Abstract
Background
Intrarenal venous flow (IRVF) patterns assessed using Doppler renal ultrasonography are real-time bedside visualizations of renal vein hemodynamics. Although this technique has the potential to detect renal congestion during sepsis resuscitation, there have been few studies on this method. We aimed to examine the relationship between IRVF patterns, clinical parameters, and outcomes in critically ill adult patients with sepsis. We hypothesized that discontinuous IRVF was associated with elevated central venous pressure (CVP) and subsequent acute kidney injury (AKI) or death.
Methods
We conducted a prospective observational study in two tertiary-care hospitals, enrolling adult patients with sepsis who stayed in the intensive care unit for at least 24 h, had central venous catheters placed, and received invasive mechanical ventilation. Renal ultrasonography was performed at a single time point at the bedside after sepsis resuscitation, and IRVF patterns (discontinuous vs. continuous) were confirmed by a blinded assessor. The primary outcome was CVP obtained at the time of renal ultrasonography. We also repeatedly assessed a composite of Kidney Disease Improving Global Outcomes of Stage 3 AKI or death over the course of a week as a secondary outcome. The association of IRVF patterns with CVP was examined using Student's t-test (primary analysis) and that with composite outcomes was assessed using a generalized estimating equation analysis, to account for intra-individual correlations. A sample size of 32 was set in order to detect a 5-mmHg difference in CVP between IRVF patterns.
Results
Of the 38 patients who met the eligibility criteria, 22 (57.9%) showed discontinuous IRVF patterns that suggested blunted renal venous flow. IRVF patterns were not associated with CVP (discontinuous flow group: mean 9.24 cm H2O [standard deviation: 3.19], continuous flow group: 10.65 cm H2O [standard deviation: 2.53], p = 0.154). By contrast, the composite outcome incidence was significantly higher in the discontinuous IRVF pattern group (odds ratio: 9.67; 95% confidence interval: 2.13–44.03, p = 0.003).
Conclusions
IRVF patterns were not associated with CVP but were associated with subsequent AKI in critically ill adult patients with sepsis. IRVF may be useful for capturing renal congestion at the bedside that is related to clinical patient outcomes.
Graphical abstract

Similar content being viewed by others
Background
Fluid overload is common during sepsis resuscitation and is associated with acute kidney injury (AKI) and mortality [1,2,3]. Recent findings have suggested that renal congestion (elevated renal interstitial pressure and impaired renal venous return) is potential mechanisms for AKI after fluid overload [4]. However, other than conventional surrogates for systemic congestion (e.g., central venous pressure (CVP), cumulative fluid balance, edema), there is a lack of reliable bedside tools that specifically inform us of real-time renal venous hemodynamics.
Recently, intrarenal venous flow (IRVF), assessed using renal venous Doppler sonography, has been introduced as a direct visualization of renal venous hemodynamics. Studies outside the critical care field have shown that interrupted, discontinuous IRVF is associated with worsening renal function, even when CVP remains within the normal range [5, 6]. These findings suggest that IRVF is a more sensitive marker of renal congestion than CVP is.
However, only limited research has applied intra-renal venous Doppler sonography in critically ill patients [7, 8], and no study has investigated the utility of IRVF patterns in patients with sepsis after the initial resuscitation phase when the resultant fluid overload was more prominent. Therefore, in this study, we investigated IRVF patterns obtained after the initial resuscitation phase (24 h after sepsis onset) and examined its relationship with CVP in critically ill adult patients with sepsis. In addition, we explored the association between IRVF and subsequent AKI or death. We hypothesized that discontinuous IRVF was associated with elevated CVP and subsequent AKI or death.
Methods
Study design and setting
This prospective observational study was conducted at two tertiary care hospitals in Japan. The institutional review board approved this study and waived the requirement for informed consent (Fujita Health University Ethics Review Committee [HM21-442], Okinawa Prefectural Chubu Hospital Research Ethics Committee [2018-129]). Both hospitals had experienced staff intensivists.
Study participants
We screened all patients admitted to the ICU between August 19, 2019, and July 5, 2020. Patients were eligible if they were adults (≥ 18 years old), were diagnosed with sepsis, stayed in the ICU at least 24 h after the diagnosis of sepsis, had central venous pressure monitored, and received invasive mechanical ventilation. Sepsis was defined based on Sepsis-3, which requires the presence of infection and sequential organ failure assessment (SOFA) scores of ≥ 2 points [9]. We excluded patients who (i) were on maintenance dialysis for chronic renal failure, (ii) were pregnant [10], (iii) had any ureteral obstructions because these affect the IRVF waveforms [11], and (vi) had earlier participation in this study.
Renal ultrasonography
The main exposure of interest was the IRVF patterns assessed using renal ultrasonography. Before this study, two investigators (KF and IN) underwent the following training sessions for the standardization of renal ultrasonography: they visited the University of Tsukuba Hospital, where the first application study of IRVF in heart failure was conducted and performed renal ultrasonography on 10 patients under the direct supervision of expert sonographers [5]. Next, the study physicians performed renal ultrasonography in 22 ICU patients at each research hospital and received feedback. These sessions were continued until each physician had independent skills.
During the study period, renal ultrasonography was performed at a single time point for each patient to capture renal congestion after sepsis resuscitation. The eligibility criterion (24 h elapsed after the diagnosis of sepsis,) was evaluated daily, and the study physicians performed renal ultrasonography at the bedside in the ICU soon after the patients fulfilled the criterion. Anonymized images and movies of renal ultrasonography were sent to the University of Tsukuba Hospital. The sonographer, blinded to all clinical information, reviewed the images and confirmed the final assessment of the IRVF pattern. Other than the study investigators, no other physician knew the results of renal ultrasonography.
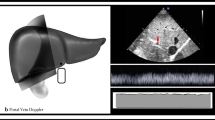
The technical aspects of renal ultrasonography are as follows. Using a sector transducer with a frequency range of 2.5 and 5 MHz [5], we examined the right kidney in the left lateral supine position, except for a patient with postural restrictions. Color Doppler images were used to determine a target, adjacent pair of inter-lobar arteries, and veins with the Doppler velocity range set at approximately 16 cm/s. Inter-lobar arterial and venous pulsed Doppler waveforms were simultaneously recorded (Fig. 1a). The IRVF waveforms (the flow away from the transducer below the baseline) were classified into two waveform patterns: continuous or discontinuous, as defined by Iida et al. [5] (Fig. 1). Specifically, a continuous pattern corresponded to the presence of continuous, uninterrupted venous flow below the baseline throughout the cardiac cycle (Fig. 1b), whereas the discontinuous pattern was a group of patterns that had at least one phase with zero velocity in venous flow during a cardiac cycle (Figs. 1c and d). The venous impedance index (VII), that is, the proportion of reduction in venous flow from its peak velocity (very low [0.2] in healthy subjects), was calculated as the peak maximum flow velocity minus the maximum flow velocity at the nadir in veins divided by the maximum flow velocity [14]. The renal venous stasis index (RVSI), as proposed by Husain et al. [15], was calculated as the index cardiac cycle time minus the renal venous flow time divided by the index cardiac cycle time. For renal arterial flow, renal resistive index (RRI) was calculated as the maximum flow velocity minus the diastolic flow velocity divided by the maximum flow velocity [12]. The RRI in healthy subjects typically ranges between 0.5 and 0.7 [13]. In patients with sinus rhythm, we measured all indices over three cardiac cycles at the end of expiration and averaged them. In patients with atrial fibrillation, we measured the indices at a cardiac cycle where two preceding cardiac cycles had nearly equal durations. Echocardiography was performed at the bedside in the ICU.
Color Doppler flow images from a right kidney. a Doppler sample volume position (*) in the inter-lobar vessels. b Intrarenal artery flow (upward Doppler signals), vein flow (downward Doppler signals) and the corresponding maximum and minimum velocity (Vmax, Vmin). The intrarenal venous flow waveforms were classified into a continuous venous flow pattern (b) or discontinuous patterns (c-1, c-2, d). Discontinuous patterns include biphasic patterns (c-1, c-2) and monophasic patterns (d) which had at least 1 phase with zero velocity during venous flow in a cardiac cycle as indicated by double-headed horizontal arrows (discontinuous time)
Central venous pressure
The primary measure of systemic congestion was central venous pressure obtained at the time of renal ultrasonography. A single investigator at each study site (KF or IN) measured CVP (a-wave, v-wave, and mean) at the ICU bedside according to the method described by Roger [16] just before or after renal sonography. We measured CVP at the end-expiratory phase in patients in the supine position after carefully zeroing at the mid-thoracic position at the level of the fifth rib. The CVP values were averaged over three cardiac cycles. We recorded the mean CVP of patients with atrial fibrillation. Peripheral edema at baseline was also assessed by palpation of the extremities and the presence of pitting edema.
Assessment of AKI and outcome measures
The secondary, exploratory outcome measure was a composite of Kidney Disease Improving Global Outcomes (KDIGO) stage 3 AKI [17] including receipt of renal replacement therapy (RRT) or death from any cause within 1 week after IRVF measurement. All patients were followed up until death from any cause, hospital discharge, or the end of the study period (September 14, 2020), whichever came first. We longitudinally assessed the AKI stage for 7 days to evaluate the association between the IRVF pattern and subsequent AKI stage trajectory. Day zero was assigned to the day of IRVF measurement, and the KDIGO stage was recorded from the day before renal sonography (day-1) to day 6. The KDIGO stage classification was based on serum creatinine level or urine output, whichever was worse [17]. If either value was missing, we classified the stage based only on the available data (creatinine or urine output). When both values were missing, we imputed the missing stage with the patient’s last observed KDIGO stage. The baseline value for the serum creatinine criteria was determined by referencing the lowest creatinine value during the previous 12 months before admission. When the pre-admission creatinine level was unavailable, the lowest creatinine value measured within the first 24 h of admission was used as the baseline. The indication, modality, and duration of renal replacement therapy were determined per routine practice at participating hospitals according to the national guidelines [18].
Sample size estimation
According to a previous study [5], we estimated that the difference between CVP in the continuous IRVF pattern group and discontinuous pattern group was 5 mmHg, and the common standard deviation of CVP in both groups was 5 mmHg. A total of 32 patients were required to confirm the difference between CVP in the IRVF groups with a 2-sided alpha level of 0.05 and power of 80%. Patients were enrolled until the size of both groups reached to at least 16.
Statistical analyses
For the primary analysis, we examined the association between IRVF pattern (continuous vs discontinuous) and CVP using Student's t-test and analysis of covariance (ANCOVA) with adjustment for the APACHE II score. As a secondary exploratory analysis, we performed a generalized estimating equation (GEE) analysis to confirm the association between the IRVF pattern (assessed at a single time point) and the composite of stage 3 AKI or death, assessed repeatedly within 1 week. The motivation for the secondary analysis was as follows: in patients with sepsis, renal congestion that evolved during fluid resuscitation probably deepens the initial worsening of AKI and delays subsequent recovery [19]. Under this hypothesis, and based on a clinical perspective, a method that is sensitive to changes in outcomes over time is desired. GEE is well suited for this purpose as it allows for modelling the correlation between outcomes evaluated at different time points. The APACHE II score, baseline KDIGO stage (on day-1), measurement period, and interaction between measurement period and group (measurement period * group) were included in the GEE model. KDIGO stages were missing in those who experienced rapid recovery from sepsis and survival discharge from the ICU to the ward during the outcome assessment time window (within 1 week after IRVF measurement). Missing values were complemented by the LOCF because the AKI trajectories of these patients were considered stable at the time of missing events. Details of the missing data and a sensitivity analysis without LOCF are presented in Additional file 2: Table S2. Similarly, we performed a GEE analysis to assess the association between baseline CVP and the primary composite outcome within one week. Kaplan–Meier estimation was used to estimate the median survival time for each IRVF group, and the log-rank test was used to assess differences between the groups. As a post hoc supplementary analysis, we added linear regression analysis to assess the potential association between CVP and RVSI, which is a continuous measure, and GEE analysis to assess RVSI and the composite outcomes.
Characteristics are presented using median and interquartile range (IQR) or mean and standard deviation (SD) for continuous variables, and frequencies and proportions for categorical variables. For differences in characteristics between the continuous and discontinuous groups, the Mann–Whitney U test was used for continuous variables, and Fisher's exact test was used for categorical variables. Statistical significance was set at a two-sided p-value of ≤ 0.05. All analyses were performed using EZR [20] version 1.56 and R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the study participants
Of 169 adult patients with sepsis who stayed in the ICU for ≥ 24 h during the study period, 50 met the inclusion criteria. Twelve patients were excluded because they were extubated, or their central venous catheters were removed before renal sonography. Finally, 38 patients who met the eligibility criteria were analyzed (Fig. 2). We successfully tracked all patients within the study period until September 14, 2020, when the last patient was discharged. In one patient (1/38 [2.6%]), renal venous Doppler flow was insufficiently detected and substituted with venous flow in the renal pelvis. The patient characteristics are summarized in Table 1. Of the 38 patients, 28 (74%) were male, and the median age was 69.5 (IQR 60–78.8). Twenty-seven patients (71%) were admitted to the ICU from the emergency room. In the entire cohort, 14 patients (36.8%) received RRT for AKI during their ICU stay, six patients (15.8%) died in the ICU, and 12 patients (31.6%) died in the hospital. The median time from ICU admission to renal ultrasonography was 2 days (IQR, 2–3 days; range, 1–13 days). Twenty-two patients (57.9%) had discontinuous intrarenal venous flow pattern. There were 2 monophasic cases and 20 biphasic cases (Fig. 1). The mean of RVSI was 0.20 with a standard deviation of 0.23. Patients with discontinuous patterns were older, had more frequent respiratory infections, and had higher APACHE II scores. Moreover, patients with discontinuous patterns more frequently had a higher cumulative fluid balance and peripheral edema. Creatinine level, AKI stage at the time of renal sonography, and positive end-expiratory pressure (PEEP) were higher in the discontinuous pattern group. Atrial fibrillation was present during renal sonography in three patients in the continuous group and two in the discontinuous group.
CVP and other parameters at renal ultrasonography
Table 2 shows CVP and findings of renal ultrasonography and echocardiography. The overall mean central venous pressure was 9.83 mmHg with a mean of 9.24 mmHg (SD: 3.19) in the discontinuous pattern group and 10.65 mmHg (SD: 2.53) in the continuous pattern group. There was no significant difference in CVP between the two groups by Student t-test (difference: − 1.4, 95% confidence interval [CI]: − 3.36–0.55, p = 0.154) and APACHE2-adjusted ANCOVA (coefficient: − 1.52, 95%CI − 3.59–0.54, p = 0.144), and the abnormal IRVF pattern was not associated with CVP (Table 2). We also performed a post hoc supplementary analysis of RVSI, a continuous measure, but the results were similar to those in Table 2, indicating no significant correlation between CVP and RVSI (coefficient: − 2.37, 95% CI − 6.59–1.86, p = 0.26). Intrarenal arterial information (renal resistive index) and echocardiographic indices of the left heart and the right heart were similar between the two groups. Patients with discontinuous patterns showed narrower IVC diameters, and their IVCs were more collapsible than those with continuous patterns.
Patient outcomes
Table 3 shows patient outcomes. ICU days, ventilator days, hospital days, and death were longer or more frequent in the discontinuous group, although not statistically significant (Table 3). The median survival time was 74 days in the continuous group and could not be calculated in the discontinuous group because the survival probability was never less than 50%. There was no statistical difference between the survival rates in the two groups (p = 0.68) (Fig. 3).
Kaplan–Meier curves for the survival probability according to intrarenal venous flow patterns. Patients were followed from the day before renal ultrasonography until death from any cause or hospital discharge or the end of the study period (September 14, 2020), whichever came first. The vertical tick marks indicate censoring
Intrarenal flow patterns and AKI
The trajectory of the proportion of patients who experienced the composite outcome (stage 3 AKI or death) is presented in Fig. 4, stratified by the IRVF pattern. The incidence of the composite outcome tended to be higher in patients with a discontinuous IRVF pattern at all measurement points. Component proportions for the composite outcomes are detailed in Additional file 1: Table S1. Table 4 shows the association between the discontinuous pattern of IRVF with AKI or death. The incidence of events leading to KDIGO stage 3 or death in the one week following IRVF measurement was significantly higher in the discontinuous pattern group (adjusted OR: 9.67, 95% CI 2.13–44.03, p = 0.003). The trend was similar in the sub-analysis, with RRT or death as the outcome (Table 4). In contrast, CVP was not significantly associated with the composite outcome (OR: 0.92, 95% CI 0.75–1.13, p = 0.439). The results were similar with and without LOCF, confirming the robustness of our conclusions (adjusted OR for the composite outcome in the discontinuous pattern group: 9.92, 95% CI 2.15–45.78, p = 0.003) (Additional file 2: Table S2). In our post hoc supplementary analysis, we did not find a significant association between RVSI and the composite outcomes (adjusted odds ratio for the composite outcome in the discontinuous pattern group: 2.25, 95% CI 0.25–20.73, p = 0.47).
Trajectory of the proportion of patients with the composite outcomes stratified by IRVF patterns and contribution of each component. The composite outcome was the combination of stage 3 acute kidney injury and death. Black bars indicate death, dark gray bars indicate stage 3 AKI patients receiving RRT, and light gray bars indicate stage 3 AKI patients without RRT. Day zero was assigned as the day of IRVF measurement. Those who died during the observation period continued to contribute to the proportions; the denominator was 16 in the continuous group and 22 in the discontinuous group on all days
Discussion
Key findings
We investigated IRVF waveform patterns obtained after the initial resuscitation phase (24 h after sepsis onset) and its relationship with CVP and clinical outcomes among critically ill adult patients with sepsis. We demonstrated that measurement of IRVF at the bedside was feasible among invasive mechanically ventilated critically ill patients, and approximately 60% of these patients showed a discontinuous pattern of IRVF on day 2 of their ICU stay. We found no association between IRVF waveform patterns and CVP. In contrast, discontinuous patterns of IRVF were associated with stage 3 AKI or death within the next 7 days. Our results suggest that IRVF could capture renal congestion after the initial resuscitation for sepsis better than CVP and has the potential to guide post-resuscitation fluid therapy in sepsis.
Relationship to previous studies
Fluid overload and congestion have been increasingly recognized as potential causes of organ dysfunction during critical illnesses. The kidney is encapsulated by a tough fibrous capsule, which is susceptible to the harmful effects of fluid overload during resuscitation [21]. Fluid overload after the initial resuscitation of critically ill patients is strongly associated with AKI and mortality in observational studies and randomized controlled trials [1,2,3]. However, although peripheral edema, cumulative fluid balance, or CVP were associated with AKI in patients with sepsis [22, 23], these markers may not directly reflect renal congestion. Furthermore, CVP is affected by many factors such as thoracic, pericardial, and abdominal pressures, making its interpretation more complex in the ICU setting [24].
Renal venous Doppler sonography directly visualizes and captures changes in IRVF patterns during renal congestion [6]. During renal congestion, intrarenal venous compliance decreases secondary to elevated renal interstitial pressure [25] or intravascular volume expansion [21], resulting in pulsatile, intermittently interrupted intrarenal venous flow (discontinuous patterns). Discontinuous IRVF patterns were associated with elevated CVP and predicted worsening renal function, hospitalization for heart failure, and death in patients with heart failure [5]. Furthermore, even when CVP remained within the normal range, discontinuous patterns were observed and associated with worse clinical outcomes [5]. These findings suggest that in patients with congestive heart failure, IRVF is a more sensitive marker of renal congestion than CVP. However, the findings in patients with congestive heart failure are not directly applicable to those with sepsis because the two patient groups have different hemodynamics.
Currently, only one study has evaluated the association between IRVF patterns and renal function, including sepsis, in an ICU setting. Spiegel et al. evaluated the IRVF waveform within 24 h of ICU admission among general ICU patients and found no association with major adverse kidney events at 30 days (OR for major adverse kidney events: 2.29, 95% CI 0.69–7.58) [7]. However, renal Doppler was performed in the early phase of critical illness (within 24 h of ICU admission), and the median amount of IV fluid administered at the time of measurement was only 636 mL, which was far less than the typical degree of fluid accumulation during sepsis [7, 26].
In the present study, we enrolled patients with sepsis who were at high risk for fluid overload and renal congestion and focused on the post-resuscitation phase of sepsis (24 h after sepsis onset). The median cumulative fluid balance was 1740 mL on renal Doppler measurements. The design of our study enabled us to evaluate the utility of IRVF in assessing renal venous congestion after the initial resuscitation of sepsis.
Clinical implications
The absence of an association between CVP and IRVF may highlight distinct hemodynamic features of renal congestion in sepsis, which are different from those in heart failure [27]. The release of inflammatory cytokines and glycocalyx destruction causes peripheral vasodilation and increases vascular permeability in sepsis [28]. As a result, the administered fluid does not remain in the intravascular space and interstitial edema such as renal congestion is not necessarily coupled with elevated central venous pressure. Indeed, discontinuous IRVF was not associated with CVP, but was associated with cumulative fluid balance and subsequent AKI in our study (cumulative fluid balance: discontinuous pattern, 2220 mL; continuous pattern, 1067 mL; p = 0.031). These results were consistent with previous findings, showing that CVP had no meaningful relationship with administered fluid volume in septic shock [29]. Our study suggests that the IRVF pattern has the potential to capture organ-specific congestion in the kidney among patients with sepsis, which CVP cannot detect [30]. In our post hoc analysis, we found no significant correlation between RVSI and composite outcomes. Although we treated RVSI as a continuous measure of IRVF, converting IRVF into a continuous variable might be difficult. A recent study by the authors revealed that IRVF patterns arose from a complex combination of renal congestion and right-sided heart hemodynamics (e.g., right ventricular filling abnormality, significant tricuspid regurgitation, and atrial fibrillation) [27]. The waveform or timing of interruptions is important to understand the underlying mechanism of interruption. Therefore, the duration or proportion of interruption (referring to RVSI) alone does not fully represent the pathology and is not necessarily proportional to the severity of renal venous congestion. Indeed, in our analyses, the composite outcomes were seen in discontinuous patterns with a short interruption time (low RVSI) and also in discontinuous patterns with a long interruption time (high RVSI), suggesting a nonlinear association. We suspected that this was the reason why the analysis using RVSI could not find a meaningful association. Thus, we believe that the IRVF pattern itself is a suitable measure of renal venous hemodynamics.
Strengths and limitations
Our study has several strengths. We demonstrated that the measurement of IRVF at the ICU bedside was feasible for physicians in invasive mechanically ventilated patients. The proportion of inadequate image quality was 2.6% [1/38], which was greatly improved from that in a previous study (inadequate image quality in 25.4% of patients) [7]. In addition, an expert sonographer who was blinded to all clinical information independently confirmed the final assessment of the IRVF pattern. The prospective nature of our study enabled us to carefully measure CVP at the bedside using a standard protocol [16].
Our study has several limitations. First, the analysis of the association of IRVF with AKI or death was exploratory, and the CI of the OR was wide due to the small number of patients. Second, renal ultrasonography was performed by physicians who were aware of the patients’ clinical information. However, we attempted to minimize bias from awareness of clinical information by adopting a blinded assessment of the IRVF pattern by an independent expert sonographer. Third, there exists between-group differences in several key prognostic factors, including age, APACHE score, and primary infectious sources (Table 1). These factors may increase mortality independent of renal congestion or AKI in the Discontinuous group and may explain the observed association. However, the major difference in the composite outcomes between the Continuous and Discontinuous groups arose from increased stage 3 AKI in the Discontinuous group (Fig. 4). The results support our notion that IRVF is not merely a marker of the severity of illness in general but rather a sensitive measure of renal congestion associated with subsequent AKI. Fourth, because we adopted repeating outcome measures, the analytical method used for the association between IRVF and the composite outcome could have led to a decrease in the p-values. Although our choice of method (GEE analyses) was intended to capture the AKI trajectory that changes over time after sepsis resuscitation, individuals who died continued to contribute to the analysis after death and may have been overrepresented, resulting in the potential unintentional decrease in the p-values. However, as we demonstrated that the proportion of death was low in both groups and the major difference in the composite outcomes between the groups arose from increased stage 3 AKI in the Discontinuous group in Additional file 1: Table S1, we believe that the possibility of an unintentional decrease in the p-value due to the mortality difference is unlikely. Fifth, differences in the administered catecholamines or intrathoracic pressure other than fluid overload might affect the IRVF waveform and explain the observed association of IRVF with AKI or death. Although we incorporated baseline kidney function and severity scores into the model to balance the two IRVF groups, a larger study with sufficient adjustment for important factors would be required to confirm our observations.
Unanswered questions and future research
A previous study showed that blunting of renal venous flow was related to a lower diuretic response, independent of underlying renal function [31]. A discontinuous IRVF pattern may indicate impaired handling of renal fluid. Indeed, in heart failure patients undergoing repeated IRVF measurements, the persistence of a discontinuous IRVF pattern is associated with renal dysfunction progression [6]. Future studies should investigate whether repeated IRVF measurements and IRVF-guided fluid therapy, particularly guided fluid discontinuation and removal, improve AKI and patient outcomes in the ICU setting. In addition, we selected patients who had a central venous catheter and received invasive mechanical ventilation, because we focused on a population with a high risk of renal congestion. Future studies should include a broader range of patients with sepsis.
We believe that with appropriate training, critical care physicians can perform renal sonography at the bedside. Based on our experiences, the key challenges of renal ultrasonography include identifying a suitable probe position and angle for visualizing the interlobar renal veins and setting the Doppler velocity in the lower range appropriate for detecting low-speed venous flow (such as 16 cm/s). Increasing the opportunity for side-by-side guidance and visual aids would expand the implementation of renal ultrasonography in the ICU.
Conclusions
The IRVF pattern assessed after the initial resuscitation phase (24 h after sepsis onset) was not associated with central venous pressure but was associated with subsequent AKI or death among critically ill adult patients with sepsis. IRVF may have the potential to capture renal congestion at the bedside. Further studies are needed to evaluate whether IRVF-guided fluid discontinuation and removal during the post-resuscitation phase of sepsis improves patient outcomes.
Availability of data and materials
The data supporting that support the findings of this study are available from Fujita Health University Hospital and Okinawa Chubu Hospital but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with the institution's permission.
Abbreviations
- AKI:
-
Acute kidney injury
- ANCOVA:
-
Analysis of covariance
- APACHE II score:
-
Acute physiology and chronic health evaluation II score
- CI:
-
Confidence interval
- CVP:
-
Central venous pressure
- GEE:
-
Generalized estimating equation
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- IV:
-
Intravenous
- IVC:
-
Inferior vena cava
- IRVF:
-
Intrarenal venous flow
- KDIGO:
-
Kidney disease improving global outcomes
- LOCF:
-
Last observation carried forward
- OR:
-
Odds ratio
- RRI:
-
Renal resistive index
- RRT:
-
Renal replacement therapy
- RVSI:
-
Renal venous stasis index
- SD:
-
Standard deviation
- SOFA:
-
Sequential organ failure assessment
- VII:
-
Venous impedance index
References
Prowle JR, Chua HR, Bagshaw SM, Bellomo R. Clinical review: volume of fluid resuscitation and the incidence of acute kidney injury—a systematic review. Crit Care. 2012;16(4):230.
Zhang J, Crichton S, Dixon A, Seylanova N, Peng ZY, Ostermann M. Cumulative fluid accumulation is associated with the development of acute kidney injury and non-recovery of renal function: a retrospective analysis. Crit Care. 2019;23(1):392.
Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients-a systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48(12):1862–70.
Mårtensson J, Bellomo R. Does fluid management affect the occurrence of acute kidney injury? Curr Opin Anaesthesiol. 2017;30(1):84–91.
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4(8):674–82.
Yamamoto M, Seo Y, Iida N, Ishizu T, Yamada Y, Nakatsukasa T, et al. Prognostic impact of changes in intrarenal venous flow pattern in patients with heart failure. J Card Fail. 2021;27(1):20–8.
Spiegel R, Teeter W, Sullivan S, Tupchong K, Mohammed N, Sutherland M, et al. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care. 2020;24(1):615.
Beaubien-Souligny W, Benkreira A, Robillard P, Bouabdallaoui N, Chassé M, Desjardins G, et al. Alterations in portal vein flow and intrarenal venous flow are associated with acute kidney injury after cardiac surgery: a prospective observational cohort study. J Am Heart Assoc. 2018;7(19): e009961.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Bateman GA, Giles W, England SL. Renal venous Doppler sonography in preeclampsia. J Ultrasound Med. 2004;23(12):1607–11.
Bateman GA, Cuganesan R. Renal vein Doppler sonography of obstructive uropathy. AJR Am J Roentgenol. 2002;178(4):921–5.
Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol. 1990;154(6):1223–7.
Ponte B, Pruijm M, Ackermann D, Vuistiner P, Eisenberger U, Guessous I, et al. Reference values and factors associated with renal resistive index in a family-based population study. Hypertension. 2014;63(1):136–42.
Ishimura E, Nishizawa Y, Kawagishi T, Okuno Y, Kogawa K, Fukumoto S, et al. Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int. 1997;51(6):1920–7.
Husain-Syed F, Birk HW, Ronco C, Schörmann T, Tello K, Richter MJ, et al. Doppler-derived renal venous stasis index in the prognosis of right heart failure. J Am Heart Assoc. 2019;8(21): e013584.
Roger C, Muller L, Riou B, Molinari N, Louart B, Kerbrat H, et al. Comparison of different techniques of central venous pressure measurement in mechanically ventilated critically ill patients. Br J Anaesth. 2017;118(2):223–31.
Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
Doi K, Nishida O, Shigematsu T, Sadahiro T, Itami N, Iseki K, et al. The Japanese clinical practice guideline for acute kidney injury 2016. J Intensive Care. 2018;6:48.
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Burnett JC Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238(4):F279–82.
Chen KP, Cavender S, Lee J, Feng M, Mark RG, Celi LA, et al. Peripheral Edema, central venous pressure, and risk of AKI in critical illness. Clin J Am Soc Nephrol. 2016;11(4):602–8.
Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17(6):R278.
Berlin DA, Bakker J. Starling curves and central venous pressure. Crit Care. 2015;19(1):55.
Cruces P, Salas C, Lillo P, Salomon T, Lillo F, Hurtado DE. The renal compartment: a hydraulic view. Intensive Care Med Exp. 2014;2(1):26.
Mele A, Cerminara E, Häbel H, Rodriguez-Galvez B, Oldner A, Nelson D, et al. Fluid accumulation and major adverse kidney events in sepsis: a multicenter observational study. Ann Intensive Care. 2022;12(1):62.
Seo Y, Iida N, Yamamoto M, Ishizu T, Ieda M, Ohte N. Doppler-derived intrarenal venous flow mirrors right-sided heart hemodynamics in patients with cardiovascular disease. Circ J. 2020;84(9):1552–9.
Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87(2):300–10.
Reddi B, Finnis M, Udy AA, Maiden M, Delaney A, Bellomo R, et al. The relationship between the change in central venous pressure and intravenous fluid volume in patients presenting to the emergency department with septic shock. Intensive Care Med. 2018;44(9):1591–2.
Itkin M, Rockson SG, Burkhoff D. Pathophysiology of the lymphatic system in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(3):278–90.
Nijst P, Martens P, Dupont M, Tang WHW, Mullens W. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail. 2017;5(9):672–81.
Acknowledgements
We thank all staff of Fujita Health University Hospital and Okinawa Prefectural Chubu Hospital. We also thank the Cardiovascular Division and Clinical Laboratory of the University of Tsukuba members who reviewed the data. We thank Takuma Ishihara from the Innovative and Clinical Research Promotion Center, Gifu University Hospital, for statistical assistance. We would like to thank Editage (www.editage.jp) for the English language editing.
Funding
This study was not financed.
Author information
Authors and Affiliations
Contributions
KF and IN conceived and drafted the initial version of the study protocol. JI, NU, TT, and MI suggested amendments and approved the final version of the protocol. KF and IN extracted data. NI and MY instructed on renal ultrasonography techniques and the quality of the collected renal ultrasonography data. YS provided knowledge of renal ultrasonography. KF, IN, and JI performed the statistical analyses. KF wrote the main manuscript text, and IN revised the text. KF prepared a graphical abstract image. KF, IN, and JI prepared Figs. 2, 3, 4 and Tables 1, 2, 3, 4. JI reviewed all the statistical analyses and critically revised the manuscript. All authors made substantial contributions to the intellectual content of the paper and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional review board of each institution approved the study protocol and opt-out approach (Fujita Health University Ethics Review Committee HM21-442, Okinawa Prefectural Chubu Hospital Research Ethics Committee 2018–129). The requirement for informed consent was waived, and the opportunity to opt out of the study was provided.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Component proportions for the composite outcomes.
Additional file 2
: Table S2. Association of IRVF with acute kidney injury, renal replacement therapy, or death without LOCF as a sensitivity analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fujii, K., Nakayama, I., Izawa, J. et al. Association between intrarenal venous flow from Doppler ultrasonography and acute kidney injury in patients with sepsis in critical care: a prospective, exploratory observational study. Crit Care 27, 278 (2023). https://doi.org/10.1186/s13054-023-04557-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04557-9