Abstract
Background
Vitamin D supplementation exerts several supporting effects on improving glycemic status, however, results are inconclusive. Thus, in the present study, we aimed to conduct an umbrella of meta-analysis regarding the impact of vitamin D on type 2 diabetes (T2DM) biomarkers.
Methods
The Scopus, PubMed, Web of Science, Embase, and Google Scholar online databases were searched up to March 2022. All meta-analyses evaluating the impact of vitamin D supplementation on T2DM biomarkers were considered eligible. Overall, 37 meta-analyses were included in this umbrella meta-analysis.
Results
Our findings indicated that vitamin D supplementation significantly decreased fasting blood sugar (FBS) (WMD = − 3.08; 95% CI: − 3.97, − 2.19, p < 0.001, and SMD = − 0.26; 95% CI: − 0.38, − 0.14, p < 0.001), hemoglobin A1c (HbA1c) (WMD = − 0.05; 95% CI: − 0.10, − 0.01, p = 0.016, and SMD = − 0.16; 95% CI: − 0.27, − 0.05, p = 0.004), insulin concentrations (WMD = − 2.62; 95% CI: − 4.11, − 1.13; p < 0.001, and SMD = − 0.33; 95% CI: − 0.56, − 0.11, p = 0.004), and homeostatic model assessment for insulin resistance (HOMA-IR) (WMD = − 0.67; 95% CI: − 1.01, − 0.32, p < 0.001, and SMD = − 0.31; 95% CI: − 0.46, − 0.16, p < 0.001).
Conclusion
This umbrella meta-analysis proposed that vitamin D supplementation may improve T2DM biomarkers.
Similar content being viewed by others
Background
Impaired glucose metabolism is associated with an increased risk of several chronic diseases, including obesity, Type 2 diabetes (T2DM), metabolic syndrome, and cardiovascular disease [1]. Both genetic predispositions and unhealthy lifestyles might incorporate into hyperglycemic complications. The actual genetic origin of hyperglycemia has not yet been identified, however there is robust evidence that obesity, unhealthy eating patterns, and sedentary lifestyles are the main modifiable non-genetic risk factors [2, 3]. Although one of the most important first-line treatments for hyperglycemia is dietary modification, however, their effectiveness is modest [4, 5]. Recently, nutritional adjuvant therapies, such as chromium [6], magnesium [7], omega-3 fatty acids [8], and vitamin C [9] have been given more attention due to the adverse effects of pharmacological treatments. Among others, vitamin D has been well studied in clinical practice for its therapeutic effects [10, 11].
Vitamin D, a lipid-soluble vitamin, is well-known for regulating bone metabolism and calcium-phosphorus homeostasis [11]. However, it exerts a variety of non-skeletal benefits, mainly managing different chronic diseases as well [12, 13]. Vitamin D deficiency is involved in abnormal glucose metabolism, altered insulin secretion and T2DM [14]. Vitamin D deficiency is very prevalent among patients with T2DM [15]. Mitri et al. [16] found that even a slight increase in vitamin D intake [from < 5 µgr/day (200 IU/days) to 12.5 µgr/day (> 500 IU/days)] reduced the risk of T2DM by 13%. Vitamin D deficiency in T2DM patients might impair insulin secretion leading to abnormal glucose metabolism and insulin resistance [17, 18]. Moreover, several studies have reported the hypoglycemic properties of vitamin D [19,20,21]. Vitamin D protects against diabetes-related complications through its antioxidant, anti-inflammatory, and immune-modulating effects which plays an important role in insulin resistance [11]. The positive benefits of vitamin D on glycemic control have been revealed in several human studies of diabetes [22,23,24]. Also, there are evidence supporting that vitamin D could decrease lipid concentrations, improve immune regulation, and reduce oxidative stress [25, 26].
The impact of vitamin D on T2DM biomarkers has been broadly examined through many meta-analyses of randomized controlled trials (RCTs), yet the fact that vitamin D supplementation is an effective strategy for controlling T2DM still remains controversial, which has led to inconsistent conclusions about the role of vitamin D on T2DM biomarkers [10, 27,28,29]. Therefore, the current study was designed as an umbrella meta-analysis to investigate the summarized effects of supplementation with vitamin D on T2DM biomarkers found by previous meta-analyses with the aim of addressing the inconsistency among current evidence.
Methods
The current umbrella review of meta-analysis, was performed in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [30], and the protocol was registered in PROSPERO (Registration ID: CRD42021292700).
.Search strategy
A comprehensive online search for relevant published records was conducted from inception until March 2022, using Scopus, Web of Science databases, Embase, PubMed, and Google Scholar. Based on MeSH and text keywords, the following pattern of search was applied: "vitamin d" OR "ergocalciferols" OR "supplementation "OR "vitamin d3″ OR "vitamin d2″ OR "intake" AND “blood glucose” OR “Glucose” OR “FBS” OR “HOMA-IR” OR “insulin sensitivity” OR Insulin” OR “HbA1c” OR “insulin resistance” AND "systematic review" OR "meta-analysis". To enhance the sensitivity of the search approach, the wild-card phrase "*" was used. Database searches were done by two authors (VM and MV). Hand searches were also conducted on the reference lists of related articles to ensure that no studies were missed. We included English-language publications.
Study selection
Meta-analyses investigating the effect of vitamin D supplementation on T2DM biomarkers (FBS, HbA1c, insulin, and HOMA-IR) providing the effect sizes (ESs) and confidence intervals (CIs) were considered eligible for including in this umbrella meta-analysis of randomized controlled trials (RCTs). Studies with the following criteria were excluded: observational studies, quasi-experimental studies, case reports, conference papers, letters, in vitro, in vivo, and ex vivo studies, controlled clinical trials, studies with insufficient data, and studies without full texts. The paper selection process was completed by two independent reviewers (ZK and VM), and any disagreements came into a consensus through discussing with a senior author (PD).
Data extraction
Two independent reviewers (ZK, and MV) extracted the following information from included studies: the first author, publication year, location of the project, study population and sample size, dosage and duration range of Vitamin D, ESs and CIs [(standardized mean difference (SMD), and weighted mean difference (WMD)] regarding study outcomes. The disagreements were consulted by a third reviewer (VM).
Quality assessment
The methodological quality of eligible articles was assessed by two independent reviewers using the assessment of multiple systematic reviews (AMSTAR2) tool (VM, and MV). The AMSTAR2 questionnaire consists of 16 questions, which reviewers are required to answer "Yes," "Partial Yes", "No", or "No Meta-analysis". “High quality”, “Moderate quality”, “Low quality”, and “Critically low quality” were the categories on the AMSTAR2 checklist [31].
Statistical analysis
Random-effect models, based on the restricted maximum likelihood method (REML), were used to estimate the overall ESs and 95% CI [32]. Heterogeneity across studies was estimated by Cochran Q and I2 statistics, in which I2 values greater than 50% or p < 0.1 were considered as significant heterogeneity. A separate analysis was carried out for each type of SMD and WMD in view of their natural differences. In order to explore sources of heterogeneity, we performed subgroup analysis applying the duration of study (≤ 15, and > 15 weeks), gender (Women, both), mean age (≤ 50, and > 50 years), sample size (≤ 500, 500–1000, and > 1000), dose (≤ 4000, and > 4000 IU/day), and health conditions (GDM, PCOS, NAFLD, obesity, diabetic nephropathy, prediabetes, and dialysis patients). The sensitivity analysis was conducted to establish how dependent the overall ES was on a specific study (Leave-one-out Method). Egger's and Begg's tests were used to examine the small-study effect. The presence of publication bias was detected using a visual inspection of the funnel plot. If publication bias was identified, the trim and fill method carried out. STATA version 16 software was used for the statistical analyses (Stata Corp, College Station, Texas, USA).
Results
Selected studies and systematic review
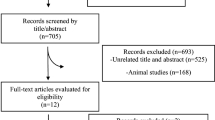
The PRISMA flow chart of the literature search process is depicted in Fig. 1. Through electronic database searches, 724 articles were initially identified, of which 246 were duplicates. After reviewing the titles and abstracts of 468 studies, 424 articles did not meet the inclusion criteria, so they were excluded from any further analysis. Eventually, 37 meta-analyses published between 2011 and 2021 were qualified to be included in the umbrella review. The characteristics of the included meta-analyses are listed in Table 1. The age range of 38,000 participants included in the current study was between 26 and 60 years with the mean of 44.7 years. Intervention duration ranged between 7 and 47 weeks.
Regarding study location, fourteen meta-analyses were performed in China [10, 27, 33,34,35,36,37,38,39,40,41,42,43,44], nine in Iran [19, 28, 45,46,47,48,49,50,51], four in the USA [52,53,54,55], two in UK [21, 56], two in Canada [11, 57], two in Poland [29, 58], two in Netherlands [59, 60], one in Italy [20], and one in Thailand [61]. Cochrane risk of bias tool was used for quality assessment. Overall, almost all randomized controlled trials (RCTs) qualified in the meta-analyses were of high quality. Detailed information is presented in Table 1 about the quality of the RCTs in the meta-analyses.
Methodological quality assessment
Table 2 presents the findings of the quality assessment of meta-analyses according to the AMSTAR2 questionnaire.
Effects of vitamin D on FBS
According to WMD analysis
The results of 14 eligible studies with 15 ESs, including 17,136 participants revealed that supplementation with vitamin D significantly decreased FBS (WMD = − 3.08; 95% CI: − 3.97, − 2.19, p < 0.001) (Fig. 2A). A significant heterogeneity was detected among meta-analyses (I2 = 92.0%, p < 0.001). Subgroup analyses indicated that the reductions in FBS levels were more pronounced in patients with a mean age of > 50 years, patients with gestational diabetes mellitus (GDM), a sample size of ≤ 1000, and studies with a duration of intervention ≤ 15 weeks, and dosage of ≤ 4000 IU/day when compared to their counterparts (Table 3).
According to SMD analysis
The results from 15 meta-analyses with 17 ESs and 12,422 participants reported that vitamin D administration significantly reduced FBS (SMD = − 0.26; 95% CI: − 0.38, − 0.14, p < 0.001), with significant inter-study heterogeneity (I2 = 67.6%, p < 0.001) (Fig. 2B). Conducting subgroup analysis indicated that the effects of vitamin D on FBS were more prominent among women and the sample size ≤ 500, intervention duration of ≤ 15 weeks, patients with GDM and polycystic ovary syndrome (PCOS), and subjects with the mean age of ≤ 50 years than the entire sample (Table 4).
Effects of vitamin D on HbA1c
According to WMD analysis
Overall, eight meta-analyses with 11 ESs (11,139 subjects) indicated that vitamin D administration significantly improved HbA1c (WMD = − 0.05; 95% CI: − 0.10, − 0.01, p = 0.016) with a high degree of study heterogeneity (I2 = 50.4%, p = 0.401) (Fig. 3A). Subgroup analysis revealed that vitamin D with a dosage of ≤ 4000 IU/day and the duration of > 15 weeks for the subjects with prediabetes and the mean age of > 50 years contributed to a robust reduction in HbA1c levels (Table 3).
According to SMD analysis
Totally, 10 meta-analyses with 13 ESs, including 11,873 participants, found that supplementation with vitamin D lowered HbA1c significantly (SMD = − 0.16; 95% CI: − 0.27, − 0.05, p = 0.004) (Fig. 3B). The between-study heterogeneity was considerable (I2 = 74.0%, p < 0.001). The intervention duration of ≤ 15 weeks among women with GDM and age ≤ 50 years contributed to a greater decrease in HbA1c (Table 4).
Effects of vitamin D on insulin
According to WMD analysis
Finding from eight meta-analyses with nine ESs including 7,723 participants demonstrated that vitamin D substantially decreased insulin level (WMD = − 2.62; 95% CI: − 4.11, − 1.13; p < 0.001) (Fig. 4A) with high heterogeneity between-meta-analyses (I2 = 82.2%, p < 0.001). Vitamin D supplement of ≤ 4000 IU/day in studies with intervention duration of ≤ 15 weeks, subjects younger than 50 years with GDM, sample size of > 500 contributed to a more robust reduction in insulin (Table 3).
According to SMD analysis
Results revealed considerable effect of vitamin D supplementation on insulin levels in 12 meta-analyses with 6,118 participants (SMD = − 0.33; 95% CI: − 0.56, − 0.11, p = 0.004; I2 = 81.8%, p < 0.001) (Fig. 4B). From these analyses, we found a significant lowering effect of vitamin D supplementation on insulin in studies with prescribed ≤ 4000 IU/day of vitamin D and treatment duration of ≤ 15 weeks, sample size less than 500 and in women with mean age of ≤ 50 (Table 4).
Effects of vitamin D on HOMA-IR
According to WMD analysis
The results of 14 meta-analyses with 17 ESs including 47,157 individuals indicated that vitamin D supplementation substantially decreased HOMA-IR (WMD = − 0.67; 95% CI: − 1.01, − 0.32, p < 0.001). The heterogeneity was considerable between studies (I2 = 96.2%, p < 0.001) (Fig. 5A) Vitamin D supplementation resulted in a significant decrease in HOMA-IR at the dosage of ≤ 4000 IU/day, in meta-analyses with intervention duration of ≤ 15 weeks, and those studies that were conducted on women with GDM with sample size ≤ 500 and mean age less than 50 years (Table 3).
According to SMD analysis
Vitamin D supplementation decreased HOMA-IR levels (SMD = − 0.31; 95% CI: − 0.46, − 0.16, p < 0.001, I2 = 75.9%, p < 0.001, 16 meta-analyses with 19 ESs). The I2 index showed considerable heterogeneity among meta-analyses (I2 = 75.9%, p < 0.001) (Fig. 5B). Vitamin D supplementation in a dosage of ≤ 4000 IU/day among > 50 years’ subjects, in studies with intervention duration of ≤ 15 weeks, in patients with GDM, T2DM, and NAFLD, and a sample size of ≤ 500 in women contributed to a more significant reduction in HOMA-IR levels based on the subgroup analyses (Table 4).
Sensitivity analysis, and publication bias
Stepwise, each study was removed from the analysis to examine the impact of each single meta-analysis on the pooled effect size based on sensitivity analysis. No study significantly changed the total effect size of the study results.
Egger’s and Begg’s tests indicated a small study effect for FBS, HbA1c (only based on WMD analysis), and HOMA-IR (p < 0.05). Moreover, no evidence of a small study effect was detected after conducting Egger’s and Begg’s tests for insulin levels (p˃0.05). Also, visual checking of the funnel plot (Additional file 1: Figs. S1–S4) revealed an asymmetric distribution of included meta-analyses, indicating publication bias. Therefore, trim and fill analysis was carried out, and did not alter the results.
Discussion
Over the past few decades, a growing body of clinical and epidemiological studies has emerged emphasizing the role of vitamin D on several diseases, such as T2DM, autoimmune disorders, cancer, and cardiovascular disease. In recent years, conflicting findings have been published on the association between circulating serum vitamin D levels with glycemic indices [62, 63]. Therefore, we performed an umbrella review to investigate the available research studies regarding the effect of vitamin D on T2DM biomarkers in adult subjects.
The current umbrella meta-analysis summarized 37 meta-analyses with a total of 36,197 adults. Our analyses shown that vitamin D supplementation significantly decreases FBS, insulin level, HbA1c, and HOMA-IR. Overall, meta-analyses using WMD for reporting the ESs, except HbA1c, revealed a stronger effect than SMD. As WMD depends on the ES of each included meta-analysis, this robust effect was not unexpected. Moreover, in meta-analyses that assessed effect of vitamin D via WMD, we found a greater reduction in FBS in subjects aged > 50 years old, and those with CVD, CKD, and GDM. Also, the vitamin D administrations significantly reduced FBS, insulin, HbA1c, and HOMA-IR at the dosages of ≤ 4000 IU/day compared to > 4000 IU/day, when administered for ≤ 15 weeks. The vitamin D administrations meaningfully reduced insulin, HOMA-IR, and HbA1c at the dosages of ≤ 4000 IU/days, when administered for shorter period of time (≤ 15 weeks). The overall quality of included meta-analyses shown in Table 2 was high to moderate. Publication bias was identified by funnel plot. Nevertheless, this bias did not affect the overall finding identified by trim and fill analysis.
Different parameters such as the latitude, skin pigmentation, duration of sun exposure, and season can affect the production of vitamin D [64, 65]. Several epidemiologic studies propose that low vitamin D levels are related to impaired insulin secretion, insulin resistance, and glucose clearance [66,67,68]. Also, several previous investigations have shown a relationship between vitamin D deficiency and the progression of T2DM as well as future macrovascular and microvascular complications [69,70,71]. Our results were consistent with the previous reports, which proposed that vitamin D might help T2DM biomarkers by increasing the absorption of glucose by the improvement of insulin sensitivity [36, 72, 73]. It should be stressed that, however, the results propose that vitamin D supplementation may be efficacious for controlling T2DM biomarkers; the effects of vitamin D on T2DM biomarkers were heterogeneous. Differences between meta-analyses in sample size, population, methodological quality, gender, duration, and dosage may partially explain this heterogeneity. Our subgroup analysis indicated that the effect of vitamin D on T2DM biomarkers was in a time-dependent manner and lower duration of supplementation (≤ 15-weeks) led to a more decrease in T2DM biomarkers in comparison with long term supplementation. There are several reasons which could explain these findings. First of all, it should not be ignored that the 15 weeks period is the time of two seasonal alterations, when the climate conditions and a smaller extent of UV exposure may have an important effect on the production of vitamin D. Besides, daily habits and diet may differ in seasons, which may contribute to the worsening of the metabolic control. Moreover, the participant’s insight of motivation and treatment may have an important effect on the treatment efficacy and mostly long-term intervention decreases the compliance rate. Finally, the fact that the prolonged duration of diseases such as T2DM or gradually worsen with the course of T2DM may help to clarify the result. However, exact interpretation must be with caution since high heterogeneity was observed in both subgroups of sample size and duration. Our study provided evidence proposing that vitamin D supplementation with a dose ≤ 4000 IU/day may be adequate to improve insulin and glucose homeostasis among adults. This is partly because most of the studies used a dose of ≤ 4000 IU/day. Nevertheless, it is possible that vitamin D has favorable effects only in vitamin D deficient participants particularly in those with poor T2DM biomarkers [59, 74].
In our meta-analyses, we observed that vitamin D significantly decreased HbA1c levels, proposing that vitamin D is helpful to delay or decrease the development and occurrence of diabetic problems. In 2007, the UK prospective diabetes study estimated a 1% decrease of HbA1c related to a 14% decrease in risk of cardiovascular events [67]. A review study reported that vitamin D had a helpful effect on glycemic indices in short-term intervention; nevertheless, no significant effect on HbA1c was detected in long term trials with an intervention period > 12 weeks [75]. However, the findings of the current umbrella review indicated that vitamin D was related to a decrease in HbA1c levels in studies with ≤ 15 week's intervention durations. Moreover, there was no significant reduction in FBS, insulin, and HOMA-IR with long-term (> 15 weeks) intervention. Furthermore, the fact of prolonged duration of diseases or gradually worsened condition may help to explain the finding. Moreover, several studies have also revealed that 25(OH) D levels are negatively related to the HOMA-IR and diabetes [76, 77]. The increased HOMA-IR is believed to be caused by the reduced insulin sensitivity. Vitamin D deficiency has been shown to impair insulin secretion in β-cells [78], and Cade et al. [79] propose that improvement of vitamin D status stimulates insulin secretion in rats with vitamin D deficiency. Insulin secretion is a highly dynamic process regulated by several factors such as calcium and hormones [80]. L-type calcium channels on islet β-cells are stimulated by 1, 25(OH) 2D which then controls calcium levels, initiates insulin signaling, and stimulates insulin secretion [80, 81].
The possible mechanisms of action of vitamin D may be through amplification of insulin secretion by the expression of vitamin D (VDR) in the pancreatic β-cells, increasing insulin sensitivity, suppressing the production of pro-inflammatory mediators and cytokines, and regulation of the intracellular and extracellular calcium flux [82,83,84,85,86,87,88,89]. The regulation of insulin secretion is greatly dependent to calcium; therefore, slightly changes in calcium flux can unfavorably affect the secretory role of β-cell [70]. This umbrella of meta-analysis used systematic methods with strong statistical power and robust search strategies, using moderate to high quality researches, which summarized the present literature regarding the effects of vitamin D on T2DM biomarkers. However, our study also has some limitations. Significant between-study heterogeneity detected, which was controlled for, applying subgroup analyses.
Conclusion
Overall, the present umbrella meta-analysis showed that vitamin D supplementation has lowering effect on FBS, HOMA-IR, HbA1c, and insulin levels. Vitamin D supplementation might be proposed as a beneficial dietary component in managing hyperglycemia and its complications. Moreover, current findings suggest to supplement with a dosage of > 4000 IU and for a treatment period of < 15 weeks. Overall, vitamin D supplementation as a complementary treatment for diabetes management is supported by the findings of this review.
Availability of data and materials
Not applicable.
References
Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–600.
Chen G-C, Qi Q. Lifestyle for the prevention of type 2 diabetes: what is the role of genetic risk information? Am J Clin Nutr. 2020;111:491–2.
Giugliano D, Ceriello A, Esposito K. Glucose metabolism and hyperglycemia. Am J Clin Nutr. 2008;87:217S-S222.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;2018(41):2669–701.
DiNicolantonio JJ, Harcombe Z, O’Keefe JH. Problems with the 2015 Dietary Guidelines for Americans: an alternative. Mo Med. 2016;113:93.
Asbaghi O, Fatemeh N, Mahnaz RK, Ehsan G, Elham E, Behzad N, et al. Effects of chromium supplementation on glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2020;161: 105098.
Asbaghi O, Moradi S, Kashkooli S, Zobeiri M, Nezamoleslami S, Hojjati Kermani MA, et al. The effects of oral magnesium supplementation on glycaemic control in patients with type 2 diabetes: a systematic review and dose–response meta-analysis of controlled clinical trials. Br J Nutr. 2022;8:1–10.
Gao L, Lin L, Shan N, Ren C-Y, Long X, Sun Y-H, et al. The impact of omega-3 fatty acid supplementation on glycemic control in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020;33:1767–73.
Mason SA, Keske MA, Wadley GD. Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People With Type 2 Diabetes: A GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2021;44:618–30.
Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10:375.
Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc. 2018;2:687–709.
Bhatt N, Ali A, Waly M. Non-skeletal benefits of vitamin D. Can J Clin Nutr. 2019;7:141–59.
Rejnmark L, Bislev LS, Cashman KD, Eiríksdottir G, Gaksch M, Grübler M, et al. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS ONE. 2017;12: e0180512.
Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–8.
Ehrampoush E, Mirzay Razzaz J, Arjmand H, Ghaemi A, Raeisi Shahraki H, Ebrahim Babaei A, et al. The association of vitamin D levels and insulin resistance. Clin Nutr. 2021;42:325–32.
Khamseh ME, Sepanlou SG, Malekzadeh R. A Response to the Letter to the Editor Regarding “Nationwide Prevalence of Diabetes and Prediabetes and Associated Risk Factors Among Iranian Adults: Analysis of Data from PERSIAN Cohort Study” to the end of Study. Diabetes Therapy. 2022;13:221–4.
von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient–a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–55.
Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–6.
Ostadmohammadi V, Milajerdi A, Ghayour-Mobarhan M, Ferns G, Taghizadeh M, Badehnoosh B, et al. The effects of vitamin D supplementation on glycemic control, lipid profiles and C-reactive protein among patients with cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des. 2019;25:201–10.
Gasparri C, Perna S, Spadaccini D, Alalwan T, Girometta C, Infantino V, et al. Is vitamin D-fortified yogurt a value-added strategy for improving human health? A systematic review and meta-analysis of randomized trials. J Dairy Sci. 2019;102:8587–603.
Ojo O, Weldon SM, Thompson T, Vargo EJ. The effect of vitamin D supplementation on glycaemic control in women with gestational diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. Int J Environ Res Public Health. 2019;16:1716.
Rajabi-Naeeni M, Dolatian M, Qorbani M, Vaezi AA. The effect of omega-3 and vitamin D co-supplementation on glycemic control and lipid profiles in reproductive-aged women with pre-diabetes and hypovitaminosis D: a randomized controlled trial. Diabetol Metab Syndr. 2020;12:1–11.
Kuchay MS, Laway BA, Bashir MI, Wani AI, Misgar RA, Shah ZA. Effect of vitamin D supplementation on glycemic parameters and progression of prediabetes to diabetes: a 1-year, open-label randomized study. Indian J Endocrinol Metab. 2015;19:387.
Bhatt SP, Misra A, Pandey RM, Upadhyay AD, Gulati S, Singh N. Vitamin D supplementation in overweight/obese Asian Indian women with prediabetes reduces glycemic measures and truncal subcutaneous fat: a 78 weeks randomized placebo-controlled trial (PREVENT-WIN Trial). Sci Rep. 2020;10:1–13.
Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90.
Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1, 25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–91.
Wei Y, Wang S, Meng Y, Yu Q, Wang Q, Xu H, et al. Effects of vitamin D supplementation in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Int J Endocrinol Metabolism. 2020;18:89.
Tabrizi R, Moosazadeh M, Lankarani KB, Akbari M, Heydari ST, Kolahdooz F, et al. The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2017;11:S975–82.
Łagowska K, Bajerska J, Jamka M. The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10:1637.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–9.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;87:358.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2019.
Guo S, Tal R, Jiang H, Yuan T, Liu Y. Vitamin D supplementation ameliorates metabolic dysfunction in patients with PCOS: a systematicreview of RCTs and insight into the underlying mechanism. Int J Endocrinol. 2020;2020:67.
Guo X-F, Wang C, Yang T, Li S, Li K, Li D. Vitamin D and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Food Fun. 2020;11:7389–99.
Mitchell BL, Smith AE, Rowlands AV, Parfitt G, Dollman J. Associations of physical activity and sedentary behaviour with metabolic syndrome in rural Australian adults. J Sci Med Sport. 2018;21:1232–7.
Hu Z, Sun X, Wang L, Wang A. Efficacy of vitamin D supplementation on glycemic control in type 2 diabetes patients: a meta-analysis of interventional studies. Medicine. 2019;98.
Miao CY, Fang XJ, Chen Y, Zhang Q. Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp Ther Med. 2020;19:2641–9.
Wang L, Wen X, Lv S, Tian S, Jiang Y, Yang X. Effects of vitamin D supplementation on metabolic parameters of women with polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Gynecol Endocrinol. 2021;37:446–55.
Wang M, Chen Z, Hu Y, Wang Y, Wu Y, Lian F, et al. The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: a systematic review and meta-analysis. Clin Nutr. 2021;40:3148–57.
Wang Y, Yang S, Zhou Q, Zhang H, Yi B. Effects of vitamin D supplementation on renal function, inflammation and glycemic control in patients with diabetic nephropathy: a systematic review and meta-analysis. Kidney Blood Press Res. 2019;44:72–87.
Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism. 2017;73:67–76.
Zhang Y, Xue Y, Zhang D, Liu Y, Xu Z, Gao J, et al. Effect of Vitamin D supplementation on glycemic control in prediabetes: a meta-analysis. Nutrients. 2021;13:4464.
Zhao J, Dong J, Wang H, Shang H, Zhang D, Liao L. Efficacy and safety of vitamin D3 in patients with diabetic nephropathy: a meta-analysis of randomized controlled trials. Chin Med J. 2014;127:2837–43.
Zou Y, Guo B, Yu S, Wang D, Qiu L, Jiang Y. Effect of vitamin D supplementation on glycose homeostasis and islet function in vitamin D deficient or insufficient diabetes and prediabetes: a systematic review and meta-analysis. J Clin Biochem Nutr. 2021;8:20–165.
Akbari M, Mosazadeh M, Lankarani KB, Tabrizi R, Samimi M, Karamali M, et al. The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2017;49:647–53.
Emadzadeh M, Sahebi R, Khedmatgozar H, Sadeghi R, Farjami M, Sharifan P, et al. A systematic review and meta-analysis of the effect of Vitamin D-fortified food on glycemic indices. BioFactors. 2020;46:502–13.
Jahanjoo F, Farshbaf-Khalili A, Shakouri SK, Dolatkhah N. Maternal and neonatal metabolic outcomes of Vitamin D supplementation in gestational diabetes mellitus: A systematic review and meta-analysis. Ann Nutr Metab. 2018;73:145–59.
Milajerdi A, Ostadmohammadi V, Amirjani S, Kolahdooz F, Asemi Z. The effects of vitamin D treatment on glycemic control, serum lipid profiles, and C-reactive protein in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Int Urol Nephrol. 2019;51:1567–80.
Sahebi R, Rezayi M, Emadzadeh M, Salehi M, Tayefi M, Parizadeh SM, et al. The effects of vitamin D supplementation on indices of glycemic control in Iranian diabetics: A systematic review and meta-analysis. Complement Ther Clin Pract. 2019;34:294–304.
Sarathy H, Pramanik V, Kahn J, Abramowitz MK, Meier K, Kishore P, et al. The effects of short-term vitamin D supplementation on glucose metabolism in dialysis patients: a systematic review and meta-analysis. Int Urol Nephrol. 2015;47:537–49.
Rezaei S, Tabrizi R, Nowrouzi-Sohrabi P, Jalali M, Shabani-Borujeni M, Modaresi S, et al. The effects of Vitamin D supplementation on anthropometric and biochemical indices in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Pharmacol. 2021;12:2918.
Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42.
Lee CJ, Iyer G, Liu Y, Kalyani RR, Ligon CB, Varma S, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: A systematic review and meta-analysis of intervention studies. J Diabetes Complications. 2017;31:1115–26.
Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, et al. Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:3551–60.
Tang H, Li D, Li Y, Zhang X, Song Y, Li X. Effects of vitamin D supplementation on glucose and insulin homeostasis and incident diabetes among nondiabetic adults: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2018;2018:9.
Manousopoulou A, Al-Daghri NM, Garbis SD, Chrousos GP. Vitamin D and cardiovascular risk among adults with obesity: a systematic review and meta-analysis. Eur J Clin Invest. 2015;45:1113–26.
Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The effect of improved serum 25-hydroxyvitamin D status on glycemic control in diabetic patients: a meta-analysis. J Clin Endocrinol Metab. 2017;102:3097–110.
Jamka M, Woźniewicz M, Jeszka J, Mardas M, Bogdański P, Stelmach-Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep. 2015;5:1–12.
Krul-Poel YH, Ter Wee MM, Lips P, Simsek S. Management of endocrine disease: the effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R1–14.
Pramono A, Jocken JW, Blaak EE, van Baak MA. The effect of vitamin D supplementation on insulin sensitivity: a systematic review and meta-analysis. Diabetes Care. 2020;43:1659–69.
Poolsup N, Suksomboon N, Plordplong N. Effect of vitamin D supplementation on insulin resistance and glycaemic control in prediabetes: a systematic review and meta-analysis. Diabet Med. 2016;33:290–9.
Mezza T, Muscogiuri G, Sorice G, Prioletta A, Salomone E, Pontecorvi A, et al. Vitamin D deficiency: a new risk factor for type 2 diabetes. Ann Nutr Metab. 2012;61:337–48.
Jafari T, Faghihimani E, Feizi A, Iraj B, Javanmard SH, Esmaillzadeh A, et al. Effects of vitamin D-fortified low fat yogurt on glycemic status, anthropometric indexes, inflammation, and bone turnover in diabetic postmenopausal women: a randomised controlled clinical trial. Clin Nutr. 2016;35:67–76.
Lips P, van Schoor NM, de Jongh RT. Diet, sun, and lifestyle as determinants of vitamin D status. Ann N Y Acad Sci. 2014;1317:92–8.
O’Mahony L, Stepien M, Gibney MJ, Nugent AP, Brennan L. The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients. 2011;3:1023–41.
Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, et al. Plasma 25-hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr. 2009;139:329–34.
Mattila C, Knekt P, Männistö S, Rissanen H, Laaksonen MA, Montonen J, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70.
Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8.
Joergensen C, Gall M-A, Schmedes A, Tarnow L, Parving H-H, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010;33:2238–43.
Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29.
Grimnes G, Emaus N, Joakimsen R, Figenschau Y, Jenssen T, Njølstad I, et al. Baseline serum 25-hydroxyvitamin D concentrations in the Tromsø Study 1994–95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet Med. 2010;27:1107–15.
Rudnicki P, Mølsted-Pedersen L. Effect of 1, 25-dihydroxycholecalciferol on glucose metabolism in gestational diabetes mellitus. Diabetologia. 1997;40:40–4.
Zhang Y, Gong Y, Xue H, Xiong J, Cheng G. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. Int J Obstetr Gynaecol. 2018;125:784–93.
Krul-Poel YH, Westra S, ten Boekel E, ter Wee MM, van Schoor NM, van Wijland H, et al. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes (SUNNY trial): a randomized placebo-controlled trial. Diabetes Care. 2015;38:1420–6.
Nigil Haroon N, Anton A, John J, Mittal M. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes: a systematic review of interventional studies. J Diabetes Metab Disord. 2015;14:1–11.
Nakamura K, Hui S-P, Ukawa S, Okada E, Nakagawa T, Imae A, et al. Serum 25-hydroxyvitamin D3 Levels and Diabetes in a Japanese Population: The DOSANCO Health Study. J Epidemiol. 2021;34:20210007.
Schleu MF, Barreto-Duarte B, Arriaga MB, Araujo-Pereira M, Ladeia AM, Andrade BB, et al. Lower levels of vitamin D are associated with an increase in insulin resistance in obese Brazilian Women. Nutrients. 2021;13:2979.
Norman AW, Frankel BJ, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–5.
Cade C, Norman AW. Rapid normalization/stimulation by 1, 25-dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D-deficient rat. Endocrinology. 1987;120:1490–7.
Jung S-R, Reed BJ, Sweet IR. A highly energetic process couples calcium influx through L-type calcium channels to insulin secretion in pancreatic β-cells. Am J Physiol-Endocrinol Metab. 2009;297:E717–27.
Kamycheva E, Jorde R, Figenschau Y, Haug E. Insulin sensitivity in subjects with secondary hyperparathyroidism and the effect of a low serum 25-hydroxyvitamin D level on insulin sensitivity. J Endocrinol Invest. 2007;30:126–32.
Wei Z, Yoshihara E, He N, Hah N, Fan W, Pinto AF, et al. Vitamin D switches BAF complexes to protect β cells. Cell. 2018;173(1135–49): e15.
Kjalarsdottir L, Tersey SA, Vishwanath M, Chuang J-C, Posner BA, Mirmira RG, et al. 1, 25-Dihydroxyvitamin D3 enhances glucose-stimulated insulin secretion in mouse and human islets: a role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J Steroid Biochem Mol Biol. 2019;185:17–26.
Zostautiene I, Jorde R, Schirmer H, Mathiesen EB, Njølstad I, Løchen M-L, et al. Genetic variations in the Vitamin D receptor predict type 2 diabetes and myocardial infarction in a community-based population: the Tromsø study. PLoS ONE. 2015;10: e0145359.
Li L, Wu B, Liu J-Y, Yang L-B. Vitamin D receptor gene polymorphisms and type 2 diabetes: a meta-analysis. Arch Med Res. 2013;44:235–41.
Park S, Kim DS, Kang S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-γ expression in nonobese Type 2 diabetic rats. J Nutr Biochem. 2016;27:257–65.
Zhou QG, Hou FF, Guo ZJ, Liang M, Wang GB, Zhang X. 1, 25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab Res Rev. 2008;24:459–64.
Chuang J-C, Cha J-Y, Garmey JC, Mirmira RG, Joyce JJJ. Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol. 2008;22:2353–63.
Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35:11–7.
Acknowledgements
The research protocol was approved and Supported by the Student Research Committee, Tabriz University of Medical Sciences (Registration code: 71788).
Funding
The authors reported no funding received for this study.
Author information
Authors and Affiliations
Contributions
VM and MV designed research; ZK and VM conducted systematic search; MV, VM and NM screened articles; ZK and VM extracted data; VM analyzed and interpreted data; VM, and ZK drew tables; NM, VM, and MV wrote the paper. PD had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The results of funnel plot for the effect of the vitamin D on glycemic indices.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Musazadeh, V., Kavyani, Z., Mirhosseini, N. et al. Effect of vitamin D supplementation on type 2 diabetes biomarkers: an umbrella of interventional meta-analyses. Diabetol Metab Syndr 15, 76 (2023). https://doi.org/10.1186/s13098-023-01010-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01010-3