Abstract
Background
Application of raw starch-degrading enzymes (RSDEs) in starch processing for biofuel production can effectively reduce energy consumption and processing costs. RSDEs are generally produced by filamentous fungi, such as Penicillium oxalicum, but with very low yields, which seriously hampers industrialization of raw starch processing. Breeding assisted by random mutagenesis is an efficient way to improve fungal enzyme production.
Results
A total of 3532 P. oxalicum colonies were generated after multiple rounds of mutagenesis, by atmospheric and room-temperature plasma (ARTP) and/or ethyl methanesulfonate (EMS). Of these, one mutant A2-13 had the highest RSDE activity of 162.7 U/mL, using raw cassava flour as substrate, a yield increase of 61.1%, compared with that of the starting strain, OXPoxGA15A. RSDE activity of A2-13 further increased to 191.0 U/mL, through optimization of culture conditions. Increased expression of major amylase genes, including the raw starch-degrading glucoamylase gene, PoxGA15A, and its regulatory gene, PoxAmyR, as well as several single-nucleotide polymorphisms in the A2-13 genome, were detected by real-time reverse transcription quantitative PCR and genomic re-sequencing, respectively. In addition, crude RSDEs produced by A2-13, combined with commercial α-amylase, could efficiently digest raw corn flour and cassava flour at 40 °C.
Conclusions
Overall, ARTP/EMS-combined mutagenesis effectively improved fungal RSDE yield. An RSDE-hyperproducing mutant, A2-13, was obtained, and its RSDEs could efficiently hydrolyze raw starch, in combination with commercial α-amylase at low temperature, which provides a useful RSDE resource for future starch processing.
Similar content being viewed by others
Background
Plant biomass biorefineries use renewable and relatively inexpensive raw materials as feedstocks for processing into value-added biofuels and chemicals, with potential benefits for industry and the environment. Biorefinery processes can help alleviate problems associated with industrial chemicals and fossil fuels, such as increasing energy costs, environmental pollution and climate change [1, 2]. A major challenge, however, for biorefineries, is the high cost of enzymes used for biomass degradation into fermentable sugars, which results in poor profitability [1].
Bioethanol production by cassava starch biorefineries has attracted considerable interest because of the competitive advantages of cassava, such as abundant availability, low cost, and non-competition with direct food/feed supplies [3]. Conventional enzymatic starch processing requires an initial high-temperature liquefaction step, using thermostable α-amylase, followed by saccharification with glucoamylase, after cooling below the starch gelatinization temperature, then fermentation to produce bioethanol [4]. Even these enzymatic processes require a large energy input and special equipment, which increases processing costs. The liquefaction step accounts for approximately 30‒40% of the total cost of bioethanol production [5].
Amylase for starch processing accounts for the majority of the industrial enzyme market and consists of four related enzymes, α-amylase (EC 3.2.1.1), glucoamylase (EC 3.2.1.3), α-glucosidase (EC 3.2.1.20), and 1,4-a-glucanbranching enzyme (EC 2.4.1.18) (https://www.cazy.org/). α-Amylase and glucoamylase are commonly used in combination for starch processing. α-amylase breaks α-1,4-glycosidic bonds into amylopectin, or amylose straight chains, to release straight-chain and branched oligosaccharides of various lengths. Glucoamylase can cleave both α-1,4- or α-1,6-glucosidic bonds at the non-reducing ends of starch chains, or dextrins, to release glucose [6, 7].
Remarkably, a few amylase proteins contain starch-binding domains (SBDs) that allow them to bind to the surface of raw starch granules [8], thereby efficiently and directly digesting granular, raw starch into glucose, even below the gelatinization temperature of starch. Those amylases are known as raw starch-degrading enzymes (RSDEs) and have potential applications in starch processing. In the natural environment, RSDEs are mainly secreted by filamentous fungi, but with low yields, which have not yet met the quantitative and cost requirements for large-scale industrialization of raw starch biorefining.
Classical physical or chemical mutagenesis is an efficient strategy for breeding of fungal strains, in particular, for improvement of enzyme yields. The main mutagens used are ethyl methanesulfonate (EMS), nitroglycerin, UV-radiation, and “atmospheric and room temperature plasma” (ARTP) [9,10,11,12]. The chemical agent EMS alkylates nucleotides at random positions, thereby resulting in transition mutations [13]. ARTP can change the structure and permeability of the cell wall and plasma membrane, by generation of plasma jets, which causes DNA damage; ARTP is a recently developed and effective mutation technique [14]. The use of EMS combined with UV treatment has been reported [9, 10], whereas EMS combined with ARTP has not, to the best of our knowledge.
In this study, we employed multiple rounds of ARTP/EMS-combined mutagenesis to improve RSDE production in P. oxalicum. The starting strain used was the engineered strain OXPoxGA15A, derived from the parental strain ∆PoxKu70 via over-expressing a raw-starch-degrading glucoamylase gene PoxGA15A [11, 15, 16]. Subsequently, culture conditions were optimized for the resulting mutant with the highest RSDE production and enzymatic hydrolysis efficiency against raw starch flour. Both genome re-sequencing and real-time reverse transcription quantitative PCR (RT-qPCR) were employed to analyze single-nucleotide polymorphisms and transcriptional levels of genes encoding major RSDEs in the isolated mutants.
Results and discussion
Development of a two-layer agar gel diffusion method for rapidly screening for RSDE-hyperproducing mutants
To optimize screening efficiency for mutants with improved RSDE production, a high-throughput screening method using two-layer agar gels was developed. A two-layer agar gel was prepared, containing raw natural cassava flour (RNCF) and ball-milled Avicel, at a series of different ratios, as the top layer, and modified minimal medium (MMM), without carbon source as the bottom layer. RNCF flour was ground directly from freshly harvested cassava tubers after drying, without any other pretreatments, such as cellulose removal. RNCF is very similar to the cassava flour used in the starch industry and different from edible raw starch available from farmer’s markets. As expected, Avicel stimulated the expression of cellulase and xylanase genes, as well as induction of the pPoxEgCel5B promoter, which controls over-expression of the RSDG gene, PoxGA15A in OXPoxGA15A [15], whereas RNCF induced the expression of amylase genes, including RSDE genes. The two-layer agar gel containing 0.5% Avicel and 1.0% RNCF exhibited clear zones around colonies of OXPoxGA15A and the parental strain ∆PoxKu70, the clear zones around the OXPoxGA15A colonies being slightly bigger (Fig. 1a). When inoculated into liquid MMM containing 0.5% Avicel and 1.0% RNCF, the RSDE activity of OXPoxGA15A was 362.6% higher than that of ∆PoxKu70 (p < 0.01; Fig. 1b). These data confirmed that the two-layer agar gel was an effective way to screen for RSDE hyperproducers, after random mutagenesis.
Phenotypic investigation of P. oxalicum strains ∆PoxKu70 and OXPoxGA15A, grown on a two-layer agar gel plate (a) and their RSDE activity in liquid medium (b). The two-layer agar gel plate consisted of an upper layer containing ball-milled Avicel (0.5% w/v) and natural raw cassava flour (RNCF, 1% w/v), and a lower layer of MMM without carbon source. Carbon sources used in liquid medium were the same as that in the top layer of the two-layer plate. RSDE activity was determined using RNCF as the substrate. ** (p ≤ 0.01) indicates significant difference between the OXPoxGA15A and ∆PoxKu70 by Student’s t test. RSDE raw starch-degrading enzyme, MMM modified minimal medium
ARTP/EMS-mediated mutagenesis and screening for RSDE hyperproducers
ARTP/EMS-mediated mutagenesis was performed in three steps, i.e., (1) three rounds of EMS, (2) one round of ARTP, and (3) one round of ARTP combined with EMS (Additional file 1: Fig. S1). Prior to EMS and/or ARTP mutagenesis, the optimal lethality of EMS against the starting strain OXPoxGA15A was determined at 1, 2, 4, 8, and 12 h after treatment. Treatment with 2% EMS for 8 h resulted in 93.6% lethality (i.e., cell death), whereas treatment for 12 h resulted in 100% lethality (Additional file 2: Fig. S2A).
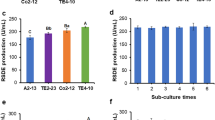
Investigation of potential P. oxalicum mutants isolated from each round of mutagenesis. a Clear zones on the two-layer agar gel plates after 8 days. Red circle indicates the isolated colony used for the next round of mutagenesis. b Diameter ratio between clear zone and colony. c RSDE activity. d RSDE activity of the strain A2-13 sub-cultured six times. RSDE activity was determined using RNCF as the substrate. ** (p ≤ 0.01) indicates significant difference between the isolates and the starting strain OXPoxGA15A by Student’s t test. RSDE raw starch-degrading enzyme, RNCF natural raw cassava flour
The lethality of ARTP against mutant E3-16 (see following description) was also determined, after 350, 400, 450, 500, and 550 s of treatment at a radio-frequency power input of 130 W and a flow rate of 10 L/min. The lethality increased with treatment time and treatment for 500 s resulted in 95.1% lethality (Additional file 2: Fig. S2B).
When cultured for 8 days after treatment, a total of 3532 colonies were observed on the two-layer agar plates, including 1616 colonies from three rounds of EMS treatment, 1120 from ARTP treatment and 796 from the ARTP/EMS combination (Additional file 1: Fig. S1). Comparison of the diameter ratio between each colony and its clear zone selected the mutants designated as E1-1, E2-3, E3-16, A1-2, and A2-13 as having the largest ratios after each round of mutagenesis; their diameter ratios ranged from 1.51 to 2.0, compared with 1.48 for the original strain, OXPoxGA15A (Fig. 2a, b). Measurement of enzymatic activities determined the RSDE activity of each mutant, using RNCF as the substrate, ranged from 103.2 to 162.7 U/mL, when cultured in liquid MMM containing 4% wheat bran plus 1% Avicel, which was consistent with its diameter ratio (Fig. 2c). A2-13 had the highest RSDE activity and the largest diameter ratio, so it was selected for further study.
RSDE production by A2-13 was 61.0% higher than that of the starting strain OXPoxGA15A, after multiple rounds of ARTP/EMS mutagenesis. This appears to be attributable to mutant selection using the two-layer agar screening plates, containing both cassava starch and Avicel. The clear zones on the screening plates indicated degradation of both starch and Avicel.
To evaluate the genetic stability of mutant A2-13, regarding enzyme production, the RSDE activities, using RNCF as the substrate, were measured after each of six successive sub-cultures. No significant change in RSDE production was observed after any of the sub-cultures (Fig. 2d).
Optimization of liquid culture conditions
The secretion of plant biomass-degrading enzymes (i.e., amylase, cellulase, and xylanase) by filamentous fungi is closely related to the characteristics of the lignocellulosic substrates in media, such as physical properties and chemical composition, as well as culture-related factors, such as the culture set-up and substrate loading [1, 17]. Therefore, to further improve RSDE production by mutant A2-13, the following liquid culture parameters were optimized: the initial pH of the medium, incubation temperature, composition of carbon source and composition ratio, nitrogen source, and concentration of spore inoculum. The optimal conditions found were as follows: initial pH of medium, 5.5; incubation temperature, 28 °C; composition of carbon source, wheat bran plus Avicel (2:3 w/w); nitrogen source, 5 g/L NH4NO3; and concentration of spore inoculum, 1% (v/v) inoculum with 108 spores/mL (Additional file 3: Fig. S3). When cultured under these optimal culture conditions, mutant A2-13 produced 191.0 U/mL of RSDEs, which was 17.4% and 89.1% higher than those of A2-13 and OXPoxGA15A under non-optimal culture conditions (p < 0.01), respectively. This was also far higher RSDE production than by P. oxalicum strain GXU20 (20 U/mL), using the same substrate [17]. The production of RSDE by A2-13 against RNCF, under the optimal conditions, is the highest reported to date. Other reports of RSDE activities using different raw starch substrates, such as processed cassava starch, potato starch, or uncooked soluble starch, are not directly comparable (Table 1).
Properties of crude RSDE produced by the mutant A2-13
To investigate the effects of ARTP/EMS-combined mutagenesis on the properties of the crude enzymes produced, the optimal pH and temperature of the crude enzymes were measured in comparison with those of OXPoxGA15A. The optimum pH and temperature, with RNCF as substrate, were 4.5 and 65 °C, respectively, which were similar to those of crude enzymes from OXPoxGA15A (Fig. 3a, b). Moreover, pH and heat stability analyses revealed that crude RSDE from A2-13 was highly stable when degrading RNCF under acidic conditions, again similar to RSDE from OXPoxGA15A. Interestingly, crude RSDE from strain A2-13 also had improved tolerance to alkaline conditions (Fig. 3c) and was more stable at low temperature (< 40 °C), but less stable at medium–high temperature (45–65 °C; Fig. 3d), compared with RSDE from OXPoxGA15A. These changes in RSDE properties are potentially very beneficial for industrial applications, such as simultaneous saccharification and fermentation of raw starch to produce bio-ethanol, in which yeasts grow at 30–35 °C [4, 11, 23, 24], using as carbon source, glucose released from raw starch by simultaneous RSDE degradation.
Effects of pH and temperature on RSDE activity of P. oxalicum strains A2-13 and OXPoxGA15A. a pH profile of RSDE activity. Enzyme activity was measured at pHs between 3.0 and 7.0 in 0.1 M citrate–phosphate buffer at 37 °C. b Temperature profile of RSDE activity. c Effect of pH on RSDE stability. d Thermal stability of RSDE activity. P. oxalicum strains were cultured for 6 days under the optimal culture conditions. In a and b, the highest RSDE activity of A2-13 and OXPoxGA15A was set as 100%. In c and d, the RSDE activity of untreated A2-13 and OXPoxGA15A was set as 100%. Each experiment was independently performed three times. Each data point represents mean ± SD
Hydrolytic analysis of raw starch with crude RSDE produced by mutant A2-13
Corn starch has been widely used as a renewable feedstock for bioethanol production in the US [25]. Cassava starch is readily available in subtropical areas, such as China, Southern Asia, and South Africa, and extensively employed for producing bioethanol and other bio-based products, because of its low cost and non-competition with direct food/feed supplies [3]. Therefore, RNCF and natural raw corn flour (RNCOF) were chosen to evaluate potential applications of A2-13, with OXPoxGA15A as control. Analysis of enzymatic hydrolysis reactions revealed that the hydrolytic efficiency of the crude A2-13 enzyme for converting RNCOF into glucose was slightly lower than the OXPoxGA15A enzyme (degree of hydrolysis (DH) 87.6% and 98.1% after 72 h, respectively Fig. 4a). In contrast, with RNCF as the substrate, the DHs were 91.8% and 62.5% after 72 h, with the OXPoxGA15A and A2-13 enzymes, respectively (Fig. 4b).
Hydrolysis efficiencies of RNCOF and RNCF by crude enzymes from P. oxalicum strains A2-13 and OXPoxGA15A. Hydrolysis of RNCOF (a) and RNCF (b) by crude enzymes from mutants A2-13 and OXPoxGA15A. Hydrolysis of RNCOF (c) and RNCF (d) by crude enzymes from mutant A2-13 combined with commercial α-amylase. Hydrolysis of RNCOF (e) and RNCF (f) by crude enzymes from OXPoxGA15A combined with commercial α-amylase. In panels a and b, 50 U crude enzyme was added per g of solid substrate. In panels c–f, the legends show the amounts of crude enzymes from P. oxalicum added. The ratio of crude enzymes from P. oxalicum and commercial α-amylase was 1:1. Values are presented as the mean ± SD of three replicates for each treatment. RNCOF natural raw corn flour, RNCF natural raw cassava flour
When hydrolyzing different substrates using the same enzyme, the substrate composition can contribute to the hydrolytic efficiency. The composition of raw starch granules is mainly amylose and amylopectin, with minor non-starch components, such as cellulose fibers, proteins, and fats [26]. Amylose is made up of long linear polymer chains of glucopyranose units, whereas amylopectin has shorter, but highly branched chains, with high molecular weights [27]. Normally, the amylose content of cassava starch is lower than that of corn starch, but the fiber content is higher [28].
Different DHs from the same substrate, using crude enzymes from strains A2-13 and OXPoxGA15A, may result from the two strains producing different RSDE mixtures. Fast degradation of raw starch requires the synergistic action of various enzymes, including α-amylases, glucoamylases, α-glucosidases, and α-1,4-glucan debranching enzymes [7]. For example, the recombinant rPoxGA15A combined with commercial α-amylase efficiently degraded raw corn starch and processed raw cassava flour, but rPoxGA15A alone did not [11].
Therefore, crude enzymes from A2-13 and OXPoxGA15A were combined with commercial α-amylase and used to hydrolyze RNCOF and RNCF. Hydrolysis of RNCOF by crude enzyme from A2-13 reached a DH of 96.0% at 72 h, with an enzyme loading of 50 U/g substrate, and a DH of 100% at 60, 48 and 36 h with 100, 150, and 200 U/g substrate, respectively (Fig. 4c). With RNCF as substrate, the DH reached 87.1% at 72 h with an enzyme loading of 50 U/g substrate, and 100% at 72, 60 and 48 h with 100, 150, and 200 U/g (Fig. 4d). The hydrolytic efficiencies against RNCOF (Fig. 4e) and RNCF (Fig. 4f) with crude enzymes from OXPoxGA15A were similar with the enzymes from A2-13, when they were combined with commercial α-amylase.
Analysis of extracellular proteins and transcriptional levels of major amylase genes in the mutant A2-13
To better understand the findings from the hydrolysis experiments, the proteins secreted by A2-13 and OXPoxGA15A, cultured under their optimal conditions, were compared by SDS-PAGE analysis. The composition of the two protein mixtures was similar, but the relative concentrations of the different components were noticeably different. Specifically, the band corresponding to raw starch-degrading enzyme PoxGA15A [11, 15] in A2-13 was denser than that in OXPoxGA15A (Fig. 5a). The PoxGA15A band (labeled by purple arrow in Fig. 5b) in A2-13 was 20% denser than that in OXPoxGA15A (p < 0.01), but the PoxAmy13A band was not significantly different between A2-13 and OXPoxGA15A (Fig. 5). A2-13 apparently produced more raw starch-degrading glucoamylase than OXPoxGA15A. Glucoamylase can release glucose from the non-reducing end of starch chains, and acts synergistically with α-amylase, which generates new non-reducing ends in starch chains, thereby improving the hydrolysis efficiency. Moreover, the crude enzymes secreted by A2-13 were a good balance between the different synergistic amylases, which can efficiently digest raw starch.
Analysis of extracellular proteins secreted by P. oxalicum strains A2-13 and OXPoxGA15A (a, b) and transcription levels of the important amylase genes and their regulatory gene via RT-qPCR analysis (c). a SDS-PAGE analysis; b Relative quantity analysis of the bands in (a). In panel a, bands marked with purple and red arrows correspond to raw starch-degrading glucoamylase PoxGA15A and α-amylase PoxAmy13A, respectively. In panel c, expression levels of the tested genes in the strain A2-13 were normalized against those in the starting strain OXPoxGA15A. The actin gene was used as reference. **p ≤ 0.01 and *p ≤ 0.05 indicate significant differences between the A2-13 and OXPoxGA15A by Student’s t test. Each experiment contained three biological replicates. Each data point represents mean ± SD. RT-qPCR real-time reverse transcription quantitative PCR, SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis, MMM modified minimal medium
The expression of the major amylase genes and their regulatory genes in A2-13 on wheat bran plus Avicel was determined by RT-qPCR, i.e., raw starch-degrading glucoamylase gene, PoxGA15A (POX01356), glucoamylase gene, POX02412, as well as α-amylase gene, PoxAmy13A (POX09352), and its key regulatory gene, PoxAmyR (POX03890). After 12 h of culture, transcription of PoxAmyR was up-regulated by 62.0%. The other three were down-regulated after 12 h of culture, by 40.8–91.9% (p < 0.05), but between 24 and 48 h, they were up-regulated by 33.9–559.5% (p < 0.05; Fig. 5c). Modification of amylase gene expression may have resulted from the change in PoxAmyR expression.
Phenotypic and growth analyses of the mutant A2-13
The phenotype and mycelial growth of A2-13 were compared with those of OXPoxGA15A. A2-13 was directly inoculated onto solid PDA medium plates containing different carbon sources: wheat bran plus Avicel, Avicel, soluble starch, glucose, and NRCA (RNCF plus Avicel) and cultured for 2‒6 days. The A2-13 colonies on wheat bran plus Avicel, Avicel, PDA, and glucose plates were smaller than those of OXPoxGA15A to varying degrees, appeared dense, thick with mycelia, and darker in color. A2-13 colonies on the other carbon sources were darker in color, but not different in size (Additional file 4: Fig. S4A). Sporulation of A2-13 varied on different carbon sources; sporulation was delayed on PDA, wheat bran plus Avicel, and glucose plates, but accelerated on the other carbon sources (Additional file 4: Fig. S4A).
Genome re-sequencing of the mutant A2-13
To further elucidate the reasons behind the observed changes in enzyme production and phenotypes, genome re-sequencing of A2-13 was carried out, with OXPoxGA15A as control. In total, 2881 Mbp of clean data for each P. oxalicum strain were generated, with an average sequencing depth of approximately 90-fold, which covered the genome of the wild-type strain HP7-1 with > 99% coverage.
Comparative genomic analyses detected 230 single-nucleotide variations (SNVs) and 131 insertion/deletions (InDels) in the genome of A2-13, compared with that of OXPoxGA15A. These SNVs were localized in 193 intergenic regions and 35 coding sequences (CDSs). Of the 35 SNVs in CDSs, 25 non-synonymous SNVs were detected, including one stop, non-synonymous mutation in gene POX03359 that encodes a hypothetical protein (Additional file 5: Table S1). There were two genes, POX02883 and POX04725, encoding putative transcription factors. Gene POX09060 encoded the homologous protein of histone methyltransferase Set1, and shared 99.9% identity with PoSet1 (PDE_02489) in P. oxalicum strain 114–2 and 64.4% with Set1 in Saccharomyces cerevisiae. Set1 performs the methylation of histone H3 lysine 4 (H3K4) [29]. In S. cerevisiae, Set1 contributes to assembly of the elongation complex associated with RNA polymerase (Pol) II, by binding nascent RNA [30]. Recently, Li et al. [31] reported that PoSet1 positively regulated the production of cellulases and xylanases in P. oxalicum, by modulating H3K4me1 and H3K4me2 signals, as well as regulating colony diameter and spore number. POX01661 encoded the subunit Tho2 of the THO complex that is required for RNA transcript elongation, mRNA biogenesis, and export in S. cerevisiae [32, 33]. POX09438 was annotated as formin Bni1p, which is involved in the polarized localization in S. cerevisiae, controlled by the Rho GTPase Cdc42, via the effector Gic2p [34]. Cdc42 is activated by the Bud site selection protein Bud3 [35] encoded by POX05164. To our surprise, a β-glucosidase gene, POX03641 was found with a unique SNV and another gene, POX02958-encoded calcium/calmodulin-dependent protein kinase type I, and two ribosomal protein-encoding genes, POX01113 and POX01133.
In addition to the SNVs in CDSs, of 193 SNVs located in intergenic regions, 38 and 10 were localized in 1500 bp upstream- and 300 bp downstream regions of the coding genes, respectively (Additional file 5: Table S1). These regions may contain gene promoters and terminators, in which SNVs may regulate the expression of the corresponding genes. A notable finding was a gene, POX02086, encoding a zinc finger protein of CCHC-type, two sugar/inositol transporter encoding genes POX05515 and POX08241 and an α-glucosidase gene POX07319.
InDels in the mutant A2-13 included 65 deletions and 76 insertions. Most of them were located in intergenic regions and introns. Of particular interest, there were six InDels located in four CDSs (POX00063, POX02669, POX07393, and POX09551) that encoded endo-β-1,4-xylanase Xyn11A, alkaline phosphatase, ABC transporter, and a hypothetical protein, respectively. InDels in POX00063 (G deleted at positions 197,194) and POX09551 (A and G inserted at positions 17,671 and 17,697) resulted in a frameshift mutation in A2-13, whereas deletion of nucleotides in POX02669 (CTCCCG deleted at position 2,276,066) and POX07393 (G and TACTCCCC deleted at positions 877 and 878) resulted in removal of a few amino acids at the C-terminus (Additional file 6: Table S2). However, it appears that changes resulting from the InDels in POX00063, POX02669, and POX07393 do not contribute to increased amylase production in A2-13. Elucidation of the biological role of POX09551 requires further study in P. oxalicum.
Conclusions
In this study, we carried out ARTP/EMS-combined mutagenesis to enhance raw starch-degrading enzyme (RSDE) production in P. oxalicum. A mutant strain, A2-13, was isolated and produced RSDEs at a concentration of 191.0 U/mL, a yield increase of 89.1% compared with that of the parental strain, OXPoxGA15A. Crude RSDE enzymes from A2-13 showed improved tolerance to alkaline conditions and high hydrolysis efficiency against raw starch, when combined with commercial α-amylase. Furthermore, the factors contributing to the improved RSDE production by A2-13 were elucidated. This study confirmed that combined ARTP/EMS is an effective tool to enhance fungal RSDE yields and provided a potential new source of RSDEs for future industrial starch processing.
Methods
P. oxalicum strains and culture conditions
All P. oxalicum strains were cultured on potato-dextrose agar (PDA) plates for spore production. The starting strain, OXPoxGA15A, was from the China Center for Type Culture Collection, Wuhan, China (accession number M 2017794; [15]). Spores were collected from P. oxalicum cultured on PDA plates for 6 days. Fresh P. oxalicum spores (1.0 × 108) were cultured in liquid MMM (g/L: (NH4)2SO4 4.0, KH2PO4 4.0, CaCl2 0.6, MgSO4·7H2O 0.60, FeSO4·7H2O 0.005, MnSO4 0.0016, ZnCl2 0.0017, CoCl2 0.002, and 1 mL/L of Tween 80) [36], supplemented with 4% w/v wheat bran, plus 1% w/v Avicel, in an orbital shaker at 180 rpm and 28 °C, for 6 days. The secreted crude enzymes were collected by centrifugation at 11,300×g for 15 min and the supernatant used for measurement of enzymatic activity and hydrolysis of natural raw starch flour.
For RT-qPCR analyses, P. oxalicum strains (1.0 × 108 spores) were pre-cultured in MMM containing glucose as the sole carbon source for 24 h, then transferred into MMM containing 2% w/v wheat bran, plus 3% w/v Avicel in a shaker, at 180 rpm and 28 °C, for 12–48 h. The mycelia were harvested by filtering for RNA extraction every 12 h.
For genome re-sequencing, P. oxalicum strains OXPoxGA15A and A2-13 were inoculated into Completed Medium containing (in g/L) yeast extract 1.0, glucose 10.0, casein hydrolysate 1.0 g, NaNO3 6.0, KCl 0.52, MgSO4·7H2O 0.52, KH2PO4 1.52, FeSO4·7H2O 0.005, MnSO4 0.0016, ZnCl2 0.0017, and CoCl2 0.002, pH 6.5, followed by shaking at 180 rpm and 28 °C, for 48 h.
A two-layer agar gel was prepared, containing raw natural cassava flour (RNCF; from a local farmer’s market in Nanning, China) and ball-milled Avicel (Sigma-Aldrich, Darmstadt, Germany), at a series of different ratios as the top layer, and MMM, without a carbon source as the bottom layer. RNCF was ground directly from freshly harvested cassava tubers after drying, without any other pretreatments, such as cellulose removal.
Extraction of total DNA and RNA
Total DNA and RNA were extracted from hyphae of P. oxalicum strains using chemical methods, as reported previously [36].
Mutagenesis
For EMS treatment, fresh spores were re-suspended in phosphate buffered saline (PBS) at 108 spores/mL. EMS (1.2%, w/v) was added to the suspension and incubated in a shaker at 180 rpm and 28 °C, for 1–12 h, to determine the optimum treatment time. An equal volume of Na2S2O3 solution was used to stop reaction. The treated spores were separated by centrifugation at 11,300×g for 10 min at 4 °C and re-suspended at different concentrations (between 102 and 108/mL) with sterile water.
For ARTP treatment, fresh spores were re-suspended in 5.0% glycerol at 106 spores/mL. Spore suspension (10 µL) was spread on carrier plates and treated for 0 to 550 s at a flow rate of ten liters per minute and radio-frequency power input of 130 W, in a Type M ARTP Mutagenesis Bio-breeding Machine (Wuxi Tmaxtree Biotechnology Co., Ltd., China), to determine the optimum treatment time. The treated spores were washed from the carrier plates using sterile water and adjected to concentrations of 102 to 108 spores/mL.
ARTP/EMS-combined treatment was performed thereafter, as described above, with the optimum treatment times of 8 h by EMS and 500 s by ARTP.
Treated spore suspension (~ 100 μL) was spread on plates containing MMM supplemented with RNCF (1% w/v) plus Avicel (0.5% w/v). After incubation for 8 days at 28 °C, the diameter ratio between each colony and its clear zone was measured and calculated. Spores without EMS and/or ARTP treatment were used as controls.
Measurement of fungal enzymatic activity
The activity of RSDEs was determined as described previously [37], using RNCF as the substrate. Briefly, crude enzymes from P. oxalicum cultures were diluted with citrate buffer (100 mM; pH 4.5). Diluted enzyme solution (50 μL) was added to RNCF (450 μL; 1.0% w/v), incubated at 65 °C for 30 min; then the reaction mixtures were transferred to boiling water for 10 min. Enzymes inactivated by boiling were used as controls. Amounts of released reducing sugars and p-nitrophenol were determined using the 3,5-dinitrosalicylic acid method [38] and spectrometry at 410 nm, respectively. One unit of enzymatic activity (U) was defined as the amount of enzyme that produced 1 μmol of reducing sugars per min from each appropriate substrate.
Optimization of culture conditions
For further enhancement of RSDE production by the mutant A2-13, culture parameters, including the initial medium pH, incubation temperature, carbon source composition, and nitrogen source, were optimized, respectively, using MMM containing wheat bran (4% w/v) plus Avicel (1% w/v). The effect of the initial medium pH on RSDE production by A2-13 was determined at pH 2.5, 3.5, 4.5, 5.5, 6.5, and 7.5, at 28 °C. Subsequently, incubation temperatures of 20, 24, 28, 32, and 36 °C were evaluated at the previously determined optimum pH, to determine the optimum incubation temperature.
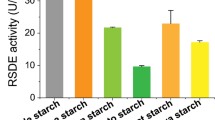
Furthermore, at the optimal pH and temperature, MMM containing wheat bran plus other carbon sources (corncob, sugarcane bagasse, rice straw, starch, and soybean cake powder) that replaced Avicel at a ratio of 4:1 (w/w) was assessed for their effects on RSDE production. Subsequently, various ratios of wheat bran to the identified carbon source ([w/w] 1:2; 2:2, 3:2, 1:4, 2:3, 1:3, 3:2, 2:1, and 4:1) were evaluated.
Similarly, several nitrogen sources were evaluated, including soybean cake powder, Urea, NH4NO3, Peptone, (NH4)2SO4, and NaNO3, and the optimum concentration of the best nitrogen source was determined.
Finally, the effects of different-sized inoculations of spores (102, 103, 104, 105, 106, and 107) on RSDE production were determined. All optimization experiments were carried out in a shaking incubator at 180 rpm. P. oxalicum was cultured for 6 days and the crude enzymes were collected for RSDE activity measurement.
Properties of crude RSDE produced by the mutant A2-13
RSDE activity against raw cassava flour (1% w/v) was measured in 0.1 M citrate–phosphate buffer at pH 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, and 7.0, at 65 °C for 30 min, as described above. RSDE activity at each pH was expressed as relative to that at the optimum pH (designated as 100%). The optimum reaction temperature was then determined at the optimum pH, by measuring the activity at various temperatures (30, 35, 40, 45, 50, 55, 60, 65, 70, 75, and 85 °C). RSDE activity at each temperature was expressed as relative to that at the optimal temperature.
To evaluate pH stability, crude RSDE was incubated at 4 °C for 24 h in buffers at various pHs, i.e., 0.1 M citrate-Na2HPO4 buffer (pH 3.0–7.0), Tris–HCl buffer (pH 7.0–9.0), and glycine–NaOH buffer (pH 9.0–11.0); then the residual RSDE activity was determined at the optimum pH and temperature for activity. Activity of untreated RSDE was defined as 100% and used as the positive control. Similarly, to ascertain temperature stability, crude RSDE was added to buffer at the optimum pH for stability, and the mixture was incubated at various temperatures from 30 to 65 °C, for 1 h. The residual RSDE activity was calculated as described above.
Enzymatic hydrolysis of raw starch
RNCF and natural raw corn flour (RNCOF, purchased from a farmer’s market in Nanning, China) were used as substrates for enzymatic hydrolysis. For raw starch hydrolysis by RSDEs from P. oxalicum A2-13 and OXPoxGA15A, hydrolysis reactions with 10% (w/v) solid substrate and an enzyme loading of 50 U/g substrate were conducted in citrate–phosphate buffer (pH 4.5) at 40 °C for 72 h. The reducing sugars produced were determined using the DNS method [38].
A combination of RSDEs from the P. oxalicum strains with commercial α-amylase (Solarbio, Beijing, China) at a ratio of 1:1 was also tested, as described above. RSDEs were added at several concentrations ([U/g substrate], 50, 100, 150, and 200).
Genome re-sequencing
Genomic DNAs of P. oxalicum strains A2-13 and OXPoxGA15A were extracted and subsequently used for construction of the read libraries with a length of 400 bp. Libraries were sequenced on a BGISEQ-500 platform at the Beijing Genomics Institute (BGI, Shenzhen, China). After removing low-quality reads and adaptors, the generated clean reads were mapped onto the genome of P. oxalicum strain HP7-1 [16] using Bowtie2 v 0.7.10 [39] to detect SNVs.
RT-qPCR assay
RT-qPCR was carried out as described previously [16]. The tested genes were detected using specific primers (Additional file 7: Table S3). The actin gene POX09428 was used as reference. The expression level of each tested gene was calculated relative to that of POX09428, and subsequently normalized against that for the parental strain ΔPoxKu70. Each experiment was repeated at least in triplicate.
Analysis by sodium dodecyl sulfate polyacrylamide gel electrophoresis
Proteins secreted by P. oxalicum strains OXPoxGA15A and A2-13 cultured in MMM, containing wheat bran plus Avicel, for 6 days, were analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The relative quantity of each protein was measured by GS-900TM calibrated densitometer (Bio-Rad, Hercules, CA, USA) with a software ImageLab.
Light microcopy
A Canon EOS 6D digital camera (Canon, Beijing, China) was used to photograph P. oxalicum colonies on agar plates. In addition, harvested mycelia were transferred to microscope slides, and photographed using an Olympus DP480 microscope (Olympus Corporation, Tokyo, Japan). Photomicrographs were analyzed using cellSens Dimension digital imaging software (Olympus).
Statistical analysis
Statistical analysis was performed using Microsoft Excel (Office 2016; Microsoft, Redmond, WA) by Student’s t test.
Accession number
Re-sequenced data are available from the Sequence Read Archive database under accession number SRA493765.
Availability of supporting data
Re-sequenced data are available from the Sequence Read Archive database under Accession number SRA493765.
Abbreviations
- ARTP:
-
Atmospheric and room-temperature plasma
- EMS:
-
Ethyl methanesulfonate
- RT-qPCR:
-
Real-time reverse transcription quantitative PCR
- SNP:
-
Single-nucleotide polymorphism
- SNVs:
-
Single-nucleotide variations
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- RSDE:
-
Raw starch-degrading enzyme
- MMM:
-
Modified minimal medium
- RNCF:
-
Natural raw cassava flour
- RNCOF:
-
Natural raw corn flour
- NRCA:
-
NRCF plus Avicel
References
Novy V, Nielsen F, Seiboth B, Nidetzky B. The influence of feedstock characteristics on enzyme production in Trichoderma reesei: a review on productivity, gene regulation and secretion profiles. Biotechnol Biofuels. 2019;12:238.
Rosales-Calderon O, Arantes V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol Biofuels. 2019;12:240.
Li SB, Cui YY, Zhou Y, Luo ZT, Liu JD, Zhao MM. The industrial applications of cassava: current status, opportunities and prospects. J Sci Food Agric. 2017;97:2282–90.
Cripwell RA, Rose SH, Favaro L, van Zyl WH. Construction of industrial Saccharomyces cerevisiae strains for the efficient consolidated bioprocessing of raw starch. Biotechnol Biofuels. 2019;12:201.
Szymanowska-Powałowska D, Lewandowicz G, Kubiak P, Błaszczak W. Stability of the process of simultaneous saccharification and fermentation of corn flour. The effect of structural changes of starch by stillage recycling and scaling up of the process. Fuel. 2014;119:328–34.
Marín-Navarro J, Polaina J. Glucoamylases: structural and biotechnological aspects. Appl Microbiol Biotechnol. 2011;89:1267–73.
Møller MS, Svensson B. Structural biology of starch-degrading enzymes and their regulation. Curr Opin Struct Biol. 2016;40:33–42.
Janeček Š, Mareček F, MacGregor EA, Svensson B. Starch-binding domains as CBM families–history, occurrence, structure, function and evolution. Biotechnol Adv. 2019;37:107451.
Adsul MG, Bastawde KB, Varma AJ, Gokhale DV. Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production. Bioresour Technol. 2007;98:1467–73.
Liu YT, Luo ZY, Long CN, Wang HD, Long MN, Hu Z. Cellulase production in a new mutant strain of Penicillium decumbens ML-017 by solid state fermentation with rice bran. N Biotechnol. 2011;28:733–7.
Xu QS, Yan YS, Feng JX. Efficient hydrolysis of raw starch and ethanol fermentation: a novel raw starch-digesting glucoamylase from Penicillium oxalicum. Biotechnol Biofuels. 2016;9:216.
Zou Z, Zhao Y, Zhang T, Xu J, He A, Deng Y. Efficient isolation and characterization of a cellulase hyperproducing mutant strain of Trichoderma reesei. J Microbiol Biotechnol. 2018;28:1473–81.
Kun RS, Gomes ACS, Hildén KS, Cerezo SS, Mäkelä MR, de Vries RP. Developments and opportunities in fungal strain engineering for the production of novel enzymes and enzyme cocktails for plant biomass degradation. Biotechnol Adv. 2019;37:107361.
Zhang X, Zhang XF, Li HP, Wang LY, Zhang C, Xing XH, Bao CY. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl Microbiol Biotechnol. 2014;98:5387–96.
Wang L, Zhao S, Chen XX, Peng QP, Li CX, Feng JX. Secretory overproduction of a raw starch-degrading glucoamylase in Penicillium oxalicum using strong promoter and signal peptide. Appl Microbiol Biotechnol. 2018;102:9291–301.
Zhao S, Yan YS, He QP, Yang L, Yin X, Li CX, Mao LC, Liao LS, Huang JQ, Xie SB, Nong QD, Zhang Z, Jing L, Xiong YR, Duan CJ, Liu JL, Feng JX. Comparative genomic, transcriptomic and secretomic profiling of Penicillium oxalicum HP7-1 and its cellulase and xylanase hyper-producing mutant EU2106, and identification of two novel regulatory genes of cellulase and xylanase gene expression. Biotechnol Biofuels. 2016;9:203.
Lin HJ, Xian L, Zhang QJ, Luo XM, Xu QS, Yang Q, Duan CJ, Liu JL, Tang JL, Feng JX. Production of raw cassava starch-degrading enzyme by Penicillium and its use in conversion of raw cassava flour to ethanol. J Ind Microbiol Biotechnol. 2011;38:733–42.
Ayodeji AO, Ogundolie FA, Bamidele OS, Kolawole AO, Ajele JO. Raw starch degrading, acidic-thermostable glucoamylase from Aspergillus fumigatus CFU-01: purification and characterization for biotechnological application. J Microbiol Biotechnol. 2017;6:90–100.
Moshi AP, Hosea KMM, Elisante E, Mamo G, Onnby L, Nges IA. Production of raw starch-degrading enzyme by Aspergillus sp and its use in conversion of inedible wild cassava flour to bioethanol. J Biosci Bioeng. 2016;121:457–63.
Kumar S, Kumar P, Satyanarayana T. Production of raw starch-saccharifying thermostable and neutral glucoamylase by the thermophilic mold Thermomucor indicae-seudaticae, in submerged fermentation. Appl Biochem Biotechnol. 2007;142:221–30.
Morita H, Fujio Y. High specific activity of raw-starch-digesting glucoamylase producing Rhizopus, sp. A-11 in liquid culture. Starch-Stärke. 1997;49:293–6.
Li H, Chi Z, Duan X, Wang L, Sheng J, Wu L. Glucoamylase production by the marine yeast Aureobasidium pullulans N13d and hydrolysis of potato starch granules by the enzyme. Process Biochem. 2007;42:462–5.
Doran-Peterson J, Jangid A, Brandon SK, DeCrescenzo-Henriksen E, Dien B, Ingram LO. Simultaneous saccharification and fermentation and partial saccharification and co-fermentation of lignocellulosic biomass for ethanol production. In: Mielenz J, editor. Biofuels. Methods in molecular biology (methods and protocols), vol. 581. Totowa: Humana Press; 2009.
Hargono JB, Kumoro AC. Production of bioethanol from sweet and bitter cassava starches by simultaneous saccharification and fermentation using Saccharomyces cerevisiae. Adv Sci Lett. 2017;23:2427–31.
Mohanty SK, Swain MR. Bioethanol production from corn and wheat: food, fuel, and future. In: Ray RC, Ramachandran S, editors. Bioethanol production from food crops: sustainable sources, interventions, and challenges, vol. 581. Salt Lake City: Academic Press; 2019.
Zhu F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr Polym. 2015;122:456–80.
Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–31.
Chisenga SM, Workneh TS, Bultosa G, Alimi BA. Progress in research and applications of cassava flour and a review. J Food Sci Technol. 2019;56:2799–813.
Binda O. On your histone mark, SET, methylate! Epigenetics. 2013;8:457–63.
Battaglia S, Lidschreiber M, Baejen C, Torkler P, Vos SM, Cramer P. RNA-dependent chromatin association of transcription elongation factors and Pol II CTD kinases. Elife. 2017;6:e25637.
Li YN, Hu YY, Zhu Z, Zhao KL, Liu GD, Wang LS, Qu YB, Zhao J, Qin YQ. Normal transcription of cellulolytic enzyme genes relies on the balance between the methylation of H3K36 and H3K4 in Penicillium oxalicum. Biotechnol Biofuels. 2019;12:198.
Gewartowski K, Cuéllar J, Dziembowski A, Valpuesta JM. The yeast THO complex forms a 5-subunit assembly that directly interacts with active chromatin. BioArchitecture. 2012;2:134–7.
Peña A, Gewartowski K, Mroczek S, Cuéllar J, Szykowska A, Prokop A, Czarnocki-Cieciura M, Aguilera A, Carrascosa JL, Valpuesta JM, Dziembowski A. Architecture and nucleic acids recognition mechanism of the THO complex, an mRNP assembly factor. EMBO J. 2012;31:1605–16.
Chen H, Kuo CC, Kang H, Howell AS, Zyla TR, Jin M, Lew DJ. Cdc42p regulation of the yeast formin Bni1p mediated by the effector Gic2p. Mol Biol Cell. 2012;23:3814–26.
Kang PJ, Lee ME, Park HO. Bud3 activates Cdc42 to establish a proper growth site in budding yeast. J Cell Biol. 2014;206:19–28.
Yan YS, Zhao S, Liao LS, He QP, Xiong YR, Wang L, Li CX, Feng JX. Transcriptomic profiling and genetic analyses reveal novel key regulators of cellulase and xylanase gene expression in Penicillium oxalicum. Biotechnol Biofuels. 2017;10:279.
He QP, Zhao S, Wang JX, Li CX, Yan YS, Wang L, Liao LS, Feng JX. Transcription factor NsdD regulates the expression of genes involved in plant biomass-degrading enzymes, conidiation, and pigment biosynthesis in Penicillium oxalicum. Appl Environ Microbiol. 2018;84(18):e01039-e1118.
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–8.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9.
Acknowledgements
Not applicable.
Funding
This work was financially supported by grants from the Guangxi Natural Science Foundation (Grant No. 2018GXNSFAA281103), the Training Program for 1000 Young and Middle-aged Backbone Teachers of Guangxi Higher Education Institution in 2019, the One Hundred Person Project of Guangxi to SZ, and the Autonomous Research Project of State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources (SKLCUSA-a201902 and SKLCUSA-a201923) to JXF.
Author information
Authors and Affiliations
Contributions
SZ supervised the study, wrote and revised the manuscript. LSG conducted mutagenesis and screening of mutants, optimization of fermentation parameters, and investigation of enzyme properties. SHL performed phenotypic analysis and took part in analysis of extracellular proteins. MZT participates measurement of enzymatic activities and ARTP mutagenesis. TZ conducted transcriptional analysis. CXL carried out bioinformatic analyses. QQZ is involved in starch hydrolysis. XML and JXF are involved in the analysis of experimental data and revised manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Fig. S1.
ARTP/EMS-mediated mutagenesis and screening for RSDE hyperproducers. The P. oxalicum isolates were grown on the two-layer agar gel plates for 8 days. *: the isolates were screened and used for the next round of mutagenesis, by comparative analysis of their RSDE activities in MMM, containing wheat bran plus Avicel as the carbon source, for 6 days. RSDE activity was determined using RNCF as the substrate. ARTP: atmospheric and room-temperature plasma; EMS: ethyl methyl sulfonate; RNCF: natural raw cassava flour; MMM: modified minimal medium.
Additional file 2: Fig. S2.
Curve showing lethality against P. oxalicum strains OXPoxGA15A and E3-16, treated by ethyl methyl sulfonate (A) and atmospheric and room-temperature plasma (B). P. oxalicum spores were spread on PDA plates and incubated at 28 °C for 4 days. Each data point represents mean ± SD. Each experiment contained three biological replicates.
Additional file 3: Fig. S3.
Effects of culture conditions and inoculated spore number on RSDE production of P. oxalicum strain A2-13. (A) Initial pH of medium; (B) incubation temperature; (C) Carbon source; (D) Proportions of wheat bran and Avicel; (E) Nitrogen source; (F) NH4NO3 concentration; (G) inoculated spore number. P. oxalicum strains were cultured at 28 °C and 180 rpm for 6 days. Each data point represents mean ± SD. Each experiment contained three biological replicates.
Additional file 4: Fig. S4.
Colonic (A) and mycelial (B) analysis of the P. oxalicum strains A2-13 and OXPoxGA15A on plates containing various carbon sources. RNCA: natural raw cassava flour plus Avicel, RNCF: natural raw cassava flour, PDA: potato dextrose agar. Scale bar = 100 μm.
Additional file 5: Table S1.
List of 230 single-nucleotide variants of the mutant A2-13 compared with the starting strain OXPoxGA15A.
Additional file 6: Table S2.
Mutation sites in coding sequences (CDS) in the mutant A2-13 compared with that in OXPoxGA15A.
Additional file 7: Table S3.
Primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gu, LS., Tan, MZ., Li, SH. et al. ARTP/EMS-combined multiple mutagenesis efficiently improved production of raw starch-degrading enzymes in Penicillium oxalicum and characterization of the enzyme-hyperproducing mutant. Biotechnol Biofuels 13, 187 (2020). https://doi.org/10.1186/s13068-020-01826-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-020-01826-5