Abstract
Background
The prevalence of dementia is expected to increase dramatically. Due to a lack of pharmacological treatment options for people with dementia, non-pharmacological treatments such as exercise programs have been recommended to improve cognition, activities of daily living, and neuropsychiatric symptoms. However, inconsistent results have been reported across different trials, mainly because of the high heterogeneity of exercise modalities. Thus, this systematic review aims to answer the questions whether exercise programs improve cognition, activities of daily living as well as neuropsychiatric symptoms in community-dwelling people with dementia.
Methods
Eight databases were searched for articles published between 2016 and 2021 (ALOIS, CENTRAL, CINAHL, Embase, MEDLINE, PsycINFO, PubMed, Web of Science). Randomized controlled trials evaluating the effects of any type of physical activity on cognition, activities of daily living, or neuropsychiatric symptoms in community-dwelling people with a formal diagnosis of dementia were included in this systematic review. Two authors independently assessed eligibility and quality of the studies. The methodology was in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
Results
Eight publications covering seven trials were included in this review with the majority investigating either a combination of strength and aerobic exercise or aerobic exercise alone. This review revealed that there is no clear evidence for the beneficial effects of exercise on cognition. None of the included trials found an impact on activities of daily living. Although different randomized controlled trials reported inconsistent results, one trial indicated that especially aerobic exercise may improve neuropsychiatric symptoms.
Conclusion
Our systematic review did not confirm the impact of exercise on cognition and activities of daily living in community-dwelling people with dementia. The results suggested that aerobic exercise might be effective to reduce neuropsychiatric symptoms. Well-designed trials including only community-dwelling people with a formal diagnosis of dementia, large samples, long-term follow-ups, and detailed description of adherence to the intervention are needed to improve the scientific evidence on the best type of exercise modality.
Trial registration
PROSPERO, CRD42021246598.
Similar content being viewed by others
Introduction
Improvements in health care in the past decades have contributed to an increase in life expectancy. Although dementia is not an inevitable part of normal aging, incidence increases with age. Currently, over 55 million people worldwide live with dementia, and prevalence of dementia is expected to increase dramatically as the population ages [1].
Due to the limited availability of pharmacological treatment, non-pharmacological interventions have been recommended as first-line approaches for over a decade to improve cognition, activities of daily living (ADLs), and neuropsychiatric symptoms (NPS) in people with dementia (pwd) [2, 3]. Among these, exercise has been recommended as an effective treatment for slowing down cognitive decline in pwd [4,5,6,7]. Furthermore, recent research partially shows physical activity to be a promising method to reduce NPS and improve ADLs [4, 5, 8]. However, these findings are not consistent, and conflicting results have been reported across different trials [9,10,11]. Although this is a field of high interest and many trials have been conducted, recent evidence seems controversial, as various systematic reviews reported conflicting results [6, 12,13,14]. As people with mild cognitive impairment (MCI), pwd living in a long-term care facility, and community-dwelling pwd have different needs and capabilities, it is necessary to distinguish between these groups, which has not been done in previous reviews. Thus, this systematic review aims to give a broad overview of the effects of exercise programs on cognition, ADLs, and NPS in community-dwelling people with a formal diagnosis of dementia. Moreover, frequency, intensity, duration, and setting of the interventions have been hardly discussed in previous reviews. Therefore, we aim to analyze how training modalities (type of training, frequency, duration, intensity, setting) influence the effects of exercise.
Methods
This systematic review was conducted and reported following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [15] and was registered at the national prospective register of systematic reviews (PROSPERO registration number: CRD42021246598).
Data sources and searches
PubMed, MEDLINE, Embase, ALOIS, Web of Science, PsychINFO, CINAHL, and CENTRAL were systematically searched using different terms for exercise and dementia. Full search strategy can be found in Additional file 2. Whenever possible, searches with additional filters such as randomized controlled trials as article type, English or German as language and publication date from 2016 until 2021, were conducted. Since a Cochrane Review on this topic has been conducted in 2015, we aim to focus on trials published after 2015.
Study selection
Randomized controlled trials (RCTs) over any length of time with the aim of improving cognition, ADLs, or NPS in pwd were eligible for this systematic review. As an intervention, we included RCTs providing any combination of resistance, endurance, or balance training. Multidomain interventions in which isolated effects of exercise cannot be measured (e.g., combination with cognitive training) had to be excluded. For control groups, usual care or social activities were included, while following regular exercise was used as exclusion criteria. Furthermore, we eliminated trials in which people with MCI or subjective cognitive impairment (SCI) or institutionalized pwd were involved. We considered people living in the community or assisted-living facilities at the time of intervention which is why acute hospitalized pwd or people living in long-term care facilities were excluded. Since most of the pwd living at home with community-dwelling people having different capabilities than institutionalized pwd, we excluded institutionalized pwd, where the disease is often more advanced [16, 17]. We had no restrictions regarding the type of dementia, as long as they had a formal medical diagnosis.
After merging search results and discarding duplicates, title, abstracts, and full texts were independently screened for inclusion by the first two authors (K.S. and A.K). In cases of disagreements, the last author was consulted for the final decision (P.K.-R.).
Data extraction and risk of bias assessment
Information from the articles was extracted by the first author, with the second author checking the collected data, which included study setting, publication year, country, way of recruitment, funding, inclusion and exclusion criteria, sample size, and drop-out rates.
Extracted data also covered participants’ baseline characteristics such as gender, age, diagnostic criteria, and diagnosis as well as Mini-Mental Status Examination (MMSE) score at baseline when available. Furthermore, a detailed description of the exercise modalities (e.g., type, frequency, duration, actual and planned intensity) and outcome data of the first follow-up was gathered. If a study combined multiple types of exercise, it was regarded as a multimodal intervention. Between-group differences in the mean changes from baseline to follow-up in domains of cognition, ADLs, and NPS were reported.
The quality of included studies was assessed by the first and the third authors using the “Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB2)” [18].
Results
Included studies
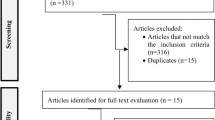
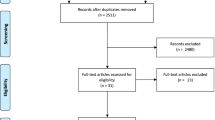
In total, 14,675 studies were identified through the search. After removing duplicates, 7651 records were screened of which 7551 were excluded as they covered irrelevant topics (Fig. 1). Of the remaining 100 articles, 92 studies were further excluded mainly because of: (1) other publication formats such as conference papers, (2) participants without a formal diagnosis [8, 19,20,21,22], (3) a combination of different interventions [23,24,25,26,27,28], (4) no assessment of target outcome [29,30,31], (5) or institutionalized pwd [32,33,34,35,36].
Consequently, eight publications of seven trials with a total of 1135 participants were included in this review. Detailed study characteristics are summarized in Table 1. The mean age ranged from 70.5 ± 7.4 [37] to 84.3 ± 7.7 years [38] and participants baseline MMSE varied between 14.7 ± 5.65 [38] and 23.94 ± 3.6 [37]. Different types of exercise were conducted, with the majority of included studies investigating either aerobic training [37, 39, 40] or a combination of aerobic, strength, and balance training (multimodal training) [41,42,43]. With the exception of Park et al. [38], all trials examined the effects of exercise on cognition. Effects on ADLs were investigated in two studies [37, 42] and NPS in four studies [37, 38, 42, 44]. Adherence to the protocol ranged from 65.63% [42] to 87% [26].
Effects of exercise on cognition
Of the seven included trials, six examined the effects of exercise on cognition in pwd, with five trials covering global cognition, measured by the Mini-Mental State Examination (MMSE) [37, 41, 43] or Alzheimer’s Disease Assessment Scale (ADAS-Cog) [40, 42]. Although Yu et al. [40] concluded that aerobic exercise is effective to reduce global cognitive decline, none of the studies demonstrated a significant superiority of the intervention group performing either aerobic [37, 39, 40] or multimodal training [41,42,43] compared to the control group receiving usual care.
Assessment tools for cognitive subdomains varied between trials. Executive function was assessed in four studies [39,40,41, 43], either using the Clock Drawing test or composite scores of multiple tests, with only one trial finding mild positive effects after 52 weeks of multimodal training [43] (Table 2). None of the trials reported an impact on the domains verbal fluency and language [37, 40, 41, 43] as well as attention and processing speed [37, 40, 41]. While the effects of exercise through either aerobic [39] or multimodal training [37] on psychomotor speed were analyzed in two trials, only Karssemeijer et al. [39] demonstrated that twelve weeks of aerobic training leads to improvements in psychomotor speed.
Effects of exercise on activities of daily living (ADLs)
The effects of moderate-to-high intensity exercise trainings over 16 weeks on ADLs were investigated in two studies [37, 42] (Table 3). Although Lamb et al. [42] found an improvement in physical fitness, these effects could not be translated into improvements in ADLs measured by Alzheimer’s Disease Cooperative Study ADL Scale (ADCS-ADL). These findings are in line with Hoffmann et al., who found no improvement in ADLs assessed with the Bristol Activities of Daily Living Scale (BADLS) [34].
Effects of exercise on neuropsychiatric symptoms (NPS)
Four studies investigated the effects of aerobic training [37], multimodal training [42, 44], and chair-based strengthening or yoga exercises [38] on NPS, which were assessed by Neuropsychiatric Inventory (NPI) in three trials [37, 42, 44], while Park et al. [38] measured agitation, depression, and anxiety by Cohen-Mansfield Agitation Inventory-Short Form (CMAI) and Hospital Anxiety and Depression Scale (HADS), respectively (Table 4). Whereas Hoffmann et al. [37] described significant differences in change in total NPS, indicating less severe NPS in the intervention group after sixteen weeks of training, neither of the other interventions led to improvements in NPS after twelve [38], 16 [42], or 52 [44] weeks.
Risk of bias in included studies
Risk of bias varied between some to high concerns in the included studies (Table 5). For the detailed description, please refer to Additional file 1. Incompleteness of outcome data, selective reporting as well as measurement of the outcome were the predominant reasons for high concerns. Concerns in the measurement of the outcome mainly occurred because outcome assessors were aware of the intervention received. As participants were also aware of the assigned intervention, the possibility of bias due to deviations from the intended intervention did lead to some concerns in all included trials. Reporting of the trial of Karssemeijer et al. [39] and Lamb et al. [42] did not raise further concerns apart from blinding and thus should be considered as the included studies with the lowest risk of bias.
Discussion
Effects on cognition
This systematic review aimed to gather the current state of research on the effects of exercise on cognition, ADLs, and NPS in community-dwelling pwd. We found that pwd receiving exercise interventions did not yield additional benefits on global cognition in any of the included trials. In line with previous reviews, exercise could thus not be described as being effective for slowing down cognitive decline in pwd [12, 45]. Moreover, the results of Lamb et al. (2018) are of high interest, as they described a worsening of cognition in the intervention group after long-term follow-up and thus do not justify recommendation of physical exercise interventions as a treatment for cognitive decline in community-dwelling pwd.
Although our findings are consistent with Forbes et al. [12], they contrast with other studies and reviews [46, 47]. Differences in the study population might explain why our findings are inconsistent with other systematic reviews, which describe exercise as an effective treatment for cognitive decline in pwd without a formal diagnosis [46, 47]. De Oliveira et al. [41] did find significant improvements in cognition after a multimodal training intervention in people with MCI, but not in those with dementia and thus concluded that physical exercise should only be recommended in the early stages of neurocognitive disorders. Therefore, it seems useful to distinguish between people with MCI and pwd, since people affected experience different symptoms and biological adaptions so that possible mediators by which physical activity improves cognition may occur differently as the disease progresses [48]. In order to establish clear evidence and recommendations for physical activity in pwd, it is necessary to analyze a homogenous group in terms of diagnosis, as trainings recommendations might not be applicable for pwd as for people with MCI [49].
However, occasional significant superiorities for intervention groups in cognitive subdomains could be identified in two trials [39, 43]. Analyzing cognition, three trials used supervised sessions in groups [37, 40, 42], one did not report how the intervention was delivered [41], one compared two different settings [43], and one performed exercise individually guided [39]. Apart from Öhman et al. [43], none of the included trials reported beneficial effects for executive functions. These inconsistencies could have been arisen through the trainings content or the duration of the trial, as this was the only trial including training of executive function as part of the intervention, which lasted 52 weeks. Nevertheless, it needs to be stated that this multimodal training did lead to improvements in executive function for the home-based intervention group, while the same intervention did not affect executive function in a group-based setting. Deriving from this, there is an indication that training intervention for community-dwelling pwd are most beneficial if they are delivered individually guided and customized through a healthcare provider or the person’s caregiver. This hypothesis may further explain deviating findings from Karssemeijer et al. [39], who described, in contrast to other trials [37, 40], a significant amelioration in psychomotor speed after 12 weeks of aerobic training in the intervention group. Since training modalities, adherence to the protocol, design of control groups, measurements of the outcome, and exercises did not differ widely between the three trials, individual training sessions seem to be preferable as they might not overwhelm pwd and allow individually adapted designs.
Effects on activities of daily living
In contrast to a previous Cochrane review [12], we could not find any beneficial effects on ADLs through physical activity in pwd. According to this review, there has been an unexplainable high heterogeneity between included studies, with only two trials [50, 51] out of six [52,53,54,55] favoring exercise over control. Furthermore, the sample size of studies included in the Cochrane review was comparatively small, ranging from six to 56 participants in the intervention group. Especially the power of the two studies observing beneficial effects on ADLs is limited, due to the sample size of eight [50] and eleven [51] participants in the intervention group. Therefore, Forbes et al. [12] suggested that these findings should be interpreted with caution and rated the quality of the evidence as low. Despite larger sample sizes in the included trials of this this review, ranging from 107 [37] to 329 [42], there were only two trials analyzing the effects of exercise on ADLs in pwd. We could not find an impact of adherence to the protocol.
Effects on neuropsychiatric symptoms
Trials investigating the effects of exercise on NPS showed inconsistent results. Since training modalities such as duration, intensity, and setting did not differ widely between trials, different types of training might explain divergences. While strength and multimodal trainings intervention showed no beneficial effects on NPS [38, 42, 44], Hoffmann et al. [37] described a reduction of NPS after 16 weeks of aerobic training. Since only one trial analyzed the impact of aerobic exercise on BSPD, it seems possible that aerobic exercise might be effective to reduce NPS in pwd and this is in line with a recently published review [56]. Even though this might seem plausible, we could not find evidence for differences between studies with active or passive control groups or deviations in exercise adherence.
Limitations
Although the search was conducted in eight different databases and 7651 trials were identified, we cannot rule out the possibility that we might have missed relevant trials due to limitations in language and year of publication. This might also be applicable for the requirement of a formal diagnosis of dementia. Some trials were excluded in this review because they included participants based on the results of screening instruments.
Conclusion
Implications for practice
There is little evidence that both strength and aerobic exercise or a combination of these cannot be recommended as a treatment option for cognitive impairment in community-dwelling pwd. Furthermore, moderate to high-intensity interventions might even worsen the cognitive decline in community-dwelling pwd after finishing the intervention [42]. In this context, it is mentionable that this was the study with the highest methodological quality and largest sample size. Furthermore, there is no evidence for the beneficial effects of exercise for ADLs. The effects on NPS are unclear, as one out of three studies found improvements after aerobic training. That is why healthcare providers and caregivers should be confident to promote the maintenance of an active and healthy lifestyle [57] among community-dwelling pwd instead, although recent recommendations [58] of moderate-intensity aerobic exercise for community-dwelling pwd are not underpinned by the results of this review. The development of best practice guidelines for healthcare providers is urgently needed. Exercise adherence does not seem to influence these outcomes.
Implications for research
As our review shows, there is a necessity for improvement in methodological approaches in the research of the effects of exercise on cognition, ADLs, and NPS in community-dwelling pwd. Due to its large sample size and high methodological quality, the trial of Lamb et al. [42] should be considered as a best practice example. Recent research recommends at least 150 min/week of moderate-intensity aerobic exercise for older people, but this might not be appropriate for community-dwelling pwd [58]. Following on from this, future RCTs should require a formal diagnosis of dementia and should distinguish between pwd and people with MCI, as the conditions lead to different capabilities and needs, so that effects of exercise could therefore result in different outcomes [41]. High methodological quality, large sample sizes and long-term follow-ups should be implemented in future trials. In respect to possible impacts of social stimulation and activities on cognition and NPS, control groups should be designed accordingly to the intervention group. Especially if supervised group sessions are analyzed in a trial, control group should receive comparable social stimulation. To give answer to the question which type of training is most beneficial for community-dwelling pwd, it would be necessary that training modalities are described in detail and to compare different exercise protocols within three-armed RCTs. To compare different exercise programs and to be able to transfer research results in practice, it is inescapable to give a detailed description of the content and exercises of the trials, as it was the case in most of the included studies.
Registration
This review was registered at the national prospective register of systematic reviews and no amendments were made (PROSPERO registration number: CRD42021246598). To view please visit https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=246598.
No protocol was published in advance.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
This paper has not been previously published and is not currently under consideration for publication elsewhere.
Abbreviations
- ADAS-Cog:
-
Alzheimer’s Disease Assessment Scale
- ADCS-ADL:
-
Alzheimer’s Disease Cooperative Study ADL Scale
- ADLs:
-
Activities of daily living
- BADLS:
-
Bristol Activities of Daily Living Scale
- CMAI:
-
Cohen-Mansfield Agitation Inventory-Short Form
- HADS:
-
Hospital Anxiety and Depression Scale
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-Mental Status Examination
- NPI:
-
Neuropsychiatric Inventory
- NPS:
-
Neuropsychiatric Symptoms
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PROSPERO:
-
National Prospective Register of Systematic Reviews
- pwd:
-
People with dementia
- RCTs:
-
Randomized controlled trials
- RoB2:
-
Revised Cochrane Risk-of-Bias Tool for Randomized Trials
- SCI:
-
Subjective cognitive impairment
References
World Health Organization. Global status report on the public health response to dementia. 2021.
National Collaborating Centre for Mental Health. Dementia: supporting people with dementia and their carers in health and social care. 2011.
Deuschl G, Maier W. S3-Leitlinie demenzen. Deutsche Gesellschaft für Neurologie, Hrsg. Leitlinien für Diagnostik und Therapie in der Neurologie, 2016:33. https://dgn.org/leitlinien/leitlinie-diagnose-und-therapievon-demenzen-2016/. Accessed 14 July 2022.
Kouloutbani K, Karteroliotis K, Politis A. The effect of physical activity on dementia. Psychiatriki. 2019;30(2):142–55.
Cass SP. Alzheimer’s disease and exercise: a literature review. Curr Sports Med Rep. 2017;16(1):19–22.
Du Z, Li Y, Li J, Zhou C, Li F, Yang X. Physical activity can improve cognition in patients with Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging. 2018;13:1593.
Song D, Yu DSF. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled trial. Int J Nurs Stud. 2019;93:97–105.
Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, et al. Aerobic exercise for Alzheimer’s disease: a randomized controlled pilot trial. PLoS ONE. 2017;12(2): e0170547.
Bürge E, Berchtold A, Maupetit C, Bourquin NM, von Gunten A, Ducraux D, et al. Does physical exercise improve ADL capacities in people over 65 years with moderate or severe dementia hospitalized in an acute psychiatric setting? A multisite randomized clinical trial. Int Psychogeriatr. 2017;29(2):323–32.
Toots A, Littbrand H, Boström G, Hörnsten C, Holmberg H, Lundin-Olsson L, et al. Effects of exercise on cognitive function in older people with dementia: a randomized controlled trial. J Alzheimers Dis. 2017;60(1):323–32.
Sanders LMJ, Hortobágyi T, Karssemeijer EGA, Van der Zee EA, Scherder EJA, van Heuvelen MJG. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: a randomized controlled trial. Alzheimers Res Ther. 2020;12(1):28.
Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4. Art. No.:CD006489. https://doi.org/10.1002/14651858.CD006489.pub4. Accessed 14 July 2022.
Ströhle A, Schmidt DK, Schultz F, Fricke N, Staden T, Hellweg R, et al. Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry. 2015;23(12):1234–49.
Huang X, Zhao X, Li B, Cai Y, Zhang S, Wan Q, et al. Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: a systematic review and network meta-analysis. J Sport Health Sci. 2022;11(2):212–23.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535.
Wimo A, Gauthier S, Prince M. Global estimates of informal care. London: Alzheimer’s Disease International (ADI); 2018.
World Health Organization. Dementia: a public health priority: World Health Organization. 2012.
Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Dimitriou T-D, Verykouki E, Papatriantafyllou J, Konsta A, Kazis D, Tsolaki M. Non-Pharmacological interventions for the anxiety in patients with dementia. a cross-over randomised controlled trial. Behav Brain Res. 2020;390:112617.
Barha CK, Hsiung G-YR, Best JR, Davis JC, Eng JJ, Jacova C, et al. Sex difference in aerobic exercise efficacy to improve cognition in older adults with vascular cognitive impairment: secondary analysis of a randomized controlled trial. J Alzheimer’s Dis. 2017;60(4):1397–410.
Goldberg SE, Van Der Wardt V, Brand A, Burgon C, Bajwa R, Hoare Z, et al. Promoting activity, Independence and stability in early dementia (PrAISED): a, multisite, randomised controlled, feasibility trial. BMC Geriatr. 2019;19(1):1–12.
Vidoni ED, Perales J, Alshehri M, Giles AM, Siengsukon CF, Burns JM. Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer’s disease (2001). J Geriatri Phys Ther. 2019;42(3):E129.
van Santen J, Dröes R-M, Twisk JW, Henkemans OAB, van Straten A, Meiland FJ. Effects of exergaming on cognitive and social functioning of people with dementia: a randomized controlled trial. J Am Med Dir Assoc. 2020;21(12):1958–67.
Pires Camargo Novelli MM, Machado SC, Balestra de Lima G, Cantatore L, Pereira de Sena B, Rodrigues RS, et al. P3‐362: the Brazilian version of Tailored Activity Program (Tap‐BR) to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden in Brazil: a randomized pilot study. Alzheimer’s & Dementia. 2016;12:P988-P.
Kim M-J, Han C-W, Min K-Y, Cho C-Y, Lee C-W, Ogawa Y, et al. Physical exercise with multicomponent cognitive intervention for older adults with Alzheimer’s disease: a 6-month randomized controlled trial. Dementia Geriatri Cogn Disord Extra. 2016;6(2):222–32.
de Oliveira AM, Radanovic M, de Homem Mello PC, Buchain PC, Dias Vizzotto A, Harder J, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program–outpatient version. Int J Geriatr Psychiatr. 2019;34(9):1301–7.
Dawson N, Judge KS, Gerhart H. Improved functional performance in individuals with dementia after a moderate-intensity home-based exercise program: a randomized controlled trial. J Geriatr Phys Ther. 2019;42(1):18–27.
Gbiri CAO, Amusa BF. Progressive task-oriented circuit training for cognition, physical functioning and societal participation in individuals with dementia. Physiother Res Int. 2020;25(4): e1866.
Khan I, Petrou S, Khan K, Mistry D, Lall R, Sheehan B, et al. Does structured exercise improve cognitive impairment in people with mild to moderate dementia? a cost-effectiveness analysis from a confirmatory randomised controlled trial: the dementia and physical activity (DAPA) trial. PharmacoEconomics-open. 2019;3(2):215–27.
Charras K, Mabire J-B, Bouaziz N, Deschamps P, Froget B, de Malherbe A, et al. Dance intervention for people with dementia: lessons learned from a small-sample crossover explorative study. Arts Psychother. 2020;70: 101676.
Sobol NA, Dall CH, Høgh P, Hoffmann K, Frederiksen KS, Vogel A, et al. Change in fitness and the relation to change in cognition and neuropsychiatric symptoms after aerobic exercise in patients with mild Alzheimer’s disease. J Alzheimer’s Dis. 2018;65(1):137–45.
Sanders L, Hortobágyi T, Karssemeijer E, Van der Zee E, Scherder E, Van Heuvelen M. Effects of low-and high-intensity physical exercise on physical and cognitive function in older persons with dementia: a randomized controlled trial. Alzheimer’s Res Ther. 2020;12(1):1–15.
Huang N, Li W, Rong X, Champ M, Wei L, Li M, et al. Effects of a modified Tai Chi program on older people with mild dementia: a randomized controlled trial. J Alzheimer’s Dis. 2019;72(3):947–56.
Fleiner T, Dauth H, Zijlstra W, Haussermann P. A structured physical exercise program reduces professional caregiver’s burden caused by neuropsychiatric symptoms in acute dementia care: randomized controlled trial results. J Alzheimer’s Dis. 2020;74(2):429–33.
Enette L, Vogel T, Merle S, Valard-Guiguet A-G, Ozier-Lafontaine N, Neviere R, et al. Effect of 9 weeks continuous vs interval aerobic training on plasma BDNF levels, aerobic fitness, cognitive capacity and quality of life among seniors with mild to moderate Alzheimer’s disease: a randomized controlled trial. Eur Rev Aging Phys Act. 2020;17(1):1–16.
Bossers WJ, van der Woude LH, Boersma F, Hortobágyi T, Scherder EJ, van Heuvelen MJ. Comparison of effect of two exercise programs on activities of daily living in individuals with dementia: a 9-week randomized, controlled trial. J Am Geriatr Soc. 2016;64(6):1258–66.
Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J Alzheimers Dis. 2016;50(2):443–53.
Park J, Tolea MI, Sherman D, Rosenfeld A, Arcay V, Lopes Y, et al. Feasibility of conducting nonpharmacological interventions to manage dementia symptoms in community-dwelling older adults: a cluster randomized controlled trial. Am J Alzheimers Dis Other Demen. 2020;35:1533317519872635.
Karssemeijer EGA, Aaronson JA, Bossers WJR, Donders R, Olde Rikkert MGM, Kessels RPC. The quest for synergy between physical exercise and cognitive stimulation via exergaming in people with dementia: a randomized controlled trial. Alzheimers Res Ther. 2019;11(1):3.
Yu F, Vock DM, Zhang L, Salisbury D, Nelson NW, Chow LS, et al. Cognitive effects of aerobic exercise in Alzheimer’s disease: a pilot randomized controlled trial. J Alzheimers Dis. 2021;80(1):233–44.
de Oliveira Silva F, Ferreira JV, Placido J, Sant’Anna P, Araujo J, Marinho V, et al. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: a randomized controlled trial. Maturitas. 2019;126:28–33.
Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675 ((no pagination)).
Ohman H, Savikko N, Strandberg TE, Kautiainen H, Raivio MM, Laakkonen ML, et al. Effects of exercise on cognition: the finnish alzheimer disease exercise trial: a randomized, controlled trial. J Am Geriatr Soc. 2016;64(4):731–8.
Öhman H, Savikko NRN, Strandberg TE, Kautiainen H, Raivio MM, Laakkonen ML, et al. Effects of frequent and long-term exercise on neuropsychiatric symptoms in patients with Alzheimer’s disease – secondary analyses of a randomized, controlled trial (FINALEX). Eur Geriatr Med. 2017;8(2):153–7.
Li X, Guo R, Wei Z, Jia J, Wei C. Effectiveness of exercise programs on patients with dementia: a systematic review and meta-analysis of randomized controlled trials. BioMed Res Int. 2019;2019:2308475.
Law C-K, Lam FM, Chung RC, Pang MY. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: a systematic review. J Physiother. 2020;66(1):9–18.
Liang JH, Xu Y, Lin L, Jia RX, Zhang HB, Hang L. Comparison of multiple interventions for older adults with Alzheimer disease or mild cognitive impairment: a PRISMA-compliant network meta-analysis. Medicine. 2018;97(20):e10744.
Maass A, Düzel S, Brigadski T, Goerke M, Becke A, Sobieray U, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142–54.
Öhman H, Savikko N, Strandberg TE, Pitkälä KH. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement Geriatr Cogn Disord. 2014;38(5–6):347–65.
Santana-Sosa E, Barriopedro M, López-Mojares LM, Pérez M, Lucia A. Exercise training is beneficial for Alzheimer’s patients. Int J Sports Med. 2008;29(10):845–50.
Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26(5):381–8.
Frances T. The effect of regular exercise on muscle strength and functional abilities of late stage Alzheimer’s disease. Va Nurses Today. 1995;3:25–6.
Conradsson M, Littbrand H, Lindelöf N, Gustafson Y, Rosendahl E. Effects of a high-intensity functional exercise programme on depressive symptoms and psychological well-being among older people living in residential care facilities: a cluster-randomized controlled trial. Aging Ment Health. 2010;14(5):565–76.
Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158–65.
Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci. 2012;26(1):12–9.
Kouloutbani K, Venetsanou F, Markati A, Karteroliotis KE, Politis A. The effectiveness of physical exercise interventions in the management of neuropsychiatric symptoms in dementia patients: a systematic review. Int Psychogeriatrics. 2022;34(2):177–90.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–46.
World Health Organization. WHO guidelines on physical activity and sedentary behaviour. 2020.
Acknowledgements
The authors are grateful to the participating experts and study participants of digiDEM Bayern.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research work is funded by the Bavarian State Ministry of Health and Care (StMGP) as part of the Bavarian Digital Registry – digiDEM Bayern (funding code: G42d-G8300-2017/1606–83; date: 01.01.2019).
Author information
Authors and Affiliations
Contributions
The present manuscript was written by K.S. A.K. was involved in the screening process and in the review of the extracted data. Risk of bias assessment was performed by K.S. and N.D. P.K.-R. was a major contributor in writing the manuscript and in the screening process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Risk of bias assessment. This additional file provides the detailed description of risk of bias assessment after discussion of both authors (K.S. and N.D.).
Additional file 2.
Search strategy. This file provides the full search strategy for each database with detailed description (e.g. additional filters and dates of search).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Steichele, K., Keefer, A., Dietzel, N. et al. The effects of exercise programs on cognition, activities of daily living, and neuropsychiatric symptoms in community-dwelling people with dementia—a systematic review. Alz Res Therapy 14, 97 (2022). https://doi.org/10.1186/s13195-022-01040-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-022-01040-5