Abstract
Background
The prevalence of antimicrobial resistance (AMR) among Gram-negative bacteria is alarmingly high. Reintroduction of colistin as last resort treatment in the infections caused by drug-resistant Gram-negative bacteria has led to the emergence and spread of colistin resistance. This study was designed to determine the prevalence of drug-resistance among beta-lactamase-producing strains of Escherichia coli and Klebsiella pneumoniae, isolated from the clinical specimens received at a tertiary care centre of Kathmandu, Nepal during the period of March to August, 2019.
Methods
A total of 3216 different clinical samples were processed in the Microbiology laboratory of Kathmandu Model Hospital. Gram-negative isolates (E. coli and K. pneumoniae) were processed for antimicrobial susceptibility test (AST) by using modified Kirby-Bauer disc diffusion method. Drug-resistant isolates were further screened for extended-spectrum beta-lactamase (ESBL), metallo-beta-lactamase (MBL), carbapenemase and K. pneumoniae carbapenemase (KPC) production tests. All the suspected enzyme producers were processed for phenotypic confirmatory tests. Colistin resistance was determined by minimum inhibitory concentration (MIC) using agar dilution method. Colistin resistant strains were further screened for plasmid-mediated mcr-1 gene using conventional polymerase chain reaction (PCR).
Results
Among the total samples processed, 16.4% (529/3216) samples had bacterial growth. A total of 583 bacterial isolates were recovered from 529 clinical samples. Among the total isolates, 78.0% (455/583) isolates were Gram-negative bacteria. The most predominant isolate among Gram-negatives was E. coli (66.4%; 302/455) and K. pneumoniae isolates were 9% (41/455). In AST, colistin, polymyxin B and tigecycline were the most effective antibiotics. The overall prevalence of multidrug-resistance (MDR) among both of the isolates was 58.0% (199/343). In the ESBL testing, 41.1% (n = 141) isolates were confirmed as ESBL-producers. The prevalence of ESBL-producing E. coli was 43% (130/302) whereas that of K. pneumoniae was 26.8% (11/41). Similarly, 12.5% (43/343) of the total isolates, 10.9% (33/302) of E. coli and 24.3% of (10/41) K. pneumoniae were resistant to carbapenem. Among 43 carbapenem resistant isolates, 30.2% (13/43) and 60.5% (26/43) were KPC and MBL-producers respectively. KPC-producers isolates of E. coli and K. pneumoniae were 33.3% (11/33) and 20% (2/10) respectively. Similarly, 63.6% (21/33) of the E. coli and 50% (5/10) of the K. pneumoniae were MBL-producers. In MIC assay, 2.2% (4/179) of E. coli and 10% (2/20) of K. pneumoniae isolates were confirmed as colistin resistant (MIC ≥ 4 µg/ml). Overall, the prevalence of colistin resistance was 3.1% (6/199) and acquisition of mcr-1 was 16.6% (3/18) among the E. coli isolates.
Conclusion
High prevalence of drug-resistance in our study is indicative of a deteriorating situation of AMR. Moreover, significant prevalence of resistant enzymes in our study reinforces their roles in the emergence of drug resistance. Resistance to last resort drug (colistin) and the isolation of mcr-1 indicate further urgency in infection management. Therefore, extensive surveillance, formulation and implementation of effective policies, augmentation of diagnostic facilities and incorporation of antibiotic stewardship programs can be some remedies to cope with this global crisis.
Similar content being viewed by others
Background
Extensive and irrational use of antibiotics has led to the emergence and spread of antimicrobial resistance (AMR)—a condition in which pathogenic strains of bacteria develop resistance to the therapeutic antibiotics prescribed against it [1]. Realising the AMR as a global crisis, World Health Organization (WHO) and US Centres of Disease Control and Prevention (CDC) have already warned of an imminent global disaster and possibility of returning to the post-antibiotic era [2]. Gram-negative bacteria, mainly Enterobacterales, Acinetobacter baumannii and Pseudomonas aeruginosa are able to produce enzymes such as extended-spectrum beta-lactamases (ESBLs), AmpC beta-lactamases, carbapenemase and metallo-beta-lactamases (MBL), which enable the host bacteria to develop resistance to most classes of antibiotics in use [3]. All of these enzymes possess a similar mechanism to hydrolyse β-lactam ring of the antibiotics. ESBLs are class A β-lactamases that are responsible for resistant against oxy-imino cephalosporins (cefotaxime, ceftazidime, ceftriaxone, cefuroxime, and cefepime) and monobactams (aztreonam) [4]. Carbapenemase are the members of class A, B and D β-lactamases. Class A and D carbapenemase bring out the serine based hydrolytic mechanism while the class B carbapenemase are metallo- β-lactamases (MBL) that contain zinc based hydrolytic mechanism [5]. A novel kind of MBL, known as New Delhi metallo-β-Lactamase (NDM) possesses the ability to resist virtually all β-lactam antibiotics (except aztreonam) and carbapenems [6]. Similarly, K. pneumoniae carbapenemase (KPC), a derivative of carbapenemase (class A β-lactamases) also has become prominent because of their ability to inactivate carbapenems [7]. Although KPC is prevalent among K. pneumoniae, the enzyme has been frequently isolated from other Gram-negative bacilli [8].
Multidrug-resistant (MDR) bacteria—better known as superbugs—seriously limit the treatment options and thus are associated with increased mortalities, morbidities and economic burden [9]. On the other hand, narrowed treatment option is forcing clinicians to rely upon the “last line” drugs, primarily colistin (a polymyxin E antibiotics), which is reintroduced to counter the rapidly surging carbapenemase-producing Gram-negative bacteria [10]. Polymyxins (polymyxin B and polymyxin E) are cyclic lipopeptide discovered in the late 1940s [11] that were introduced in the treatment of infections caused by Gram-negative bacteria. However, they were no longer used due to their neuro- and nephro-toxicity and also due to the availability of comparatively ‘safer’ drugs such as beta-lactams [12]. Although their toxic effects were standstill, polymyxins were re-introduced in 1990s to counter the uncontrolled emanation of carbapenem resistant bacteria [13]. Polymyxins are now extensively used in modern clinics due to paucity of novel, effective and safer antibiotics [14]. Like with all other antibiotics, bacteria have managed to develop resistant to colistin, as a large number of studies suggest the emergence and globalization of colistin-resistance [10].
Until the first report of a variant of noble plasmid-mediated mobilized colistin resistance gene (mcr-1) in late 2015 in China, polymyxin (particularly, colistin) resistance was solely attributed to the regulatory changes mediated by the chromosomal genes (phoPQ, pmrAB, and mgrB) [2]. Since the first identification of mcr-1, several variants (from mcr-1 to mcr-9) have been reported from more than 40 countries across five different continents [15]. The mcr-1 encodes for phosphoethanolamine (pEtN) transferase enzyme (discovered in late 2015), which modifies the outer membrane lipopolysaccharides by adding pEtN to the phosphate groups in Lipid A thereby decreasing the net negative charges [2]. The resulting modification reduces the binding affinity of polymyxins to the bacterial cell wall [16, 17]. Unlike chromosomal mutation, acquisition of mcr is a matter of serious concern because of its potential transferability, as the gene is spread rapidly through the horizontal transfer at a higher rate than occurring through spontaneous mutation [18]. In addition, plasmids resistant to multiple classes of antibiotics can be transferred to other bacteria [19, 20]. Elevated endemicity of mcr genes all over the world in a short span of time is attributable to their ability to proliferate at a higher pace [21].
Since mcr gene was first isolated in an E. coli from animal sources in China, the plasmid-mediated colistin resistance may have transmitted from animals (colistin was extensively used as growth promoters for long times) to humans [22]. E. coli is the most prevalent species harbouring the mcr gene, accounting for approximately 91% of the entire load of mcr-positive bacteria, which is followed by Salmonella enterica (~ 7%) and K. pneumoniae (~ 2%) [23]. Higher burden of mcr among S. enterica than K. pneumoniae also supports the fact that the former is the food-borne pathogen and is very likely to be transmitted via food chain [24]. Moreover, these drug-resistant bacteria are isolated from humans, animals, and environments so that the perspective of ‘One Health’ has been jeopardized [25].
Implementation of effective surveillance programs and infection controls are considered as the two pillars to check the growth and spread of AMR [26]. However, in the developing countries like Nepal, circulation and co-circulation of resistant genes may go undetected, underreported and poorly characterized due to poor diagnostic facilities [27, 28]. In addition, irrational use of antibiotics among humans and animals (often as growth promoters) is putting pressure of potential outbreaks in the future [10]. Moreover, there are a limited number of studies on colistin resistance and the prevalence of resistance can vary and change over the time within and between the countries. Therefore, this study was conducted in a tertiary care center with an attempt to determine the prevalence of beta-lactamases including ESBL, MBL, KPC and colistin resistance among Gram-negative MDR pathogens. At the same time, we also aimed to explore the possible role of mcr genes in conferring resistance to colistin. Furthermore, antibiogram of the resistant strains to a variety of antibiotics was carried out to recognize the possible therapeutic options for combating superbugs.
Methods
Study design and study samples
This cross-sectional study was conducted from March to August, 2019 at Kathmandu Model Hospital, Nepal. A total of 3216 clinical samples consisting of urine (n = 1776), blood (n = 875), pus (n = 156), sputum (n = 187), body fluids (n = 88), wound swab (n = 51), tissue including femur, tibia, intestine region, and appendicular sites (n = 18), catheter and other tips (n = 12), and other samples including stool, urethral and vaginal swabs, bone, and bone marrow aspirate (n = 53) was collected and processed during the study period. Patients of all age-groups and gender who were admitted in or visiting the hospital for treatment were included in this study. All the samples with completely filled demographic information and having no visible signs of contamination were included in the study. However, others were rejected and requested for repetition, if possible.
Sample collection and transport
All the samples were aseptically collected following the standard microbiological procedure. Individual collection procedures varied in accordance to the type of samples. Generally, samples were collected in a dry, wide-mouthed, and leak-proof container and were sent to Microbiology Department without delay. In case of unwarranted delay, clinical specimens were refrigerated at 4 to 6 °C.
Culture, isolation and identification of bacteria
Each sample was processed by following the standard microbiological guidelines [29, 30].
Urine sample
Urine samples were inoculated into Cysteine Lactose Electrolyte Deficient (CLED) agar using sterile and standard calibrated loop. The plates were incubated at 37 °C overnight.
Blood and endotracheal and catheter tips
Blood specimen was inoculated aseptically into BHI broth at the ratio of 1: 10 and was incubated at 37 °C for 7 days and routinely inspected twice a day for at least first three days for microbial growth. Then broth from the culture bottle showing visible signs of microbial growth was sub-cultured in Blood agar (BA), MacConkey agar (MA) and Salmonella-Shigella agar (SS) and plates were incubated at 37 °C for 24 h [31].
Sputum and throat swab
Sputum samples were inoculated into BA, chocolate agar (CA) and MA plates. For sputum, in CA plate a 5 g\(\mu\) optochin disc and a 10U bacitracin disc were added to screen S. pneumoniae and H. influenzae respectively whereas for throat swab, 0.05U bacitracin disc was added to the plate to screen Streptococcus pyogenes. CA and BA were incubated at 37 °C overnight in 5–10% CO2 environment whereas the MA plate was incubated at 37 °C in an aerobic condition [31].
Pus, pus swab, and wound swab
These samples were inoculated into BA and MA plates, and inoculated at 37 °C for 24 h. In case of swab, an initial inoculum was made by rubbing the swab over the media plate in order to transfer maximum number of organisms. Then the streaking was performed [31].
Body fluids: Body fluid samples were centrifuged before culture. The sediment after centrifugation was inoculated into BA, MA and CA plates. The BA and CA plates were incubated in 5–10% CO2 enriched atmosphere and MA plates were incubated aerobically at 37 °C overnight [31].
Identification of the bacterial isolates
Following incubation, culture plates were observed for possible microbial growth. Isolates were presumably identified on the basis of Gram’s staining and colony characteristics. Further confirmation of the isolates were based on biochemical tests such as IMViC (Indole production, Methyl red test, Voges-Proskauer test and Citrate utilization), H2S production, catalase test, coagulase test, and oxidase test [30, 32].
Antibiotic susceptibility test (AST)
E. coli and K. pneumoniae isolates were further subjected to in-vitro antibiotic susceptibility assay by using modified Kirby-Bauer disk diffusion method as recommended by Clinical Laboratory Standard Institute [33]. Nitrofurantoin (300 µg), cefotaxime (30 µg), cotrimoxazole (25 µg), cefixime (5 µg), amoxycillin (10 µg), ofloxacin (5 µg), levofloxacin (5 µg), gentamicin (10 µg), moxifloxacin (5 µg), ceftazidime (30 µg), amoxycillin/clavulanate (20/10 µg), amikacin (30 µg), ciprofloxacin (5 µg), chloramphenicol (30 µg), azithromycin (15 µg), cefoperazone/sulbactam (75/30 µg), meropenem (10 µg), imipenem (10 µg), ertapenem (10 µg), piperacillin/tazobactam (100/10 µg), doxycycline (30 µg), cefepime (5 µg), ampicillin/sulbactam (10/10 µg), polymyxin-B (100 µg), colistin (10 µg), and tigecycline (15 µg) discs were tested for susceptibility assay. In this method, broth culture of test bacteria (comparable to McFarland tube no.0.5; inoculums density 1.5 × 108 bacteria/ml) was uniformly carpeted on the surface of Mueller Hinton agar (MHA). Then, antibiotics discs were placed onto the lawn culture of the test bacteriaby sterile forceps. The inoculated and seeded MHA plates were incubated at 37 ℃ for 24 h. After incubation, zone of inhibition was measured and results were interpreted as sensitive, intermediate and resistant [33]. Isolates showing resistance to at least one agent of three or more classes of antimicrobial agents were termed as multidrug-resistant (MDR) [34].
Screening of the ESBL production
ESBL-producers were screened by using Ceftazidime (30 µg) and Cefotaxime (30 µg) in the AST. Isolates showing reduced susceptibility to one or both of these drugs with diameter of the zone of inhibition for ceftazidime ≤ 22 mm and cefotaxime ≤ 27 mm were considered as potential ESBL-producers [33]. Suspected strains were further processed using confirmatory assay.
Confirmation of the ESBL-producers by phenotypic method
Combination disc test (CDT) as prescribed by the CLSI was used for the confirmation of ESBL-producing strains. In this method, cefotaxime (30 µg) and ceftazidime (30 µg) discs alone and in combination with clavulanic acid (10 µg) (ceftazidime plus clavulanic acid, 30/10 µg and cefotaxime plus clavulanic acid, 30/10 µg) were used. The zone of inhibition of cephalosporin disc alone was compared with their respective cephalosporin/clavulanic acid (combined) disc. An increase in zone of inhibition by ≥ 5 mm in the presence of clavulanic acid was considered as confirmed ESBL production [33].
Screening for carbapenemase and/or KPC producers
In AST, isolates showing resistance to carbapenem drugs (imipenem 10 µg, meropenem 10 µg, and ertapenem 10 µg) were suspected as potential carbapenemase-producers [27].
Phenotypic confirmatory test for carbapenemase and/or KPC producers
Inhibitor-based method was followed for the confirmation of carbapenemase and KPC production. In this method, combined disc test of carbapenem with and without phenyl boronic acid (PBA) was employed. An increase in the diameter of zone of inhibition by ≥ 5 mm in combined disc (carbapenem disc supplemented with PBA) than single disc (only carbapenem disc) was considered as confirmed test for carbapenem or KPC production [35, 36].
Phenotypic confirmatory test for MBL production
Confirmation of MBL production was made by inhibition method in which Ethylene Diamine Tetra Acetic Acid (EDTA) was used as an inhibitor. Two imipenem (10 µg) discs were placed on MHA and 10 µl of 0.5 M EDTA solution was added to one of the discs to obtain the desired concentration. After overnight incubation, an increase in the diameter of zone of inhibition by 7 mm in combined disc (imipenem disc supplemented with EDTA) than single one (only imipenem disc) was considered as confirmed test for MBL production [37].
Determination of MIC of colistin
Agar dilution method was used to determine the minimum inhibitory concentration of colistin. Different concentrations of colistin ranging from 2 µg/ml to 32 µg/ml were prepared in the agar medium. Bacterial inoculum was applied readily onto the agar surface and the plates were incubated at 37 °C upto 18 h. The MIC end point was determined as the lowest concentration of antibiotics that completely inhibits the visible growth. Isolates having a MIC of ≤ 4 μg/mL is considered colistin susceptible while MIC of > 4 μg/mL is considered colistin resistant [22, 38].
Plasmid and genomic DNA extraction
Isolated colonies of bacteria were inoculated in Luria–Bertani (LB) broth and incubated overnight at 37 °C. After incubation, alkaline-lysis method was adopted to extract the DNA. Plasmid DNA of E. coli and K. pneumoniae was extracted by using phenol–chloroform method [39]. Extracted plasmid DNA and genomic DNA was then suspended in TE buffer and preserved at − 20 °C until further processing [39].
PCR amplification of mcr-1 gene
Amplification of mcr-1 gene was carried out by conventional PCR using primers: 5′-CGGTCAGTCCGTTTGTTC-3′ as forward primer and 5′-CTTGGTCGGTCTGTAGGG-3′ as reverse primer [2]. A PCR mixture having the final volume of 25 µl (3 µl of template DNA, 0.5 µl each forward and reverse primers and 21 µl of PCR master mix) was used for the reaction mixture.
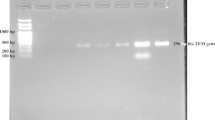
The thermal condition for amplification was initial heat activation of 95ºC for 15 min followed by 35 cycles of denaturation at 94 ℃ for 30 s; annealing at 57ºC for 90 s; extension at 72 ℃ for 90 s; and final extension at 72 ℃ for 10 min. The amplified products were subjected to gel electrophoresis (2.0% agarose gel stained with ethidium bromide) at 100 V for 60 min and visualized under UV transilluminator [22].
Quality control during antimicrobial susceptibility and MIC assays
Each batch of media, reagents and antibiotic discs were checked for their lot number, expiry date, and proper storage. Similarly, purity plates were used to ensure the pure culture of test organisms. Control strain of E. coli ATCC 25922 was used during AST.
Data analysis
All the data were entered in the worksheet of Statistical Package for Social Sciences (SPSS) software (Version 25). The results have been presented in the form of tables and figures. Chi-square (χ2) test was applied to test the association between the variables. A p value of < 0.05 was considered as statistically significant.
Results
Distribution of bacterial isolates
Among the total samples processed, 16.4% (529/3216) had bacterial growth.. Higher percent of bacterial isolates were obtained from urine samples (304/529; 57.5%) followed by pus (81/529; 15.3%) and blood samples (49/529; 9.3%). More than half of the isolates (300/529; 56.7%) were recovered from the female population. The bacterial infection was in high percentage in the age group 16–45 years (260/529; 49.1%) followed by the older age group above 60 years (158/529; 29.9%) (Table 1).
Out of 529 culture positive specimens, 50 samples showed polymicrobial growth and 473 samples showed monomicrobial growth. Due to polymicrobial infections, 583 isolates were recovered from 529 samples. Among total isolates, 78.0% (455/583) were Gram-negative bacteria. The most predominant isolate among Gram-negatives was E. coli (66.4%; 302/455) followed by K. pneumoniae (9.0%; 41/455) and Acinetobacter calcoaceticus baumannii complex (6.2%; 28/455), Pseudomonas aeruginosa (4.6%; 21/455), Salmonella enterica Typhi (3.7%, 17/455) and Salmonella enterica Paratyphi (2.9%; 13/455). Small portion of the isolates included other bacteria such as Citrobacter koseri, Serratia marscescens, Acinetobacter lwoffii, Klebsiella oxytoca, and Neisseria gonorrheae. Distribution of the isolates is depicted in the Fig. 1.
In this way, 9.4% (301/3216) and 1.3% (35/3216) isolates of E. coli and K. pneumoniae respectively were obtained from the total sample processed. Majority of these isolates were obtained from urine (78.9%; 265/336) followed by pus (9.2%; 30/336) and wound swab (3.9%; 13/336). Respectively, 240 and 25 isolates of E. coli and K. pneumoniae were from urine samples. Similarly, large number of samples processed and the isolates recovered were predominantly obtained from female patients of the age group of 16–45 years (Additional file 1; Table 2).
Antibiotic susceptibility pattern of E. coli and K. pneumoniae
All of the tested isolates of E. coli and K. pneumoniae were susceptible to colistin, polymixin B and tigecycline. E. coli isolates were also susceptible to antibiotics nitrofurantoin (93.5%; only for uropathogens), gentamycin (87.8%) and amikacin (89.8%). However, all of the tested E. coli isolates were resistant to azithromycin. Similarly, K. pneumoniae isolates were resistant to amoxycillin and azithromycin. Resistance of K. pneumoniae towards cephalosporin antibiotics include: cefepime (57.1%), ceftazidime (55.6%) and cefotaxime (51.2%). None of the isolates was resistant to ciprofloxacin, colistin, polymyxin-B and tigecycline (Table 3).
Prevalence of MDR, ESBL producing bacteria, and carbapenem resistant bacteria
The overall prevalence of MDR among both of the isolates was 58.0% (199/343). Individually, 59.2% (179/302) of the total E. coli isolates and 48.7% (20/41) of the K. pneumoniae isolates were reported as MDR (Table 4).
Of 343 bacterial isolates, 41.1% (n = 141) isolates were confirmed as ESBL-producers. The prevalence of ESBL-producing E. coli was 43% (130/302) whereas that of K. pneumoniae was 26.8% (11/41) (Table 4).
The prevalence of carbapenem resistance among both the isolates was 12.5% (43/343). Comparatively, higher percentage of carbapenem resistance was documented among E. coli (10.9%; 33/302) than K. pneumoniae (24.3%; 10/41) (Table 4).
Distribution of KPC and MBL among carbapenem resistant E. coli and K. pneumoniae
Among 43 carbapenem resistant isolates, 30.2% (13/43) and 60.5% (26/43) were KPC and MBL-producers respectively. Both KPC and MBL production was reported higher among E. coli in comparison to K. pneumoniae. 33.3% (11/33) and 20% (2/10) of the isolates of E. coli and K. pneumoniae were KPC-producers respectively. Similarly, 63.6% (21/33) and 50% (5/10) of the E. coli and K. pneumoniae were MBL-producers, respectively (Fig. 2).
Heat map showing the distribution of MDR, carbapenem resistant and ESBL, KPC and MBL producers among E. coli (A) and K. pneumoniae (B) in different clinical samples. The red box indicates more prevalent in the clinical specimen compared to green box. The colour gradient from red to green displays a linear scale of the percent distribution from high to low as a measure of the MDR, carbapenem resistant and ESBL, KPC and MBL producers
Determination of MIC and colistin resistant E. coli and K. pneumoniae isolates
In MIC assay, 2.2% (4/179) of E. coli and 10% (2/20) of K. pneumoniae isolates were confirmed as colistin resistant (MIC ≥ 4 µg/ml). Overall, the prevalence of colistin resistance among the tested isolates was 3.01% (6/199) (Table 5). MIC of colistin-resistant isolates of E. coli and K. pneumoniae ranged from ≤ 4 µg/ml to 8 µg/ml and from ≤ 8 µg/ml to 16 µg/ml respectively.
PCR amplification of mcr-1 in colistin resistant and sensitive E. coli and K. pneumoniae
Out of four phenotypically colistin-resistant E. coli, 3 (75.0%) and 2 (50%) of them harboured plasmid-mediated and chromosomal mcr-1 gene respectively (Table 5). In contrast, none of the phenotypically colistin resistant K. pneumoniae harboured mcr-1 gene.
Among the 193 colistin sensitive MDR isolates, PCR amplification for mcr-1 was performed with 18 E. coli isolates exhibiting an MIC breakpoint of 2 μg/ml. Out of 18 MDR E. coli with MIC 2 µg/ml, 16.6% (3/18) and 61.1% (11/18) harboured plasmid-mediated and chromosomal mcr-1 genes respectively (Table 5).
Antibiotic resistance profiles of colistin resistant and sensitive isolates
Antibiotic resistance profiles of colistin resistance E. coli and K. pneumoniae were determined. Colistin resistant 100% E. coli isolates were resistant to cefotaxime, cefixime, amoxycillin, ofloxacin, levofloxacin, amoxycillin/clavulanate, and doxycycline where as colistin resistant 100% K. pneumoniae isolates were resistant to nitrofurantoin, cefotaxime, cefixime, amoxycillin, ofloxacin, and levofloxacin (Fig. 3, Table 6).
Discussion
Drug-resistance, especially pan-drug resistance and MDR is emerging as a major challenge in the treatment of infections caused by Gram-negative bacteria. As surveillance of AMR and early response to the infection control are crucial steps to address the issues, this study also aimed to determine the status of MDR among E. coli and K. pneumoniae isolates and to investigate possible acquisition of mcr-1 in colistin resistant isolates. In this study, most of the isolates were resistant to commonly prescribed broad-spectrum antibiotics. In addition, prevalence of colistin resistance and acquisition of mcr-1 among the drug-resistant isolates was also observed in this study.
In this study, 16.4% (529/3216) specimens showed bacterial growth in which prevalence of Gram-negative bacteria from different clinical specimens was much higher than the Gram-positives. This finding concords with previous studies reported from Nepal [9, 10, 40,41,42,43,44]. Higher prevalence of E. coli in comparison to other species could be due to being normal flora of human gut which is highly opportunistic in immunocompromised patients. When E. coli reaches out to the tissues other than its common site, it serves as an opportunistic pathogen. A number of virulence factors encoded by pathogenic strains of E. coli enable them to colonize the human body in spite of effective host defence [45].
In this study, all of the isolates of E. coli and K. pnemoniae were susceptible to colistin, polymyxin B and tigecycline, which is comparable to some previous findings [46, 47]. These classes of antibiotics can be effective drugs in the management of Gram-negatives. Conversely, all of the isolates were resistant towards azithromycin. Previous exposure of the isolates to these antibiotics as well as the state of resistance genes of corresponding antibiotics may be the reasons for their susceptibility patterns [48].
In this study, increased resistance to third-generation cephalosporins was observed, as more than half of the isolates were non-susceptible to those drugs. Similar findings have been reported by some previous studies [47, 49, 50]. Higher rate of resistance to cephalosporins can be attributable to their irrational prescription and uses [51].
Resistance rate of E. coli to fluoroquinolones in this study ranged from 47 to 55% which are in agreement with earlier studies from Nepal [52, 53] and India [54]. Resistance to fluroquinolones among MDR Gram-negative bacteria is common and is expected to sustain and perhaps accelerate even if other antibiotics are used [55]. The prevalence of fluroquinolone resistance is related to the intensity of antibiotics used, which may reduce the efficacy of drug in a progressive manner [56].
In this study, almost one fourth of E. coli isolates were resistant to carbapenem antibiotics. The resistance rate towards these antibiotics ranged from < 3.0% to 21.0% in some of the previous studies from Nepal [50, 52, 53] whereas 100% sensitivity towards imipenem was reported in some other studies [47, 57]. Production of beta-lactamase enzymes and the upregulation of efflux pump are suggested as the reasons for reduced susceptibility [58]. However, comparatively low resistance to carbapenem antibiotics reported in this study could be due to the lower use of these antibiotics in the treatment of infections [59].
In AST assay of K. pneumoniae, two-third of the isolates were susceptible to fluoroquinolones and gentamicin. This finding is similar to another study reported from Nepal [60]. However, some other studies have reported lower sensitivity rates (less than 50.0%) [57, 61]. This variation may be due to the difference in the specimens included in the study as well as the exposure of isolates towards the antibiotics. All of the K. pneumoniae isolates were resistant to amoxicillin. Similar findings were reported in other studies [52, 60]. In addition, reduced sensitivity towards cephalosporin and carbapenem antibiotics was observed in this study. High resistant rate towards cephalosporins was also reported in previous studies [50, 52, 62, 63]. Multiple factors such as extensive use of drugs, production of beta-lactamases, or efflux pumps (which actively pump out these antibiotics) are attributable to the rise in the resistance against carbapenems [26, 64].
Among the total (343) isolates of E. coli and K. pneumoniae, more than half (58.0%) were MDR. MDR strains were predominant among the isolates of E. coli in comparison to K. pneumoniae. This result was in consistent with previous findings which also reported the rate of MDR in a range of 41.0%–67.7% [9, 20, 28, 52, 60] while lower than some other findings [8, 10]. In this study, the prevalence of MDR E. coli and K. pneumoniae was 59.3% and 48.8% respectively. Common risk factors associated with development of MDR are poor hygiene, misuse of antibiotics and absence of antimicrobial surveillance program [65, 66]. Higher rate of antibiotic resistance among E. coli and Klebsiella spp. is associated with their ability to produce different kinds of β-lactamases primarily ESBL, AmpC and MBL, and carriage of resistance trait for quinolones and aminoglycosides in the plasmid [67]. In several hospitals in Nepal, the antibiotics used for the treatment of infected patients are effective in curing only a half of the cases whereas other half of the treatment course shows no response [68]. In addition, development of partial resistance by bacteria, most antibiotics intended to cure people are becoming less effective which might also be the reason of increasing prevalence of MDR reported in this study [26].
In this study, the prevalence of ESBL producing strains was found to be 41.1% among Gram-negative isolates. This result is comparable to some previous reports from Nepal [52, 69]. The prevalence of ESBL production was reported low in other studies [70,71,72]. The difference in the prevalence of ESBL production can be partially due to geographical variations, type of specimens processed, and local practices of antibiotics prescription and use [73].
In our study, among 343 isolates of E. coli and K. pneumoniae, 12.5% were resistant to carbapenem. In earlier study reported from Kathmandu Model Hospital, the prevalence of carbapenem resistant ranged from 4.5% to 20.0% among the members of Enterobacteriaceae [74]. However, higher rate of carbapenem resistant among Enterobacteriaceae was reported in other studies [75, 76]. The difference in utilization of carbapenem antibiotics to treat infections in different study settings may be responsible for these variations [75].
Carbapenem resistant isolates were subjected to KPC and MBL production test phenotypically. In this assay, 30.2% and 60.5% isolates were KPC and MBL-producers respectively. Our findings are comparable to a previous finding [77]. The prevalence of KPC production in E. coli and K. pneumoniae was 33.3% and 20.0% respectively in our study. Similarly, MBL production was reported 63.6% in E. coli and 50.0% in K. pneumoniae. In a previous study, KPC production in E. coli was reported as 14.4% and 7.1% in K. pneumoniae [8]. Accordingly, another study reported comparatively lower incidence of MBL producing-E. coli and K. pneumoniae in different clinical samples in Central Nepal [78]. Similarly, lower rate of MBL (9.0%) and KPC (6.5%) production was reported in E. coli [79]. Another study from Iran reported 80.5% of K. pneumoniae isolates as KPC-producers [80]. This study revealed higher prevalence of MBL and KPC production in E. coli and K. pneumoniae which may be due to dissemination of plasmid encoded carbapenem resistance genes [59].
This study reported comparatively low prevalence (3.0%) of colistin resistant E. coli and K. pneumoniae isolates (3.0%). Similar result was reported in previous findings of 0.3% in Switzerland [81], 0.7% in Spain [82] and in a previous report of global antimicrobial surveillance programs [83, 84]. Low prevalence of the resistant isolates in our study may be attributable to the presence of low number of mcr-1 positive bacteria and lesser use of colistin use for the treatment of community acquired infections [81]. However, other studies from India [85] and Thailand [86] reported the prevalence rate of colistin resistant as high as 32.0% and 71.3% respectively. In this study, rates of colistin resistance were different among the bacterial species. Higher prevalence of colistin resistance was reported in K. pneumoniae (10.0%) when compared to E. coli (2.2%). Different studies also showed that higher prevalence of colistin resistant K. pneumoniae than E. coli [87, 88]. The main risk factor for the development of colistin resistance is related to the extensive and irrational use of colistin in antimicrobial therapy [89].
MIC range of colistin for E. coli ranged from ≤ 2 µg/ml to 8 µg/ml which was lesser than that of K. pneumoniae (≤ 2 µg/ml to 16 µg/ml). This finding is in agreement with a previous study from China [90]. MIC range of mcr-1 positive Enterobacteriaceae typically have a moderate level 4–16 mg/l of colistin resistant strains [91]. MIC of colistin resistant isolates carrying mcr-1 was lower in this study. Exceptionally, MIC range of colistin in colistin resistant K. pneumoniae without mcr-1 was high in this study. This result suggests that colistin resistance in K. pneumoniae might be associated with chromosomal mutations in mgrB, phoP/phoQ, pmrA, pmrB, pmrC and crrABC [92]. High MIC may also be due to strong selective pressure in the isolates. These strains may carry another variant of mcr gene [93].
Strength and limitations
This is the first study from Nepal which also determined the prevalence of mcr-1 among colistin susceptible isolates. In addition, this study is one among a handful of studies that attempted to investigate the role of the beta-lactamases (ESBL, MBL, and carbapenemase) and acquisition of mcr-1 among Gram-negative isolates. The findings of this study can be an important reference tool for policy makers and clinicians which can ameliorate the practice of antibiotic prescription and use in the country. However, this study suffers from some limitations. Firstly, this study was conducted among small population of a tertiary care centre so that the reported rates cover a limited geographical region and may not reflect overall picture of epidemiology across the country. Secondly, this study tested the acquisition of only one variant (mcr-1) of mcr gene; isolation and characterization of all variants (mcr-1 to mcr-9) is suggested in future studies to predict the role of genes in conferring resistance. In addition, this study could not predict the origin and possible transferability of resistant strains. The insertion sequence ISApl1 plays an important role in the mobilization of this mcr-1 gene. However, in this study only mcr-1 gene was analysed. Therefore, further molecular analysis can better predict other possible mechanisms responsible for colistin resistance and role of insertion sequence in dissemination of this gene.
Conclusion
High burden of MDR strains in our study could be due to the pervasive and irrational practices of antibiotic prescription and use, as half of the Gram-negative isolates were found as drug-resistant. Moreover, the presence of colistin resistance and acquisition of mcr-1 among clinical isolates warrants an imminent threat of no antibiotic era. Therefore, prompt action is recommended for proper infection control. Augmentation of diagnostic facilities, AMR surveillance, and antibiotic stewardship programs can be some effective measures to address the problem of drug-resistance in the country.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AMR:
-
Antimicrobial Resistance
- AST:
-
Antimicrobial susceptibility testing
- CLSI:
-
Clinical and Laboratory Standard Institute
- CPE:
-
Cytopathic effect
- CRE:
-
Carbapenem resistant E. coli
- ESBL:
-
Extended spectrum β-lactamase
- GNB:
-
Gram-negative bacteria
- ICU:
-
Intensive care unit
- kDa:
-
Kilo-Dalton
- LPS:
-
Lipopolysaccharide
- mcr-1 :
-
Mobilized colistin resistance
- MDR:
-
Multidrug resistance
- MIC:
-
Minimum inhibitory concentration
- ORF:
-
Open reading frame
- PCR:
-
Polymerase chain reaction
- UTI:
-
Urinary tract infection
- WHO:
-
World Health Organization
- WGS:
-
Whole Genome Sequencing
References
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109:309–18.
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8.
Shad A. MCR-1 colistin resistance in Escherichia coli wildlife: a continental mini-review. J Drug Metab Toxicol. 2018;9:243. https://doi.org/10.4172/2157-7609.1000243.
Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents. 2010;35:316–21.
Bush K, Bradford PA. Epidemiology of beta-lactamase-producing pathogens. Clin Microbiol Rev. 2020. https://doi.org/10.1128/CMR.00047-19.
Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54.
Swathi CH, Chikala R, Ratnakar KS, Sritharan V. A structural, epidemiological & genetic overview of Klebsiella pneumoniae carbapenemases (KPCs). Indian J Med Res. 2016;144:21–31.
Dhungana K, Awal BK, Dhungel B, Sharma S, Banjara MR, Rijal KR. Detection of Klebsiella pneumoniae carbapenemase (KPC) and metallo betalactamae (MBL) producing Gram negative bacteria isolated from different clinical samples in a transplant Center, Kathmandu. Nepal ASMI. 2019;2(12):60–9.
Kayastha K, Dhungel B, Karki S, Adhikari B, Banjara MR, Rijal KR, Ghimire P. Extended-Spectrum beta-Lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting International Friendship Children’s Hospital, Kathmandu, Nepal. Infect Dis (Auckl). 2020;13:1178633720909798.
Muktan B, Thapa Shrestha U, Dhungel B, Mishra BC, Shrestha N, Adhikari N, Banjara MR, Adhikari B, Rijal KR, Ghimire P. Plasmid mediated colistin resistant mcr-1 and co-existence of OXA-48 among Escherichia coli from clinical and poultry isolates: first report from Nepal. Gut Pathog. 2020;12:44.
Ayoub Moubareck C. Polymyxins and bacterial membranes: a review of antibacterial activity and mechanisms of resistance. Membranes (Basel). 2020;10:181.
Yu Z, Qin W, Lin J, Fang S, Qiu J. Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed Res Int. 2015;2015:679109.
Dandachi I, Sokhn ES, Dahdouh EA, Azar E, El-Bazzal B, Rolain JM, Daoud Z. Prevalence and characterization of multi-drug-resistant gram-negative bacilli isolated from lebanese poultry: a nationwide study. Front Microbiol. 2018;9:550.
Lenhard JR, Bulman ZP, Tsuji BT, Kaye KS. Shifting gears: the future of polymyxin antibiotics. Antibiotics (Basel). 2019;8:42.
Luo Q, Wang Y, Xiao Y. Prevalence and transmission of mobilized colistin resistance (mcr) gene in bacteria common to animals and humans. Biosafety Health. 2020;2:71–8.
Trimble MJ, Mlynarcik P, Kolar M, Hancock RE. Polymyxin: alternative mechanisms of action and resistance. Cold Spring Harb Perspect Med. 2016;6: a025288
Sun J, Zhang H, Liu YH, Feng Y. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018;26:794–808.
Fodor A, Abate BA, Deak P, Fodor L, Gyenge E, Klein MG, Koncz Z, Muvevi J, Otvos L, Szekely G, et al. Multidrug resistance (MDR) and collateral sensitivity in bacteria, with special attention to genetic and evolutionary aspects and to the perspectives of antimicrobial peptides-a review. Pathogens. 2020;9:522.
Thapa Shrestha U, Shrestha S, Adhikari N, Rijal KR, Shrestha B, Adhikari B, Banjara MR, Ghimire P. plasmid profiling and occurrence of beta-lactamase enzymes in multidrug-resistant uropathogenic Escherichia coli in Kathmandu, Nepal. Infect Drug Resist. 2020;13:1905–17.
Aryal SC, Upreti MK, Sah AK, Ansari M, Nepal K, Dhungel B, Adhikari N, Lekhak B, Rijal KR. Plasmid-mediated AmpC beta-lactamase CITM and DHAM genes among gram-negative clinical isolates. Infect Drug Resist. 2020;13:4249–61.
Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther. 2012;10:917–34.
Bista S, Thapa Shrestha U, Dhungel B, Koirala P, Gompo TR, Shrestha N, Adhikari N, Joshi DR, Banjara MR, Adhikari B, et al. Detection of plasmid-mediated colistin resistant mcr-1 gene in Escherichia coli isolated from infected chicken livers in Nepal. Animals (Basel). 2020;10:2060
Anyanwu MU, Jaja IF, Nwobi OC. Occurrence and characteristics of mobile colistin resistance (mcr) gene-containing isolates from the environment: a review. Int J Environ Res Public Health. 2020;17:1028.
Zurfluh K, Stephan R, Widmer A, Poirel L, Nordmann P, Nuesch HJ, Hachler H, Nuesch-Inderbinen M. Screening for fecal carriage of MCR-producing Enterobacteriaceae in healthy humans and primary care patients. Antimicrob Resist Infect Control. 2017;6:28.
Argudin MA, Deplano A, Meghraoui A, Dodemont M, Heinrichs A, Denis O, Nonhoff C, Roisin S. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics (Basel). 2017;6:12.
Acharya KP, Wilson RT. Antimicrobial resistance in Nepal. Front Med. 2019. https://doi.org/10.3389/fmed.2019.00105.
Gurung S, Kafle S, Dhungel B, Adhikari N, Thapa Shrestha U, Adhikari B, Banjara MR, Rijal KR, Ghimire P. Detection of OXA-48 gene in carbapenem-resistant Escherichia coli and Klebsiella pneumoniae from urine samples. Infect Drug Resist. 2020;13:2311–21.
Sah RSP, Dhungel B, Yadav BK, Adhikari N, Thapa Shrestha U, Lekhak B, Banjara MR, Adhikari B, Ghimire P, Rijal KR. Detection of TEM and CTX-M genes in Escherichia coli isolated from clinical specimens at tertiary care heart hospital, Kathmandu. Diseases. 2021;9:15.
American Society for Microbiology. Manual of Clinical Microbiology. 2nd edition: ASM Press, 2011.
Forbes BA. DS, Weissfelt SA. Bailey and Scott;s diagnostic Microbiology: Mosby Publication; 2007.
Chesbrough M. District laboratory practice in tropical countries, part II. 2nd ed. New York: Cambridge University Press; 2006.
Collee JG, Mackie TJ, McCartney JE. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. p. 131–49.
Clinical and Laboratory Standards Institute (2018). Performance standards for antimicrobial susceptibility testing. 28th edition Informational supplement M100-S28. Wayne PCalsi.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Giske CG, Gezelius L, Samuelsen O, Warner M, Sundsfjord A, Woodford N. A sensitive and specific phenotypic assay for detection of metallo-beta-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin Microbiol Infect. 2011;17:552–6.
Tsakris A, Kristo I, Poulou A, Themeli-Digalaki K, Ikonomidis A, Petropoulou D, Pournaras S, Sofianou D. Evaluation of boronic acid disk tests for differentiating KPC-possessing Klebsiella pneumoniae isolates in the clinical laboratory. J Clin Microbiol. 2009;47:362–7.
Sachdeva R, Sharma B, Sharma R. Evaluation of different phenotypic tests for detection of metallo-beta-lactamases in imipenem-resistant Pseudomonas aeruginosa. J Lab Physicians. 2017;9:249–53.
Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1):5–16.
Sambrook J, Russell D. Molecular cloning: A Laboratory Manual (3rd edition). New York: Cold Spring Harbor Lab Press; 2001.
Guragain N, Pradhan A, Dhungel B, Banjara MR, Rijal KR, Ghimire P. Extended spectrum B-lactamase producing Gram Negative bacterial isolates from urine of patients visiting Everest Hospital, Kathmandu. Nepal TUJM. 2019;6(1):26–31.
Raut S, Rijal KR, Khatiwada S, Karna S, Khanal R, Adhikari J, Adhikari B. Trend and characteristics of Acinetobacter baumannii infections in patients attending universal college of medical sciences, Bhairahawa, western Nepal: a longitudinal study of 2018. Infect Drug Resist. 2020;13:1631–41.
Thakur P, Ghimire P, Rijal KR, Singh GK. Antimicrobial resistance pattern of Escherichia coli isolated from urine samples in patients visiting tertiary health care centre in eastern Nepal. Sunsari Tech Coll J. 2012;1(1):22–6.
Upadhyaya G, Bhattarai A, Rijal KR, Ghimire P, Upadhyaya B. Urinary tract infections in Kidney transplant patients of Kathmandu Valley. Int J Microbiol Res Rev. 2013;3(1):1–6.
Tamang K, Shrestha P, Koirala A, Khadka J, Gautam N, Rijal KR. Prevalence of bacterial uropathogens among diabetic patients attending padma nursing Hospital of Western Nepal. Himalayan J Sci Techonl. 2017;1:15–9.
Bien J, Sokolova O, Bozko P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Int J Nephrol. 2012;2012:681473.
Ansari S, Nepal HP, Gautam R, Shrestha S, Neopane P, Gurung G, Chapagain ML. Community acquired multi-drug resistant clinical isolates of Escherichia coli in a tertiary care center of Nepal. Antimicrob Resist Infect Control. 2015;4:15.
Parajuli NP, Maharjan P, Joshi G, Khanal PR. Emerging perils of extended spectrum beta-lactamase producing enterobacteriaceae clinical isolates in a teaching hospital of Nepal. Biomed Res Int. 2016;2016:1782835.
Soares GM, Figueiredo LC, Faveri M, Cortelli SC, Duarte PM, Feres M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J Appl Oral Sci. 2012;20:295–309.
Yadav KK, Adhikari N, Khadka R, Pant AD, Shah B. Multidrug resistant Enterobacteriaceae and extended spectrum beta-lactamase producing Escherichia coli: a cross-sectional study in National Kidney Center, Nepal. Antimicrob Resist Infect Control. 2015;4:42.
Adhikari RP, Shrestha S, Rai JR, Amatya R. Antimicrobial resistance patterns in clinical isolates of Enterobacteriaceae. Nepalese Med J. 2018;1(2):74–8.
Mshana SE, Kamugisha E, Mirambo M, Chakraborty T, Lyamuya EF. Prevalence of multiresistant gram-negative organisms in a tertiary hospital in Mwanza, Tanzania. BMC Res Notes. 2009;2:49.
Nepal K, Pant ND, Neupane B, Belbase A, Baidhya R, Shrestha RK, Lekhak B, Bhatta DR, Jha B. Extended spectrum beta-lactamase and metallo beta-lactamase production among Escherichia coli and Klebsiella pneumoniae isolated from different clinical samples in a tertiary care hospital in Kathmandu, Nepal. Ann Clin Microbiol Antimicrob. 2017;16:62.
Shrestha B, Tada T, Shrestha S, Kattel HP, Ohara H, Kirikae T, Rijal BP, Sherchand JB, Pokhrel BM. Carbapenem-resistant Escherichia coli among the hospitalized patients with urinary tract infections in a tertiary care center of Nepal. PMJN. 2014;14(1):29–33.
Yadav M, Khumanthem SD, Kshtrimayum MD. Antibiotic resistance trends of uropathogenic Escherichia coli isolated from inpatients in a tertiary care hospital in north east India. Inter J Recent Scientific Res. 2017;8(7):18496–500.
Friedrich LV, White RL, Bosso JA. Impact of use of multiple antimicrobials on changes in susceptibility of Gram-negative aerobes. Clin Infect Dis. 1999;28:1017–24.
Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46:2540–5.
Bhandari P, Thapa G, Pokhrel BM, Bhatta DR, Devkota U. Nosocomial isolates and their drug resistant pattern in ICU Patients at National Institute of Neurological and Allied Sciences. Nepal Inter J Microb. 2015;2015:6.
Rodriguez-Martinez JM, Poirel L, Nordmann P. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53:4783–8.
Codjoe FS, Donkor ES. Carbapenem resistance: a review. Med Sci (Basel). 2017;6:1.
Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes. 2012;5:38.
Bhatta DR, Hamal D, Shrestha R, Supram HS, Joshi P, Nayak N, Gokhale S. Burden of multidrug resistant respiratory pathogens in intensive care units of tertiary care hospital. Asian J Med Sci. 2019;10(2):14–9.
Estabraghi E, Salehi TZ, Amini K, Jamshidian M. Molecular identification of extended-spectrum β-lactamase and integron genes in Klebsiella pneumoniae. J Nepal Med Assoc. 2016;54(202):72–8.
Rizwan MAM, Najmi A, Singh K. Escherichia coli and Klebsiella pneumoniae sensitivity/resistance pattern towards antimicrobial agents in primary and simple urinary tract infection patients visiting university hospital of Jamia Hamdard New Delhi. Drug Res. 2018;68(07):415–20.
Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med. 2009;360:439–43.
Jaggi N, Sissodia P, Sharma L. Control of multidrug resistant bacteria in a tertiary care hospital in India. Antimicrob Resist Infect Control. 2012;1:23.
Kanafani ZA, Mehiosibai SA, Araj GF, Kanaan M, Kanj SS. Epidemiology and risk factors for extended-spectrum beta-lactamase-producing organisms: a case control study at a tertiary care center in Lebanon. Am J Infect Control. 2005;33(6):326–32.
Poudyal S, Bhatta DR, Shakya G, Upadhyaya BR, Dumre SP, Buda G, Kandel BP. Extended spectrum beta-lactamase producing multidrug resistant clinical bacterial isolates at National Public Health Laboratory, Nepal. Nepal Med Coll J. 2011;13(1):34–8.
Ghimire S, Nepal S, Bhandari S, Nepal P, Palaian S. A prospective surveillance of drug prescribing and dispensing in a teaching hospital in western Nepal. J Pak Med Assoc. 2009;59:726–31.
Lohani B, Thapa M, Sharma L, Adhikari H, Sah AK, Khanal AB, Basnet RB, Aryal M. Predominance of CTX-M type extended spectrum β-lactamase (ESBL) producers among clinical isolates of Enterobacteriaceae in a tertiary care hospital, Kathmandu. Nepal Open Microbiol J. 2019;13:28–33.
Pathak P, Jaishi N, Yadav BK, Shah PK. Prevalence of extended spectrum beta lactamase (ESBL) and metallo beta lactamase (MBL) mediated resistance in gram negative bacterial pathogens. TUJM. 2017;4(1):49–54.
Bhandari R, Pant ND, Poudel A, Sharma M. Assessment of the effectiveness of three different cephalosporin/clavulanate combinations for the phenotypic confirmation of extended-spectrum beta-lactamase producing bacteria isolated from urine samples at National Public Health Laboratory, Kathmandu, Nepal. BMC Res Notes. 2016;9:390.
Shakya P, Shrestha D, Maharjan E, Sharma VK, Paudyal R. ESBL production among E. coli and Klebsiella spp. causing urinary tract infection: a hospital based study. Open Microbiol J. 2017;11:23–30.
Nag VL, Ayyagari A, Venakatesh V, Ghar M, Yadav V, Prasad KN. Drug resistant Haemophilus influenzae from respiratory tract infection in a tertirary care hospital North India. Indian J Chest Dis Allied Sci. 2001;43(1):13–7.
Karn S, Panta ND, Neupane S, Khatiwada S, Basnyat S, Shrestha B. Prevalence of carbapenem resistant bacterial strains isolated from different clinical samples: study from a tertiary care hospital in Kathmandu. Nepal J Biomed Sci. 2016;3(1):11–5.
Gashaw M, Berhane M, Bekele S, Kibru G, Teshager L, Yilma Y, Ahmed Y, Fentahun N, Assefa H, Wieser A, Gudina EK, Ali S. Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: a cross sectional study. Antimicrob Resist Infect Control. 2018;7:138.
Pokharel K, Dawadi BR, Bhatt CP, Gupte S, Jha B. Resistance pattern of carbapenem on Enterobacteriaceae. J Nepal Med Assoc. 2018;56(214):931–5.
Kumar S, Mehra SK. Performance of modified hodge test and combined disc test for detection of carbapenemases in clinical isolates of Enterobacteriaceae. Int J Curr Microbiol App Sci. 2015;4(5):255–61.
Bora A, Sanjana R, Jha BK, Mahaseth SN, Pokharel K. Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in central Nepal. BMC Res Notes. 2014;7:557.
Chaudhary U, Agrawal S, Raghuramahan K. Identification of extended spectrum beta lactamases, AmpC and carbapenemase production among isolates of Escherichia coli in North Indian tertiary care center. Avicenna J Med. 2018;8:46–50.
Bina M, Pournajaf A, Mirkalantari S, Talebi M, Irajian G. Detection of the Klebsiella pneumoniae carbapenemase (KPC) in K. pneumoniae isolated from the clinical samples by the phenotypic and genotypic methods. Iran J Pathol. 2015;10(3):199–205.
Liassine N, Aaaouvie L, Descombes MC, Tendon VD, Kieffer N, Poirel L, Nordmann P. Very low prevalence of MCR-1/MCR-2 plasmid-mediated colistin-resistance in urinary tract Enterobacteriaceae in Switzerland. Inter J Infect Dis. 2016;51:4–5.
Prim N, Rivera A, Rodriguez-Navarro J, Espanol M, Turbau M, Coll P, Mirelis B. Detection of mcr-1 colistin resistance gene in polyclonal Escherichia coli isolates in Barcelona, Spain, 2012 to 2015. Euro Surveill. 2016;21(13):30183.
Bradford PA, Kazmierczak KM, Biedenbach DJ, Wise MG, Hackel M, Sahm DF. Correlation of beta-lactamase production and colistin resistance among Enterobacteriaceae isolates from a global surveillance program. Antimicrob Agents Chemother. 2015;60:1385–92.
Castanheira M, Griffin MA, Deshpande LM, Mendes RE, Jones RN, Flamm RK. Detection of mcr-1 among Escherichia coli clinical isolates collected worldwide as part of the SENTRY antimicrobial surveillance program in 2014 and 2015. Antimicrob Agents Chemother. 2016;60:5623–4.
Manohar P, Shanthini T, Ayyanar R, Bozdogan B, Wilson A, Tamhankar AJ, Nachimuthu R, Lopes BS. The distribution of carbapenem- and colistin-resistance in Gram-negative bacteria from the Tamil Nadu region in India. J Med Microbiol. 2017;66:874–83.
Eiamphungporn W, Yainoy S, Jumderm C, Tan-arsuwongkul R, Tiengrim S, Thamlikitkul V. Prevalence of the colistin resistance gene mcr-1 in colistin-resistant Escherichia coli and Klebsiella pneumoniae isolated from humans in Thailand. J Global Antimicrob Res. 2018;2018(15):32–5.
Kaza P, Mahindroo J, Veeraraghavan B, Mavuduru RS, Mohan B, Taneja N. Evaluation of risk factors for colistin resistance among uropathogenis isolates of Escherichia coli and Klebsiella pneumoniae: a case-control study. J Med Microbiol. 2019;68(6):837–47.
Moosavian M, Emam N. The first report of emerging mobilized colistin-resistance (mcr) genes and ERIC-PCR typing in Escherichia coli and Klebsiella pneumoniae clinical isolates in southwest Iran. Infect Drug Resist. 2019;12:1001–10.
Ah YM, Kim AJ, Lee JY. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents. 2014;44:8–15.
Quan J, Li X, Chen Y, Jiang Y, Zhou Z, Zhang H, Sun L, Ruan Z, Feng Y, Akova M, Yu Y. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis. 2017;17:400–10.
Walkty A, Karlowsky JA, Adam HJ, Lagace-Wiens P, Baxter M, Mulvey MR, McCracken M, Poutanen SM, Roscoe D, Zhanel GG. Frequency of mcr-1-mediated colistin resistance among Escherichia coli clinical isolates obtained from patients in Canadian hospitals (CANWARD 2008–2015). CMAJ Open. 2016;4(4):641–5.
Poirel L, Kieffer N, Nordmann P. In vitro study of ISApl1-mediated mobilization of the colistin resistance gene mcr-1. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/AAC.00127-17.
Dalmolin TV, Lima-Morales DD, Barth AL. Plasmid-mediated colistin resistance: what do we know? J Infectiol. 2018;1(2):16–22.
Acknowledgements
We express our sincere gratitude to all the faculties and laboratory assistants of Central Department of Microbiology, Tribhuvan University, Kathmandu, Nepal and Kathmandu Model Hospital, Nepal.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceiving and designing the study: DK, BD, MRB and KRR; supervision: MRB, BS, and PG. providing the primers for PCR: PRJ; lab work and data collection: DK, SB, AK; data curation: DK, SB, AK, MRB and KRR; data analysis: KRR, BD and MRB; drafting the manuscript: BD and KRR; reviewing, editing and finalizing the draft: KRR, PG, MRB and BD. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Institutional Review committee of Kathmandu Model Hospital (Reg. No: 006-2019). Written informed consent was obtained from each patient enrolled in the study. This study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All the authors declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Characteristics of infected patients and samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Karki, D., Dhungel, B., Bhandari, S. et al. Antibiotic resistance and detection of plasmid mediated colistin resistance mcr-1 gene among Escherichia coli and Klebsiella pneumoniae isolated from clinical samples. Gut Pathog 13, 45 (2021). https://doi.org/10.1186/s13099-021-00441-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-021-00441-5