Abstract
Background
The mosquito Aedes aegypti is an urban vector of dengue and other arboviruses. During epidemics of these viruses, pyrethroid insecticides are used for the control of adult mosquitoes. The worldwide resistance of Ae. aegypti to these insecticides is a cause of failure of vector control campaigns. The primary target of pyrethroids is the voltage-gated sodium channel. Point mutations in the gene coding for this channel, called knockdown resistance (kdr) mutations, are associated with pyrethroid resistance. Two kdr mutations, V1016I and F1534C, have increased in frequency in natural populations of Ae. aegypti in the Americas during the last decade. Their association with pyrethroid resistance has been largely demonstrated in field populations throughout the Americas, and in in vitro assays. Diagnostics for kdr polymorphism allow early detection of the spread of insecticide resistance, which is critical for timely decisions on vector management. Given the importance of resistance management, high-throughput methods for kdr genotyping are valuable tools as they can be used for resistance monitoring programs. These methods should be cost-effective, to allow regional-scale surveys. Despite the extensive presence of Ae. aegypti and incidence of dengue in Argentina, the presence, abundance, and distribution of kdr mutations in populations of this mosquito have yet to be reported for the country.
Methods
Aedes aegypti samples were collected as immature stages or adults from Buenos Aires Metropolitan Area and northern localities of Tartagal (Salta Province) and Calilegua (Jujuy Province). Immature stages were maintained in the laboratory until they developed into adults. A high-resolution melting assay, based on an analysis of melting temperatures, was developed for the simultaneous genotyping of V1016I and F1534C kdr mutations. We used this method to infer the presence and frequencies of kdr alleles in 11 wild populations from Argentina.
Results
We demonstrated the presence of kdr mutations in Ae. aegypti in Argentina in regions where this species is under different selection pressures due to the use of pyrethroids. The populations under analysis are located in geographically distant regions of the species’ distribution in Argentina: the northern provinces of Salta and Jujuy and the Buenos Aires Metropolitan Area. Higher frequencies of resistant-associated alleles were detected in the northern region. We report a multiplex high-throughput assay based on a high-resolution melting polymerase chain reaction method for the simultaneous genotyping of V1016I and F1534C kdr mutations. This assay was shown to be cost-effective, and thus provides an interesting molecular tool for kdr genotyping in A. aegypti control campaigns.
Conclusions
We report, to the best of our knowledge for the first time, the presence of kdr mutations in populations of Ae. aegypti from geographically distant locations of Argentina that differ with respect to their epidemiological situation and history of mosquito control. We have developed a high-throughput method for the genotyping of kdr mutations in Ae. aegypti from the Americas. Given its affordability and short running time, this method can be used in control campaigns to monitor the presence and spread of kdr alleles. The information provided here is relevant for the rational design of control strategies in the context of integrated vector management.
Graphical Abstract

Similar content being viewed by others
Background
Around 390 million people worldwide are infected with dengue every year. In the past decades, the number of reported cases in the Americas has increased more than tenfold, to more than 16 million, from the 1.5 million cases recorded in the 1980s [1]. Dengue, Zika, chikungunya, and the re-emerging virus yellow fever, are transmitted by females of the mosquito species Aedes aegypti, which is considered one of the most successful invasive species worldwide [2]. Urbanization, human population growth and climate change are factors that promote the expansion and abundance of Ae. aegypti [3]. This mosquito was reintroduced into Argentina in 1986 and it is now established in all the northern and central provinces of the country; its southern boundary is formed by the provinces of Buenos Aires [4], Neuquén [5] and Río Negro [6]. Since Ae. aegypti’s reintroduction, four major and widely distributed dengue epidemics have occurred in Argentina (in 2009, 2016, 2020 and 2023), which were of considerable public health concern. There were more than 93,000 cases in the most recent outbreak, which occurred in 2023 (https://www.argentina.gob.ar/salud/epidemiologia/boletines).
Control of Ae. aegypti expansion involves environmental management through the elimination of domestic and peri-domestic breeding sites and the use of larvicides such as insect growth regulators, organophosphate neurotoxins and the biolarvicide Bacillus thuringiensis israelensis [7]. It is recommended that the use of adulticides should be restricted to periods of arbovirus epidemics [7]; for these, the neurotoxic pyrethroids are the preferred compounds, given their favorable toxicological properties. Even though the organophosphate malathion is used in some countries in cases of high pyrethroid resistance, its application for vector control is banned in Argentina, and pyrethroids are the only insecticides allowed in the country for domestic use and public health applications [8].
The target site of pyrethroid insecticides is the voltage-gated sodium channel (Nav), a membrane protein present in excitable cells. Given their rapid lethal action, the effect of pyrethroids is known as knockdown; the single nucleotide polymorphisms of the sodium channel gene that confer resistance to pyrethroids are known as knockdown resistance (kdr) mutations [9]. In the Americas, four kdr mutations related to the loss of pyrethroid susceptibility have been identified in Ae. aegypti: Val to Leu in position 410 (V410L), Ile to Met in position 1011 (I1011M), Val to Ile in position 1016 (V1016I), and Phe to Cys in position 1534 (F1534C) [10,11,12]. The frequency of I1011M seems to have decreased in the last 20 years, and its role in the pyrethroid resistance of natural populations is not clear [13]. Conversely, in the last decade, V1016I and F1534C mutations have increased in frequency in natural populations in the Americas [14,15,16]. In addition, 410Lkdr is strongly associated with 1016Ikdr and 1534Ckdr in field populations. Results of a recent study [12] showed that 410Lkdr augmented resistance to a pyrethroid in the field, with a significant interaction with spraying application distance.
With respect to positions 1016 and 1534, three alleles of the Nav gene of Ae. aegypti (aedaenav) are widely distributed in South and North America [14, 16, 17]: 1016V + 1534F (susceptible); 1016 V + 1534Ckdr (resistant 1; R1); 1016kdr I + 1534Ckdr (double mutation, resistant 2; R2). The R3 allele (1016Ikdr + 1534F) was detected with a very low frequency (≤ 0.1% of the samples) in Brazil [10], Mexico [15] and Florida [17]. Conversely, the R1 and R2 genotypes are predominant in all the regions studied in the Americas.
The role of kdr mutations in pyrethroid resistance in Ae. aegypti has been well established. In the last 10 years, kdr alleles in natural populations propagated in parallel with increasing levels of resistance to pyrethroids [16,17,18]. More recently, a comparison of the homozygous R1R1, R2R2 and the heterozygous R1R2 laboratory lines of Ae. aegypti with a SS susceptible line confirmed that the three kdr genotypes conferred deltamethrin resistance to the insects, and that the R2R2 genotype conferred the highest level of resistance [19]. Electrophysiological recordings from Xenopus laevis oocytes showed reduced affinity to type I (permethrin) but not type II (deltamethrin) pyrethroids in oocytes expressing the R1 allele (1016V + 1534Ckdr) sodium channel compared to those expressing the SS allele (1016V + 1534F) [20]. In parallel, channels expressing the R3 (1016Ikdr + 1534F) allele did not present reduced sensitivity to pyrethroids when expressed in X. laevis oocytes. Moreover, when the R2 allele was expressed in X. laevis oocytes, a higher resistance to both type I and type II pyrethroids was observed compared to when the R1 allele was expressed [21].
Resistance monitoring is critical for the success of vector control campaigns, and should be undertaken for operational decision making. Diagnostics for kdr polymorphisms allow early detection of the spread of insecticide resistance, given that they can be used to detect an individual carrying the mutation before it is fixed in the population through the selection pressure exerted by the insecticide. Studies on kdr distribution and abundance have been performed in the Americas, e.g. Venezuela [22], Mexico [14], USA [16] and Brazil [18, 19], for resistance monitoring. Despite the extensive presence of Ae. aegypti and the incidence of dengue in Argentina, the existence, abundance and distribution of kdr mutations in this mosquito have yet to be reported for the country.
Given the importance of resistance management, cost-effective and high-throughput methods for kdr genotyping are considered invaluable for resistance monitoring programs. For Ae. aegypti, the methods that have been implemented to date are based either on allele-specific polymerase chain reaction (PCR) [23] or on the use of TaqMan probes [24]. While the running costs associated with allele-specific PCR are minor compared to those associated with TaqMan assays [25], the results of the former may be less reproducible than those of the latter when the assays are carried out by different laboratories, given the need for careful setting up of the PCR conditions to avoid amplification of nonspecific products. On the other hand, high-resolution melting (HRM) is a reproducible probe-free high-throughput method for genotyping. Well-calibrated equipment and a third generation fluorescent double-stranded DNA dye are used in this method [26]. HRM has economic and practical advantages with respect to other methods for the detection of kdr in Anopheles gambiae [25]. Single-site HRM was used for genotyping kdr mutations in Asian populations of Ae. aegypti [27], but the development of a multiplex HRM-based assay (mHRM) for kdr genotyping would allow the genotyping of two mutations in a single reaction tube, which would decrease both costs and running time.
Here, we report, to the best of our knowledge for the first time, the presence of kdr mutations in Ae. aegypti from Argentinean populations under different selection pressure with pyrethroids. To detect these, we developed a mHRM assay for the simultaneous genotyping of sites 1016 and 1534, which may be a valuable tool for the monitoring of kdr in Ae. aegypti mosquitoes from the Americas.
Methods
Mosquito sampling and DNA extraction
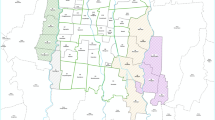
Mosquito sampling was performed in 2018 and 2019 in the provinces of Salta (Tartagal; S22°30′58.9ʺ, W63°48.079′), Jujuy (Calilegua National Park; S23°38′20″, W64°50′17) and Buenos Aires [La Plata (S34°55′17.2ʺ, W 57°57.272ʹ); Merlo (34°39′55″S, 58°43′39″O); Arturo Seguí (34°53′16″S, 58°07′36″O); Lomas de Zamora (34°46′00″S, 58°24′00″O); Avellaneda (34°40′00″S 58°21′00″O); Quilmes (34°43′00″S, 58°16′00″O); La Matanza (34°43′00″S, 58°38′00″O) and Tigre (34°25′00″S, 58°35′00″O)] (Fig. 1). These localities were selected to compare geographically distant regions within the distribution of A. aegypti to estimate the dispersion of kdr mutations in the country. We also considered the history of dengue epidemics and the consequent control of adult mosquitoes through spatial spraying with adulticides, which has been carried out more recently in Buenos Aires Metropolitan Area (Área Metropolitana de Buenos Aires; AMBA) than in the northern provinces. Furthermore, AMBA is the most populated region of the country, and thus has a greater number of inhabitants that are potentially exposed to arboviruses.
The tested mosquitoes were captured as immature stages or adults from the field. Immature stages were collected from artificial containers. All immature stages were reared to adults in the laboratory facilities of Centro de Estudios Parasitológicos y de Vectores (CEPAVE). Adults were captured in the field with aspirators, nets, or traps. Morphological identification was determined under a stereoscopic microscope by using dichotomous taxonomic keys [28]. Mosquito samples were conserved in ethanol at − 80 °C until genomic DNA was extracted from individual mosquitoes. For DNA extractions, an adapted protocol based on magnetic nanoparticles was used [29]. DNA from each individual was eluted in 50 µL of pure water.
Development of the HRM assay
Primer v.0.4.0 software (http://bioinfo.ut.ee/primer3–0.4.0/primer3/) was used to design the primers flanking the IIS6 and IIIS6 Nav segments, which include positions 1016 and 1534, respectively, in the aedaenav gene (AAEL023266; see primer sequences in Table 1). Amplicon melting temperatures (MTs) were predicted with uMELT online software (https://www.dna.utah.edu/umelt/umelt.html). GC tails were added to forward and reverse primers of the 1534 amplicon to differentiate at least 2 °C MT of both amplicons. Both the real-time PCR and HRM steps were performed on an ArialMx Real-Time PCR system (Agilent). PCR was performed in 12 μL containing 6 μL Brillant HRM Ultra-fast Loci Master Mix (Agilent), 200 nM of each primer and 2 μL of genomic DNA diluted 1/10. The PCR amplification protocol began with a denaturation step at 95 °C for 3 min, followed by 40 cycles of 5 s denaturation at 95 °C and 30 s annealing-extension at 62 °C. After amplification, the reaction tubes were cooled to 55 °C, then warmed from 55 to 95 °C at the rate of 0.2 °C/s. The melting curves were analyzed with AriaMX 1.5 software (Agilent). For the setting up of the reaction, samples previously genotyped by Dr. A. J. Martin’s lab from Brazilian populations were used: SS (1016 VV + 1534 FF), SR2 (1016 VIkdr + 1534 FCkdr) and R2R2 (1016 Ikdr Ikdr + 1534 Ckdr C kdr). Samples of known genotypes determined by Sanger sequencing were also included in every mHRM plate for comparisons with samples of unknown genotype.
Sanger sequencing
Genotypes of random selected samples were confirmed by sequencing of the relevant regions of the aedaenav gene. For Sanger sequencing, the genomic DNA was used as a template for the PCR reactions using GoTaq (Promega). The cycling consisted of initial denaturation for 5 min at 95 °C, followed by 40 cycles of 30 s at 95 °C (denaturation) and 30 s at 62 °C, and a final extension step at 72 °C for 5 min. Twenty-one samples were sequenced for the 1016 amplicon and 14 for the 1534 amplicon by using the Sanger method at Macrogen (Seoul, Korea) with the Ae1016GqPCRRv and Ae1534qPCRFwGC primers, respectively (Table 1).
Statistical analysis
One-way ANOVA followed by Tukey’s multiple comparison test was used to compare MTs among different variants for positions 1016 or 1534. The frequency of each mutation was calculated by adding the number of homozygotes multiplied by 2 to the number of heterozygotes and dividing the total by the sample size multiplied by 2. The 95% confidence interval was calculated using the adjusted Wald formula [30]. Hardy–Weinberg equilibrium was evaluated, and a chi-square test with 1 or 3 df (when three or six genotypes were evidenced, respectively) was used to test the null hypothesis of equilibrium.
Results
Fragments containing the 1016 and 1534 positions of the aedaenav gene (vectorbase number AAEL023266) were simultaneously amplified in duplex PCR from genomic DNA, using two primer pairs in the reaction tube. By the addition of GC tails in the 5’ regions of forward and reverse primers flanking the 1534 position (Table 1), a difference of around 10 °C in the MT was achieved between 1016 (MT around 73 °C) and 1534 (MT around 83 °C) amplicons (Fig. 2a). This difference allowed the individual analysis of both polymorphic sites using two primer pairs in a single reaction. Figure 2a shows representative curves of homozygous samples in both positions (SS and R2R2), and the heterozygous SR2. For representative curves of the heterozygous SR1, R1R2 and the homozygous R1R1 see Additional file 1.
a–e Results of genotyping positions 1016 and 1534 in the voltage-gated sodium channel (Nav) gene of Aedes aegypti (aedaenav) with high-resolution melting (HRM). Detection of kdr single nucleotide polymorphism by melt curve analysis. Alleles are distinguished by changes in the melting temperature (MT). a Raw results of multiplex HRM (mHRM). Peaks on the left and on the right indicate, respectively, IIS6 and IIIS6 Nav segment amplicons. b, c Derivative and difference melting plots for IIS6 with variation in the 1016 position. d, e Derivative and difference melting plots for IIIS6 with variation in the 1534 position. Red kdr homozygous standard (R2R2), yellow heterozygous standard (SR2), green wild type homozygous standard (SS)
As expected for a substitution of a guanine (in the wild type 1016S) by an adenine (in the 1016Ikdr variant), the MT of the IIS6 amplicons was highest for the 1016VV genotype (73.61 °C ± 0.02 °C; n = 10) and lowest for 1016 IkdrI kdr (72.74 °C ± 0.07 °C; n = 6); for the heterozygote 1016 VIkdr, the MT was intermediate (73.09 °C ± 0.02 °C; n = 6) (Fig. 2b, c). On the other hand, for the IIIS6 segment, the MTs were 82.45 °C (± 0.01 °C, n = 6) for 1534 FF and 83.07 °C (± 0.01 °C, n = 12) for 1534 CkdrCkdr. The MT was intermediate for the heterozygote 1534 FCkdr (82.68 °C ± 0.02 °C, n = 6) (Fig. 2d, e).
MTs were obtained for the IIS6 and IIIS6 paired amplicons with their respective variations in the 1016 and 1534 positions, and significant differences were observed in the MTs for both positions between both the homozygous and the heterozygous samples (P < 0.05; n = 6–12) (Fig. 3). The genotyping of heterozygous samples was less straightforward for particular samples, and we observed that both the quality and quantity of DNA extracted from individual mosquitoes were key to accurate genotyping.
a, b Changes in MTs with variation in the position of Nav in Aedes aegypti. a Position 1016. Homozygous Val (VV), present in genotypes SS, SR1 and R1R1; heterozygous Val Ile (VI), present in genotypes SR2 and R1R2; homozygous Ile (II), present in genotype R2R2. b Position 1534. Homozygous Phe (FF), present in genotypes SS; heterozygous Phe Cys (FC), present in genotypes SR1 and SR2; homozygous Cys (CC), present in genotypes R1R1, R1R2 and R2R2. Different letters indicate significant differences (P < 0.05; ANOVA, Tukey’s multiple comparison test)
The mHRM method developed here presented advantages compared to the other options [24, 31], such as higher throughput, and a one-step and closed-tube reaction, given that both alleles can be detected in a single reaction (Table 2).
We used mHRM for the analysis of Ae. aegypti from eight populations from the center of Argentina (AMBA) and two populations from the north-west (Tartagal-Salta Province and Calilegua National Park-Jujuy Province) (Fig. 1). R1 and S alleles were present at different frequencies in all the localities under study, whereas R2 was only found in the northern region (Tartagal and Calilegua) (Table 3; Fig. 1). To confirm the mHRM results by a gold standard sequencing method, a number of samples of genomic DNA were PCR amplified for IIS6 and IIIS6 Nav segments with specific primers and subjected to direct Sanger sequencing. For the 1016 position, the results for all of the samples subjected to Sanger sequencing were in accordance with those obtained using mHRM (n = 21), whereas for position 1534, there was agreement between the Sanger sequencing and HRM results for 12 of 14 samples (the two samples that did not agree were for heterozygous samples). All the sequenced IIS6 fragments were identical, with the exception of samples from Tartagal that had the kdr substitution in the 1016 position (Additional file 2: A). In two samples from La Plata Zoo and one from Arturo Segui, a silent substitution of thymidine to cytosine in position 1528 (Additional file 2: B) was detected. This polymorphism in the 1528 position has been previously reported in samples from Africa and the Americas [15].
For AMBA, we analyzed samples from both rural/peri-urban locations and populated urban localities. In the former, the most common allele was S (98.3% in Arturo Seguí; 95% in La Plata Cemetery and 92.5% in La Plata Zoo) (Fig. 1; Table 3). In the urban localities, a higher frequency of R1 was detected, although it was the least common allele in Quilmes (31.7%), but the most common one in Tigre (73.4%). In the other localities analyzed, the rate of the R1 allele was around 50% (55.4% in Lomas de Zamora, 55.6% in Avellaneda, 48.1% in La Matanza and 53.85% in Merlo) (Fig. 1; Table 3). In Tartagal, a locality with a more intense use of pyrethroids historically, the R2 allele was detected in 36.0% of the samples, but the most common allele was R1 (56.0%); the S allele was found in only 8.0% of the individuals. A similar pattern was detected in Parque Nacional Calilegua; the R2 allele was present in 48% of the samples, the R1 allele in 34%, and the S allele in only 18%. Genotype frequencies, confidence intervals for the six possible genotypes in each population and the results of the tested deviation from Hardy–Weinberg equilibrium are presented in Table 3. For both the La Matanza and Quilmes populations, from AMBA, the assumption of Hardy–Weinberg equilibrium was rejected (P < 0.05), as the heterozygous SR1 genotype for both populations was higher than expected according to the hypothesis. For the northern populations, the Hardy-Weinberg equilibrium was rejected for Tartagal (P < 0.05), as R1R2 was the most abundant genotype. R1R2 was also the most abundant genotype in Calilegua, but the Hardy–Weinberg equilibrium hypothesis was not rejected in this case (P = 0.07).
Discussion
Pyrethroid resistance in Ae. aegypti seriously compromises dengue control campaigns. Alternative adulticides, such as the organophosphate malathion, are not permitted for the treatment of human dwellings in Argentina, given environmental and sanitary considerations [8]. The rational design of vector control campaigns needs to include resistance-management strategies to prolong the period of efficacy of pyrethroids. In this context, the monitoring of resistance-conferring alleles, such as kdr mutations, should be routinely performed to aid decision-making for control campaigns. Notwithstanding the wide distribution of kdr alleles in natural populations of Ae. aegypti worldwide, and the epidemiologic situation in Argentina, the presence and/or distribution of kdr have not been previously reported for this country.
There are a number of assays available for genotyping kdr alleles in Ae. aegypti. The most widely used one of these for populations in the Americas is based on TaqMan probes [24]. Allele-specific PCR has also been developed for this; although cheaper, it has a lower throughput, and the reaction conditions, which must be carefully determined to avoid inaccurate results, were not reproducible in different labs [25]. Singleplex HRM has been used to genotype mutations in Asian populations of Ae. aegypti [31], which achieved double the genotyping effort when compared with the mHRM proposed here. Multiplex allele-specific PCR may be a suitable option for less well-equipped laboratories, though the costs of skilled labor should be taken into consideration. All life stages of mosquitoes (from eggs to adults), and even dead individuals, can be sampled during routine surveillance, without necessitating the collection or rearing of insects. Using a high-throughput genotyping technique such as the mHRM presented here, results can be obtained in a few hours (Table 2). Careful sample conservation and the use of high-yielding DNA extraction methods positively affect the quality of the results. Furthermore, we observed that it is important to use a high-quality HRM master mix, given that preliminary assays with other commercially available options gave a number of uninterpretable results. Also, it is important to calibrate the qPCR equipment with the same reagents that will be used for the assays. Where genotyping results are ambiguous, which was mainly found for heterozygous samples, a complementary method (such as TaqMan probes or Sanger sequencing) can also be used. In sum, the use of available complementary methods, including the mHRM presented here, will improve genotyping efforts in terms of time and cost.
Despite the lack of detailed information on insecticide application by region in Argentina, we can assume that there is a direct relationship between dengue epidemics and the control of adult mosquitoes by spatial spraying with adulticides, as recommended by the National Health Ministry during dengue outbreaks. The use of pyrethroids was prolonged in the northern region (Salta and Jujuy) because dengue epidemics have been recorded there since 1998 [32], with cases reported almost every year [33] up until the present (Bulletin of the Ministry of Health Argentina 2023). Accordingly, we observed a higher frequency of the R2 genotype both in a sylvatic and in an urban collection site in this region. In agreement with these findings, Harburger et al. [34] reported pyrethroid resistance in Ae. aegypti adults from Argentina for the first time in Salta Province (Salvador Mazza). Given that the Calilegua mosquitoes were collected in a protected sylvatic area, where insecticides are not used, it is possible that individuals invaded the national park from nearby urban locations.
AMBA has a more recent history of dengue outbreaks, which represented 35% of the most important dengue outbreaks registered in Argentine in 2016 [35], 2020 and 2023 (Bulletin of the Ministry of Health Argentina), despite it being the most populated region of Argentina. Interestingly, a positive correlation exists between the specific years of treatment with pyrethroids and the emergence of the 1016Ikdr mutation. Also, rural or peri-urban populations that were not treated with pyrethroids during ultralow volume spraying had a higher proportion of the wild type (sensitive) allele. These results are in agreement with the sequential selection of kdr mutations in Ae. aegypti [21]. Given that the V1016Ikdr mutation alone does not confer insecticide resistance, it is possible that mutation F1534Ckdr emerges earlier in response to selective pressure due to pyrethroid use. The 1016Ikdr + 1534Ckdr alleles, which emerged more recently in the Americas, lead to a greater and broader spectrum of pyrethroid resistance.
Although 1016Ikdr and 1534Ckdr are mutations of high epidemiological relevance, other kdr mutations that have an impact on pyrethroid sensitivity have been described for A. aegypti [36]. Among these, 410Lkdr, detected in samples from Brazil, Mexico and USA, was strongly associated with the 1016Ikdr and 1534Ckdr alleles [10,11,12], indicating that it may have a wide distribution throughout the Americas. Given the strong association of the 410Lkdr allele with 1016Ikdr and 1534Ckdr, it may not be necessary to genotype the 410 site for resistance monitoring purposes [10]. However, the frequency of 410Lkdr over time and throughout the Americas, and its association with other kdr mutations and with levels of insecticide resistance, are all of relevance for the study of pyrethroid resistance in mosquitoes [12]. The setting up of mHRM conditions for the simultaneous detection of three or more single nucleotide polymorphisms is challenging, yet feasible [37]. Hence, further studies should be undertaken for the development of mHRM that can simultaneously detect 410Lkdr, 1016Ikdr, 1534Ckdr and/or other polymorphisms of interest in the Ae. aegypti sodium channel gene.
Even though the frequency results obtained here should be interpreted with caution due to the low number of individuals analyzed, they indicate, to our knowledge for the first time, the extensive presence of kdr alleles in field populations of Ae. aegypti from Argentina. To confirm this finding, further, nationwide, studies are necessary. However, we have shown here that these alleles are present in regions that are more than 1500 km apart, which suggests that they have a wide distribution.
Information on kdr alleles in Ae. aegypti populations in Buenos Aires and northern provinces of Argentina should be taken into consideration in the rational design of vector control campaigns. The results presented here, and especially those for the populations sampled from the densely populated AMBA region, should be taken as a warning sign when designing Ae. aegypti control campaigns. Despite the fact that the rate of insecticide resistance for a given population will be influenced by its entire genetic background, including metabolic resistance mechanisms, detected kdr mutations are useful molecular markers for the early detection of pyrethroid resistance in vector control campaigns. In this context, we have developed a new high-throughput method that may have economic and practical benefits for the management of insecticide resistance in Ae. aegypti in many regions of the Americas. In the context of the lack of approved alternative compounds, decision-making with regard to planned pyrethroid treatments should be both careful and rational to enable their utility to be prolonged.
Conclusions
We report here, to our knowledge for the first time, the presence of kdr mutations in Ae. aegypti populations from geographically distant regions of Argentina, which have different epidemiological situations and different histories of mosquito control efforts. We have developed a high-throughput multiplex HRM method for the genotyping of these mutations. The method developed here could be incorporated into programs designed to assess the presence of resistance-associated alleles and control their spread, and may lead to reduced costs and running time. Optimal results would be obtained through the use of complementary, available genotyping methods. It is important to use these methods to obtain a complete picture of the frequencies of kdr alleles throughout the area of distribution of Ae. aegypti in Argentina for the effective short-term management of resistance, and to remain vigilant in assessing the dynamics of these frequencies over the longer term. In parallel, scientific effort needs to be urgently focused on the development of novel vector control alternatives which should have a low environmental impact.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Change history
14 August 2023
A Correction to this paper has been published: https://doi.org/10.1186/s13071-023-05894-y
References
World Health Organization (WHO). Dengue and severe dengue. 2021. https://www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-dengue.
Powell JR, Gloria-Soria A, Kotsakiozi P. Recent history of Aedes aegypti: vector genomics and epidemiology records. Bioscience. 2018;68:854–60.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7.
Díaz-Nieto LM, Maciá A, Perotti MA, Berón CM. Geographical limits of the southeastern distribution of Aedes aegypti (Diptera, Culicidae) in Argentina. PLoS Negl Trop Dis. 2013;7:1–6.
Grech M, Visintin A, Laurito M, Estallo E, Lorenzo P, Roccia I, et al. New records of mosquito species (Diptera: Culicidae) from Neuquén and La Rioja provinces, Argentina. Rev Saude Publica. 2012;46:387–9.
Rubio A, Cardo MV, Vezzani D, Carbajo AE. Aedes aegypti spreading in South America: new coldest and southernmost records. Mem Inst Oswaldo Cruz. 2020;115:1–4.
Cameron MM, Bell M, Howard AFV. Integrated vector management. Biol Environ Control Dis Vectors. 2013;24:175–89.
Gurtler R, Cecere MC. Chagas disease vector control. In: Guarneri A, Lorenzo M, editors. Triatominae—the biology of Chagas disease vectors. Springer: Berlin; 2021. p. 491–594.
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17.
Melo Costa M, Campos KB, Brito LP, Roux E, Melo Rodovalho C, Bellinato DF, et al. kdr genotyping in Aedes aegypti from Brazil on a nation-wide scale from 2017 to 2018. Sci Rep. 2020;10:1–12. https://doi.org/10.1038/s41598-020-70029-7.
Villanueva-Segura OK, Ontiveros-Zapata K, Lopez-Monroy B, Ponce-Garcia G, Gutierrez-Rodriguez SM, et al. Distribution and frequency of the kdr mutation V410L in natural populations of Aedes aegypti (L.) (Diptera: Culicidae) from eastern and southern Mexico. J Med Entomol. 2020;57:218–22.
Hernandez JR, Longnecker M, Fredregill CL, Debboun M, Pietrantonio PV. Kdr genotyping (V1016I, F1534C) of the Nav channel of Aedes aegypti (L.) mosquito populations in Harris County (Houston), Texas, USA, after Permanone 31–66 field tests and its influence on probability of survival. PLoS Negl Trop Dis. 2021;15:e0009833. https://doi.org/10.1371/journal.pntd.0009833.
Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Correction to: contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2021;15:1–2.
Martins AJ, Lima JBP, Peixoto AA, Valle D. Frequency of Val1016Ile mutation in the voltage-gated sodium channel gene of Aedes aegypti Brazilian populations. Trop Med Int Heal. 2009;14:1351–5.
Cosme LV, Gloria-Soriaid A, Caccone A, Powell JR, Martins AJ. Evolution of kdr haplotypes in worldwide populations of aedes Aegypti: independent origins of the F1534C kdr mutation. PLoS Negl Trop Dis. 2020;14:1–18. https://doi.org/10.1371/journal.pntd.0008219.
Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black WC IV. Coevolution of the Ile 1016 and Cys 1534 mutations in the voltage gated sodium channel gene of Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2015;9:1–22.
Linss JG, Brito L, Garcia G, Araki A, Bruno R, Lima JB, et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys kdr mutations in Aedes aegypti Brazilian natural populations. Parasit Vectors. 2014;7:25. https://doi.org/10.1186/1756-3305-7-25.
Estep AS, Sanscrainte ND, Waits CM, Bernard SJ, Lloyd AM, Lucas KJ, et al. Quantification of permethrin resistance and kdr alleles in Florida strains of Aedes aegypti (L.) and Aedes albopictus (Skuse). PLoS Negl Trop Dis. 2018;12:1–17.
Brito LP, Carrara L, de Freitas RM, Lima JBP, Martins AJ. Levels of resistance to pyrethroid among distinct kdr alleles in Aedes aegypti laboratory lines and frequency of kdr alleles in 27 natural populations from Rio de Janeiro, Brazil. Biomed Res Int. 2018;2018:1–10.
Hu Z, Du Y, Nomura Y, Dong K. A sodium channel mutation identified in Aedes aegypti selectively reduces cockroach sodium channel sensitivity to type I, but not type II pyrethroids. Insect Biochem Mol Biol. 2011;41:9–13. https://doi.org/10.1016/j.ibmb.2010.09.005.
Chen M, Du Y, Wu S, Nomura Y, Zhu G, Zhorov BS, et al. Molecular evidence of sequential evolution of DDT-and pyrethroid-resistant sodium channel in Aedes aegypti. PLoS Negl Trop Dis. 2019;13:e0007432.
Alvarez LC, Ponce G, Saavedra-Rodriguez K, Lopez B, Flores AE. Frequency of V1016I and F1534C mutations in the voltage-gated sodium channel gene in Aedes aegypti in Venezuela. Pest Manag Sci. 2015;71:863–9.
Villanueva-Segura K, Ponce-Garcia G, Lopez-Monroy B, Mora-Jasso E, Perales L, Gonzalez-Santillan FJ, et al. Multiplex PCR for simultaneous genotyping of kdr mutations V410L, V1016I and F1534C in Aedes aegypti (L.). Parasit Vectors. 2020;13:325. https://doi.org/10.1186/s13071-020-04193-0.
de Macoris ML, Martins AJ, Andrighetti MTM, Lima JBP, Valle D. Pyrethroid resistance persists after ten years without usage against Aedes aegypti in governmental campaigns: lessons from São Paulo State Brazil. PLoS Negl Trop Dis. 2018;12:1–18.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111.
Liew M, Pryor R, Palais R, Meadows C, Erali M, Lyon E, et al. Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clin Chem. 2004;50:1156–64.
Wuliandari JR, Lee SF, White VL, Tantowijoyo W, Hoffmann AA, Endersby-Harshman NM. Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in aedes aegypti from Yogyakarta, Indonesia. Insects. 2015;6:658–85.
Darsie R. The mosquitoes of Argentina. Part I. Keys for identification of adult females and fourth stage larvae in English and Spanish (Diptera: Culicidae). Mosq Syst. 1985;17:153–253.
Oberacker P, Stepper P, Bond DM, Höhn S, Focken J, Meyer V, et al. Bio-on-magnetic-beads (BOMB): open platform for high-throughput nucleic acid extraction and manipulation. PLoS Biol. 2019;17:1–16.
Agresti A, Coull BA. Approximate is better than “Exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–26. https://doi.org/10.1080/00031305.1998.10480550.
Saingamsook J, Saeung A, Yanola J, Lumjuan N, Walton C, Somboon P. A multiplex PCR for detection of knockdown resistance mutations, V1016G and F1534C, in pyrethroid-resistant Aedes aegypti. Parasit Vectors. 2017;10:1–9.
Das S, Sarfraz A, Jaiswal N, Das P, Avilés G, Rangeón G, et al. Dengue history in Argentina. Emerg Infect Dis. 1999;5:575–8. https://doi.org/10.1016/j.jiph.2017.02.004.
Avilés G, Paz MV, Rangeon G, Ranaivoarisoa MY, Verzeri N, Roginski S, et al. Laboratory surveillance of dengue in Argentina, 1995–2001. Emerg Infect Dis. 2003;9:738–42.
Harburguer L, Gonzalez PV, Zerba E. First report of adult Aedes aegypti (Diptera: Culicidae) resistance to pyrethroids in Argentina. J Med Entomol. 2022;59:372–5.
Bolzan A, Insua I, Pamparana C, Giner MC, Medina A, Zucchino B. Dinámica y caracterización epidemiológica del brote de dengue en Argentina año 2016: el caso de la Provincia de Buenos Aires. Rev Chil Infectol. 2019;36:16–25.
Zhorov BS, Dong K. Pyrethroids in an alphaFold2 model of the insect sodium channel. Insects. 2022. https://doi.org/10.3390/insects13080745.
Zhang L, Ma X, You G, Zhang X, Fu Q. A novel multiplex HRM assay to detect clopidogrel resistance. Sci Rep. 2017;7:16021. https://doi.org/10.1038/s41598-017-16310-8.
Acknowledgements
The authors would like to thank Claudia Rodriquez Torres, Pedro Mendoza Zélis, Elisa de Sousa and Luciana Juncal for generously providing the silica-coated magnetic nanoparticles for the DNA extractions; Laura Morote for helping to design the figures; Darío Balcazar at the molecular laboratory at CEPAVE; Graciela Minardi at the statistical methodology laboratory at CEPAVE; Melisa B. Bonica; and María Eugenia Cano, who collected mosquito samples in the context of her PhD thesis. The Argentinean groups are members of the Argentinean Network for Research in Pesticide Resistance (RAREP).
Funding
This work was funded by PICT2018-0862 and PICT2020-0518 to SO. ANB has a research fellowship from CONICET. SO, MVM and MSS are researchers at CONICET. MIS is a technical professional at CONICET.
Author information
Authors and Affiliations
Contributions
ANBI: performed all of the HRM experiments, analyzed the data, contributed to manuscript writing. MVM: conceptualization, mosquito sampling, manuscript writing. MIS: sequence analysis. MSS: mosquito sampling, funding acquisition. AJM: provided the standard samples for setting up the HRM; critical revision of the manuscript. SO: conceptualization, funding acquisition, writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Detection of kdr single nucleotide polymorphism by melt curve analysis. Raw curves showing both 1016 (left peak) and 1534 (right peak) positions. Red kdr homozygous standard (R2R2), yellow heterozygous standard (SR2), green homozygous standard (SS). A Representative curve for the SR1 genotype (violet). B Representative curve for the R1R1 genotype (blue). C Representative curve for the R1R2 genotype (grey).
Additional file 2.
Clustal alignments of sequences obtained by Sanger sequencing and gene AAEL023266 in Vectorbase for regions 1016 and 1534.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barrera-Illanes, A.N., Micieli, M.V., Ibáñez-Shimabukuro, M. et al. First report on knockdown resistance mutations in wild populations of Aedes aegypti from Argentina determined by a novel multiplex high-resolution melting polymerase chain reaction method. Parasites Vectors 16, 222 (2023). https://doi.org/10.1186/s13071-023-05840-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05840-y