Abstract
Background
Until now, Mendelian randomization (MR) studies have investigated the causal association of risk factors with Alzheimer’s disease (AD) using large-scale AD genome-wide association studies (GWAS), GWAS by proxy (GWAX), and meta-analyses of GWAS and GWAX (GWAS+GWAX) datasets. However, it currently remains unclear about the consistency of MR estimates across these GWAS, GWAX, and GWAS+GWAX datasets.
Methods
Here, we first selected 162 independent educational attainment genetic variants as the potential instrumental variables (N = 405,072). We then selected one AD GWAS dataset (N = 63,926), two AD GWAX datasets (N = 314,278 and 408,942), and three GWAS+GWAX datasets (N = 388,324, 455,258, and 472,868). Finally, we conducted a MR analysis to evaluate the impact of educational attainment on AD risk across these datasets. Meanwhile, we tested the genetic heterogeneity of educational attainment genetic variants across these datasets.
Results
In AD GWAS dataset, MR analysis showed that each SD increase in years of schooling (about 3.6 years) was significantly associated with 29% reduced AD risk (OR=0.71, 95% CI: 0.60–0.84, and P=1.02E−04). In AD GWAX dataset, MR analysis highlighted that each SD increase in years of schooling significantly increased 84% AD risk (OR=1.84, 95% CI: 1.59–2.13, and P=4.66E−16). Meanwhile, MR analysis suggested the ambiguous findings in AD GWAS+GWAX datasets. Heterogeneity test indicated evidence of genetic heterogeneity in AD GWAS and GWAX datasets.
Conclusions
We highlighted significant difference and genetic heterogeneity in clinically diagnosed AD GWAS and self-report proxy phenotype GWAX. Our MR findings are consistent with recent findings in AD genetic variants. Hence, the GWAX and GWAS+GWAX findings and MR findings from GWAX and GWAS+GWAX should be carefully interpreted and warrant further investigation using the AD GWAS dataset.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is the most common neurodegenerative disease [1, 2]. From 2009 to 2019, large-scale genome-wide association studies (GWAS) have been conducted using clinically diagnosed AD and cognitively normal controls, and successfully identified multiple common AD genetic variants with genome-wide significance P < 5.00E−08 [3,4,5,6,7,8,9], especially two large-scale GWAS meta-analyses from the International Genomics of Alzheimer’s Project (IGAP) including the IGAP 2013 (n= 74,046, 25,580 cases, and 48,466 controls) [6], and the IGAP 2019 (n= 94,437, including 35,274 cases and 59,163 controls) [9]. However, these genetic variants could only explain about 40% of the genetic variance of AD [10,11,12]. Hence, the majority of AD genetic risk remains undiscovered [10,11,12]. Until recently, GWAS for family history of AD, known as GWAS by AD proxy phenotype (GWAX) using UK Biobank individuals is widely used to increase the sample size into the traditional GWAS, which may contribute to identify more additional novel genetic variants [13,14,15]. These meta-analyses of AD GWAS and GWAX (GWAS+GWAX) have reported novel findings [13,14,15].
Importantly, all these AD GWAS, GWAX, and GWAS+GWAX summary datasets are publicly available, which provides strong data support to investigate the causal association between AD and previously reported risk factors using a Mendelian randomization (MR) design [16,17,18]. Meanwhile, there is no strict standard to limit the use of AD GWAS, GWAX, or GWAS+GWAX datasets in MR studies. Hence, some MR studies are based on the AD GWAS datasets from IGAP 2013 or IGAP 2019 [19,20,21,22,23,24,25], of which three MR studies had evaluated the impact of multiple modifiable risk factors on AD [16,17,18]. In 2017, Larsson and colleagues selected 24 potentially modifiable risk factors and found that only increased educational attainment was significantly associated with a reduced risk of AD [16]. In 2020, Wang and colleagues analyzed 45 potentially modifiable risk factors, and eventually highlighted educational attainment and urate levels [17]. In 2021, Andrews and colleagues selected 22 risk factors, and eventually identified educational attainment using polygenic risk scores (PRS) and MR [18]. Meanwhile, others MR studies are based on the AD GWAS+GWAX datasets [26,27,28,29,30,31,32]. However, it currently remains unclear about the consistency of MR estimates from AD GWAS, GWAX, and GWAS+GWAX datasets.
Here, we selected the educational attainment as the risk factor, and evaluated its impact on AD risk using large-scale AD GWAS, GWAX, or GWAS+GWAX datasets, as the causal association between educational attainment and AD had been well established in recent MR studies [16,17,18, 33].
Methods
Study design
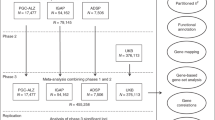
MR is based on three principal assumptions. First, the instrumental variables (genetic variants) should be significantly associated with the exposure (educational attainment), generally achieving the genome-wide significant level (P<5.00E-08) [19]. Second, instrumental variables should not be associated with confounders of the exposure (educational attainment) and outcome (AD) [19]. Third, instrumental variables should affect the risk of the outcome (AD) only through exposure (educational attainment) [19]. The second and third assumptions are collectively known as independence from pleiotropy. Figure 1 provides a flow chart about our MR study design.
Educational attainment genetic variants
We selected 162 independent educational attainment genetic variants with the genome-wide significance (P < 5.00E−08) to be the potential instrumental variables, as provided in supplementary Table 1 [34]. Educational attainment is a continuous variable, which is determined by the number of years of schooling completed at or above age 30 [34]. These 162 genetic variants are identified by a large-scale GWAS in 405,072 individuals of European descent including 293,723 individuals in discovery stage (SSGAC) and 111,349 individuals in the independent replication stage (UK Biobank) [34].
AD GWAS dataset
We selected the clinically diagnosed AD GWAS dataset from IGAP 2019 stage 1 including 21,982 AD cases and 41,944 cognitively normal controls of European descent [9]. This GWAS dataset is based on a meta-analysis using participants from four AD consortia including Alzheimer Disease Genetics Consortium, Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE), The European Alzheimer’s Disease Initiative (EADI), and Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES) [9]. Table 1 provides the demographic profile about the AD GWAS dataset.
AD GWAX datasets
We selected two GWAX datasets for AD proxy phenotype including GWAX 2018 [13], and GWAX 2021 [15], respectively. Both studies are based on the UK Biobank participants [35]. UK Biobank is a large national and international health resource including the genetic and phenotype data from 502,536 community-dwelling individuals aged between 37 and 73 years recruited in the United Kingdom between 2006 and 2010 [35]. In UK Biobank, a proxy phenotype for AD case-control status was assessed via self-report [13]. Participants were asked to report “Has/did your father or mother ever suffer from Alzheimer’s disease/dementia?” [13]. Table 1 provides the demographic profile about these two AD GWAX datasets.
AD GWAS+GWAX datasets
We selected three large-scale AD GWAS+GWAX datasets including GWAS+GWAX 2018 (a meta-analysis using IGAP 2013 and UK Biobank) [13], GWAS+GWAX 2019 (a meta-analysis using IGAP 2013, PGC-ALZ, ADSP, and UK Biobank) [14], and GWAS+GWAX 2021 (a meta-analysis using IGAP 2019 and UK Biobank) [15], respectively. All these three GWAS+GWAX datasets included the IGAP and UK Biobank participants, and are not independent of each other. Table 1 provides the demographic profile about the three GWAS+GWAX datasets.
Establishing the Wald estimator
For the same effect allele from each educational attainment genetic variant Gj(j = 1, …, 162), we assume that we have successfully extracted their corresponding summary results in educational attainment GWAS dataset including the beta coefficients and their standard errors (\({\hat{\beta}}_{Xj}, se\left({\hat{\beta}}_{Xj}\right)\)), and in the AD GWAS, GWAX and GWAS+GWAX datasets including the beta coefficients and their standard errors (\({\hat{\beta}}_{Yj}, se\left({\hat{\beta}}_{Yj}\right)\)). For a given genetic variant, the causal effect of educational attainment on AD can be consistently estimated as a simple ratio, also called the Wald estimator \({\hat{\theta}}_j=\frac{{\hat{\beta}}_{Yj}}{{\hat{\beta}}_{Xj}}\) and its approximate variance \({v}_j=\frac{se{\left({\hat{\beta}}_{Yj}\right)}^2}{{{\hat{\beta}}_{Xj}}^2}\) [19, 36].
MR analysis
Using the Wald estimator from each educational attainment genetic variant, we conducted the MR analysis using four MR methods including inverse-variance weighted (IVW), weighted median, MR-Egger, and MR-PRESSO (Mendelian Randomization Pleiotropy RESidual Sum and Outlier) to combine the variant-specific estimates and get the overall estimate [36,37,38,39]. IVW is the main MR analysis method, which combines the variant-specific Wald estimators by taking the inverse of their approximate variances as the corresponding weights [37]. Weighted median could derive consistent estimates when up to 50% of instruments are not valid [37]. MR-Egger could test the presence of potential pleiotropy and account for this potential pleiotropy using the MR-Egger intercept test [36]. MR-PRESSO could detect and correct for the horizontal pleiotropy via outlier removal (the MR-PRESSO outlier test) [40]. The odds ratio (OR) as well as 95% confidence interval (CI) of AD corresponds to about per 3.6 years increase (about 1 standard deviation (SD)) in EduYears. All the statistical tests were completed using R Packages “MendelianRandomization” [39] and “MR-PRESSO” [40]. The significance threshold is P < 0.05.
Heterogeneity test and paired-samples T test
We performed a heterogeneity test of the Wald estimators using the Cochran’s Q statistic and the I2 statistic [41]. Cochran’s Q statistic approximately follows a χ2 distribution with k-1 degrees of freedom (k is the number of the selected studies) [42]. \({I}^2=\left(\mathrm{Q}-\left(\mathrm{k}-1\right)\right)\left/ Q\times 100\%\right.\) ranges from 0 to 100%, with 0–25%, 25–50%, 50–75%, and 75–100% corresponding to low, moderate, large and extreme heterogeneity, respectively [42]. Importantly, the Cochran’s Q statistic and I2 assume that the subjects are independent of one another and were selected at random from a larger population. Hence, we only test the heterogeneity in AD GWAS and GWAX including two comparisons GWAS vs. GWAX 2018, and GWAS vs. GWAX 2021. All statistical tests were completed using R Package “meta: General Package for Meta-Analysis.”
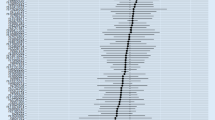
In line with the heterogeneity test, we further conducted the paired-samples T test to evaluate the average differences of Wald estimators in GWAS vs. GWAX 2018, and GWAS vs. GWAX 2021. Analysis of variance (ANOVA) is widely used to analyze the differences among means from multiple independent (unrelated) groups. However, the selected AD GWAS, GWAX, and GWAS+GWAX datasets are not completely independent with each other. Therefore we could not provide the overall differences among the mean effect sizes across the AD GWAS, GWAX, and GWAS+GWAX datasets. Here, we provide a combined plot using all shared genetic variants to visualize the differences in effect sizes and directions across the six datasets.
Results
MR analysis in AD GWAS dataset
We extracted the GWAS summary statistics of 159 educational attainment genetic variants in the AD GWAS dataset, as provided in supplementary Table 2. Using the MR-Egger intercept test, we did not identify any significant pleiotropic variant. Using MR-PRESSO Global Test, we found two genetic variants rs268134 and rs28420834 to be the pleiotropic variants (Table 2). Hence, we excluded both variants in MR analysis. IVW showed that each SD increase in years of schooling (about 3.6 years) was significantly associated with 29% reduced AD risk (OR=0.71, 95% CI: 0.60–0.84, and P=1.02E−04). Interestingly, evidence from weighted median, MR-Egger, and MR-PRESSO further supported this finding, as provided in Table 3. Meanwhile, all the MR estimates from these four methods are consistent in terms of direction.
MR analysis in AD GWAX datasets
We extracted the GWAS summary statistics of 147 and 159 educational attainment genetic variants in GWAX 2018, and GWAX 2021, respectively, as provided in supplementary Table 3-4. In GWAX 2018, no pleiotropic variant is identified using both the MR-Egger intercept test and MR-PRESSO Global Test (Table 2). MR analysis indicated no significant causal association between educational attainment and AD, as provided in Table 3. However, two methods showed an increased trend of AD risk with high educational attainment including IVW (OR=1.09, 95% CI: 1.00–1.19, and P=0.051), and MR-PRESSO (OR=1.09, 95% CI: 1.00–1.20, and P=0.053). In GWAX 2021, rs268134 is identified to be a pleiotropic variant and then excluded in MR analysis (Table 2). IVW highlighted that each SD increase in years of schooling could significantly increase 84% AD risk (OR=1.84, 95% CI: 1.59–2.13, and P=4.66E−16). The MR estimates from weighted median, MR-Egger and MR-PRESSO were consistent with the IVW estimate in terms of direction and magnitude (Table 3).
MR analysis in AD GWAS+GWAX datasets
We extracted the GWAS summary statistics of 147, 155, and 159 educational attainment genetic variants in GWAS+GWAX 2018, GWAS+GWAX 2019, and GWAS+GWAX 2021, respectively, as provided in supplementary Table 5-7. Only in GWAS+GWAX 2021, MR-PRESSO indicated rs268134 to be a pleiotropic variant, and then excluded in MR analysis (Table 2). In GWAS+GWAX 2018, MR analysis showed no significant causal association between educational attainment and AD (Table 3). In GWAS+GWAX 2019, we found that high educational attainment could reduce the risk of AD (Table 3). In GWAS+GWAX 2021, MR analysis further supported the increased risk of AD with high educational attainment using four MR methods, as provided in Table 3. IVW showed that each SD increase in years of schooling could significantly increase 22% AD risk (OR=1.22, 95% CI: 1.08–1.36, and P=1.00E−03). The MR estimates from weighted median, MR-Egger and MR-PRESSO were consistent with the IVW estimate in terms of direction and magnitude (Table 3).
MR analysis in AD GWAS, GWAX, and GWAS+GWAX datasets using the same genetic variants
These above MR analyses were based on different educational attainment genetic variants as the instrumental variables in AD GWAS, GWAX, and GWAS+GWAX datasets. We further conducted a secondary MR analysis using the same educational attainment genetic variants in each AD GWAS, GWAX, and GWAS+GWAX datasets. Finally, we selected 143 same educational attainment genetic variants available across all datasets by excluding rs268134 and rs28420834, as both are pleiotropic variants. Interestingly, the secondary MR analysis supported these above findings, as provided in Table 4. Figure 2 is a combined plot, which visualizes the differences in effect sizes and directions across the six datasets using the 143 genetic variants.
Heterogeneity test and paired-samples T test
Using the 143 same educational attainment genetic variants, we tested the genetic heterogeneity of the Wald estimators in AD GWAS and GWAX datasets. Using GWAS vs. GWAS 2018 comparison, 47 (33%) and 29 (20%) of 143 genetic variants showed evidence of heterogeneity with I2 > 25% and I2 > 50%, respectively. GWAS vs. GWAX 2021 comparison indicated that 57 (40%) and 44 (30%) of 143 genetic variants showed heterogeneity with I2 > 25% and I2 > 50%. paired-samples T test showed that the average Wald estimator in GWAS (mean = − 0.39) was significantly smaller than the average Wald estimators in GWAX 2018 (mean = 0.13, and P = 2.17E−06) and GWAX 2021 (mean = 0.65, and P = 3.16E−14), respectively.
Discussion
Until now, MR methods had been widely used to determine the causal association between AD and previously reported risk factors using the AD GWAS, GWAX, or GWAS+GWAX summary datasets [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. However, the consistency of MR estimates from AD GWAS, GWAX, or GWAS+GWAX datasets currently remains unclear. Here, we first evaluated the causal association of a well-established risk factor educational attainment with the risk of AD using large-scale GWAS, GWAX, or GWAS+GWAX datasets [16,17,18, 33]. MR analysis in the AD GWAS dataset showed that high educational attainment could significantly reduce the risk of AD, which is consistent with recent findings [16,17,18, 33]. However, MR analysis indicated no significant causal association between educational attainment and AD in AD GWAX 2018 dataset, and even showed that high educational attainment increased the risk of AD in the AD GWAX 2021 dataset. Meanwhile, MR analysis in AD GWAS+GWAX datasets suggested the ambiguous findings about the causal association between educational attainment and AD. Hence, all these above findings indicated the inconsistency of MR estimates in AD GWAS, GWAX, and GWAS+GWAX datasets.
Interestingly, our findings are consistent with recent findings in AD genetic variants. Andrews and colleagues recently summarized and discussed 40 AD susceptibility loci with genome-wide significance, which were identified by at least one of the four studies [10], including GWAS 2013 [6], GWAS+GWAX 2018 [13], GWAS+GWAX 2019 [14], and GWAS 2019 [9]. They found that only 15 were replicated across all the four studies, and 9 were replicated in two or three studies at full genome-wide significance [10].
We further test the genetic heterogeneity of educational attainment genetic variants across the GWAS, GWAX, and GWAS+GWAX datasets. Interestingly, heterogeneity test indicated evidence of genetic heterogeneity across the GWAS, GWAS, and GWAS+GWAX datasets. Importantly, GWAS vs. GWAX 2021 comparison highlighted the largest number of genetic variants with heterogeneity. Hence, the genetic heterogeneity may have caused the opposite directions about the causal association between educational attainment and AD in clinically diagnosed AD and self-report proxy phenotype.
Our findings are consistent with recent hypothesis that the phenotypic heterogeneity may cause the genetic heterogeneity, and further reduce the statistical power for GWAX and GWAS+GWAX [10, 13]. It is known that the AD GWAX is based on the self-report AD proxy phenotype from UK Biobank participants [10, 13]. GWAX theoretically could increase the large-scale sample size into traditional AD GWAS, and further improve the statistical power [10, 13]. In fact, not all UK Biobank participants could discriminate AD from other dementia subtypes, and exactly reflect the clinically diagnosed AD status, considering the different presentations and genetic architectures [13]. Hence, the incorrect AD classification may reduce the statistical power to detect the true AD risk loci, and further influence the loci uncovered using GWAX and GWAS+GWAX [13]. This hypothesis may explain why only a small fraction of AD susceptibility loci could be replicated across the AD GWAS, GWAX, and GWAS+GWAX datasets. We think that this hypothesis and our findings from heterogeneity test may explain the inconsistency of MR estimates in AD GWAS, GWAX, and GWAS+GWAX datasets. Meanwhile, the biological factors and medical interventions may also have dramatically different effects on different people [43].
Our MR study may have several strengths. First, we selected one large-scale educational attainment GWAS dataset, and six large-scale AD GWAS, GWAX, or GWAS+GWAX datasets, which may provide ample power to detect the causal association between educational attainment and the risk of AD, as reported by recent MR studies [16,17,18, 33]. Importantly, all these participants are of European descent, which may further reduce the influence from population stratification. Third, we selected multiple MR methods and tested the pleiotropy. Hence, the MR assumptions did not seem to be violated. Fourth, educational attainment is well-established AD risk factor, as reported by recent MR studies [16,17,18, 33]. Hence, evaluating the impact of educational attainment on AD risk may exactly reflect the consistency of MR estimates from AD GWAS, GWAX, or GWAS+GWAX datasets.
Limitations
Our MR study may have some limitations. First, we only selected one AD risk factor educational attainment to evaluate the consistency of MR estimates across AD GWAS, GWAX, or GWAS+GWAX datasets. In fact, several risk factors have been identified to be causally associated with AD risk [23, 26, 27, 44,45,46,47,48,49,50,51,52]. Hence, our findings should be further verified using other well-established AD risk factors. Second, the educational attainment of GWAS is based on the meta-analysis of SSGAC (293,723 individuals) and UK Biobank (111,349) [34]. Hence, the educational attainment GWAS dataset and GWAX, or GWAS+GWAX may have the overlapped individuals, and may not be independent. Hence, our MR findings from GWAX, and GWAS+GWAX should be further evaluated using independent datasets.
Conclusions
In summary, our MR analysis highlighted the difference and genetic heterogeneity in clinically diagnosed AD and self-report proxy phenotype using large-scale AD GWAS, GWAX, and GWAS+GWAX summary datasets. Hence, the GWAX and GWAS+GWAX findings and MR findings from GWAX and GWAS+GWAX should be carefully interpreted and warrant further investigation using the AD GWAS dataset.
Availability of data and materials
All relevant data are within the paper. The authors confirm that all data underlying the findings are either fully available without restriction through consortia websites, or may be made available from consortia upon request. IGAP consortium data are available at http://web.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php;https://www.niagads.org/datasets/ng00075.
References
Liu G, Yao L, Liu J, Jiang Y, Ma G, Chen Z, et al. Cardiovascular disease contributes to Alzheimer's disease: evidence from large-scale genome-wide association studies. Neurobiol Aging. 2014;35(4):786–92.
Hu Y, Sun JY, Zhang Y, Zhang H, Gao S, Wang T, et al. rs1990622 variant associates with Alzheimer's disease and regulates TMEM106B expression in human brain tissues. BMC Med. 2021;19(1):11.
Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–40.
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43(5):429–35.
Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41(10):1094–9.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452–8.
Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088–93.
Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43(5):436–41.
Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–30.
Andrews SJ, Fulton-Howard B, Goate A. Interpretation of risk loci from genome-wide association studies of Alzheimer's disease. Lancet Neurol. 2020;19(4):326–35.
Bellenguez C, Grenier-Boley B, Lambert JC. Genetics of Alzheimer's disease: where we are, and where we are going. Curr Opin Neurobiol. 2020;61:40–8.
Sims R, Hill M, Williams J. The multiplex model of the genetics of Alzheimer's disease. Nat Neurosci. 2020;23(3):311–22.
Marioni RE, Harris SE, Zhang Q, McRae AF, Hagenaars SP, Hill WD, et al. GWAS on family history of Alzheimer's disease. Transl Psychiatry. 2018;8(1):99.
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. 2019;51(3):404–13.
Schwartzentruber J, Cooper S, Liu JZ, Barrio-Hernandez I, Bello E, Kumasaka N, et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer's disease risk genes. Nat Genet. 2021;53(3):392–402.
Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS. Modifiable pathways in Alzheimer's disease: Mendelian randomisation analysis. BMJ. 2017;359:j5375.
Wang Z, Meng L, Shen L, Ji HF. Impact of modifiable risk factors on Alzheimer's disease: A two-sample Mendelian randomization study. Neurobiol Aging. 2020;91:167 e111–9.
Andrews SJ, Fulton-Howard B, O'Reilly P, Marcora E, Goate AM. Causal Associations Between Modifiable Risk Factors and the Alzheimer's Phenome. Ann Neurol. 2021;89(1):54–65.
Liu G, Zhao Y, Jin S, Hu Y, Wang T, Tian R, et al. Circulating vitamin E levels and Alzheimer's disease: a Mendelian randomization study. Neurobiol Aging. 2018;72:189 e181–9.
Wang L, Qiao Y, Zhang H, Zhang Y, Hua J, Jin S, et al. Circulating Vitamin D Levels and Alzheimer's Disease: A Mendelian Randomization Study in the IGAP and UK Biobank. J Alzheimers Dis. 2020;73(2):609–18.
He Y, Zhang H, Wang T, Han Z, Ni QB, Wang K, et al. Impact of Serum Calcium Levels on Alzheimer's Disease: A Mendelian Randomization Study. J Alzheimers Dis. 2020;76(2):713–24.
Fani L, Georgakis MK, Ikram MA, Ikram MK, Malik R, Dichgans M. Circulating biomarkers of immunity and inflammation, risk of Alzheimer's disease, and hippocampal volume: a Mendelian randomization study. Transl Psychiatry. 2021;11(1):291.
Lord J, Jermy B, Green R, Wong A, Xu J, Legido-Quigley C, et al. Mendelian randomization identifies blood metabolites previously linked to midlife cognition as causal candidates in Alzheimer's disease. Proc Natl Acad Sci U S A. 2021;118(16):e2009808118.
Anderson EL, Richmond RC, Jones SE, Hemani G, Wade KH, Dashti HS, et al. Is disrupted sleep a risk factor for Alzheimer's disease? Evidence from a two-sample Mendelian randomization analysis. Int J Epidemiol. 2021;50(3):817–28.
Zhuang Z, Yang R, Wang W, Qi L, Huang T. Associations between gut microbiota and Alzheimer's disease, major depressive disorder, and schizophrenia. J Neuroinflammation. 2020;17(1):288.
Li X, Tian Y, Yang YX, Ma YH, Shen XN, Chen SD, et al. Life Course Adiposity and Alzheimer's Disease: A Mendelian Randomization Study. J Alzheimers Dis. 2021;82(2):503–12.
Ma YH, Yang YX, Shen XN, Chen SD, Tan L, Dong Q, et al. Evaluation relationships between subjective wellbeing, personality traits, and Alzheimer's disease: A two-sample Mendelian randomization study. J Psychiatr Res. 2021;137:498–505.
Sproviero W, Winchester L, Newby D, Fernandes M, Shi L, Goodday SM, et al. High Blood Pressure and Risk of Dementia: A Two-Sample Mendelian Randomization Study in the UK Biobank. Biol Psychiatry. 2021;89(8):817–24.
Shen LX, Yang YX, Kuo K, Li HQ, Chen SD, Chen KL, et al. Social Isolation, Social Interaction, and Alzheimer's Disease: A Mendelian Randomization Study. J Alzheimers Dis. 2021;80(2):665–72.
Yeung CHC, Schooling CM. Systemic inflammatory regulators and risk of Alzheimer's disease: a bidirectional Mendelian-randomization study. Int J Epidemiol. 2021;50(3):829–40.
Zhuang Z, Gao M, Yang R, Liu Z, Cao W, Huang T. Causal relationships between gut metabolites and Alzheimer's disease: a bidirectional Mendelian randomization study. Neurobiol Aging. 2021;100:119 e115–8.
Kwok MK, Schooling CM. Herpes simplex virus and Alzheimer's disease: a Mendelian randomization study. Neurobiol Aging. 2021;99:101 e111–3.
Anderson EL, Howe LD, Wade KH, Ben-Shlomo Y, Hill WD, Deary IJ, et al. Education, intelligence and Alzheimer's disease: evidence from a multivariable two-sample Mendelian randomization study. Int J Epidemiol. 2020;49(4):1163–72.
Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533(7604):539–42.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304–14.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40.
Liu G, Zhang S, Cai Z, Ma G, Zhang L, Jiang Y, et al. PICALM Gene rs3851179 Polymorphism Contributes to Alzheimer's Disease in an Asian Population. Neuromolecular Med. 2013;15(2):384–8.
Dahl A, Cai N, Ko A, Laakso M, Pajukanta P, Flint J, et al. Reverse GWAS: Using genetics to identify and model phenotypic subtypes. PLoS Genet. 2019;15(4):e1008009.
Zhou M, Li H, Wang Y, Pan Y. Causal effect of insulin resistance on small vessel stroke and Alzheimer's disease: A Mendelian randomization analysis. Eur J Neurol. 2021. https://doi.org/10.1111/ene.15190. Online ahead of print.
Liu H, Zhang Y, Hu Y, Zhang H, Wang T, Han Z, et al. Mendelian randomization to evaluate the effect of plasma vitamin C levels on the risk of Alzheimer's disease. Genes Nutr. 2021;16(1):19.
Huang SY, Yang YX, Kuo K, Li HQ, Shen XN, Chen SD, et al. Herpesvirus infections and Alzheimer's disease: a Mendelian randomization study. Alzheimers Res Ther. 2021;13(1):158.
Zhang Z, Wang M, Liu X. C-reactive protein and risk of Alzheimer's disease. Neurobiol Aging. 2022;109:259–63.
Wang RZ, Yang YX, Li HQ, Shen XN, Chen SD, Cui M, et al. Genetically determined low income modifies Alzheimer's disease risk. Ann Transl Med. 2021;9(15):1222.
Wu BS, Zhang YR, Li HQ, Kuo K, Chen SD, Dong Q, et al. Cortical structure and the risk for Alzheimer's disease: a bidirectional Mendelian randomization study. Transl Psychiatry. 2021;11(1):476.
Zhang Z, Wang M, Yuan S, Cai H, Zhu SG, Liu X. Genetically Predicted Coffee Consumption and Risk of Alzheimer's Disease and Stroke. J Alzheimers Dis. 2021;83(4):1815–23.
Handy A, Lord J, Green R, Xu J, Aarsland D, Velayudhan L, et al. Assessing Genetic Overlap and Causality Between Blood Plasma Proteins and Alzheimer's Disease. J Alzheimers Dis. 2021;83(4):1825–39.
Li GH, Cheung CL, Cheung EY, Chan WC, Tan KC. Genetically Determined TSH Level Within Reference Range Is Inversely Associated With Alzheimer Disease. J Clin Endocrinol Metab. 2021;106(12):e5064–74.
Acknowledgements
We thank the International Genomics of Alzheimer's Project (IGAP) and Complex Trait Genetics lab (CTGlab), Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University & VU Medical Centre Amsterdam for providing summary results data. The investigators within IGAP contributed to the design and implementation of IGAP and/or provided data but did not participate in analysis or writing of this report. IGAP was made possible by the generous participation of the control subjects, the patients, and their families. The i-Select chips was funded by the French National Foundation on AD and related disorders. EADI was supported by the LABEX (laboratory of excellence program investment for the future) DISTALZ grant, Inserm, Institut Pasteur de Lille, Université de Lille 2, and the Lille University Hospital. GERAD was supported by the Medical Research Council (Grant n° 503480), Alzheimer's Research UK (Grant n° 503176), the Wellcome Trust (Grant n° 082604/2/07/Z) and German Federal Ministry of Education and Research (BMBF): Competence Network Dementia (CND) grant n° 01GI0102, 01GI0711, 01GI0420. CHARGE was partly supported by the NIH/NIA grant R01 AG033193 and the NIA AG081220 and AGES contract N01-AG-12100, the NHLBI grant R01 HL105756, the Icelandic Heart Association, and the Erasmus Medical Center and Erasmus University. ADGC was supported by the NIH/NIA grants: U01 AG032984, U24 AG021886, U01 AG016976, and the Alzheimer’s Association grant ADGC-10-196728. This research has been conducted using the UK Biobank resource (https://www.ukbiobank.ac.uk). We thank the individual patients who provided the sample that made data available; without them, the study would not have been possible.
Funding
This work was supported by funding from the National Natural Science Foundation of China (Grant No. 82071212, and 81901181), the Mathematical Tianyuan Fund of the National Natural Science Foundation of China (Grant No. 12026414), Beijing Natural Science Foundation (Grant No. JQ21022), and Beijing Ten Thousand Talents Project (Grant No. 2020A15). This work was also partially supported by funding from the Science and Technology Beijing One Hundred Leading Talent Training Project (Z141107001514006), the Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150802), the Funds of Academic Promotion Programme of Shandong First Medical University & Shandong Academy of Medical Sciences (No. 2019QL016, No. 2019PT007).
Author information
Authors and Affiliations
Contributions
G.Y.L., H.J.L., and Y.H. designed the study; G.Y.L., H.J.L., and Y.H. analyzed the data; all authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article contains human participants collected by several studies performed by previous studies. All participants gave informed consent in all the corresponding original studies, as described in the Materials and methods. Here, our study is based on the publicly available, large-scale datasets, and not the individual-level data. Hence, ethical approval was not sought.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. 162 independent SNPs that reached genome-wide significance (P < 5×10-8) in the pooled-sex EduYears meta-analysis of the discovery and replication samples (N =405,072). Supplementary Table 2. GWAS summary statistics of 159 educational attainment genetic variants in AD GWAS dataset. Supplementary Table 3. GWAS summary statistics of 147 educational attainment genetic variants in AD GWAX 2018 dataset. Supplementary Table 4. GWAS summary statistics of 159 educational attainment genetic variants in AD GWAX 2021 dataset. Supplementary Table 5. GWAS summary statistics of 147 educational attainment genetic variants in AD GWAS+GWAX 2018 dataset. Supplementary Table 6. GWAS summary statistics of 159 educational attainment genetic variants in AD GWAS+GWAX 2019 dataset. Supplementary Table 7. GWAS summary statistics of 159 educational attainment genetic variants in AD GWAS+GWAX 2021 dataset.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, H., Hu, Y., Zhang, Y. et al. Mendelian randomization highlights significant difference and genetic heterogeneity in clinically diagnosed Alzheimer’s disease GWAS and self-report proxy phenotype GWAX. Alz Res Therapy 14, 17 (2022). https://doi.org/10.1186/s13195-022-00963-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-022-00963-3