Abstract
Background
Extended spectrum beta-lactamase (ESBL) and metallo beta-lactamase (MBL) production in Klebsiella pneumoniae and Escherichia coli are the commonest modes of drug resistance among these commonly isolated bacteria from clinical specimens. So the main purpose of our study was to determine the burden of ESBL and MBL production in E. coli and K. pneumoniae isolated from clinical samples. Further, the antimicrobial susceptibility patterns of E. coli and K. pneumoniae were also determined.
Methods
A cross-sectional study was conducted at Om Hospital and Research Centre, Kathmandu, Nepal by using the E. coli and K. pneumoniae isolated from different clinical samples (urine, pus, body fluids, sputum, blood) from May 2015 to December 2015. Antimicrobial susceptibility testing was performed by Kirby-Bauer disc diffusion technique. Extended spectrum beta-lactamase production was detected by combined disc method using ceftazidime and ceftazidime/clavulanic acid discs and cefotaxime and cefotaxime/clavulanic acid discs. Similarly, metallo beta-lactamase production was detected by combined disc assay using imipenem and imipenem/ethylenediaminetetracetate discs. Bacteria showing resistance to at least three different classes of antibiotics were considered multidrug resistant (MDR).
Results
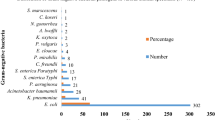
Of total 1568 different clinical samples processed, 268 (17.1%) samples were culture positive. Among which, E. coli and K. pneumoniae were isolated from 138 (51.5%) and 39 (14.6%) samples respectively. Of the total isolates 61 (34.5%) were ESBL producers and 7 (4%) isolates were found to be MBL producers. High rates of ESBL production (35.9%) was noted among the clinical isolates from outpatients, however no MBL producing strains were isolated from outpatients. Among 138 E. coli and 39 K. pneumoniae, 73 (52.9%) E. coli and 23 (59%) K. pneumoniae were multidrug resistant. The lowest rates of resistance was seen toward imipenem followed by piperacillin/tazobactam, amikacin and cefoperazone/sulbactam.
Conclusions
High rate of ESBL production was found in the E. coli and K. pneumoniae isolated from outpatients suggesting the dissemination of ESBL producing isolates in community. This is very serious issue and can’t be neglected. Regular monitoring of rates of ESBL and MBL production along with multidrug resistance among clinical isolates is very necessary.
Similar content being viewed by others
Background
The drug resistance among the gram negative bacteria is present as a serious global problem [1]. ESBLs are the important members of beta-lactamases produced mainly by gram negative bacteria [2] and are responsible for mediating resistance to extended-spectrum cephalosporins and monobactam aztreonam [3]. These enzymes are commonly detected in the members of the enterobacteriaceae like Klebsiella pneumoniae and Escherichia coli [3]. ESBL producing bacteria do not show resistance only to penicillins, most cephalosporins and aztreonam but also to other classes of antibiotics such as aminoglycosides, cotrimoxazole, tetracycline and fluoroquinolones [4, 5]. Further, the easy transmission of the ESBLs coding plasmids between the species has become a major threat mainly in hospitalized patients, often the infections caused by organisms producing ESBL being involved in outbreaks [6, 7]. Carbapenems are the drugs of choice for the treatment of the infections caused by ESBL producing bacteria [8]. However over the past few years, carbapenem resistance due to metallo-beta-lactamases (MBLs) production has been increasingly reported among clinical isolates from all around the world [9]. Metallo-beta-lactamase needs bivalent metal ions mainly zinc for its activation and resistance to carbapenems is mainly mediated by this enzyme [10].
The rapid increasing rate of MBL production among the members of enterobacteriaceae, mainly E. coli and K. pneumoniae, which are the most common causes of infections among human is present as a serious global public health problem [9]. There are limited treatment options for the infections caused by ESBL and MBL producing bacteria [11] due to which the treatments of such infections are very difficult often resulting into treatment failure. The regular surveillance of the drug resistance among the clinical isolates will be helpful to know the actual gravity of the situation, hence to formulate the necessary policy to reduce the incidence of drug resistance among the bacteria. Further, the knowledge about the local antimicrobial susceptibility patterns will be helpful to start timely proper preliminary treatment. So, in this study we determined the burden of ESBL and MBL production in E. coli and K. pneumoniae isolated from clinical samples. In addition, we also determined their antimicrobial susceptibility patterns.
Methods
A cross-sectional study was conducted at Om Hospital and Research Centre, Kathmandu, Nepal, a 150 bedded hospital by using the E. coli and K. pneumoniae isolated from total of 1568 different clinical samples (urine, pus, body fluids, sputum, blood) from May 2015 to December 2015. The colonies grown after culturing of samples using standard microbiological techniques were identified with the help of biochemical tests [12, 13]. Antimicrobial susceptibility testing was performed by Kirby-Bauer disc diffusion technique following clinical and laboratory standards institute guidelines [14]. For performing antimicrobial susceptibility testing 90 mm diameter petri-plates were used and in one plate 6 antibiotic discs were tested. The diameter of the susceptibility zone was measured with the help of Vernier caliper.
Screening and confirmation of ESBL producers
The isolates were screened for possible ESBL production using ceftazidime (30 μg) and cefotaxime (30 μg). According to the CLSI guidelines, the isolates showing reduced susceptibility to at least one of these drugs with zone of inhibition for ceftazidime ≤ 22 mm and cefotaxime ≤ 27 mm were considered as the possible ESBL producing strains. The suspected ESBL producing strains were confirmed for ESBL production by combined disc assay using ceftazidime (30 μg) and ceftazidime/clavulanic acid (30/10 μg) discs and cefotaxime (30 μg) and cefotaxime/clavulanic acid discs (30/10 μg). The zones of inhibition for the ceftazidime and cefotaxime discs were compared to those of the ceftazidime/clavulanic acid and cefotaxime/clavulanic acid discs. An increase in zone diameter of ≥ 5 mm in the presence of clavulanic acid was confirmed as positive for ESBL production [14]. Bacteria showing resistance to at least three different classes of antibiotics were considered multidrug resistant [15].
Detection of MBL producers
The isolates showing resistance to imipenem were subjected to confirmation for MBL production by combined disc assay using imipenem and imipenem/ethylenediaminetetracetate discs [16].
Results
Of total 1568 different clinical samples processed, 268 (17.1%) samples showed bacterial growth. Among which, E. coli and K. pneumoniae were isolated from 138 (51.5%) and 39 (14.6%) samples respectively. Out of 138 E. coli, 84% isolates were from urine followed by pus (7.9%). Likewise, out of 39 K. pneumoniae, 51.3% isolates were from urine followed by sputum (17.9%) (Table 1).
Distribution of the isolates on the basis of type and department of the patients
A total of 128 (72.3%) isolates were from outpatients and 49 (27.7%) from inpatients. Among the isolates, 78.3% and 21.7% of E. coli were obtained from outpatients and inpatients respectively. Similarly, 51.3 and 48.7% of K. pneumoniae were obtained from outpatients and inpatients respectively. Highest percentage of the isolates were obtained from general medicine department (35.5%) followed by obstetrics and gynecology (31.1%). Maximum percentage (35.5%) of E. coli were isolated from general medicine followed by obstetrics and gynecology (33.3%). Most of the K. pneumoniae were isolated from intensive care unit (25.6%) (Table 2).
Antibiotic resistance patterns of the isolates
The lowest rate of resistance was seen toward imipenem followed by piperacilli/tazobactam, amikacin and cefoperazone/sulbactam. All isolates were found to be resistant to amoxicillin (Table 3). Out of total 177 isolates, 96 (54.2%) were multidrug resistant. Among 138 E. coli and 39 K. pneumoniae, 73 (52.9%) E. coli and 23 (59%) K. pneumoniae were multidrug resistant.
ESBL and MBL production among the isolates
Out of 177 isolates, 121 isolates of E. coli and 33 isolates of K. pneumoniae were suspected as ESBL producers on primary screening test. Among them, 46 isolates of E. coli and 15 isolates of K. pneumoniae were confirmed as ESBL producers. Of the total isolates 61 (34.5%) were ESBL producers. Of total 61 ESBL positive strains detected, 51 were detected by both ceftazidime and ceftazidime/clavulanic acid discs and cefotaxime and cefotaxime/clavulanic acid discs, while 4 were detected by ceftazidime and ceftazidime/clavulanic acid discs only and 6 by cefotaxime and cefotaxime/clavulanic acid discs only.
Similarly, 9 isolates (3 E. coli and 6 K. pneumoniae) were suspected as MBL producers on the basis of resistance to imipenem. 7 (2 E. coli and 5 K. pneumoniae) (4%) isolates were found to be MBL producers.
Distribution of ESBL and MBL producing isolates on the basis of samples
The distribution of ESBL producing strains according to different samples is presented in Table 4. Similarly, 1 of MBL producing bacterium was isolated from each urine, catheter tip and pus samples, while 2 MBL producing bacteria were isolated from each sputum and suction tip.
Distribution of ESBL and MBL producing bacteria on the basis of type and department of patients
Most of the ESBL producing isolates were from outpatients. Among the outpatients, most of the isolates were from general medicine (43.3%) followed by obstetrics and gynecology department (27.9%). Similarly, among the inpatients, ESBL producing bacteria were more frequent in intensive care unit (16.4%) than wards (8.2%) (Table 5). MBL producing strains were not isolated from outpatients. 3 MBL producing bacteria were isolated from intensive care unit and 4 from wards.
Resistance patterns of ESBL and MBL producing isolates
No ESBL producing strains were found to be resistant to imipenem. Similarly, among other antibiotics tested lowest rate of resistance was seen toward cefoperazone/sulbactam, piperacillin/tazobactam and amikacin (Table 6). MBL producing strains showed high rates of resistance to the antibiotics tested.
Discussion
Similar rate of growth positivity as in our study was also reported by Poudyal et al. (16.9%) [17]. In our study, E. coli (51.5%) and K. pneumoniae (14.7%) were most frequently isolated gram negative bacteria. Similar isolation rates for E. coli and K. pneumoniae were also reported by Poudyal et al. [17]. E. coli and K pneumoniae are among the commonest bacteria isolated from clinical specimens. Majority of the isolates in our study were from urine samples. This may be due to the larger number of urine samples included in our study. Further, E. coli and K. pneumoniae are common cause of urinary tract infection [18].
Higher numbers of isolates were from outpatient department in comparison to inpatient department. This may be attributed to the larger number of samples being included from outpatients.
Misuse of antibiotic is responsible for higher incidence of antibiotic resistance among bacteria [19]. We found the incidence of multidrug resistant bacteria to be 54.2%, with 52.9% of E. coli and 59% of K. pneumoniae being multidrug resistant. Different studies in Nepal have found the rates of multidrug resistance among E. coli to be ranging from 38.2 to 95.52% and those for K. pneumoniae to be 25–100% [17, 20, 21]. Common risk factors associated with infection by multidrug resistant bacteria are hospitalization and previous use of antibiotics [22].
In our study the rate of ESBL production was 34.5 with 33.3% of the E. coli and 38.5% of the K. pneumoniae being ESBL positive. The prevalence of ESBL producing E. coli and K. pneumoniae was found as low as 18.2 and 4.1% respectively in a study conducted by Raut et al. [23] and as high as 80% for E. coli [17] and 90.9% for K. pneumoniae [24]. The worldwide prevalence of ESBL production among the clinical isolates is found to be ranging from < 1 to 74% [25].
In our study, cefotaxime-clavulante combination disc identified numerically more confirmed ESBL producers in comparison to ceftazidime-clavulanate, which was analogous to the findings by Poudyal et al. [17] and Ranjini et al. [26].
Lower rate of MBL production in comparison to the study by Bora et al. (E. coli = 18.98%, K. pneumoniae = 21.08%) was reported in our study [9]. However, in other studies conducted in different countries showed the rates of MBL production to be ranging from 13.4 to 61.5% for E. coli and 33–36% for K. pneumoniae [27,28,29].
In our study high rate of ESBL production was observed among the bacteria isolated from out patients, which is very serious and shows the dissemination of ESBL producing bacteria to the community. However, no MBL producing organism was isolated from outpatients.
The prevalence of drug resistant bacteria may not only vary from countries to countries but also from institutions to institutions and this can be partially explained by the difference in local antibiotic prescribing habits and difference in effectiveness of infection control program in different health institutes.
In our study, the highest rate of susceptibility of the bacteria was found toward imipenem followed by pipercillin/tazobactum, amikacin and cefoperazone/sulbactum. These findings were in harmony with the findings of other studies conducted by Ansari et al. [30], Kader and Kumar [31] and Shashwati et al. [32].
Conclusions
ESBL and MBL production along with multidrug resistance among E. coli and K. pneumoniae are presenting as the serious problem in Nepal. The high rate of ESBL production among the isolates from outpatients is very serious issue, which suggests the dissemination of ESBL producing isolates in community. Regular monitoring of rate of ESBL and MBL production along with multidrug resistance among these clinical isolates is very necessary. Further, to control the emergence of drug resistance strict policy to rationalize the use of antibiotics is necessary. On the basis of the drug resistance patterns we found in our study imipenem followed by piperacilli/tazobactam, amikacin and cefoperazone/sulbactam may be used for the preliminary treatment of the infections caused by E. coli and K. pneumoniae in our setting.
Abbreviations
- ESBL:
-
extended spectrum beta-lactamase
- MBL:
-
metallo beta-lactamase
- MDR:
-
multidrug resistant
- ICU:
-
intensive care unit
References
Gniadkowski M. Evolution and epidemiology of extended-spectrum beta-lactamases (ESBLs) and ESBL-producing microorganisms. Clin Microbiol Infect. 2001;7(11):597–608.
Haque R, Salam MA. Detection of ESBL producing nosocomial gram negative bacteria from a tertiary care hospital in Bangladesh. Pak J Med Sci. 2010;26(4):887–91.
El-Baky RMA, El-Azeim NHA, Gad GFM. Prevalence of extended-spectrum beta-lactamase, AmpC Beta-lactamase, and metallo-beta-lactamase among clinical isolates of Pseudomonas aeruginosa. J Adv Biotechnol Bioeng. 2013;1:22–9.
Colodner R. Extended-spectrum beta-lactamases: a challenge for clinical microbiologists and infection control specialists. Am J Infect Control. 2005;33(2):104–7.
Rawat D, Nair D. Extended-spectrum ß-lactamases in gram negative bacteria. J Global Infect Dis. 2010;2:263–74.
Rangachari RK, Mathavi SK, Priyadharsini I. Detection of extended spectrum beta-lactamase producing gram negative bacilli in urinary isolates. Int J Biol Med Res. 2010;1(4):130–2.
Tonkic M, Goic-Barisic I, Punda-Polic V. Prevalence and antimicrobial resistance of extended-spectrum beta-lactamases-producing Escherichia coli and Klebsiella pneumoniae strains isolated in a university hospital in Split, Croatia. Int Microbiol. 2005;8(2):119–24.
Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86(3):250–9.
Bora A, Sanjana R, Jha BK, Mahaseth SN, Pokharel K. Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in central Nepal. BMC Res Notes. 2014;7:557.
Manoharan A, Chatterjee S, Mathai D, SARI Study Group. Detection and characterization of metallo beta lactamases producing Pseudomonas aeruginosa. Indian J Med Microbiol. 2010;28(3):241–4.
Mishra SK, Acharya J, Kattel H, Pokhrel BM, Rijal BP. Extended-spectrum beta-lactamase and metallo-beta-lactamase-producing bacterial strains among the patients attending a tertiary care center in Nepal. Int J Infect Dis. 2012;16:e425.
Cheesbrough M. District laboratory practice in tropical countries, part II. 2nd ed. New York: Cambridge University Press; 2006.
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. Bergey’s manual of determinative bacteriology. Baltimore: Williamsons and Wilkins; 1994.
Clinical and Laboratory Standards Institute. CLSI document M100-S25. Performance standards for antimicrobial susceptibility testing: Twenty fifth informational supplement ed. Wayne: CLSI; 2015.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40(10):3798–801.
Poudyal S, Bhatta DR, Shakya G, Upadhyaya B, Dumre SP, Buda G, et al. Extended Spectrum beta-lactamase producing multidrug resistant clinical bacterial isolates at National Public Health Laboratory, Nepal. Nepal Med Coll J. 2011;13(1):34–8.
Behzadi P, Behzadi E, Yazdanbod H, Aghapour R, Akbari Cheshmeh M, Salehian Omran D. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar). 2010;5(2):111–5.
Neupane S, Pant ND, Khatiwada S, Chaudhary R, Banjara MR. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob Resist Infect Control. 2016;5:5.
Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes. 2012;5:38.
Yadav KK, Adhikari N, Khadka R, Pant AD, Shah B. Multidrug resistant Enterobacteriaceae and extended spectrum β-lactamase producing Escherichia coli: a cross-sectional study in National Kidney Center, Nepal. Antimicrob Resist Infect Control. 2015;4:42.
Chaudhary NK, Murthy MS. Prevalence of multidrug resistance in uropathogenic Klebsiella species with reference to extended spectrum β-lactamases production. Res J Pharm Biol Chem Sci. 2013;4(3):728–35.
Raut S, Gokhale S, Adhikari B. Prevalence of extended spectrum beta-lactamases among Escherichia coli and Klebsiella spp. isolates in Manipal Teaching Hospital, Pokhara, Nepal. J Microbiol Infect Dis. 2015;5(2):69–75.
Pathak J, Pokharel N. Multidrug resistant and extended spectrum β-lactamase (ESBL) isolates from different clinical specimens. Int J Sci and Res Pub. 2015;5:1–5.
Thokar MA, Fomda BA, Maroof P, Ahmed K, Bashir D, Bashir G. Proliferation of extended spectrum ß–lactamase (ESBL) producing Gram negative bacteria, diagnostic inputs and impact on selection of Antimicrobial therapy. Phys Acad. 2010;4(3):25–31.
Ranjini CY, Kasukurthi LR, Madhumati B, Rajendran R. Prevalence of multidrug resistance and extended spectrum beta-lactamases among uropathogenic Escherichia coli isolates in a tertiary care hospital in South India: an alarming trend. Community Acquir Infect. 2015;2:19–24.
Enwuru NV, Enwuru CA, Ogbonnia SO, Adepoju-Bello AA. Metallo-Β-lactamase production by Escherichia Coli and Klebsiella species isolated from Hospital and Community subjects in lagos, Nigeria. Nat Sci. 2011;9(11):1–5.
Mate PH, Devi KS, Devi KM, Damrolien S, Devi NL, Devi PP. Prevalence of carbapenem resistance among Gram-Negative bacteria in a Tertiary Care Hospital in North-East India. IOSR J Dent Med Sci. 2014;13(12):56–60.
Wadekar MD, Anuradha K, Venkatesh D. Phenotypic detection of ESBL and MBL in clinical isolates of Enterobacteriaceae. Int J curr Res Aca Rev. 2013;1(3):89–95.
Ansari S, Nepal HP, Gautam R, Shrestha S, Neopane P, Gurung G, et al. Community acquired multi-drug resistant clinical isolates of Escherichia coli in a tertiary care center of Nepal. Antimicrob Resist Infect Control. 2015;4:15.
Kader AA, Kumar AK. Prevalence of extended spectrum beta-lactamase among multidrug resistant gram-negative isolates from a general hospital in Saudi Arabia. Saudi Med J. 2004;25:570–4.
Shashwati N, Kiran T, Dhanvijay AG. Study of extended spectrum β-lactamase producing Enterobaceriaceae and antibiotic co-resistance in a tertiary care teaching hospital. J Nat Sci Biol Med. 2014;5:30–5.
Authors’ contributions
NDP and KN designed and conceived the study, carried out the research works, analyzed data, and prepared the final manuscript. AB, BN and RB carried out the research works and analyzed the data. RKS, BL, DRB and BJ monitored the study. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank, Golden Gate International College, Kathmandu, Nepal and OM Hospital and Research Center, Kathmandu, Nepal for providing the opportunity to conduct this research. The authors would also like to thank all the patients and the technical staffs for their help during the study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data related to this study can be made available by the authors of this article if requested.
Consent for publication
Not applicable.
Funding
To conduct this study no fund was obtained from any sources.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nepal, K., Pant, N.D., Neupane, B. et al. Extended spectrum beta-lactamase and metallo beta-lactamase production among Escherichia coli and Klebsiella pneumoniae isolated from different clinical samples in a tertiary care hospital in Kathmandu, Nepal. Ann Clin Microbiol Antimicrob 16, 62 (2017). https://doi.org/10.1186/s12941-017-0236-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-017-0236-7