhe J, Scheeren AM, Koot HM. A continuous false belief task reveals egocentric biases in children and adolescents with autism spectrum disorders. Autism. 2012;16(4):357–66. https://doi.org/10.1177/1362361311434545.
Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444. https://doi.org/10.1302/0301-620X.51B3.444.
Bellani M, Moretti A, Perlini C, Brambilla P. Language disturbances in ADHD. Epidemiol Psychiatric Sci. 2011;20(4):311–5. https://doi.org/10.1017/S2045796011000527.
Bellgrove MA, Hawi Z, Gill M, Robertson IH. The cognitive genetics of attention deficit hyperactivity disorder (ADHD): sustained attention as a candidate phenotype. Cortex. 2006;42(6):838–45. https://doi.org/10.1016/S0010-9452(08)70426-X.
Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43(13):1847–57. https://doi.org/10.1016/j.neuropsychologia.2005.03.011.
Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–6. https://doi.org/10.1016/j.neuropsychologia.2004.05.007.
Bidwell LC, Willcutt EG, DeFries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biol Psychiat. 2007;62(9):991–8. https://doi.org/10.1016/j.biopsych.2007.04.003.
Biederman J, Spencer TJ, Newcorn JH, Gao H, Milton DR, Feldman PD, Witte MM. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology. 2007;190(1):31–41. https://doi.org/10.1007/s00213-006-0565-2.
Bishop D. Child communication checklist - second edition (second). Sydney: Pearson Clinical; 2003.
Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15(2):59–71. https://doi.org/10.1007/s11065-005-6252-y.
Boedhoe PSW, van Rooij D, Hoogman M, Twisk JWR, Schmaal L, Abe Y, van den Heuvel OA. Subcortical brain volume, regional cortical thickness, and cortical surface area across cisorders: findings from the ENIGMA ADHD, ASD, and OCD working groups. Am J Psychiatry. 2020;177(9):834–43. https://doi.org/10.1176/appi.ajp.2020.19030331.
Bora E, Pantelis C. Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): comparison with healthy controls and autistic spectrum disorder. Psychol Med. 2016;46(4):699–716. https://doi.org/10.1017/S0033291715002573.
Borsboom D, Rhemtulla M, Cramer AOJ, Van Der Maas HLJ, Scheffer M. Kinds versus continua: a review of psychometric approaches to uncover the structure of psychiatric constructs. Psychol Med. 2016;2016:1567–79. https://doi.org/10.1017/S0033291715001944.
Broadbent J, Gali I, Stokes MA. Validation of autism spectrum quotient adult version in an Australian sample. Autism Research and Treatment. 2013. Retrieved from https://link.gale.com/apps/doc/A369914176/AONE?u=monash&sid=AONE&xid=0e5c5b53.
Broche-Pérez Y, Herrera Jiménez LF, Omar-Martínez E. Neural substrates of decision-making. Neurologia. 2016;31(5):319–25. https://doi.org/10.1016/j.nrleng.2015.03.009.
Brunsdon VEA, Happé F. Exploring the “fractionation” of autism at the cognitive level. Autism. 2014;18(1):17–30. https://doi.org/10.1177/1362361313499456.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22. https://doi.org/10.1016/S1364-6613(00)01483-2.
Byrne EM, Zhu Z, Qi T, Skene NG, Bryois J, Pardinas AF, Wray NR. Conditional GWAS analysis to identify disorder-specific SNPs for psychiatric disorders. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-020-0705-9.
Bzdok D, Altman N, Krzywinski M. Statistics versus machine learning. Nat Methods. 2018;15(4):233–4. https://doi.org/10.1038/nmeth.4642.
Cambridge Cognition. Cambridge gambling task (CGT). Retrieved from http://www.cambridgecognition.com/cantab/cognitive-tests/executive-function/cambridge-gambling-task-cgt/ (2018a).
Cambridge Cognition. Cambridge neuropsychological test automated battery [CANTAB]. In: Cognitive assessment software; 2018b.
Cambridge Cognition. Spatial Working Memory (SWM); 2018c.
Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(3):374–83. https://doi.org/10.1097/00004583-199703000-00016.
Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Moffitt TE. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2(2):119–37. https://doi.org/10.1177/2167702613497473.
Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57(11):1416–23. https://doi.org/10.1016/j.biopsych.2004.12.005.
Cervantes-Henríquez ML, Acosta-López JE, Martinez AF, Arcos-Burgos M, Puentes-Rozo PJ, Vélez JI. Machine learning prediction of ADHD severity: association and linkage to ADGRL3, DRD4, and SNAP25. J Atten Disord. 2021. https://doi.org/10.1177/10870547211015426.
Chamberlain SR, Robbins TW, Winder-Rhodes S, Mller U, Sahakian BJ, Blackwell AD, Barnett JH. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biol Psychiat. 2011;69(12):1192–203. https://doi.org/10.1016/j.biopsych.2010.08.019.
Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33(5):631–46. https://doi.org/10.1016/j.neubiorev.2008.08.016.
Chien YL, Chou MC, Chiu YN, Chou WJ, Wu YY, Tsai WC, Gau SSF. ADHD-related symptoms and attention profiles in the unaffected siblings of probands with autism spectrum disorder: focus on the subtypes of autism and asperger’s disorder. Mol Autism. 2017. https://doi.org/10.1186/s13229-017-0153-9.
Chien YL, Gau SS-F, Shang C-Y, Chiu Y-N, Tsai W-C, Wu Y-Y. Visual memory and sustained attention impairment in youths with autism spectrum disorders. Psychol Med. 2015;45(11):2263–73. https://doi.org/10.1017/S0033291714003201.
Choi SW, O’Reilly PF. PRSice-2: polygenic risk score software for biobank-scale data. GigaScience. 2019;8(7):1–6. https://doi.org/10.1093/gigascience/giz082.
Clark LA, Watson D. Constructing validity: new developments in creating objective measuring instruments. Psychol Assess. 2019;31(12):1412–27. https://doi.org/10.1037/pas0000626.
Clarke P. When can group level clustering be ignored? Multilevel models versus single-level models with sparse data. J Epidemiol Commun Health. 2008;62(8):752–8. https://doi.org/10.1136/jech.2007.060798.
Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Tamminga CA. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–84. https://doi.org/10.1176/appi.ajp.2015.14091200.
Coghill D, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol Med. 2014;44(9):1989–2001. https://doi.org/10.1017/S0033291713002547.
Coghill D, Sonuga-Barke EJS. Annual research review: categories versus dimensions in the classification and conceptualisation of child and adolescent mental disorders—implications of recent empirical study. J Child Psychol Psychiatry. 2012;53(5):469–89. https://doi.org/10.1111/j.1469-7610.2011.02511.x.
Conners CK, Erhardt D, Sparrow E. Conners adult ADHD rating scales (CAARS). Toronto: Pearson; 1999.
Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257–68. https://doi.org/10.1023/A:1022602400621.
Conners CK. Conners’ rating scales—revised: user’s manual. multi-health systems, incorporated; 1997.
Constantino JN. Social responsiveness scale, second edition (SRS-2). Torrence: Western Psychological Services; 2011.
Conway C, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, Eaton NR. A hierarchical taxonomy of psychopathology can transform mental health research. Perspect Psychol Sci. 2019;14(3):419–36. https://doi.org/10.1177/1745691618810696.
Conway C, Simms LJ. Maximizing the applied value of structural models of psychopathology: introduction to a special issue of personality and mental health. Personal Ment Health. 2020;14(1):3–8. https://doi.org/10.1002/pmh.1474.
Cools R. Neuropsychopharmacology of cognitive flexibility. Brain mapping: an encyclopedic reference, vol. 3. Amsterdam: Elsevier Inc; 2015. https://doi.org/10.1016/B978-0-12-397025-1.00253-0.
Cooper M, Martin J, Langley K, Hamshere M, Thapar A. Autistic traits in children with ADHD index clinical and cognitive problems. Eur Child Adolesc Psychiatry. 2014;23(1):23–34. https://doi.org/10.1007/s00787-013-0398-6.
Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166(2–3):210–22. https://doi.org/10.1016/j.psychres.2008.02.005.
Coret MC, McCrimmon AW. Test review: Wiig, E. H., Semel, E., & Secord, W. A. (2013). Clinical evaluation of language fundamentals–fifth edition (CELF-5). J Psychoeduc Assess. 2013;33(5):495–500. https://doi.org/10.1177/0734282914557616.
Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Evaluat. 2005. https://doi.org/10.7275/jyj1-4868.
Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55(98):343–61. https://doi.org/10.1016/S0301-0082(98)00011-2.
Craig F, Margari F, Legrottaglie AR, Palumbi R, de Giambattista C, Margari L. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2016;12:1191–202. https://doi.org/10.2147/NDT.S104620.
Cupertino RB, Soheili-Nezhad S, Grevet EH, Bandeira CE, Picon FA, Tavares MEA, Sprooten E. Reduced fronto-striatal volume in attention-deficit/hyperactivity disorder in two cohorts across the lifespan. NeuroImage Clin. 2020;28:102403. https://doi.org/10.1016/j.nicl.2020.102403.
Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. https://doi.org/10.1002/wps.20087.
Dajani DR, Llabre MM, Nebel MB, Mostofsky SH, Uddin LQ. Heterogeneity of executive functions among comorbid neurodevelopmental disorders. Sci Rep. 2016;6:1–10. https://doi.org/10.1038/srep36566.
Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verbal Learn Verbal Behav. 1980;19(4):450–66.
Das Neves MDCL, Tremeau F, Nicolato R, Lauar H, Aurelio M, Romano-Silva MA, Correa H. Facial emotion recognition deficits in relatives of children with autism are not associated with 5HTTLPR. Revista Brasileira de Psiquiatria. 2011;33(3), 261–267.
DeFilippis M. Depression in children and adolescents with autism spectrum disorder. Children. 2018;5(9):112. https://doi.org/10.3390/children5090112.
Dekkers TJ, Popma A, Agelink van Rentergem JA, Bexkens A, Huizenga HM. Risky decision making in attention-deficit/hyperactivity disorder: a meta-regression analysis. Clin Psychol Rev. 2016;45:1–16. https://doi.org/10.1016/j.cpr.2016.03.001.
Delosis. Psytools. London; 2018.
Demetriou EA, DeMayo MM, Guastella AJ. Executive function in autism spectrum disorder: history, theoretical models, empirical findings, and potential as an endophenotype. Front Psychol. 2019;10(November):1–17. https://doi.org/10.3389/fpsyt.2019.00753.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Neale BM. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. https://doi.org/10.1038/s41588-018-0269-7.
Demopoulos C, Hopkins J, Davis A. A comparison of social cognitive profiles in children with autism spectrum disorders and attention-deficit/hyperactivity disorder: a matter of quantitative but not qualitative difference? J Autism Dev Disord. 2013;43(5):1157–70. https://doi.org/10.1007/s10803-012-1657-y.
Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J Child Psychol Psychiatry. 2011;52(11):1164–73. https://doi.org/10.1111/j.1469-7610.2010.02374.x.
Di X, Azeez A, Li X, Haque E, Biswal BB. Disrupted focal white matter integrity in autism spectrum disorder: a voxel-based meta-analysis of diffusion tensor imaging studies. Progress Neuro-Psychopharmacol Biol Psychiatry. 2018;82:242–8. https://doi.org/10.1016/j.pnpbp.2017.11.007.
Dowson JH, McLean A, Bazanis E, Toone B, Young S, Robbins TW, Sahakian BJ. Impaired spatial working memory in adults with attention-deficit/ hyperactivity disorder: comparisons with performance in adults with borderline personality disorder and in control subjects. Acta Psychiatr Scand. 2004;110(1):45–54. https://doi.org/10.1111/j.1600-0447.2004.00292.x.
Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39(1):25–7. https://doi.org/10.1038/ng1933.
Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiat. 2003;53(10):871–8. https://doi.org/10.1016/S0006-3223(02)01904-2.
D’Agati E, Curatolo P, Mazzone L. Comorbidity between ADHD and anxiety disorders across the lifespan. Int J Psychiatry Clin Pract. 2019;23(4):238–44. https://doi.org/10.1080/13651501.2019.1628277.
D’Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology. 2013;27(2):152–60. https://doi.org/10.1037/a0031721.
Eid M, Geiser C, Koch T. Measuring method effects: from traditional to design-oriented approaches. Curr Dir Psychol Sci. 2016;25(4):275–80. https://doi.org/10.1177/0963721416649624.
Enders CK. Applied missing data analysis. New York: The Guilford Press; 2010.
Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Bolla K. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160(6):1061–70. https://doi.org/10.1176/appi.ajp.160.6.1061.
Euesden J, Lewis CM, O’Reilly PF. PRSice: polygenic risk score software. Bioinformatics. 2015;31(9):1466–8. https://doi.org/10.1093/bioinformatics/btu848.
Evans DW, Uljarević M, Lusk LG, Loth E, Frazier T. Development of two dimensional measures of restricted and repetitive behavior in parents and children. J Am Acad Child Adolescent Psychiatry. 2017;56(1):51–8. https://doi.org/10.1016/j.jaac.2016.10.014.
Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36(9):774–88.
van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36(4):1093–106. https://doi.org/10.1016/j.neubiorev.2012.01.003.
Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci USA. 2012;109(17):6769–74. https://doi.org/10.1073/pnas.1115365109.
Faja S, Murias M, Beauchaine TP, Dawson G. Reward-based decision making and electrodermal responding by young children with autism spectrum disorders during a gambling task. Autism Res. 2013;6(6):494–505. https://doi.org/10.1002/aur.1307.
Falck-Ytter T, Pettersson E, Bölte S, D’Onofrio B, Lichtenstein P, Kennedy DP. Difficulties maintaining prolonged fixation and attention-deficit/hyperactivity symptoms share genetic influences in childhood. Psychiatry Res. 2020;293(August):4–6. https://doi.org/10.1016/j.psychres.2020.113384.
Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Franke B. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015. https://doi.org/10.1038/nrdp.2015.20.
Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562–75. https://doi.org/10.1038/s41380-018-0070-0.
Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–23. https://doi.org/10.1016/j.biopsych.2004.11.024.
Feczko E, Fair DA. Methods and challenges for assessing heterogeneity. Biol Psychiat. 2020;88(1):9–17. https://doi.org/10.1016/j.biopsych.2020.02.015.
Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, Fair DA. The heterogeneity problem: approaches to identify psychiatric subtypes. Trends Cogn Sci. 2019;23(7):584–601. https://doi.org/10.1016/j.tics.2019.03.009.
Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJMJ, Gillan CM, Potenza MN. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014;19(1):69–89. https://doi.org/10.1017/S1092852913000801.
Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37(2):163–80. https://doi.org/10.1017/S0033291706008750.
Fortenbaugh FC, Degutis J, Esterman M. Recent theoretical, neural, and clinical advances in sustained attention research. Ann N Y Acad Sci. 2018;1396(1):70–91. https://doi.org/10.1111/nyas.13318.Recent.
Freeman NC, Gray KM, Taffe JR, Cornish KM. Development of a new attention rating scale for children with intellectual disability: the scale of attention in intellectual disability (SAID). Am J Intellect Dev Disabil. 2015;120(2):91–109. https://doi.org/10.1352/1944-7558-120.2.91.
Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, Hewitt JK. Stability and change in executive function abilities from late adolescence to early adulthood: a longitudinal twin study. Dev Psychol. 2016;52(2):326–40. https://doi.org/10.1037/dev0000075.
Gargaro BA, Rinehart NJ, Bradshaw JL, Tonge BJ, Sheppard DM. Autism and ADHD: how far have we come in the comorbidity debate? Neurosci Biobehav Rev. 2011;35(5):1081–8. https://doi.org/10.1016/j.neubiorev.2010.11.002.
Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Buxbaum JD. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46(8):881–5. https://doi.org/10.1038/ng.3039.
Gerenser J. Language disorders in children with autism. Handbook of child language disorders. New York: Psychology Press; 2009.
Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention defcit hyperactivity disorder and autism ? J Child Psychol Psychiatry. 2004;45(4):836–54.
Ghirardi L, Brikell I, Kuja-Halkola R, Freitag CM, Franke B, Asherson P, Larsson H. The familial co-aggregation of ASD and ADHD: a register-based cohort study. Mol Psychiatry. 2018;23(2):257–62. https://doi.org/10.1038/mp.2017.17.
Ghirardi L, Pettersson E, Taylor MJ, Freitag CM, Franke B, Asherson P, Kuja-Halkola R. Genetic and environmental contribution to the overlap between ADHD and ASD trait dimensions in young adults: a twin study. Psychol Med. 2019;49(10):1713–21. https://doi.org/10.1017/S003329171800243X.
Gleitman H, Fridlund A, Reisberg D. Memory. In: Psychology. New York: W.W. Norton; 1999. p. 260–99.
Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska directed emotional faces: a validation study. Cogn Emot. 2008;22(6):1094–118. https://doi.org/10.1080/02699930701626582.
Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, Landa RJ. Subtle executive impairment in children with autism and children with ADHD. J Autism Dev Disord. 2005;35(3):279–93. https://doi.org/10.1007/s10803-005-3291-4.
Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–6.
Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry Allied Disciplines. 2000;41(5):645–55.
Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–76. https://doi.org/10.1146/annurev.psych.58.110405.085530.
Green JL, Rinehart N, Anderson V, Nicholson JM, Jongeling B, Sciberras E. Autism spectrum disorder symptoms in children with ADHD: a community-based study. Res Dev Disabil. 2015;47:175–84. https://doi.org/10.1016/j.ridd.2015.09.016.
Groen Y, Gaastra GF, Lewis-Evans B, Tucha O. Risky behavior in gambling tasks in individuals with ADHD—a systematic literature review. PLoS ONE. 2013;8(9): e74909. https://doi.org/10.1371/journal.pone.0074909.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, Børglum AD. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431–44. https://doi.org/10.1038/s41588-019-0344-8.
Grzadzinski R, Dick C, Lord C, Bishop S. Parent-reported and clinician-observed autism spectrum disorder (ASD) symptoms in children with attention deficit/hyperactivity disorder (ADHD): implications for practice under DSM-5. Molecular Autism. 2016;7:7. https://doi.org/10.1186/s13229-016-0072-1.
Harms MB, Martin A, Wallace GL. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev. 2010;20(3):290–322. https://doi.org/10.1007/s11065-010-9138-6.
Hawi Z, Cummins TDR, Tong J, Arcos-Burgos M, Zhao Q, Matthews N, Bellgrove MA. Rare DNA variants in the brain-derived neurotrophic factor gene increase risk for attention-deficit hyperactivity disorder: a next-generation sequencing study. Mol Psychiatry. 2017;22(4):580–4. https://doi.org/10.1038/mp.2016.117.
Hawi Z, Cummins TDR, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry. 2015;20(3):289–97. https://doi.org/10.1038/mp.2014.183.
Helzer JE, Kraemer HC, Krueger RF. The feasibility and need for dimensional psychiatric diagnoses. Psychol Med. 2006;36(12):1671–80. https://doi.org/10.1017/S003329170600821X.
Hoekstra RA, Bartels M, Verweij CJH, Boomsma DI. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 2007;161(4):372–7. https://doi.org/10.1001/archpedi.161.4.372.
Holmes J, Bryant A, Gathercole SE. Protocol for a transdiagnostic study of children with problems of attention, learning and memory (CALM). BMC Pediatr. 2019;19(1):1–11. https://doi.org/10.1186/s12887-018-1385-3.
Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, Franke B. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4(4):310–9. https://doi.org/10.1016/S2215-0366(17)30049-4.
Hoogman M, Bralten J, Mennes M, Zwiers M, Van Hulzen K, Schweren L, Franke B. Subcortical volumes across the life span in ADHD: an ENIGMA collaboration. Eur Neuropsychopharmacol. 2015;25:S189–S189.
Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, Franke B. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. 2019;176(7):531–42. https://doi.org/10.1176/appi.ajp.2019.18091033.
Hoogman M, van Rooij D, Klein M, Boedhoe P, Ilioska I, Li T, Franke B. Consortium neuroscience of attention deficit/hyperactivity disorder and autism spectrum disorder: the ENIGMA adventure. Hum Brain Mapp. 2020. https://doi.org/10.1002/hbm.25029.
Howland RH. Potential adverse effects of discontinuing psychotropic drugs part 3: antipsychotic, dopaminergic, and mood-stabilizing drugs. J Psychosocial Nurs Mental Health Services. 2010;48(11):11–4. https://doi.org/10.3928/02793695-20100708-01.
Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. Int J Methods Psychiatr Res. 2007;16(S1):S16–23. https://doi.org/10.1002/mpr.217.
Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43(3):302–13. https://doi.org/10.1111/j.1469-8986.2006.00403.x.
Insel T. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171(4):395–7. https://doi.org/10.1176/appi.ajp.2014.14020138.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine D, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010. https://doi.org/10.1176/appi.ajp.2010.09091379.
Iossifov I, Ronemus M, Levy DL, Wang Z, Hakker I, Rosenbaum J, Wigler M. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74(2):285–99. https://doi.org/10.1016/j.neuron.2012.04.009.De.
Isaksson J, Tammimies K, Neufeld J, Cauvet É, Lundin K, Buitelaar JK, Zwiers MP. EU-AIMS Longitudinal European Autism Project (LEAP): The autism twin cohort. Mol Autism. 2018. https://doi.org/10.1186/s13229-018-0212-x.
Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. NeuroReport. 2002;13(18):2453–7. https://doi.org/10.1097/00001756-200212200-00016.
Jang J, Matson JL, Williams LW, Tureck K, Goldin RL, Cervantes PE. Rates of comorbid symptoms in children with ASD, ADHD, and comorbid ASD and ADHD. Res Dev Disabil. 2013;34(8):2369–78. https://doi.org/10.1016/j.ridd.2013.04.021.
Johansen EB, Aase H, Meyer A, Sagvolden T. Attention-deficit/hyperactivity disorder (ADHD) behaviour explained by dysfunctioning reinforcement and extinction processes. Behav Brain Res. 2002;130(1–2):37–45. https://doi.org/10.1016/S0166-4328(01)00434-X.
Johnson KA, Kelly SP, Robertson IH, Barry E, Mulligan A, Daly M, Bellgrove MA. Absence of the 7-repeat variant of the DRD4 VNTR is associated with drifting sustained attention in children with ADHD but not in controls. Am J Med Genet B Neuropsychiatr Genet. 2008;147(6):927–37. https://doi.org/10.1002/ajmg.b.30718.
Johnson BP, Lum JAG, Rinehart NJ, Fielding J. Ocular motor disturbances in autism spectrum disorders: systematic review and comprehensive meta-analysis. Neurosci Biobehav Rev. 2016;69:260–79. https://doi.org/10.1016/j.neubiorev.2016.08.007.
Johnson KA, Robertson IH, Barry E, Mulligan A, Dáibhis A, Daly M, Bellgrove MA. Impaired conflict resolution and alerting in children with ADHD: evidence from the attention network task (ANT). J Child Psychol Psychiatry. 2008;49(12):1339–47. https://doi.org/10.1111/j.1469-7610.2008.01936.x.
Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Dáibhis A, Bellgrove MA. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45(10):2234–45. https://doi.org/10.1016/j.neuropsychologia.2007.02.019.
Johnston K, Madden AK, Bramham J, Russell AJ. Response inhibition in adults with autism spectrum disorder compared to attention deficit/hyperactivity disorder. J Autism Dev Disord. 2011;41(7):903–12. https://doi.org/10.1007/s10803-010-1113-9.
Kercood S, Grskovic JA, Banda D, Begeske J. Working memory and autism: a review of literature. Res Autism Spectrum Disord. 2014;8(10):1316–32. https://doi.org/10.1016/j.rasd.2014.06.011.
Kim YS, Fombonne E, Koh YJ, Kim SJ, Cheon KA, Leventhal BL. A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. J Am Acad Child Adolesc Psychiatry. 2014;53(5):500–8. https://doi.org/10.1016/j.jaac.2013.12.021.
Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38(2):179–89. https://doi.org/10.1017/S0048577201981399.
Kohls G, Peltzer J, Schulte-Rüther M, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Konrad K. Atypical brain responses to reward cues in autism as revealed by event-related potentials. J Autism Dev Disord. 2011;41(11):1523–33. https://doi.org/10.1007/s10803-011-1177-1.
Kotov R, Krueger RF, Watson D, Cicero DC, Conway CC, Deyoung CG, Wright AGC. The hierarchical taxonomy of psychopathology (HiTOP): a quantitative nosology based on consensus of evidence. Annu Rev Clin Psychol. 2021;17:83–108. https://doi.org/10.1146/annurev-clinpsy-081219-093304.
Kotov R, Waszczuk MA, Krueger RF, Forbes MK, Watson D, Clark LA, Zimmerman M. The hierarchical taxonomy of psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126(4):454–77. https://doi.org/10.1037/abn0000258.
Kovacs M, Beck AT. An empirical-clinical approach toward a definition of childhood depression. Depression Childhood Diagnosis Treatment Conceptual Models. 1977;1–25.
Kraemer HC, Noda A, O’Hara R. Categorical versus dimensional approaches to diagnosis: methodological challenges. J Psychiatr Res. 2004;38(1):17–25. https://doi.org/10.1016/S0022-3956(03)00097-9.
Krueger RF, Bezdjian S. Enhancing research and treatment of mental disorders with dimensional concepts: toward DSM-V and ICD-11. World Psychiatry. 2009;8(1):3–6. https://doi.org/10.1002/j.2051-5545.2009.tb00197.x.
Krueger RF, Deyoung CG. The RDoC initiative and the structure of psychopathology. Psychophysiology. 2016;53(3):351–4. https://doi.org/10.1111/psyp.12551.
Krueger RF, Kotov R, Watson D, Forbes MK, Eaton NR, Ruggero CJ, Zimmermann J. Progress in achieving quantitative classification of psychopathology. World Psychiatry. 2018;17(3):282–93. https://doi.org/10.1002/wps.20566.
Kuijper SJM, Hartman CA, Bogaerds-Hazenberg STM, Hendriks P. Narrative production in children with autism spectrum disorder (ASD) and children with attention-deficit/hyperactivity disorder (ADHD): similarities and differences. J Abnorm Psychol. 2017;126(1):63–75. https://doi.org/10.1037/abn0000231.
Kuntsi J, Wood AC, Johnson KA, Andreou P, Arias-Vasquez A, Buitelaar JK, Asherson P. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry. 2010;67(11):1159–67.
Kushki A, Anagnostou E, Hammill C, Duez P, Brian J, Iaboni A, Lerch JP. Examining overlap and homogeneity in ASD, ADHD, and OCD: a data-driven, diagnosis-agnostic approach. Transl Psychiatry. 2019. https://doi.org/10.1038/s41398-019-0631-2.
Kvist AP, Nielsen HS, Simonsen M. The importance of children’s ADHD for parents’ relationship stability and labor supply. Soc Sci Med. 2013;88:30–8. https://doi.org/10.1016/j.socscimed.2013.04.001.
Larson MJ, South M, Krauskopf E, Clawson A, Crowley MJ. Feedback and reward processing in high-functioning autism. Psychiatry Res. 2011;187(1–2):198–203. https://doi.org/10.1016/j.psychres.2010.11.006.
Latzman RD, DeYoung CG, Afzali MH, Allen TA, Althoff RR, DeYoung CG, Zald DH. Using empirically-derived dimensional phenotypes to accelerate clinical neuroscience: the hierarchical taxonomy of psychopathology (HiTOP) framework. Neuropsychopharmacology. 2020;45(7):1083–5. https://doi.org/10.1038/s41386-020-0639-6.
Lau WKW, Leung MK, Lau BWM. Resting-state abnormalities in autism spectrum disorders: a meta-analysis. Sci Rep. 2019;9(1):1–8. https://doi.org/10.1038/s41598-019-40427-7.
Lencer R, Mills LJ, Alliey-Rodriguez N, Shafee R, Lee AM, Reilly JL, Bishop JR. Genome-wide association studies of smooth pursuit and antisaccade eye movements in psychotic disorders: findings from the B-SNIP study. Transl Psychiatry. 2017;7(10): e1249. https://doi.org/10.1038/tp.2017.210.
Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry. 1997;36(6):737–44. https://doi.org/10.1097/00004583-199706000-00009.
Lisdahl KM, Sher KJ, Conway KP, Gonzalez R, Feldstein Ewing SW, Nixon SJ, Heitzeg M. Adolescent brain cognitive development (ABCD) study: overview of substance use assessment methods. Dev Cogn Neurosci. 2018;32:80–96. https://doi.org/10.1016/j.dcn.2018.02.007.
Lopez BR, Lincoln A, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. J Autism Dev Disord. 2005;35(4):445–60.
Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, Veenstra-VanderWeele J. Autism spectrum disorder. Nat Rev Dis Primers. 2020. https://doi.org/10.1038/s41572-019-0138-4.
De Los Reyes A, Salas S, Menzer MM, Daruwala SE. Criterion validity of interpreting scores from multi-informant statistical interactions as measures of informant discrepancies in psychological assessments of children and adolescents. Psychol Assess. 2013;25(2):509–19. https://doi.org/10.1037/a0032081.
De Los Reyes A, Thomas SA, Goodman KL, Kundey SMA. Principles underlying the use of multiple informants’ reports. Annu Rev Clin Psychol. 2013;9(1):123–49. https://doi.org/10.1146/annurev-clinpsy-050212-185617.
Loth E, Charman T, Mason L, Tillmann J, Jones EJH, Wooldridge C, Buitelaar JK. The EU-AIMS Longitudinal European Autism Project (LEAP): Design and methodologies to identify and validate stratification biomarkers for autism spectrum disorders. Mol Autism. 2017;8(1):24. https://doi.org/10.1186/s13229-017-0146-8.
Lubke GH, Muthén B. Investigating population heterogeneity with factor mixture models. Psychol Methods. 2005;10(1):21–39. https://doi.org/10.1037/1082-989X.10.1.21.
Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev. 2010;34(5):744–54. https://doi.org/10.1016/j.neubiorev.2009.11.021.
MacDonald SWS, Nyberg L, Bäckman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29(8):474–80. https://doi.org/10.1016/j.tins.2006.06.011.
Marsh HW, Morin AJS, Parker PD, Kaur G. Exploratory structural equation modeling: an integration of the best features of exploratory and confirmatory factor analysis. Ann Rev Clin Psychol. 2014;10:85–110. https://doi.org/10.1146/annurev-clinpsy-032813-153700.
Martin AF, Jassi A, Cullen AE, Broadbent M, Downs J, Krebs G. Co-occurring obsessive–compulsive disorder and autism spectrum disorder in young people: prevalence, clinical characteristics and outcomes. Eur Child Adolesc Psychiatry. 2020;29(11):1603–11. https://doi.org/10.1007/s00787-020-01478-8.
Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(4):377–84. https://doi.org/10.1097/01.chi.0000153228.72591.73.
Masi L. ADHD and comorbid disorders in childhood psychiatric problems, medical problems, learning disorders and developmental coordination disorder. Clin Psychiatry. 2015;1(1):1–9. https://doi.org/10.21767/2471-9854.100005.
Mayes SD, Calhoun SL, Mayes RD, Molitoris S. Autism and ADHD: overlapping and discriminating symptoms. Res Autism Spectrum Disorders. 2012;6(1):277–85. https://doi.org/10.1016/j.rasd.2011.05.009.
McNeish DM. Modeling sparsely clustered data: design-based, model-based, and single-level methods. Psychol Methods. 2014;19(4):552–63. https://doi.org/10.1037/met0000024.
Michelini G, Palumbo IM, DeYoung CG, Latzman RD, Kotov R. Linking RDoC and HiTOP: a new interface for advancing psychiatric nosology and neuroscience. Clin Psychol Rev. 2021;86: 102025. https://doi.org/10.1016/j.cpr.2021.102025.
Miettunen J, Nordström T, Kaakinen M, Ahmed AO. Latent variable mixture modeling in psychiatric research: a review and application. Psychol Med. 2016;2016:457–67. https://doi.org/10.1017/S0033291715002305.
Miller HL, Ragozzino ME, Cook EH, Sweeney JA, Mosconi MW. Cognitive set shifting deficits and their relationship to repetitive behaviors in autism spectrum disorder. J Autism Dev Disord. 2015;45(3):805–15. https://doi.org/10.1007/s10803-014-2244-1.
Miller GA, Rockstroh B. Endophenotypes in psychopathology research: where do we stand? Annu Rev Clin Psychol. 2013;9:177–213. https://doi.org/10.1146/annurev-clinpsy-050212-185540.
Morandini HAE, Silk TJ, Griffiths K, Rao P, Hood S, Zepf FD. Meta-analysis of the neural correlates of vigilant attention in children and adolescents. Cortex. 2020;132:374–85. https://doi.org/10.1016/j.cortex.2020.08.008.
Morris SE, Cuthbert BN. Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14(1):29–37.
Mosconi MW, Sweeney JA. Sensorimotor dysfunctions as primary features of autism spectrum disorders. Sci China Life Sci. 2015;58(10):1016–23. https://doi.org/10.1007/s11427-015-4894-4.
Mostofsky SH, Lasker AG, Cutting LE, Denckla MB, Zee DS. Oculomotor abnormalities in attention deficit hyperactivity disorder: a preliminary study. Neurology. 2001;57(3):423–30. https://doi.org/10.1212/WNL.57.3.423.
Mowinckel AM, Pedersen ML, Eilertsen E, Biele G. A meta-analysis of decision-making and attention in adults with ADHD. J Atten Disord. 2015;19(5):355–67. https://doi.org/10.1177/1087054714558872.
Mulligan A, Anney RJL, O’Regan M, Chen W, Butler L, Fitzgerald M, Gill M. Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with donduct, oppositional defiant, language and motor disorders. J Autism Dev Disord. 2009;39(2):197–209. https://doi.org/10.1007/s10803-008-0621-3.
Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218–28. https://doi.org/10.1038/nrn1345.
National Institute of Mental Health. RDoC changes to the matrix (CMAT) workgroup update: proposed positive valence domain revisions; 2018
Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. J Abnorm Psychol. 2004;113(4):614–25. https://doi.org/10.1037/0021-843X.113.4.614.
Nigg JT, Karalunas SL, Feczko E, Fair DA. Toward a revised nosology for attention-deficit/hyperactivity disorder heterogeneity. Biol Psychiatry Cognit Neurosci Neuroimaging. 2020;5(8):726–37. https://doi.org/10.1016/j.bpsc.2020.02.005.
Noordhof A, Krueger RF, Ormel J, Oldehinkel AJ, Hartman CA. Integrating autism-related symptoms into the dimensional internalizing and externalizing model of psychopathology. The TRAILS Study. J Abnorm Child Psychol. 2015;43(3):577–87. https://doi.org/10.1007/s10802-014-9923-4.
Norbury CF, Gemmell T, Paul R. Pragmatics abilities in narrative production: a cross-disorder comparison. J Child Lang. 2014;41(3):485–510. https://doi.org/10.1017/S030500091300007X.
Oerlemans AM, Droste K, Van Steijn DJ, De Sonneville LMJ, Buitelaar JK, Rommelse NNJ. Co-segregation of social cognition, executive function and local processing style in children with ASD, their siblings and normal controls. J Autism Dev Disord. 2013;43(12):2764–78. https://doi.org/10.1007/s10803-013-1807-x.
Oerlemans AM, Hartman CA, De Bruijn YGE, Van Steijn DJ, Franke B, Buitelaar JK, Rommelse NNJ. Simplex and multiplex stratification in ASD and ADHD families: a promising approach for identifying overlapping and unique underpinnings of ASD and ADHD? J Autism Dev Disord. 2015;45(3):645–57. https://doi.org/10.1007/s10803-014-2220-9.
Oerlemans AM, van der Meer JMJ, van Steijn DJ, de Ruiter SW, de Bruijn YGE, de Sonneville LMJ, Rommelse NNJ. Recognition of facial emotion and affective prosody in children with ASD (+ADHD) and their unaffected siblings. Eur Child Adolesc Psychiatry. 2014;23(5):257–71. https://doi.org/10.1007/s00787-013-0446-2.
Oldham S, Arnatkeviciute A, Smith RE, Tiego J, Bellgrove MA, Fornito A. The efficacy of different preprocessing steps in reducing motion-related confounds in diffusion MRI connectomics. Neuroimage. 2020;222:117252. https://doi.org/10.1016/j.neuroimage.2020.117252.
Open Brain Consent. Make open data sharing a no-brainer for ethics committees; 2020.
van Ouden HEM, Daw ND, Fernandez G, Elshout JA, Rijpkema M, Hoogman M, Cools R. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090–100. https://doi.org/10.1016/j.neuron.2013.08.030.
O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 1989;485(7397):246–50. https://doi.org/10.1038/nature10989.
Parkes L, Fulcher B, Yücel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;171:415–36. https://doi.org/10.1016/j.neuroimage.2017.12.073.
Patrick CJ, Hajcak G. RDoC: translating promise into progress. Psychophysiology. 2016;53(3):415–24. https://doi.org/10.1111/psyp.12612.
Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. J Abnorm Psychol. 2013;122(3):902–16. https://doi.org/10.1037/a0032807.
Peirce JW. PsychoPy—psychophysics software in Python. J Neurosci Methods. 2007;162(2):8–13.
Perkins ER, Latzman RD, Patrick CJ. Interfacing neural constructs with the hierarchical taxonomy of psychopathology: ‘why’ and ‘how.’ Personal Ment Health. 2020;14:106–22. https://doi.org/10.1002/pmh.1460.
Peterson CC, Garnett M, Kelly A, Attwood T, Pineda-Alhucema W, Aristizabal E, Vélez JI. Everyday social and conversation applications of theory-of-mind understanding by children with autism-spectrum disorders or typical development. Eur Child Adolesc Psychiatry. 2009;28(2):105–15. https://doi.org/10.1007/s11065-018-9381-9.
Pineda-Alhucema W, Aristizabal E, Escudero-Cabarcas J, Acosta-López JE, Vélez JI. Executive function and theory of mind in children with ADHD: a systematic review. Neuropsychol Rev. 2018;28(3):341–58. https://doi.org/10.1007/s11065-018-9381-9.
van der Plas E, Dupuis A, Arnold P, Crosbie J, Schachar R. Association of sutism spectrum disorder with obsessive-compulsive and attention-deficit/hyperactivity traits and response inhibition in a community sample. J Autism Dev Disord. 2016;46(9):3115–25. https://doi.org/10.1007/s10803-016-2853-y.
Podsakoff PM, MacKenzie SB, Podsakoff NP. Sources of method bias in social science research and recommendations on how to control it. Annu Rev Psychol. 2012;63(1):539–69. https://doi.org/10.1146/annurev-psych-120710-100452.
Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–42. https://doi.org/10.1093/ije/dyt261.
Quay HC. Inhibition and attention deficit hyperactivity disorder. J Abnorm Child Psychol. 1997;25(1):7–13. https://doi.org/10.1023/A:1025799122529.
Rahko JS, Vuontela VA, Carlson S, Nikkinen J, Hurtig TM, Kuusikko-Gauffin S, Kiviniemi VJ. Attention and working memory in adolescents with autism spectrum disorder: a functional MRI study. Child Psychiatry Hum Dev. 2016;47(3):503–17. https://doi.org/10.1007/s10578-015-0583-6.
Rashid B, Calhoun V. Towards a brain-based predictome of mental illness. Hum Brain Mapp. 2020;41(12):3468–535. https://doi.org/10.1002/hbm.25013.
Reckase MD. Multidimensional item response theory. New York: Springer; 2009.
Reiersen AM, Todd RD. Co-occurrence of ADHD and autism spectrum disorders: phenomenology and treatment. Expert Rev Neurotherapeutics. 2008;8(4):657–69.
Rhodes SM, Coghill D, Matthews K. Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology. 2004;175(3):319–30. https://doi.org/10.1007/s00213-004-1833-7.
Rhodes SM, Coghill D, Matthews K. Neuropsychological functioning in stimulant-naive boys with hyperkinetic disorder. Psychol Med. 2005;35(8):1109–20. https://doi.org/10.1017/S0033291705004599.
Rietz EDu, Pettersson E, Brikell I, Ghirardi L, Chen Q, Hartman C, Kuja-Halkola R. ADHD is more closely linked to neurodevelopmental than externalizing and internalizing disorders: a genetically informed multivariate Swedish population study. MedRxiv. 2020. https://doi.org/10.1101/2020.02.26.20028175.
Riley AW, Forrest CB, Starfield B, Rebok GW, Robertson JA, Green BF. The parent report form of the CHIP—child edition. Reliabil Valid. 2004;42(3):210–20. https://doi.org/10.1097/01.mlr.0000114909.33878.ca.
Roak BJO, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Eichler EE. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43(6):585–9. https://doi.org/10.1038/ng.835.Exome.
Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA. 2009;106(50):21437–41.
Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55(6):594–602. https://doi.org/10.1016/j.biopsych.2003.11.012.
Rommelse NNJ, Altink ME, Oosterlaan J, Buschgens CJM, Buitelaar J, Sergeant JA. Support for an independent familial segregation of executive and intelligence endophenotypes in ADHD families. Psychol Med. 2008;38(11):1595–606. https://doi.org/10.1017/S0033291708002869.
Rommelse NNJ, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19(3):281–95. https://doi.org/10.1007/s00787-010-0092-x.
Van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, Buitelaar JK. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. Am J Psychiatry. 2018;175(4):359–69. https://doi.org/10.1176/appi.ajp.2017.17010100.
Rovira P, Demontis D, Sánchez-Mora C, Zayats T, Klein M, Mota NR, Ribasés M. Shared genetic background between children and adults with attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2020;45(10):1617–26. https://doi.org/10.1038/s41386-020-0664-5.
Sabaroedin K, Tiego J, Parkes L, Sforazzini F, Finlay A, Johnson B, Fornito A. Functional connectivity of corticostriatal circuitry and psychosis-like experiences in the general community. Biol Psychiatry. 2019;86(1):16–24. https://doi.org/10.1016/j.biopsych.2019.02.013.
Saccenti E, Hendriks MHWB, Smilde AK. Corruption of the Pearson correlation coefficient by measurement error and its estimation, bias, and correction under different error models. Sci Rep. 2020;10(1):1–19. https://doi.org/10.1038/s41598-019-57247-4.
Sanchez-Roige S, Palmer AA. Emerging phenotyping strategies will advance our understanding of psychiatric genetics. Nat Neurosci. 2020. https://doi.org/10.1038/s41593-020-0609-7.
Sanders S, Murtha M, Gupta A, Murdock JD, Raubeson MJ, Willsey AJ, State MW. De novo mutations revealed by whole exome sequencing are strongly associated with autism. Nature. 2012;485(7397):237–41. https://doi.org/10.1038/nature10945.De.
Satterfield JH, Cantwell DP, Satterfield BT. Pathophysiology of the hyperactive child syndrome. Arch Gen Psychiatry. 1974;31(6):839–44. https://doi.org/10.1001/archpsyc.1974.01760180079010.
Satterstrom FK, Walters RK, Singh T, Wigdor EM, Lescai F, Demontis D, Daly MJ. Autism spectrum disorder and attention deficit hyperactivity disorder have a similar burden of rare protein-truncating variants. Nat Neurosci. 2019;22(12):1961–5. https://doi.org/10.1038/s41593-019-0527-8.
Schachar RJ, Crosbie J, Barr CL, Ornstein TJ, Kennedy J, Malone M, Pathare T. Inhibition of motor responses in siblings concordant and discordant for attention deficit hyperactivity disorder. Am J Psychiatry. 2005;162(6):1076–82. https://doi.org/10.1176/appi.ajp.162.6.1076.
Scheeren AM, de Rosnay M, Koot HM, Begeer S. Rethinking theory of mind in high-functioning autism spectrum disorder. J Child Psychol Psychiatry. 2013;54(6):628–35. https://doi.org/10.1111/jcpp.12007.
Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):720–4. https://doi.org/10.1016/j.biopsych.2006.04.042.
Schmitt LM, Bojanek E, White SP, Ragozzino ME, Cook EH, Sweeney JA, Mosconi MW. Familiality of behavioral flexibility and response inhibition deficits in autism spectrum disorder (ASD). Mol Autism. 2019;10(1):47. https://doi.org/10.1186/s13229-019-0296-y.
Schmitz N, Rubia K, Van Amelsvoort T, Daly E, Smith A, Murphy DGM. Neural correlates of reward in autism. Br J Psychiatry. 2008;192(1):19–24. https://doi.org/10.1192/bjp.bp.107.036921.
Schoechlin C, Engel RR. Neuropsychological performance in adult attention-deficit hyperactivity disorder: meta-analysis of empirical data. Arch Clin Neuropsychol. 2005;20(6):727–44. https://doi.org/10.1016/j.acn.2005.04.005.
Schuh JM, Eigsti IM. Working memory, language skills, and autism symptomatology. Behav Sci. 2012;2(4):207–18. https://doi.org/10.3390/bs2040207.
Sciberras E, Mueller KL, Efron D, Bisset M, Anderson V, Schilpzand EJ, Nicholson JM. Language problems in children with ADHD: a community-based study. Pediatrics. 2014;133(5):793–800. https://doi.org/10.1542/peds.2013-3355.
Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals preschool-2 (CELF P2). Sydney: AU: Pearson Clinical; 2004.
Seng G, Tseng W, Chiu Y, Tsai W, Wu Y, Gau SS. Executive functions in youths with autism spectrum disorder and their unaffected siblings. Psychol Med. 2020. https://doi.org/10.1017/S0033291720001075.
Sengupta SM, Grizenko N, Fortier MÈ, Ter-Stepanian M, Joober R. Facing the methodological challenge in dissecting the genetics of ADHD: a case for deep phenotyping and heterogeneity reduction. J Can Acad Child Adolescent Psychiatry. 2020;29(3):188–201.
Sergeant J. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24:7–12.
Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton Mifflin; 2002.
Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Dev Neuropsychol. 2002;21(1):43–71. https://doi.org/10.1207/S15326942DN2101.
Slaats-Willemse D, Swaab-Barneveld H, De Sonneville LEO, Buitelaar JAN. Familial clustering of executive functioning in affected sibling pair families with ADHD. J Am Acad Child Adolescent Psychiatry. 2005;44(4):385–91. https://doi.org/10.1097/01.chi.0000153227.34473.c7.
van der Sluis S, Posthuma D, Nivard MG, Verhage M, Dolan CV. Power in GWAS: lifting the curse of the clinical cut-off. Mol Psychiatry. 2013;18(1):2–3. https://doi.org/10.1038/mp.2012.65.
van der Sluis S, Verhage M, Posthuma D, Dolan CV. Phenotypic complexity, measurement bias, and poor phenotypic resolution contribute to the missing heritability problem in genetic association studies. PLoS ONE. 2010. https://doi.org/10.1371/journal.pone.0013929.
Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the children’s depression inventory. J Abnorm Child Psychol. 1986;14(1):25–39. https://doi.org/10.1007/BF00917219.
De Sonneville LMJ. Amsterdam neuropsychological tasks: a computer-aided assessment program. Comput Psychol. 1999;6:187–203.
Sparrow SS, Cicchetti DV, Saulnier CA. Vineland adaptive behavior scales, third edition (Vineland-3) (Third). Sydney: Pearson; 2016.
Spence SH. A measure of anxiety symptoms among children. Behav Res Ther. 1998;36:545–66.
Sprenger L, Bühler E, Poustka L, Bach C, Heinzel-Gutenbrunner M, Kamp-Becker I, Bachmann C. Impact of ADHD symptoms on autism spectrum disorder symptom severity. Res Dev Disabil. 2013;34(10):3545–52. https://doi.org/10.1016/j.ridd.2013.07.028.
Stanton K, Delucia Elizabeth A, Brown Matthew FD, McDonnell C. Advancing understanding of the classification of broad autism phenotype and attention-deficit/hyperactivity disorder symptom dimensions within the hierarchical taxonomy of psychopathology. Personal Mental Health. 2020. https://doi.org/10.1002/pmh.
Stavropoulos KKM, Carver LJ. Reward anticipation and processing of social versus nonsocial stimuli in children with and without autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(12):1398–408. https://doi.org/10.1111/jcpp.12270.
Steele SD, Minshew NJ, Luna B, Sweeney JA. Spatial working memory deficits in autism. J Autism Dev Disord. 2007;37(4):605–12. https://doi.org/10.1007/s10803-006-0202-2.
van Steensel FJA, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev. 2011;14(3):302–17. https://doi.org/10.1007/s10567-011-0097-0.
Van Steijn DJ, Richards JS, Oerlemans AM, De Ruiter SW, Van Aken MAG, Franke B, Rommelse NNJ. The co-occurrence of autism spectrum disorder and attention-deficit/ hyperactivitydisorder symptoms in parents of children with ASD or ASD with ADHD. J Child Psychol Psychiatry. 2012;53(9):954–63. https://doi.org/10.1111/j.1469-7610.2012.02556.x.
Stergiakouli E, Davey Smith G, Martin J, Skuse DH, Viechtbauer W, Ring SM, St Pourcain B. Shared genetic influences between dimensional ASD and ADHD symptoms during child and adolescent development. Mol Autism. 2017;8(1):1–13. https://doi.org/10.1186/s13229-017-0131-2.
Stevens T, Peng L, Barnard-Brak L. The comorbidity of ADHD in children diagnosed with autism spectrum disorder. Res Autism Spectrum Disord. 2016;31:11–8. https://doi.org/10.1016/j.rasd.2016.07.003.
Stoltenberg C, Schjølberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, Susser E. The Autism Birth Cohort (ABC): a paradigm for gene-environment-timing research. Mol Psychiatry. 2011;15(7):676–80. https://doi.org/10.1038/mp.2009.143.THE.
Strange BC. Once-daily treatment of ADHD with guanfacine: patient implications. Neuropsychiatr Dis Treat. 2008;4(3):499–506. https://doi.org/10.2147/ndt.s1711.
Strømme P, Diseth TH. Prevalence of psychiatric diagnoses in children with mental retardation: data from a population-based study. Dev Med Child Neurol. 2000;42(4):266–70. https://doi.org/10.1017/S0012162200000451.
Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen G, O’Donovan MC. Psychiatric genomics: an update and an agenda. Am J Psychiatry. 2018;175(1):15–27. https://doi.org/10.1176/appi.ajp.2017.17030283.Psychiatric.
Sørensen L, Sonuga-Barke E, Eichele H, van Wageningen H, Wollschlaeger D, Plessen KJ. Suboptimal decision making by children with ADHD in the face of risk: poor risk adjustment and delay aversion rather than general proneness to taking risks. Neuropsychology. 2017;31(2):119–28. https://doi.org/10.1037/neu0000297.
Taurines R, Schwenck C, Westerwald E, Sachse M, Siniatchkin M, Freitag C. ADHD and autism: differential diagnosis or overlapping traits? A selective review. ADHD Attent Deficit Hyperact Disord. 2012;4(3):115–39. https://doi.org/10.1007/s12402-012-0086-2.
Totsika V, Hastings RP, Emerson E, Lancaster GA, Berridge DM. A population-based investigation of behavioural and emotional problems and maternal mental health: associations with autism spectrum disorder and intellectual disability. J Child Psychol Psychiatry. 2011;52(1):91–9. https://doi.org/10.1111/j.1469-7610.2010.02295.x.
Tye C, Johnson KA, Kelly SP, Asherson P, Kuntsi J, Ashwood KL, McLoughlin G. Response time variability under slow and fast-incentive conditions in children with ASD, ADHD and ASD+ADHD. J Child Psychol Psychiatry. 2016;57(12):1414–23. https://doi.org/10.1111/jcpp.12608.
Uddin LQ. Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder. Biol Psychiatry. 2020. https://doi.org/10.1016/j.biopsych.2020.05.010.
Uljarevic M, Hamilton A. Recognition of emotions in autism: a formal meta-analysis. J Autism Dev Disord. 2013;43(7):1517–26. https://doi.org/10.1007/s10803-012-1695-5.
Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cognit Sci. 2008;12(11):418–24. https://doi.org/10.1016/j.tics.2008.07.005.
Verbruggen F, Logan GD, Stevens MA. STOP-IT: windows executable software for the stop-signal paradigm. Behav Res Methods. 2008;40(2):479–83. https://doi.org/10.3758/brm.40.2.479.
Vogan VM, Francis KE, Morgan BR, Smith ML, Taylor MJ. Load matters: Neural correlates of verbal working memory in children with autism spectrum disorder. J Neurodev Disord. 2018;10(1):1–12. https://doi.org/10.1186/s11689-018-9236-y.
WHOQOL Group W. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment. Geneva: WHO; 1996.
Waddington F, Hartman C, de Bruijn Y, Lappenschaar M, Oerlemans A, Buitelaar J, Rommelse N. An emotion recognition subtyping approach to studying the heterogeneity and comorbidity of autism spectrum disorders and attention-deficit/hyperactivity disorder. J Neurodev Disord. 2018;10(1):31. https://doi.org/10.1186/s11689-018-9249-6.
Waddington F, Hartman C, de Bruijn Y, Lappenschaar M, Oerlemans A, Buitelaar J, Rommelse N. Visual and auditory emotion recognition problems as familial cross-disorder phenomenon in ASD and ADHD. Eur Neuropsychopharmacol. 2018;28(9):994–1005. https://doi.org/10.1016/j.euroneuro.2018.06.009.
Walton KM. Leisure time and family functioning in families living with autism spectrum disorder. Autism. 2019;23(6):1384–97. https://doi.org/10.1177/1362361318812434.
Wang E, Sun L, Sun M, Huang J, Tao Y, Zhao X, Song Y. Attentional selection and suppression in children with attention-deficit/hyperactivity disorder. Biol Psychiatry Cognit Neurosci Neuroimaging. 2016;1(4):372–80. https://doi.org/10.1016/j.bpsc.2016.01.004.
Wang X, Xu Q, Bey AL, Lee Y, Jiang YH. Transcriptional and functional complexity of Shank3 provides a molecular framework to understand the phenotypic heterogeneity of SHANK3 causing autism and Shank3 mutant mice. Molecular Autism. 2014;5(1):1–14. https://doi.org/10.1186/2040-2392-5-30.
Waszczuk MA, Eaton NR, Krueger RF, Shackman AJ, Waldman ID, Zald DH, Kotov R. Redefining phenotypes to advance psychiatric genetics: Implications from hierarchical taxonomy of psychopathology. J Abnorm Psychol. 2020;129(2):143–61. https://doi.org/10.1037/abn0000486.
Wechsler D. Wechsler adult intelligence scale—fourth edition Australian and New Zealand language adapted edition (Fourth). Sydney: Pearson; 2008.
Wechsler D. Wechsler Intelligence Scale for Children, Fifth Edition: Australian and New Zealand Standardised Edition (Fifth). Sydney: Pearson; 2016.
Wechsler D. Wechsler Abbreviated Scale of Intelligence - Second Edition (Second). Sydney: Pearson; 2011.
Wechsler D. Wechsler Preschool and Primary Scale of Intelligence–Fourth Edition (WPPSI-IV): Technical and interpretative manual. San Antonio, TX: The Psychological Corporation; 2012.
Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition (WAIS-IV) Australian and New Zealand Language Adapted Edition. Sydney, AU: Pearson; 2008.
Wiig EH, Secord WA, Semel E. CELF-5 screening test. Sydney: Pearson Clinical; 2013.
Wiig EH, Semel E, Secord WA. Clinical evaluation of language fundamentals australian and new zealand—fifth edition (CELF-5). Sydney: AU: Pearson Clinical; 2017.
Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–46. https://doi.org/10.1016/j.biopsych.2005.02.006.
Wodka EL, Mahone EM, Blankner JG, Larson JCG, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. J Clin Exp Neuropsychol. 2007;29(4):345–56. https://doi.org/10.1080/13803390600678046.
Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, Schachar R. Response inhibition and psychopathology: a meta-analysis of Go/No-Go task performance. J Abnorm Physiol. 2014;123(2):429–39. https://doi.org/10.1037/a0036295.
Xu MK, Gaysina D, Barnett JH, Scoriels L, Van De Lagemaat LN, Wong A, Jones PB. Psychometric precision in phenotype definition is a useful step in molecular genetic investigation of psychiatric disorders. Transl Psychiatry. 2015;5(6):1–8. https://doi.org/10.1038/tp.2015.86.
Yang J, Wray NR, Visscher PM. Comparing apples and oranges: equating the power of case-control and quantitative trait association studies. Genet Epidemiol. 2010;34(3):254–7. https://doi.org/10.1002/gepi.20456.
Yip BHK, Bai D, Mahjani B, Klei L, Pawitan Y, Hultman CM, Sandin S. Heritable variation, with little or no maternal effect, accounts for recurrence risk to autism spectrum disorder in Sweden. Biol Psychiat. 2018;83(7):589–97. https://doi.org/10.1016/j.biopsych.2017.09.007.
Zablotsky B, Bramlett MD, Blumberg SJ. The co-occurrence of autism spectrum disorder in children with ADHD. J Atten Disord. 2017. https://doi.org/10.1177/1087054717713638.
Zaboski BA, Storch EA. Comorbid autism spectrum disorder and anxiety disorders: a brief review. Future Neurol. 2018;13(1):31–7. https://doi.org/10.2217/fnl-2017-0030.
Zamboulis C, Reid JL. Withdrawal of Guanfacine after long-term treatment in essential hypertension. Eur J Clin Pharmacol. 1981;19:19–24.
Zhou ZW, Fang YT, Lan XQ, Sun L, Cao QJ, Wang YF, Zhang H. Inconsistency in abnormal functional connectivity across datasets of ADHD-200 in children with attention deficit hyperactivity disorder. Front Psychiatry. 2019;10:1–15. https://doi.org/10.3389/fpsyt.2019.00692.
Ziegler S, Pedersen ML, Mowinckel AM, Biele G. Modelling ADHD: a review of ADHD theories through their predictions for computational models of decision-making and reinforcement learning. Neurosci Biobehav Rev. 2016;71:633–56. https://doi.org/10.1016/j.neubiorev.2016.09.002.
Zimmerman IL, Steiner VG, Pond RE. Preschool language scales—fifth edition (PLS-5). 5th ed. Bloomington: Pearson; 2011.
Acknowledgements
A huge thank you to all the families who have participated so far.
Funding
The MAGNET Project is supported by a grant from the Federal Department of Health of Australia under Medical Research Future Fund (MRFF) Emerging Priorities and Consumer-Driven Research initiative. RK is supported by an Australian Government Research Training Program (RTP) Scholarship. B.P.J is supported by the Peter Doherty Early Career Fellowship (APP1112348) from the National Health and Medical Research Council (NHMRC) of Australia and the Medical Research Future Fund (EPCD000002) from the Department of Health Australia. M.A.B is supported by a Senior Research Fellowship (Level B) from the NHMRC of Australia.
Author information
Authors and Affiliations
Contributions
RK, BJ, JT, OM, AF, KK, MK, VPM, ZH, KF, KH, KW, AU, AF, KG, DC, AN, DP, EL, LM, DM, JB and MAB contributed to study design, developed data acquisition and/or analysis protocols. RK, BJ, OM, AF, KK, MK, VPM, DM, E-CM, and KF collected data. RK wrote the first and final draft. BJ, JT, OM, KK, VPM, AA, TC, KH, KW, and MAB contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Monash University Human Research Ethics Committee (CF16/1537-2016000806), Department of Education and Training Victoria Human Research Ethics Committee (2017_003570), and Monash Health Human Research Ethics Committee (RES-19-0000-372A). It will comply with the conditions of these ethics committee approvals, and the NHMRC National Statement on ethical Conduct in Human Research (2018).
Consent for publication
Consent for publication was obtained from all participants prior to the study.
Competing interests.
No competing interests to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. MAGNET Project study protocol summary.
Additional file 2
. Best clinical estimate protocol.
Additional file 3
. Protocol for testing non-verbal children and children with ID.
Additional file 4
. Clinical assessments protocol.
Additional file 5
. Research visit and neurocognitive testing protocol summary.
Additional file 6
. Oculomotor testing protocol.
Additional file 7
. Saliva collection protocol.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Knott, R., Johnson, B.P., Tiego, J. et al. The Monash Autism-ADHD genetics and neurodevelopment (MAGNET) project design and methodologies: a dimensional approach to understanding neurobiological and genetic aetiology. Molecular Autism 12, 55 (2021). https://doi.org/10.1186/s13229-021-00457-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13229-021-00457-3