Abstract
Background
Mildly elevated lactate levels (i.e., 1–2 mmol/L) are increasingly recognized as a prognostic finding in critically ill patients. One of several possible underlying mechanisms, microcirculatory dysfunction, can be assessed at the bedside using sublingual direct in vivo microscopy. We aimed to evaluate the association between relative hyperlactatemia, microcirculatory flow, and outcome.
Methods
This study was a predefined subanalysis of a multicenter international point prevalence study on microcirculatory flow abnormalities, the Microcirculatory Shock Occurrence in Acutely ill Patients (microSOAP). Microcirculatory flow abnormalities were assessed with sidestream dark-field imaging. Abnormal microcirculatory flow was defined as a microvascular flow index (MFI) < 2.6. MFI is a semiquantitative score ranging from 0 (no flow) to 3 (continuous flow). Associations between microcirculatory flow abnormalities, single-spot lactate measurements, and outcome were analyzed.
Results
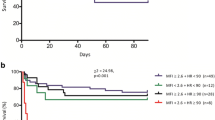
In 338 of 501 patients, lactate levels were available. For this substudy, all 257 patients with lactate levels ≤ 2 mmol/L (median [IQR] 1.04 [0.80–1.40] mmol/L) were included. Crude ICU mortality increased with each lactate quartile. In a multivariable analysis, a lactate level > 1.5 mmol/L was independently associated with a MFI < 2.6 (OR 2.5, 95% CI 1.1–5.7, P = 0.027).
Conclusions
In a heterogeneous ICU population, a single-spot mildly elevated lactate level (even within the reference range) was independently associated with increased mortality and microvascular flow abnormalities. In vivo microscopy of the microcirculation may be helpful in discriminating between flow- and non-flow-related causes of mildly elevated lactate levels.
Trial registration
ClinicalTrials.gov, NCT01179243. Registered on August 3, 2010.
Similar content being viewed by others
Background
An elevated lactate level, classically defined as an arterial lactate level > 2 mmol/L, is a well-known predictor of adverse outcome in terms of organ dysfunction and mortality in different subgroups of critically ill patients [1,2,3]. Surviving Sepsis Campaign guidelines consider a threshold of 1 mmol/L as an indicator of tissue hypoperfusion, but they suggest resuscitation to normalize arterial lactate levels in patients with lactate levels > 4 mmol/L in order to improve outcome, based on the principles of early goal-directed therapy [4,5,6]. Similarly, in nonseptic patients, the value of lactate levels in goal-directed resuscitation, as well as the additive value of serial lactate measurements, is recognized [7,8,9,10]. Recent studies indicate that small increases in lactate levels are already associated with an unfavorable clinical course. This association has been demonstrated for “relative hyperlactatemia” with thresholds as low as 1.1 mmol/L [11,12,13,14]. Although lactate is easily measured in daily practice, unraveling the underlying causative mechanism is often much more difficult. Organ hypoperfusion is regarded as an important cause of hyperlactatemia, although several other mechanisms also play a significant role, ranging from accelerated aerobic glycolysis to decreased lactate metabolism and mitochondrial and microcirculatory dysfunction [15]. Sublingual direct in vivo microscopy is a suitable method of detecting microcirculatory derangements at the bedside [16]. Several studies have demonstrated an association between lactate levels and microcirculatory alterations in subgroups of critically ill patients as well as in experimental settings [17,18,19,20,21,22,23,24,25]. We previously demonstrated that both microcirculatory derangements and arterial lactate levels were independent predictors of mortality in selected high-risk patients [26].
The aforementioned studies were primarily focused on the early phase of intensive care unit (ICU) admission. The significance of minimally elevated lactate levels as well as concomitant microcirculatory dysfunction at a later time point is unclear. Therefore, we aimed to investigate the significance of a single-spot arterial lactate measurement and simultaneous in vivo microscopy in a heterogeneous ICU population recruited from 36 ICUs worldwide.
Methods
Patients and setting
This study was a post hoc analysis of a prospective observational point prevalence study of the prevalence and significance of microcirculatory alterations in a heterogeneous ICU population (Microcirculatory Shock Occurrence in Acutely ill Patients [microSOAP; ClinicalTrials.gov identifier NCT01179243; registered on August 3, 2010 [26]). Thirty-six ICUs worldwide participated in this study. Being a point prevalence study, data collection on patient characteristics and laboratory values, as well as simultaneous sublingual sidestream dark-field (SDF) imaging, was performed on a single day for all patients in a given ICU or ICU subunit. Lactate levels were measured within a maximum of 4 h before or after SDF imaging. For this substudy, patients with an arterial lactate level ≤ 2 mmol/L were included.
Ethics approval
Every participating center obtained ethics approval according to local legislation. A copy of the ethics approval was sent to the study coordinator before the start of the study (see Additional file 1). Written informed consent was obtained from all included subjects, unless the local ethics committee specifically allowed a waiver in this respect.
SDF imaging
SDF imaging is a noninvasive technique consisting of a camera incorporated in a handheld device that emits stroboscopic green light with a wavelength within the absorption spectrum of hemoglobin (Hb) [12]. The light emitted by the SDF camera (MicroScan; MicroVision Medical, Amsterdam, The Netherlands) is absorbed by Hb, visualizing erythrocytes as black cells on the screen. Offline software-assisted analysis of SDF images (AVA 3.0; MicroVision Medical) yields information on convective oxygen transport and diffusion distance. The semiquantitative microvascular flow index (MFI), ranging from 0 (no flow) to 3 (continuous flow), and the percentage of perfused vessels (PPV) provides information on convection, whereas total vessel density (TVD) and perfused vessel density (PVD) provide information on diffusion [27]. A single measurement consisting of three sublingual SDF image sequences of 10–20 seconds was obtained for every patient. SDF imaging as well as subsequent image analysis were performed in line with international consensus [27, 28].
Statistical analysis
Analysis was focused on associations between lactate levels, mortality, organ dysfunction, and microcirculatory alterations. An abnormal microcirculatory blood flow was predefined as a sublingual MFI < 2.6 for vessels < 20 μm, being the lowest reported lower bound of the 95% CI of healthy volunteers. We defined this value a priori for the analysis of the original microSOAP data. A post hoc analysis confirmed this cutoff value as the Youden index in an ROC curve [26]. This cutoff value has been validated as clinically relevant [26, 29]. To determine cutoff values for lactate levels for both abnormal MFI and mortality, the AUC was calculated. These cutoff values were subsequently tested in logistic regression analysis.
Backward stepwise logistic regression was employed to detect determinants of a capillary MFI < 2.6. Predictors with P < 0.25 in univariable logistic regression were used for multivariable modeling (see Additional file 2 for additional information on the statistical analysis). Tested predictors included Sequential Organ Failure Assessment (SOFA) score on the day of SDF imaging, Acute Physiology and Chronic Health Evaluation II (APACHE II) score on ICU admission, length of stay in the ICU prior to SDF imaging (≤24 h and > 24 h), admission diagnosis, the presence of sepsis at the time of SDF imaging, Hb ≤ 5.37 mmol/L, arterial lactate level > 1.5 mmol/L, heart rate, mean arterial pressure, fluid balance, and vasopressor use. In case of nonlinearity of the logit, variables were dichotomized. The resulting models were tested for multicollinearity. Hosmer and Lemeshow goodness of fit was used to test the fit of the model. Furthermore, the associations between lactate levels, microcirculatory dysfunction, mortality, and organ dysfunction (SOFA, cumulative vasopressor index [CVI] [30]) were described by dividing the lactate measurements into quartiles. To test for differences between normally distributed variables, Student’s t test or the Mann-Whitney U test was performed. To compare dichotomous variables, Fisher’s exact test was applied. Distributions across more than two groups were tested using the nonparametric Kruskal-Wallis test. The data were analyzed using IBM SPSS Statistics version 23.0 (IBM, Armonk, NY, USA) and Prism 5.04 (GraphPad Software, Inc., La Jolla, CA, USA) software and are presented as the median [IQR] or mean ± SD, unless indicated otherwise. P < 0.05 was considered statistically significant.
Results
General characteristics
Out of 501 patients, arterial lactate levels were available for 338 (67%) of patients. In 257 out of these 338 patients (76%), arterial lactate levels were ≤ 2 mmol/L. These patients, with median APACHE of 16 [10–23] and median SOFA of 5 [3–8], were included for further analysis (Table 1). Surgery (35.4%) and sepsis (17.5%) were the main reasons for ICU admission. Median arterial lactate levels were 1.04 [0.80–1.40] mmol/L. ICU and hospital mortality were 20.6% and 27.2%, respectively.
Lactate levels and mortality
Increases in ICU mortality were observed for every lactate quartile (≤0.80 mmol/L, 12.9%; 0.81–1.04 mmol/L, 15.3%; 1.05–1.40 mmol/L, 15.4%; > 1.40 mmol/L, 39.7%; P < 0.001). Similar trends were observed for hospital mortality (24.3% in the lowest quartile, 44.4% in the highest quartile; P = 0.005) (see Fig. 1). The AUC was 0.65 (95% CI 0.56–0.73, P = 0.001) with a cutoff value of 1.42 mmol/L for ICU mortality (sensitivity 40%, specificity 81%). The same cutoff value was seen for hospital mortality with a sensitivity of 47% and a specificity of 81% (AUC 0.59, 95% CI 0.51–0.67, P = 0.025). Mortality was at least almost twice as high for patients with an arterial lactate level > 1.5 mmol/L as compared with patients with a lower lactate level (ICU mortality 41.2% vs. 15.5%, P < 0.001; hospital mortality 45.1% vs. 22.9%, P = 0.001).
Lactate levels and microcirculatory flow abnormalities
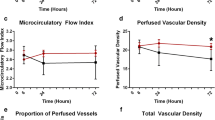
Patients with a capillary MFI < 2.6 (14%) had slightly but nonsignificantly higher lactate levels than patients with a higher MFI (1.11 [0.90–1.60] vs. 1.00 [0.80–1.40] mmol/L, P = 0.117). A nonsignificant trend toward a higher prevalence of an abnormal microcirculation in the highest lactate quartile was observed (P = 0.169) (Fig. 1). Hb was significantly lower in patients with an MFI < 2.6 (Hb 5.4 [5.2–6.8] vs. Hb 6.3 [5.5–7.1], P = 0.011). No significant differences with respect to illness severity scores, hemodynamics, vasopressor use or dose, reason for ICU admission, or time in ICU prior to SDF imaging were observed. Comparing patients with lactate levels ≤ 1.5 mmol/L and > 1.5 mmol/L, no significant differences were observed for small vessel MFI; large vessel MFI; and small vessel TVD, PVD, PPV, (perfused) De Backer score, and heterogeneity index.
Multivariable logistic regression analysis for MFI < 2.6
In multivariable logistic regression analysis, the only remaining significant predictors for an abnormal MFI were an Hb ≤ 5.37 mmol/L (OR 4.6, 95% CI 2.1–10.2; P < 0.001), a stay in the ICU < 24 h prior to SDF (OR 2.9, 95% CI 1.3–6.6, P = 0.008), and an arterial lactate level > 1.5 mmol/L (OR 2.5, 95% CI 1.1–5.7, P = 0.027). The AUC for this three-variable model was 0.74 (95% CI 0.65–0.83, P = 0.001). The Hosmer and Lemeshow chi-square statistic was 2.015 (P =0.847) (see also Additional file 2).
Lactate levels and organ dysfunction
A higher lactate level was not accompanied by a significantly higher SOFA score or CVI (P = 0.078 and P = 0.063, respectively) (Figs. 2 and 3).
Different phenotypes
Although an abnormal MFI and elevated lactate levels appear to be associated, several different phenotypes exist. For individual patients, a higher lactate level was not necessarily associated with adverse outcome or an abnormal microcirculation or vice versa, pointing toward a multifactorial etiology and significance of both hyperlactatemia and microvascular derangements (Fig. 4).
Discussion
In the present study, a single-spot arterial lactate level > 1.5 mmol/L was associated with increased mortality as well as with microcirculatory abnormalities and organ dysfunction. This “relative hyperlactatemia” is an emerging concept [11,12,13,14, 31]. Lactate levels on admission as low as 1.1 mmol/L already appeared to be associated with adverse outcome [12]. Our observations add to the idea that the prognostic relevance of mildly elevated lactate levels is not restricted to the early phase of ICU admission. The twofold increase in mortality in patients with a lactate level > 1.5 mmol/L is in agreement with results of previous studies focused on the first day of ICU admission [13, 14]. Researchers in a few studies have reported lactate levels and their association with outcome during the later phase of ICU stay, showing contradictory results. Some have observed an association between hyperlactatemia after initial stabilization with higher mortality rates, whereas others found that not lactate itself but impaired lactate clearance was associated with adverse outcome [32, 33]. In this respect, it is notable that we were able to demonstrate this association in a highly heterogeneous study population, in terms of both the timing of the lactate measurement as well as the underlying diagnosis and disease severity. Not only mortality but also organ dysfunction in terms of SOFA score appeared to be more severe for increasing lactate levels, albeit that this was statistically nonsignificant. A previous study was able to show associations between incremental lactate levels > 2 mmol/L and SOFA scores [2]. However, in that study, the investigators evaluated the time course of lactate measurements, whereas in the present study, we evaluated the implications of a single lactate measurement.
Several mechanisms may be involved in the increase of lactate levels. One of these, microcirculatory flow abnormalities, was indeed associated with mildly elevated lactate levels in the present study. PVD, and therefore effective diffusion distance, did not differ between patients with and without mildly elevated lactate levels. Therefore, impaired convective oxygen transport, but not diffusion distance, might have contributed to anaerobic glycolysis. Several researchers have also observed an association between impairment of microvascular flow and elevations in arterial lactate, whereas others have been able to demonstrate associations between lactate levels and parameters of vessel density in a variety of disease states [17, 19,20,21,22,23,24,25].
Alternatively, several non-flow-related factors may lead to increased nonanaerobic lactate formation under conditions of stress by promoting conversion of glucose to lactate via pyruvate instead of pyruvate entering the citric acid cycle [15, 34]. Indeed, lactate formation in endotoxemia results predominantly from increased aerobic lactate formation [35]. On top of that, exogenous adrenergic stress resulting from β-adrenergic drugs can also increase aerobic lactate formation [36].
Besides ongoing lactate formation, impaired lactate clearance has to be kept in mind as a cause of mildly elevated lactate levels. Levraut and coworkers observed that in stable septic patients in whom arterial lactate levels were < 2 mmol/L after the initial resuscitation phase, impaired clearance of exogenous sodium lactate but not baseline lactate values could discriminate between survivors and nonsurvivors [32, 37]. It is conceivable that a similar mechanism was involved in our patients.
Altogether, the direct observation of the microcirculation in conjunction with lactate measurements confirms the idea that impaired organ perfusion is only one of many explanations for elevated lactate levels with potential consequences for therapeutic strategies in the ICU [29, 38].
Our study has several limitations. At first glance, the absolute numbers of lactate and MFI seem to indicate that the study population was not severely ill. However, owing to the design of the study, patients with a longer stay in the ICU before study inclusion were overrepresented. Therefore, the median APACHE II score of 16 seems to be a better indicator of considerable severity of illness of the population at ICU admission. The lack of macrohemodynamic monitoring limited in-depth statistical analysis of factors associated with relative hyperlactatemia. Furthermore, no detailed information on factors influencing lactate clearance or drugs influencing lactate metabolism (e.g., metformin) was available. In addition, the presence of microvascular flow abnormalities in other organs not detected by sublingual in vivo microscopy cannot be ruled out [39]. Serial measurements of both microcirculation and lactate could have shed more light on the time course of organ dysfunction in patients with relative hyperlactatemia [30, 40, 41]. Although independently associated in the multivariate analysis, it is conceivable that a factor not accounted for in our model influenced both lactate and MFI. Last, it should be stated that the observed association between relatively low lactate levels and outcome does not automatically imply clinical relevance. Not only is the predictive value of this multivariate model relatively low with an AUC of 0.74, but it also remains to be established whether interventions aimed at achieving a further reduction of lactate will be beneficial to patients.
Conclusions
Our data indicate that even single-spot lactate levels within the usual reference range are associated with an unfavorable clinical course. However, the question remains how the clinician must incorporate these findings into an individualized approach to treating otherwise seemingly stable ICU patients. In vivo microscopy of the (sublingual) microcirculation may be helpful for detection of organ perfusion-related causes of mildly elevated lactate levels with potential consequences for a therapeutic strategy.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- CVI:
-
Cumulative vasopressor index
- Hb:
-
Hemoglobin
- ICU:
-
Intensive care unit
- MFI:
-
Microvascular flow index
- microSOAP:
-
Microcirculatory Shock Occurrence in Acutely ill Patients
- PPV:
-
Percentage of perfused vessels
- PVD:
-
Perfused vessel density
- SDF:
-
Sidestream dark-field imaging
- SOFA:
-
Sequential Organ Failure Assessment
- TVD:
-
Total vessel density
References
Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007;33:970–7.
Jansen TC, van Bommel J, Woodward R, Mulder PGH, Bakker J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. 2009;37:2369–74.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Heffner AC, Kline JA, et al. Prognostic value and agreement of achieving lactate clearance or central venous oxygen saturation goals during early sepsis resuscitation. Acad Emerg Med. 2012;19:252–8.
Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61.
Blow O, Magliore L, Claridge JA, Butler K, Young JS. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma. 1999;47:964–9.
Pölönen P, Ruokonen E, Hippeläinen M, Pöyhönen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg. 2000;90:1052–9.
Vincent JL, Quintairos e Silva A, Couto Jr L, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20:257.
Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38:4–10.
Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14:R25.
Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001;27:74–83.
Rishu AH, Khan R, Al-Dorzi HM, Tamim HM, Al-Qahtani S, Al-Ghamdi G, et al. Even mild hyperlactatemia is associated with increased mortality in critically ill patients. Crit Care. 2013;17:R197.
Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3:12.
Wilson RF, et al. Shock. In: Wilson RF, editor. Critical care manual: applied physiology and principles of therapy. Philadelphia: F.A. Davis; 1992. p. 223–86.
De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34:403–8.
Dubin A, Pozo MO, Ferrara G, Murias G, Martins E, Canullán C, et al. Systemic and microcirculatory responses to progressive hemorrhage. Intensive Care Med. 2009;35:556–64.
Hernandez G, Boerma EC, Dubin A, Bruhn A, Koopmans M, Edul VK, et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care. 2013;28:538.e9–14.
De Backer D, Dubois MJ, Schmartz D, Koch M, Ducart A, Barvais L, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg. 2009;88:1396–403.
De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147:91–9.
Yeh YC, Wang MJ, Chao A, Ko WJ, Chan WS, Fan SZ, et al. Correlation between early sublingual small vessel density and late blood lactate level in critically ill surgical patients. J Surg Res. 2013;180:317–21.
Hernandez G, Bruhn A, Castro R, Pedreros C, Rovegno M, Kattan E, et al. Persistent sepsis-induced hypotension without hyperlactatemia: a distinct clinical and physiological profile within the spectrum of septic shock. Crit Care Res Prac. 2012;2012:536852.
Jung C, Ferrari M, Rödiger C, Fritzenwanger M, Goebel B, Lauten A, et al. Evaluation of the sublingual microcirculation in cardiogenic shock. Clin Hemorheol Microcirc. 2009;42:141–8.
Filbin MR, Hou PC, Massey M, Barche A, Kao E, Bracey A, et al. The microcirculation is preserved in emergency department low-acuity sepsis patients without hypotension. Acad Emerg Med. 2014;21:154–62.
Vellinga NAR, Boerma EC, Koopmans M, Donati A, Dubin A, Shapiro NI, et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med. 2015;43:48–56.
De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101.
Boerma EC, Mathura KR, van der Voort PHJ, Spronk PE, Ince C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit Care. 2005;9:R601–6.
Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013;39:612–9.
Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–7.
Walker CA, Griffith DM, Gray AJ, Datta D, Hay AW. Early lactate clearance in septic patients with elevated lactate levels admitted from the emergency department to intensive care: time to aim higher? J Crit Care. 2013;28:832–7.
Levraut J, Ichai C, Petit I, Ciebiera JP, Perus O, Grimaud D. Low exogenous lactate clearance as an early predictor of mortality in normolactatemic critically ill septic patients. Crit Care Med. 2003;31:705–10.
Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009;13:R90.
Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R502–16.
Michaeli B, Martinez A, Revelly JP, Cayeux MC, Chioléro RL, Tappy L, et al. Effects of endotoxin on lactate metabolism in humans. Crit Care. 2012;16:R139.
Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, Hien TT, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348:219–23.
Levraut J, Ciebiera JP, Chave S, Rabary O, Jambou P, Carles M, et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than overproduction. Am J Respir Crit Care Med. 1998;157:1021–6.
Hanson JP, Lam SWK, Mohanty S, Alam S, Pattnaik R, Mahanta KC, et al. Fluid resuscitation of adults with severe falciparum malaria: effects on acid-base status, renal function, and extravascular lung water. Crit Care Med. 2013;41:972–81.
Boerma EC, van der Voort PHJ, Spronk PE, Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med. 2007;35:1055–60.
Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221–6.
Lee TR, Kang M, Cha WC, Shin TG, Sim MS, Jo I, et al. Better lactate clearance associated with good neurologic outcome in survivors who treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Crit Care. 2013;17:R260.
Acknowledgements
We thank Prof. J. L. Vincent, Department of Intensive Care, Erasme University Hospital, Brussels, Belgium, for allowing us to use the SOAP acronym in our study; F. Messie, Osix, Huizen, The Netherlands, for software engineering; A. Carsetti, MD, R. Domizi, MD, and C. Scorcella, MD, Department of Biomedical Sciences and Public Health, Università Politecnica delle Marche, Ancona, Italy; G. Veenstra, MD, and B. Scheenstra, MD, ICU, Medical Center Leeuwarden, Leeuwarden, The Netherlands, for their contributions to SDF analysis; D. M. J. Milstein, PhD, and K. Yuruk, MD, Department of Translational Physiology, Academic Medical Center, Amsterdam, The Netherlands for their help with SDF imaging; P. Ormskerk, RN, and D. van Duijn, RN, Intensive Care Adults, Erasmus MC University Medical Center Rotterdam, The Netherlands, for their help with study logistics; and D. S. Martin, MD, PhD, P. Meale, RGN, University College London, ICU, Royal Free Hospital, London, UK, and A. Vivian-Smith, RGN, ICU, Royal London Hospital, London, UK, for their assistance with UK ethics approval.
Participating centers and members of the microSOAP study group:
ICU, Medical Center Leeuwarden, Leeuwarden, The Netherlands (E. C. Boerma, MD, PhD, M. Koopmans, RN; N. A. R. Vellinga, MD)
ICU, St. Antonius Hospital, Nieuwegein, The Netherlands (M. van Iterson, MD, PhD)
ICU, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands (P. H. J. van der Voort, MD, PhD)
ICU, Erasmus Medical Center, Rotterdam, The Netherlands (J. Bakker, MD, PhD; J. van Bommel, MD, PhD)
ICU, Gelre Ziekenhuizen, Apeldoorn, The Netherlands (P. E. Spronk, MD, PhD, FCCP)
Departamento de Medicina Intensiva, Escuela de Medicina, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile (C. Ruiz, MD; G. Hernandez, MD, PhD)
Departamento de Anestesiologia, Dor e Terapia Intensiva, Hospital Sao Paulo, Universidade Federal de São Paulo Sao Paulo, Brazil (F. R. Machado, MD, PhD; A. T. Bafi, MD)
Servicio de Terapia Intensiva, Sanatorio Otamendi y Miroli, Buenos Aires, Argentina (A. Dubin, MD, PhD; V. S. Kanoore Edul, MD)
ICU, Hospital San Martín, La Plata, Argentina (H. S. Canales, MD)
ICU, Hospital Español ‘Juan J Crotoggini’, Montevideo, Uruguay (F. J. Hurtado, MD; G. Lacuesta, MD; M. Baz, MD)
ICU, Cooper University Hospital, Cooper Medical School of Rowan University, Camden, NJ, USA (S. M. Hollenberg MD, FACC, FCCM, FAHA, FCCP; U. Patel, MD)
ICU, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, MA, USA (N. I. Shapiro, MD, MPH)
Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, USA (H. Gomez, MD; M. Pinsky, MD, CM, Dr hc, MCCM, FCCP)
Critical Care Medicine/Neurocritical Care, Mercy Hospital St. Louis/St. Louis University Hospital, St. Louis, MO, USA (F. G. Sadaka, MD; K. Krause, RN)
ICU, University of California, San Diego, San Diego, CA, USA (R. Mehta, MD, PhD)
Department of Internal Medicine I, Universitätsklinikum Jena, Friedrich-Schiller-University, Jena, Germany (C. Jung, MD)
Department of Surgical Intensive Care, University Hospital Aachen, Aachen, Germany (M. Fries, MD, PhD)
Adult Critical Care Unit, Royal London Hospital, London, UK (R. M. Pearse, MBBS, FRCA, FFICM; A. Smith, RGN)
ICU, Royal Free Hospital, London, UK (D. S. Martin, MD, PhD; P. Meale, RGN)
ICU, The Royal Marsden Hospital, Chelsea, London, UK (S. Jhanji, MD, PhD)
ICU, Derriford Hospital, Plymouth University Peninsula School of Medicine, Plymouth, UK (G. Minto, MD, FRCA)
ICU, New Cross Hospital, Wolverhampton, UK (G. Martinelli, MD; M. Lombrano, MD)
ICU, Royal Devon and Exeter Hospital, Exeter, UK (S. M. A. Hubble, MD; C. Thorn, PhD)
Critical Care Department, Joan XXIII University Hospital, Tarragona, Spain (A. H. Rodriguez, MD, PhD; I. Martin-Loeches, MD, PhD [current affiliation: Critical Care Centre, Corporacio Sanitaria I Universitaria Parc Tauli – Hospital de Sabadell, Barcelona, Spain])
Department of Intensive Care Medicine, Waikato Hospital, Hamilton, New Zealand (F. M. P. van Haren, MD, PhD [current affiliation: Intensive Care Unit, Canberra Hospital, Canberra, Australia])
Intensive Care Department, Lithuanian University of Health Sciences, Kaunas, Lithuania (A. Pranskunas, MD, PhD; V. Pilvinis, MD, PhD)
Clinica di Anestesia e Rianimazione, Azienda Ospedaliera-Universitaria Ospedali Riuniti, Ancona, Italy (A. Donati, MD)
Dipartimento di Anestesia, Rianimazione e Terapia Intensiva, Azienda Unità Locale Socio Sanitaria 9 (ULSS 9) Veneto, Treviso, Italy (C. Sorbara, MD; A. Forti, MD, A. Comin, PhD)
ICU, Santa Maria degli Angeli Hospital, Pordenone, Italy (M. L. Chierego, MD; T. Pellis, MD)
ICU, The University of Queensland and Royal Brisbane and Women’s Hospital, Brisbane, Australia (A. Holley, MD, FACEM, FCICM; J. Paratz, MD, PhD)
Departement d’Anesthesie-Reanimation, Hôpitaux Universitaires Paris-Sud, Université Paris-Sud, Hôpital de Bicêtre Assistance Publique – Hôpitaux de Paris (AP-HP), Le Kremlin-Bicêtre, France, Paris, France (J. Duranteau, MD, PhD; A. Harrois, MD)
Department of Anesthesiology, Critical Care and Mobile Emergency and Resuscitation Service (SMUR), Hôpital Lariboisière (AP-HP)/Université Paris 7 Diderot, Paris, France (D. Payen, MD, PhD; C. Damoisel, MD)
Medical ICU, University Hospital Basel, Basel, Switzerland (G. M. Balestra, MD; E. Bucher, MD)
Ispat Hospital, Rourkela, Orissa, India (R. Pattnaik)
Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand (A. M. Dondorp, MD, PhD; M. T. Herdman, MD, PhD)
ICU, Hacettepe University, Ankara, Turkey (U. Aypar MD, B. Ayhan, MD)
ICU, Kosuyolu University, Istanbul, Turkey (T. Ayazoglu-Akarsu, MD)
The work was performed in the ICUs of the hospitals named above in the microSOAP study group affiliations and was coordinated from the ICU of Medical Center Leeuwarden, Leeuwarden, The Netherlands.
Collaborating authors:
J. van Bommel
G. Hernandez
F. Machado
V. Kanoore Edul
G. Lacuesta
M. Baz
U. Patel
M. Pinsky
K. Krause
A. Smith
P. Meale
M. Lombrano
C. Thorn
I. Martin-Loeches
V. Pilvinis
A. Forti
A. Comin
T. Pellis
J. Paratz
A. Harrois
C. Damoisel
E. Bucher
R. Pattnaik
M. T. Herdman
B. Ayhan
Funding
This study was supported in part by an unrestricted grant from the local hospital fund, Medical Center Leeuwarden, Leeuwarden, The Netherlands. The funder had no role in the study design; data acquisition, analysis, interpretation, and review; or approval of the manuscript. No financial compensation was received by participating centers or persons who made additional contributions.
Availability of data and materials
The datasets generated during and/or analyzed during the present study is not publicly available, owing to further research based on this dataset, but they are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Contributions
NARV, ECB, MK, ADo, ADu, NIS, RMP, JB, and CI conceived of and designed the study. NARV, ECB, MK, ADo, ADu, NIS, MRP, TB, MF, TAA, AP, SHo, GB, MvI, PHJvdV, FS, GMi, UA, FJH, GMa, DP, FvH, AH, AMD, HG, RLM, AHR, CR, HSC, JD, PES, SJ, SHu, MC, CJ, DM, CS, JB, CI acquired data. NARV, ECB, MK, and CI were responsible for data analysis. NARV, ECB, MK, ADo, ADu, NIS, RMP, JB, and CI interpreted the data. NARV, ECB, and CI wrote the manuscript draft. All authors revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All centers obtained medical ethics approval via their local ethics committees. Written informed consent (or a waiver, if applicable) was obtained in accordance with local applicable law.
Consent for publication
Not applicable.
Competing interests
The institution of NARV and ECB has received grant support from a local hospital fund (Medical Center Leeuwarden, unrestricted grant). NIS has served as a board member for the Cumberland Data and Safety Monitoring Board. The institution of NIS has received grant support from Biosite, Cheetah Medical, Rapid Pathogen Screening, and Thermo Fisher Scientific. RMP has consulted for Massimo and Edwards Lifesciences and has lectured for Nestle Health Sciences. The institution of RMP has received grant support from Nestle Health Sciences, LiDCO, and Cephalon (some are equipment loans, not funding). DP has consulted for Vygon Italia. RLM has consulted for AbbVie, CSL Behring, AM-Pharma, Grifols, Ardea Biosciences, and GlaxoSmithKline; has provided expert testimony for Nell DyMott; has lectured for AbbVie; and has stock options with Astute Medical. The institution of RLM has received grant support from Spectral Medical, AlloCure, and Eli Lilly. AHR has served as a board member for MSD; has consulted and lectured for Pfizer, Astellas Pharma, Novartis, and Brahms; and has received support for travel from Pfizer, MSD, Astellas Pharma, Novartis, and Brahms. HSC has disclosed government work. The institution of JD has lectured for LFB Biopharmaceuticals. SHu is employed by Royal Devon and Exeter Hospital NHS Trust (intensive care consultant). CJ reports receiving (all outside the submitted work) grants and personal fees from Actelion Pharmaceuticals, Bayer Healthcare, and ZOLL Medical, as well as personal fees from Boehringer Ingelheim, Vifor Pharma, Pfizer, Abbott Vascular, Boston Scientific, and Novartis. CS has disclosed government work. CI has developed SDF imaging and is listed as inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the Academic Medical Center, Amsterdam, The Netherlands. CI has been a consultant for MVM in the past but has not been involved with this company for more than 5 years, except for still holding shares. Braedius Medical, a company owned by a relative of CI, has developed and designed a handheld microscope called CytoCam-IDF imaging. CI has no financial relationship with Braedius Medical of any sort (i.e., has never owned shares or received consultancy or speaker fees from Braedius Medical).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Declarations of ethical approval. (DOCX 23 kb)
Additional file 2:
Supplemental material. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vellinga, N.A.R., Boerma, E.C., Koopmans, M. et al. Mildly elevated lactate levels are associated with microcirculatory flow abnormalities and increased mortality: a microSOAP post hoc analysis. Crit Care 21, 255 (2017). https://doi.org/10.1186/s13054-017-1842-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-017-1842-7