Abstract
Venous congestion is an under-recognized contributor to mortality in critically ill patients. Unfortunately, venous congestion is difficult to measure, and right heart catheterization (RHC) has been considered the most readily available means for measuring venous filling pressure. Recently, a novel “Venous Excess Ultrasound (VExUS)” score was developed to noninvasively quantify venous congestion using inferior vena cava (IVC) diameter and Doppler flow through the hepatic, portal, and renal veins. A preliminary retrospective study of post-cardiac surgery patients showed promising results, including a high positive-likelihood ratio of high VExUS grade for acute kidney injury. However, studies have not been reported in broader patient populations, and the relationship between VExUS and conventional measures of venous congestion is unknown. To address these gaps, we prospectively assessed the correlation of VExUS with right atrial pressure (RAP), with comparison to inferior vena cava (IVC) diameter. Patients undergoing RHC at Denver Health Medical Center underwent VExUS examination before their procedure. VExUS grades were assigned before RHC, blinding ultrasonographers to RHC outcomes. After controlling for age, sex, and common comorbidities, we observed a significant positive association between RAP and VExUS grade (P < 0.001, R2 = .68). VExUS had a favorable AUC for prediction of a RAP ≥ 12 mmHg (0.99, 95% CI 0.96–1) compared to IVC diameter (0.79, 95% CI 0.65–0.92). These results suggest a strong correlation between VExUS and RAP in a diverse patient population, and support future studies of VExUS as a tool to assess venous congestion and guide management in a spectrum of critical illnesses.
Similar content being viewed by others
Background
There has been increasing recognition in recent years that vascular congestion is a common and under-appreciated contributor to patient morbidity in many settings, including the intensive care unit (ICU) [1,2,3,4,5,6,7]. The pathophysiologic parameter of interest is mean systemic filling pressure (pMSF), defined as the vascular pressure under static conditions, best conceived of as pressure required to return venous circulation to the heart [2]. An elevated pMSF limits circulation by decreasing the organ perfusion pressure (OPP), defined as mean arterial pressure (MAP)—pMSF [2, 8]. However, pMSF is particularly difficult to quantify; the standard approach remains invasive measurement of right atrial filling pressure (RAP) by right heart catheterization (RHC), which can serve as an approximation of pMSF [2, 8, 9]. Unfortunately, RHC is not universally available, and carries procedural risk with reported complication rates as high as 1%, even in experienced centers [10]. The cost and time required for high-quality RHC limits repeated assessment, especially in patients in whom placement of an indwelling Swan-Ganz catheter is not feasible. Ultrasonographic measurement of inferior vena cava (IVC) diameter is commonly used to approximate pMSF, but has been demonstrated to have multiple clinical limitations and only moderate sensitivity and specificity, especially in patients with chronically elevated right heart filling pressure or undergoing positive pressure ventilation [11].
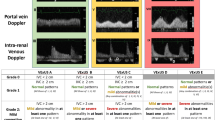
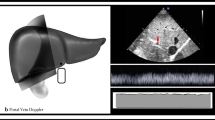
These limitations highlight the need for an inexpensive, noninvasive, and repeatable bedside technique for estimation of venous congestion. To that end, Beaubien-Souligny et al. developed an ultrasound technique to estimate pMSF: “Venous Excess Ultrasound (VExUS).” The VexUS exam is a novel 4-point ultrasound exam of venous flow through the IVC, hepatic vein, portal vein, and renal vasculature. These measurements, when combined, provide an overall “VExUS grade” of venous congestion [12]. VExUS was initially derived from 706 serial ultrasound examinations of 145 cardiac surgery patients [12]. The procedure is conducted at the bedside, with ultrasonographic hepatic and portal vein views being acquired in the subcostal window, and the renal vasculature captured best in the posterior axillary line. The examination is noninvasive and can be completed in 5–10 min by an experienced practitioner. Interestingly, in the initial validation study, VExUS demonstrated a greater positive-likelihood ratio for prediction of acute kidney injury than invasively measured central venous pressure, a finding that has generated considerable interest among cardiovascular, critical care, and nephrology investigators [12, 13]. A recent review described the application of the technique in a spectrum of critically ill patients [13]. However, it is important to note that the initial study was a post hoc analysis [12], and VExUS has not been compared with RHC, a conventional measure of venous congestion. Furthermore, there is controversy regarding the reliability and utility of VExUS measurements when compared to more widely-used methods of ultrasonographic volume assessment such as IVC diameter. To address this gap in the literature, we assessed the association between VExUS and invasively measured RAP, as compared to IVC diameter.
Methods
We conducted a prospective assessment of the diagnostic accuracy of VExUS grade for elevated RAP, adhering to the STARD and STROBE guidelines for diagnostic accuracy and cohort studies [6, 14]. A consecutive cohort of patients undergoing ambulatory and inpatient RHC at the Denver Health Medical Center from 12/20/2022–3/1/2023 underwent VExUS examination immediately prior to RHC. VExUS examinations were conducted and graded as previously described (see Additional file 1) [12]. Inclusion criteria included age > 18 years, plan to undergo RHC within 3 h, and ability and willingness to provide informed consent. Exclusion criteria included pregnancy or incarceration. No sample size calculation was performed for this proof-of-concept study.
Ultrasonographers were internal and emergency medicine residents with institutional training in ultrasound. Ultrasonographers and researchers were not part of the clinical team. All ultrasonographers completed a 4-h video series on VExUS developed by the Beaubien-Souligny group [15], before undergoing in-person training by an Emergency Medicine attending physician with a subspeciality training in ultrasonography, familiar with the VExUS technique. Prior to analysis, one of the clinicians that developed the VExUS score reviewed a representative subset of ultrasonographer scans by videoconference to assess image quality and confirm grading accuracy.
VExUS results were graded and recorded before publication of RHC results. Investigators were blinded to the outcome of RAP at the time of VExUS assessment and grading, and clinicians recording RAP were blinded to VExUS grade. Data were manually extracted from patient charts, including past medical history, demographic information, and pertinent laboratory and imaging results (see Additional file 2). Patients in whom VExUS exams could not be completed or interpreted due to poor image quality, or whose RHC were not completed were excluded. Multivariable linear regression was used to assess the relationship between the independent variable of VExUS grade and the dependent variable of RAP, controlling for age, sex, and Charlson comorbidity index (CCI) [16]. Linear model diagnostics are presented in Additional file 1. Covariates of age, sex, and CCI were selected a-priori by investigators for their clinical significance, and to minimize over-fitting. Specific conditions known to be associated with elevated RAP were avoided due to concern for colinearity, in favor of the broader variable CCI. Validation of linear models was conducted using standard techniques. Normality of residuals was assessed by visual inspection of QQ plots and the Shapiro–Wilk test, the Breusch–Pagan test was used to assess heteroscedasticity. D’Agostino and Anscombe tests were used to assess skewness and kurtosis. The Rainbow test was used to assess linearity, and Student’s T testing was used to evaluate outliers. Receiver operating characteristic curves were constructed for both VExUS grade and IVC diameter for identification of RAP ≥ 12 mmHg. Twelve mmHg was determined a-priori to be a clinically significant measure of venous congestion by a group of senior intensive care and cardiology-trained attending physicians.
Results
Sixty patients were screened for study inclusion, of which 56 were included and underwent VExUS examination. Results from 4 patients were excluded due to poor image quality, and one for procedure cancelation (see Additional file 1). Descriptive statistics for the cohort are presented in Table 1. No patients were on vasoactive medications, and no patients were using any form of positive pressure ventilation at the time of VExUS exam or RHC. All scans were completed in the allotted time frame prior to RHC, and were well-tolerated by patients.
After controlling for age, sex, and CCI, there was a significant positive association between RAP and VExUS grade, as shown in Fig. 1 (P < 0.001, R2 = 0.68). VExUS had a favorable AUC for prediction of a RAP ≥ 12 mmHg (0.99, 95% CI 0.96–1) compared to IVC diameter (0.79, 95% CI 0.65–0.92). A VExUS grade of 3 had a sensitivity of 1 (95% CI 0.69–1), and a specificity of 0.85 (95% CI 0.71–0.94) for RAP ≥ 12 mmHg.
Discussion
We report a strong, positive statistical association between RAP and VExUS score after controlling for confounding variables. This finding suggests a probable physiologic correlation between RAP and VExUS. In this pilot study, we observed consistent correlation between a simple-to-perform bedside ultrasound technique and invasively measured RAP. We also found that VExUS may have a greater positive predictive value than IVC diameter when used to assess elevated venous pressures. Given that elevated venous pressures are associated with poor outcomes and longer stays in the ICU [17], a noninvasive approach for estimating this parameter may prove to be a valuable tool for clinicians at the bedside. There could be several potential uses for this novel technique in the ICU [18], including guidance of diuretic therapy among patients with cardiogenic shock [19], personalized calibration of fluid resuscitation for patients with septic shock [1], and screening for evidence of pulmonary hypertension or worsening right ventricular dysfunction. The exam may be useful where invasive hemodynamic assessment is unavailable, broadening access to important diagnostic data (Additional file 1).
This pilot study suggests a link between VExUS and pMSF, as estimated by invasively measured RAP, but further investigation and validation of the technique is required. The results reported here suggest that VExUS may be useful as a noninvasive monitor of RAP, with potentially broad clinical utility. However, the current study has several limitations, including selection bias (critically ill patients are less likely to undergo RHC due to their clinical instability, for example), and a limited sample size. A further limitation to be addressed in future studies is the lack of comparison of RAP to IVC collapsibility, a guideline-recommended component of noninvasive evaluation of RAP [20], and a lack of feasibility data. Follow-up studies should include a wider range of patient pathologies to better evaluate relationships between RHC pressures and VExUS grade among patients with comorbidities such as cirrhosis, valvular disease, diastolic dysfunction, and other potential hemodynamic confounders of VExuS imaging. Before widespread use of VExUS as a proxy for RHC can be recommended, VExUS must also be evaluated for practical feasibility, interrater reliability, and inter-user reproducibility. Given the rapid proliferation of handheld ultrasound as a diagnostic tool, it would also be valuable to evaluate whether VExUS exams can be reliably acquired and interpreted using handheld ultrasound, in addition to traditional ultrasound machines. The authors hope to address these questions in a future prospective cohort study (Additional file 2).
Availability of data and materials
All datasets and imaging results are available upon request to the corresponding author.
References
Kattan E, Castro R, Miralles-Aguiar F, Hernandez G, Rola P. The emerging concept of fluid tolerance: a position paper. J Crit Care. 2022;71:154070. https://doi.org/10.1016/j.jcrc.2022.154070.
Persichini R, Lai C, Teboul JL, Adda I, Guerin L, Monnet X. Venous return and mean systemic filling pressure: physiology and clinical applications. Crit Care. 2022;26(1):150. https://doi.org/10.1186/s13054-022-04024-x.
Chen KP, Cavender S, Lee J, et al. Peripheral Edema, Central venous pressure, and risk of AKI in critical Illness. Clin J Am Soc Nephrol. 2016;11(4):602–8. https://doi.org/10.2215/CJN.08080715.
Spiegel R, Teeter W, Sullivan S, et al. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care. 2020;24(1):615. https://doi.org/10.1186/s13054-020-03330-6.
Beaubien-Souligny W, Benkreira A, Robillard P, et al. Alterations in portal vein flow and intrarenal venous flow are associated with acute kidney injury after cardiac surgery: a prospective observational cohort study. J Am Heart Assoc. 2018;7(19):e009961. https://doi.org/10.1161/JAHA.118.009961.
Chen CY, Zhou Y, Wang P, Qi EY, Gu WJ. Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: a meta-analysis. Crit Care. 2020;24(1):80. https://doi.org/10.1186/s13054-020-2770-5.
Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–96. https://doi.org/10.1016/j.jacc.2008.05.068.
Wijnberge M, Sindhunata DP, Pinsky MR, et al. Estimating mean circulatory filling pressure in clinical practice: a systematic review comparing three bedside methods in the critically ill. Ann Intensive Care. 2018;8(1):73. https://doi.org/10.1186/s13613-018-0418-2.
De Backer D, Vincent JL. Should we measure the central venous pressure to guide fluid management? Ten answers to 10 questions. Crit Care. 2018;22(1):43. https://doi.org/10.1186/s13054-018-1959-3.
Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48(12):2546–52. https://doi.org/10.1016/j.jacc.2006.07.061.
Ciozda W, Kedan I, Kehl DW, Zimmer R, Khandwalla R, Kimchi A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc Ultrasound. 2016;14(1):33. https://doi.org/10.1186/s12947-016-0076-1.
Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12(1):16. https://doi.org/10.1186/s13089-020-00163-w.
Jury D, Shaw AD. Utility of bedside ultrasound derived hepatic and renal parenchymal flow patterns to guide management of acute kidney injury. Curr Opin Crit Care. 2021;27(6):587–92. https://doi.org/10.1097/MCC.0000000000000899.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. https://doi.org/10.7326/0003-4819-147-8-200710160-00010.
Rola P. The VExUS Course. Video. Accessed 2023, https://vimeo.com/ondemand/thevexuscourse
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. https://doi.org/10.1093/aje/kwq433.
Li DK, Wang XT, Liu DW. Association between elevated central venous pressure and outcomes in critically ill patients. Ann Intensive Care. 2017;7(1):83. https://doi.org/10.1186/s13613-017-0306-1.
Rola P, Miralles-Aguiar F, Argaiz E, et al. Clinical applications of the venous excess ultrasound (VExUS) score: conceptual review and case series. Ultrasound J. 2021;13(1):32. https://doi.org/10.1186/s13089-021-00232-8.
Beaubien-Souligny W, Bouchard J, Desjardins G, et al. Extracardiac signs of fluid overload in the critically Ill cardiac patient: a focused evaluation using bedside ultrasound. Can J Cardiol. 2017;33(1):88–100. https://doi.org/10.1016/j.cjca.2016.08.012.
Porter TR, Shillcutt SK, Adams MS, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28(1):40–56. https://doi.org/10.1016/j.echo.2014.09.009.
Funding
This study received no funding from any source.
Author information
Authors and Affiliations
Contributions
Author AL wrote the bulk of the manuscript, with input from all coauthors on study design. Authors AL, KM, KL, GS, and JB contributed to data collection.
Corresponding author
Ethics declarations
Ethical Approval
This study had approval from the local Colorado Multiple Institutional Review Board (#22–2024).
Competing interests
None of the authors have any competing interests or competing interests to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Appendix of additional information including Research Protocol, validation of chart review, VExUS scanning protocol, and model diagnostics.
Additional file 2
. Glossary of definitions for data extracted from electronic medical record.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Longino, A., Martin, K., Leyba, K. et al. Correlation between the VExUS score and right atrial pressure: a pilot prospective observational study. Crit Care 27, 205 (2023). https://doi.org/10.1186/s13054-023-04471-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04471-0