Abstract
Background
In septic patients, hyperoxia may help with its bactericidal effects, but it may cause systemic impairments. The role of hyperoxia and the appropriate oxygen target in these patients is unknown. The aim of this systematic review was to summarize the available literature.
Methods
We conducted a systematic search screening PubMed and Cochrane Library. Studies on adult patients with sepsis or septic shock and admitted to ICU addressing the topic of hyperoxia were included and described.
Results
We included 12 studies, for a total of 15.782 included patients. Five studies were randomized controlled trials (RCTs) or analyses from RCTs, three were prospective observational studies, and four were retrospective observational studies. The definition of hyperoxia was heterogeneous across the included studies. Mortality was the most frequent outcome: six studies showed an increased rate or risk of mortality with hyperoxia, three found no differences, and one a protective effect of hyperoxia. At the critical appraisal assessment stage, no major methodological flaws were detected, except for a single-center, pilot study, with a lack of adjustment for confounders and imbalance between the groups.
Conclusion
The optimum range of oxygen level able to minimize risks and provide benefits in patients with sepsis or septic shock seems still unknown. Clinical equipoise between hyperoxia and normoxia is uncertain as conflicting evidence exists. Further studies should aim at identifying the best range of oxygenation and its optimal duration, investigating how effects of different levels of oxygen may vary according to identified pathogens, source of infection, and prescribed antibiotics in critically ill patients with sepsis and septic shock.
Similar content being viewed by others
Background
Sepsis and septic shock are leading causes of mortality and morbidity in patients admitted to the intensive care unit (ICU). In the pathophysiology of septic shock, an imbalance occurs between oxygen supply and oxygen consumption [1]. Therefore, many ICU patients with sepsis require vasopressors, invasive ventilation, and the provision of supplemental oxygen. However, the appropriate regimen of oxygen administration is unknown [2]. The Surviving Sepsis Campaign Guidelines [3] stated that there is insufficient evidence to make a recommendation on the use of conservative oxygen targets in adults with sepsis-induced hypoxemic respiratory failure, thus not providing any threshold for arterial oxygen partial pressure (PaO2) or arterial oxygen saturation (SaO2). Although oxygen therapy is essential in most critically ill patients, they may be exposed to high level of oxygen and develop a hyperoxia status, potentially determining harm. The effects of a high PaO2 are controversial: on the one hand, oxygen has bactericidal properties, but on the other hand, hyperoxemia seems also able to cause systemic complications. Indeed, an excess of oxygen availability may result in the production of reactive oxygen (ROS) [4, 5] alteration of mitochondrial respiration, activation of apoptosis pathway, atelectasis [6], and vasoconstriction [7]. Moreover, in vitro studies showed that exposure to different levels of oxygen may modify the sensitivity of bacteria to antibiotics [8]. Therefore, oxygen levels may influence the outcome of septic patients through several mechanisms.
In literature, many studies have been published in recent years, evaluating the effects of hyperoxemia in the setting of critical care, some showing that hyperoxia may increase mortality, especially in settings like traumatic brain injury, and others the return of spontaneous circulation after cardiac arrest [9,10,11]. However, the role of hyperoxia in patients with sepsis or septic shock remains unclear. Therefore, we aimed at summarizing the available evidence on the role of hyperoxia in critically ill patients with sepsis or septic shock and the association between hyperoxia and mortality and other clinical outcomes (e.g., hemodynamics, renal function, etc.), as investigated by the available literature.
Main text
For the purpose of this review, we performed a systematic search in PubMed and The Cochrane Library database, lastly updated on 17 April 2023. We included the following search key terms: “sepsis” or “septic shock,” “hyperoxia” and “critical care” and related synonyms, alternatives, and plural. The full search strategy is available in Supplementary Material 1. The reference list of relevant articles was also screened (i.e., the snowballing method). The systematic review was conducted as per PRISMA guidelines [12].
Studies were independently screened from titles and abstract by two authors (F.R.C., A.M.) to identify all the relevant records and screened from full text against inclusion and exclusion pre-defined criteria by the same authors. Differences were resolved by consensus with a third author (M. I.). Eligibility criteria included studies assessing the effects of hyperoxia in adults (≥ 18 years) admitted to the critical care for sepsis or septic shock. We included studies independently of definition of hyperoxia. Studies including less than 10 patients, case reports, abstracts, review articles, and articles in languages different than English were excluded. We also excluded studies conducted on pediatric patients and animal studies. No studies were excluded for their outcomes. Authors, publications, date of publication, hyperoxia definition, and primary and secondary outcomes were extracted from each original article and were tabulated. The included studies were then assessed using JBI’s Critical Appraisal Checklists (https://jbi.global/critical-appraisal-tools) [13,14,15], according to their designs.
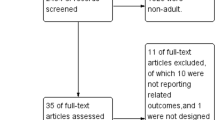
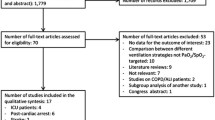
A total of 725 records were retrieved. After the screening of the records and removal of duplicates, 33 records were evaluated from full text, of whom 21 were excluded and 12 studies were included, for a total of 15,782 included patients. All patients received supplemental oxygen, and the majority were mechanically ventilated. At the critical appraisal assessment stage, no major methodological flaws were detected, except for a single-center, hypothesis-generating pilot observational study, with lack of adjustment for confounders and unclear balance of patients’ characteristics between the groups [16]. No studies were excluded at this stage.
The inclusion/exclusion process is presented with details as a PRISMA flow diagram, as shown in Fig. 1. The included studies comprised 1 randomized clinical trial (RCT), 4 secondary analyses from RCTs, 3 prospective observational studies, and 4 retrospective observational studies. The comparison group, present in 10 studies, was normoxia, and the most frequently investigated outcomes were mortality, intensive care unit-acquired weakness, atelectasis formation, length of stay in the ICU, incidence of renal-replacement therapy and acute kidney Injury (AKI), days to suspension of vasopressor or inotropic agents, and the percentage of resolution of primary and secondary infections, mechanical ventilation duration, vascular effects, oxidative stress, and the incidence of sepsis-associated encephalopathy (SAE). The main characteristics of the included studies are described in Table 1. The PRISMA checklist is available as Supplementary Material 2.
Oxygen therapy in sepsis and septic shock
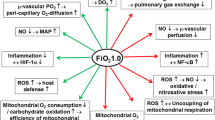
Sepsis is a medical emergency; therefore, early diagnosis and appropriate management improve outcome [28,29,30]. Treatment is based on early and appropriate antimicrobial therapy, source control, fluid resuscitation, and eventually (e.g., septic shock) the use of vasoactive medications and mechanical ventilation [31]. Patients often receive oxygen supplementation [32]. However, the Surviving Sepsis Campaign Guidelines [28] do not provide indication on targets for the partial pressure of oxygen in arterial blood or arterial oxygen saturation. The physiologic effects of hyperoxia and its role on clinical outcomes have been graphically summarized in Fig. 2.
Proposed physiologic effects and clinical impact of hyperoxia in patients with sepsis. The figure summarizes the physiologic effects of hyperoxia and its role on clinical outcomes in patients with sepsis or septic shock. ICUAW, intensive care unit-acquired weakness; LOS, length of stay; ROS, reactive oxygen species
Definitions of hyperoxia
A high incidence of hyperoxia has been described in septic patients, reaching an average of 92.8% in a prospective study [16] conducted on 83 septic patients, despite being included according to an old definition. The effects of hyperoxia in patients with sepsis or septic shock have received increasing interest over the last three decades [33].
Hyperoxia was differently defined across the included studies. Some studies considered as belonging to the “hyperoxia group” the patients receiving a fixed FiO2 of 1.0 [17, 20, 27]. Some trials used a threshold of PaO2 > 100 mmHg [16, 23], PaO2 > 120 mmHg [26], PaO2 > 150 mmHg [21], or SpO2 > 96% [22] to define hyperoxia status. The absence of a uniform definition may be one of the main issues on the topic, both in the clinical setting and in the research field.
Pathophysiology
Oxidative cellular damage has been widely studied in medical research and has been associated with an impaired mitochondrial activity and the production of reactive oxygen species (ROS) [4, 5]. Breathing with excess oxygen may increase the formation of ROS, such as hydroxyl radical (OH•) and peroxynitrite (ONOO−), able to interact with lipids, proteins, and nucleic acids [34], thus determining a direct oxidative stress [35] and an indirect damage through radical-mediated mechanisms, inducing cells to undergo necrosis or apoptosis. Moreover, neutrophils can use oxygen to form superoxide and other reactive oxygen species that, despite beneficial in the killing of microorganisms, may become risky in the context of a dysregulated host response such as sepsis. From a hemodynamic perspective, hyperoxia induces systemic vasoconstriction through the ROS [36] production and the low bioavailability of NO [37]. ROS production has also been considered among the possible mechanism of ICUacquired weakness [38]. Absorption atelectasis [39] are important pulmonary effects, along with pulmonary cellular damage [40] and decreased mucus clearance [40]. Indeed, when using high FiO2, alveolar nitrogen, that is an inert gas, is gradually replaced by oxygen and washed out, thus determining alveolar collapse once that oxygen is absorbed into the blood.
Overall, the pathophysiological effects of hyperoxia in sepsis are controversial. On the one hand, supplemental oxygen can be life-saving in such patients, and even hyperoxia may be useful due to its bactericidal effects, but on the other hand, a use of high FiO2 may cause systemic impairments.
Moreover, the devices adopted to deliver oxygen may also have non-oxygen-related effects that may be considered as confounders of the net effect of oxygen per se. Indeed, septic patients have an increased respiratory drive and usually high spontaneous efforts [41,42,43], and it has been shown that the use of HFNC may reduce respiratory drive in such patients, compared with low-flow oxygen therapy, and contribute to maintain a state of normoxia [44, 45] by determining washout of dead space, compensating excessive carbon dioxide production due to a hypermetabolic state, and provide expiratory positive pressure [46, 47], overall reducing the work of breathing [44].
Mortality in sepsis/septic shock
Mortality was an assessed outcome in 10 of the included studies. Of these, 6 found a higher mortality rate or an increased risk of mortality among patients with sepsis/septic shock and hyperoxia [24, 25], 3 found no difference in mortality between the two groups [16, 22, 26], and 1 found a reduced risk of mortality [23] among patients with hyperoxia.
A secondary analysis of a prospective observational study [23], which included 454 postsurgical patients with sepsis or septic shock and need for invasive mechanical ventilation, showed that hyperoxia, defined as PaO2 > 100 mmHg during the first 48 h after major surgery, was associated with a lower risk of 90-day mortality (OR 0.61, 95% CI: 0.39–0.95, p = 0.029), compared to PaO2 < 100 mmHg, independently of age, presence of chronic renal failure, procalcitonin levels, or APACHE II score. Patients were first treated with empirical antibiotic therapy waiting for susceptibility testing to be completed, with subsequent targeted therapy selected according to the results. Specifically, linezolid or teicoplanin was used for methicillin-resistant Staphylococcus aureus and at least one of the following antibiotics for Pseudomonas aeruginosa: imipenem, cefepime, or piperacillin-tazobactam, in association with amikacin or ciprofloxacin.
Two retrospective cohort studies did not find any significant association between hyperoxia and ICU mortality in mechanically ventilated septic patients. In the first [26] one, hyperoxia was defined as PaO2 > 120 mmHg during the first 24 h of ICU stay, and the study included 488 patients with septic shock, defined according to SEPSIS-3 criteria. The second one [22] evaluated 83 patients treated with conventional oxygenation targets (SpO2 target of ≥ 96%) and 130 patients with permissive hypoxia (SpO2 target of 88–92% or PaO2 target of 60 mmHg; reduction of FiO2 if PaO2 > 110 mmHg). There was no statistically significant difference in ICU mortality (p = 0.18).
Stolmeijer et al. conducted a single-center prospective observational study [16] including a small sample size of 83 septic patients and found no significant differences between hyperoxia and normoxia groups in terms of in-hospital and 28-day mortality. However, the outcomes of this study must be considered in the context of limitations typical of the study design.
No association has been found between survival and hyperoxia in a recent post hoc analysis [19] of the ALBIOS RCT. The authors included 1632 septic patients who survived the first 48 h after randomization and stratified them into two groups based on their mean PaO2 levels during the first 48 h (PaO2 0–48 h) with a cutoff of 100 mmHg (mean PaO2 0–48 h > 100 mmHg: hyperoxemia group n = 971; PaO2 0–48 h ≤ 100: normoxemia group n = 661). The data analysis did not show any significant difference between the two groups regarding mortality at 90 and 28 days. However, a subgroup analysis performed in the same study and including patients with lung as the primary site of infection (n = 663) showed a reduced risk of mortality at 90 days in patients with hyperoxemia.
Four of the included studies found an increase in mortality in the group of patients with hyperoxia. The multicentric RCT HYPERS2S [17] by Asfar et al. compared the effects of hyperoxia (FiO2 1.0 for 24h after inclusion) with normoxia in 434 patients with septic shock who were on mechanical ventilation. The study was prematurely terminated due to a higher 28-day mortality in the group receiving hyperoxia. In this study, hyperoxia was associated with higher risk of mortality, although not statistically significant; 28-day mortality was recorded for 434 patients; 93 (43%) of 217 patients had died in the hyperoxia group versus 77 (35%) of 217 patients in the normoxia group (HR 1.27 (95% CI 0.94–1.72); p = 0.12).
A post hoc analysis [20] of the same study compared mortality rates in the 397 patients in whom lactate levels were available at baseline to compare a Sepsis-3 [48] shock subset (lactate > 2 mmol/L) of patients to those with vasopressor-dependent hypotension only (lactate ≤ 2 mmol/L). Hyperoxia treatment for 24 h compared to “normoxia” was associated with a higher mortality rate in patients with septic shock defined as per the Sepsis-3 definition (57.4% vs. 44.3%, p = 0.054). In patients with lactate ≤ 2 mmol/L, hyperoxia had no effect on mortality (p = 0.680).
Young et al. undertook a post hoc analysis [24] of the ICU-ROX trial, on the subcohort of 251 patients with sepsis. Indeed, the ICU-ROX trial had compared conservative oxygen therapy (FiO2 reduced as much as possible down to a minimum of 0.21, maintaining SpO2 < 97%), with usual oxygen therapy (no specific thresholds for FiO2 or SpO2) in 1000 mechanically ventilated patients admitted to ICU. In the secondary analysis, the conservative oxygen therapy group did not result in a statistically significant reduction of 90-day mortality (95% CI − 4.6 to 18.6% points; p = 0.24) compared with the usual oxygen group in septic patients. However, the authors discussed that the analysis was underpowered to detect the effect on 90-day mortality.
A single-center retrospective observational study [21], conducted on 49 septic patients subjected to assisted-mechanical ventilation before hospital admission, showed that hyperoxia, defined as PaO2 > 150 mmHg at ICU admission, was associated with mortality at day 28 in septic patients, using a propensity score analysis including SOFA score, pre-hospital duration, lactate, and pre-hospital fluid volume expansion (p = 0.02, OR [CI95] = 1.59 [1.20–2.10]) [21]. However, a strong limit of the study was the unknown duration of hyperoxemia, impossible to determine because the included patients had been treated by a mobile intensive care unit and subjected to invasive mechanical ventilation prior to hospital admission. Further studies are currently ongoing on the topic, also investigating mortality as a primary outcome, and would reasonably contribute to producing useful data on the topic (NCT04198077).
Finally, another observational cohort study [25] was conducted on a sample of 11740 septic patients undergoing oxygen therapy in the ICU or perioperative period, selected from the MIMIC IV and eICU databases. The authors observed a directly proportional correlation between oxygen therapy and the incidence of sepsis-associated encephalopathy (SAE). SAE refers to cognitive dysfunction attributable to a systemic inflammatory response in the absence of direct CNS infections (defined in this study as GCS < 15 and/or patients diagnosed with delirium). The authors observed higher mortality rates among septic patients who developed SAE compared to those who did not and higher PaO2 and PaO2/FiO2 values among non-survivors of patients who developed SAE. The observational nature of the study is certainly a limitation; however, they observed that the range of PaO2 (97–339) mmHg, PaO2/FiO2 (189–619), and SpO2 ≥ 93% reduced the incidence of SAE and may reduce the hospital mortality of SAE. Instead, hypoxia (SPO2 < 93%, PaO2 < 97 mmHg, and PaO2/FiO2 < 189) and hyperoxia (PaO2 > 339 mmHg and PaO2/FiO2 > 619) were associated with increased incidence of SAE. Thus, lower or higher oxygenation could induce SAE.
Other outcomes
Ten additional outcomes were evaluated among the included studies: intensive care unit-acquired weakness, atelectasis formation, length of stay in the ICU, incidence of renal-replacement therapy and acute kidney injury (AKI), days to suspension of vasopressor or inotropic agents, and the percentage of resolution of primary and secondary infections, mechanical ventilation duration, vascular effects, and oxidative stress.
In the HYPERS2S RCT, a higher number of patients with intensive care unit-acquired weakness (24 [11%] vs 13 [6%]; p = 0.06) and atelectasis (26 [12%] vs 13 [6%]; p = 0.04) within the first 3 days was found in the hyperoxia group [17] compared with the normoxia group. There were no significant differences in the secondary outcomes: length of stay in the ICU (p = 0.49) and requirements for renal replacement treatment (p = 0.74). In the secondary analysis of a prospective observational study written by Martín-Fernández et al., hyperoxemia (PaO2 > 100 mmHg) was associated with a lower length of ICU stay (5 [9] vs. 8 [13] days, p < 0.001) and reduced mechanical ventilation duration (1 [4] vs. 2 [8] days, p < 0.001).
In a single-center retrospective study [22], a reduced ICU stay (11.0 [IQR: 6.0–19.0] days vs. 9.0 [IQR: 4.0–15.0] days, p = 0.02) and in mechanical ventilation duration (11.0 [IQR: 6.0–19.0] days vs. 7.0 [IQR: 3.0–14.0] days, p = 0.01) were found between the conventional oxygenation target and conservative targets groups.
In the recent post hoc analysis of an RCT [19], AKI, the percentage of patients undergoing renal replacement therapy, the suspension times of vasopressor or inotropic agents, the resolution of the primary infection, and mortality in UTI were not significantly different between the study groups. Conversely, a reduction in mechanical ventilation time and intensive care stay was found in patients with normoxemia compared to the hyperoxemia group.
Rossi et al. [27] in a prospective study evaluated the vascular effects during mechanical ventilation in 14 septic patients. After a 20-min period of hyperoxic ventilation (FiO2 1.0), two-dimensional images of the brachial artery cross-sectional area and brachial blood flow velocities were recorded using conventional ultrasonography and pulsed Doppler simultaneously with invasive arterial pressure measurements. They observed a reduction in brachial cross-sectional areas and an increase in MAP of about 7%, an increase in pulse pressure and in resistance index, and a decrease in distensibility coefficient and in cross-sectional, showing that vasomotor tone increases. Vasoconstriction as a response to hyperoxia seems to result in a paradoxical decrease in arterial oxygen delivery, due to an impaired arterial blood flow, at least for the upper limbs.
In a sub-study of the ICU-ROX RCT [18] on 27 septic patients, the correlation between hyperoxemia (SpO2 ≥ 97%) and increased oxidative stress was evaluated comparing levels of ascorbate (one of the most potent water-soluble antioxidants in human plasma) and protein carbonyls (a biomarker of protein oxidation). From the data analysis, it emerged that conservative oxygen therapy did not alter systemic markers of oxidative stress in critically ill ventilated patients with sepsis compared with standard oxygen therapy.
Limitations
This review has limitations. The main limitation was represented by the heterogeneity of the hyperoxia definition adopted across the studies, limiting the chance to further summarize and analyze data. Furthermore, the majority of the included studies did not provide detailed data on the causative microorganisms, antibiotic administration, or hyperoxia duration, which are expected to contribute to mortality as confounding variables, and the effects of blood oxygen levels on sensitivity to antibiotics were not investigated. Lastly, the small sample size in many of the selected trials does not allow for generalizable results.
Conclusions
Conflicting evidence emerges from the included studies, but data from RCTs issued safety concerns on the use of hyperoxia in patients with sepsis or septic shock and potential association with higher mortality. The heterogeneity of the definitions adopted for hyperoxia hampers the chance to further summarize the available data. The optimum range of oxygen level able to minimize risks and provide benefits seems still unknown. Clinical equipoise between the two conditions (i.e., hyperoxia and normoxia) is uncertain in this population of patients, thus limiting future research options. Future studies should aim at (i) identifying the best range of oxygenation and its optimal duration to maximize benefits and minimize harm and (ii) investigating how effects of different levels of oxygen may vary according to identified pathogens, source of infection, and prescribed antibiotics in critically ill patients with sepsis and septic shock.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CNS:
-
Central nervous system
- FiO2 :
-
Fraction of inspired O2
- HFNC:
-
High-flow nasal cannula
- ICU:
-
Intensive care unit
- MAP:
-
Mean arterial pressure
- PaO2 :
-
Partial pressure of oxygen
- RCT:
-
Randomized controlled trial
- ROS:
-
Reactive oxygen species
- SAE:
-
Sepsis-associated encephalopathy
- SaO2 :
-
Saturation of oxygen
References
Vincent J-L, De Backer D (2013) Circulatory shock. N Engl J Med 369(18):1726–1734. https://doi.org/10.1056/NEJMra1208943
Girardis M, Alhazzani W, Rasmussen BS (2019) What’s new in oxygen therapy? Intensive Care Med 45(7):1009–1011. https://doi.org/10.1007/s00134-019-05619-9
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M (2021) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med 47(11):1181–1247. https://doi.org/10.1007/s00134-021-06506-y
Magder S (2006) Reactive oxygen species: toxic molecules or spark of life? Crit Care 10(1):208. https://doi.org/10.1186/cc3992
Chow C-W, Herrera Abreu MT, Suzuki T, Downey GP (2003) Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol 29(4):427–431. https://doi.org/10.1165/rcmb.F278
Benoît Z, Wicky S, Fischer J-F, Frascarolo P, Chapuis C, Spahn DR, Magnusson L (2002) The effect of increased Fio2 before tracheal extubation on postoperative atelectasis. Anesth Analg 95(6):1777–1781. https://doi.org/10.1097/00000539-200212000-00058
Mak S, Azevedo ER, Liu PP, Newton GE (2001) Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest 120(2):467–473. https://doi.org/10.1378/chest.120.2.467
Gupta S, Laskar N, Kadouri DE (2016) Evaluating the effect of oxygen concentrations on antibiotic sensitivity, growth, and biofilm formation of human pathogens. Microbiol Insights 9:37–46. https://doi.org/10.4137/MBI.S40767
Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, Donati A (2014) Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care 18(6):711. https://doi.org/10.1186/s13054-014-0711-x
Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M (2016) Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA 316(15):1583. https://doi.org/10.1001/jama.2016.11993
Helmerhorst HJF, Roos-Blom M-J, van Westerloo DJ, de Jonge E (2015) Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies*. Critical Care Medicine 43(7):1508–1519. https://doi.org/10.1097/CCM.0000000000000998
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 Statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71. https://doi.org/10.1136/bmj.n71
(2020) Chapter 3: Systematic reviews of effectiveness. In JBI Manual for Evidence Synthesis; JBI. https://doi.org/10.46658/JBIMES-20-04.
(2020) Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; JBI. https://doi.org/10.46658/JBIMES-20-08.
Munn, Z.; Barker, T. H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. (2019) Methodological quality of case series studies: an introduction to the JBI Critical Appraisal Tool. JBI Database of Systematic Reviews and Implementation Reports, Publish Ahead of Print. https://doi.org/10.11124/JBISRIR-D-19-00099.
Stolmeijer R, ter Maaten JC, Zijlstra JG, Ligtenberg JJM (2014) Oxygen therapy for sepsis patients in the emergency department: a little less? Eur J Emerg Med 21(3):233–235. https://doi.org/10.1097/MEJ.0b013e328361c6c7
Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, Grimaldi D, Grelon F, Anguel N, Lasocki S, Henry-Lagarrigue M, Gonzalez F, Legay F, Guitton C, Schenck M, Doise JM, Devaquet J, Van Der Linden T, Chatellier D, Rigaud JP, Dellamonica J, Tamion F, Meziani F, Mercat A, Dreyfuss D, Seegers V, Radermacher P (2017) Hyperoxia and Hypertonic Saline in Patients with Septic Shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med 5(3):180–190. https://doi.org/10.1016/S2213-2600(17)30046-2
Carr AC, Spencer E, Mackle D, Hunt A, Judd H, Mehrtens J, Parker K, Stockwell Z, Gale C, Beaumont M, Kaur S, Bihari S, Young PJ (2020) The effect of conservative oxygen therapy on systemic biomarkers of oxidative stress in critically ill patients. Free Radic Biol Med 160:13–18. https://doi.org/10.1016/j.freeradbiomed.2020.06.018
Catalisano G, Ippolito M, Blanda A, Meessen J, Giarratano A, Todesco N, Bonato V, Restuccia F, Montomoli J, Fiore G, Grasselli G, Caironi P, Latini R, Cortegiani A (2023) Effects of hyperoxemia in patients with sepsis – a post-hoc analysis of a multicentre randomized clinical trial. Pulmonology S2531-0437(23):00042–9. https://doi.org/10.1016/j.pulmoe.2023.02.005
the HYPER2S investigators, Demiselle J, Wepler M, Hartmann C, Radermacher P, Schortgen F, Meziani F, Singer M, Seegers V, Asfar P (2018) Hyperoxia toxicity in septic shock patients according to the Sepsis-3 criteria: a post hoc analysis of the HYPER2S trial. Ann Intensive Care 8(1):90. https://doi.org/10.1186/s13613-018-0435-1
Jouffroy R, Saade A, Saint Martin LC, Philippe P, Carli P, Vivien B (2019) Prognosis value of partial arterial oxygen pressure in patients with septic shock subjected to pre-hospital invasive ventilation. Am J Emerg Med 37(1):56–60. https://doi.org/10.1016/j.ajem.2018.04.050
Nishimoto K, Umegaki T, Ohira S, Soeda T, Anada N, Uba T, Shoji T, Kusunoki M, Nakajima Y, Kamibayashi T (2021) Impact of permissive hypoxia and hyperoxia avoidance on clinical outcomes in septic patients receiving mechanical ventilation: a retrospective single-center study. BioMed Res Int 2021:1–10. https://doi.org/10.1155/2021/7332027
Martín-Fernández M, Heredia-Rodríguez M, González-Jiménez I, Lorenzo-López M, Gómez-Pesquera E, Poves-Álvarez R, Álvarez FJ, Jorge-Monjas P, Beltrán-DeHeredia J, Gutiérrez-Abejón E, Herrera-Gómez F, Guzzo G, Gómez-Sánchez E, Tamayo-Velasco Á, Aller R, Pelosi P, Villar J, Tamayo E (2022) Hyperoxemia in postsurgical sepsis/septic shock patients is associated with reduced mortality. Crit Care 26(1):4. https://doi.org/10.1186/s13054-021-03875-0
Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N, Litton E, McArthur C, McGuinness S, Panwar R (2020) Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX). Intensive Care Med 46(1):17–26. https://doi.org/10.1007/s00134-019-05857-x
Li Y, Zhao L, Yu Y, Zhang K, Jiang Y, Wang Z, Xie K, Yu Y (2022) Conservative oxygen therapy in critically ill and perioperative period of patients with sepsis-associated encephalopathy. Front Immunol. 13:1035298. https://doi.org/10.3389/fimmu.2022.1035298
Popoff B, Besnier E, Dureuil B, Veber B, Clavier T (2021) Effect of early hyperoxemia on mortality in mechanically ventilated septic shock patients according to Sepsis-3 criteria: analysis of the MIMIC-III database. Eur J Emerg Med 28(6):469–475. https://doi.org/10.1097/MEJ.0000000000000854
Rossi P, Tauzin L, Weiss M, Rostain J-C, Sainty J-M, Boussuges A (2007) Could hyperoxic ventilation impair oxygen delivery in septic patients? Clin Physiol Funct Imaging 27(3):180–184. https://doi.org/10.1111/j.1475-097X.2007.00732.x
Levy MM, Evans LE, Rhodes A (2018) The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med 44(6):925–928. https://doi.org/10.1007/s00134-018-5085-0
Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC (2010) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36(2):222–231. https://doi.org/10.1007/s00134-009-1738-3
Damiani E, Donati A, Serafini G, Rinaldi L, Adrario E, Pelaia P, Busani S, Girardis M (2015) Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS One 10(5):e0125827. https://doi.org/10.1371/journal.pone.0125827
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent J-L, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock. Crit Care Med 45(3):486–552. https://doi.org/10.1097/CCM.0000000000002255
Sjöberg F, Singer M (2013) The medical use of oxygen: a time for critical reappraisal. J Intern Med 274(6):505–528. https://doi.org/10.1111/joim.12139
Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P (2015) Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 Update. Ann Intensive Care 5(1):42. https://doi.org/10.1186/s13613-015-0084-6
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424. https://doi.org/10.1152/physrev.00029.2006
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(2):335–344. https://doi.org/10.1113/jphysiol.2003.049478
McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, Sinoway LI (2007) Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol 102(5):2040–2045. https://doi.org/10.1152/japplphysiol.00595.2006
Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA (1997) Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276(5321):2034–2037. https://doi.org/10.1126/science.276.5321.2034
Kress JP, Hall JB (2014) ICU-acquired weakness and recovery from critical illness. N Engl J Med 370(17):1626–1635. https://doi.org/10.1056/NEJMra1209390
Aboab J, Jonson B, Kouatchet A, Taille S, Niklason L, Brochard L (2006) Effect of inspired oxygen fraction on alveolar derecruitment in acute respiratory distress syndrome. Intensive Care Med 32(12):1979–1986. https://doi.org/10.1007/s00134-006-0382-4
Kallet RH, Matthay MA (2013) Hyperoxic acute lung injury. Respiratory Care 58(1):123–141. https://doi.org/10.4187/respcare.01963
Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D (2020) Respiratory drive in critically ill patients. Pathophysiology and Clinical Implications. Am J Respir Crit Care Med 201(1):20–32. https://doi.org/10.1164/rccm.201903-0596SO
Linton RAF, Poole-Wilson PA, Davies RJ, Cameron IR (1973) A comparison of the ventilatory response to carbon dioxide by steady-state and rebreathing methods during metabolic acidosis and alkalosis. Clin Sci 45(2):239–249. https://doi.org/10.1042/cs0450239
Tang G-J, Kou YR, Lin YS (1998) Peripheral neural modulation of endotoxin-induced hyperventilation. Critical Care Med 26(9):1558–1563. https://doi.org/10.1097/00003246-199809000-00024
Mauri T, Spinelli E, Pavlovsky B, Grieco DL, Ottaviani I, Basile MC, Dalla Corte F, Pintaudi G, Garofalo E, Rundo A, Volta CA, Pesenti A, Spadaro S (2021) Respiratory drive in patients with sepsis and septic shock: modulation by high-flow nasal cannula. Anesthesiology 135(6):1066–1075. https://doi.org/10.1097/ALN.0000000000004010
Maggiore SM, Grieco DL, Lemiale V (2023) The use of high-flow nasal oxygen. Intensive Care Med. https://doi.org/10.1007/s00134-023-07067-y
Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, Pesenti A (2017) Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 195(9):1207–1215. https://doi.org/10.1164/rccm.201605-0916OC
Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, Bottino N, Lissoni A, Spadaro S, Volta CA, Gattinoni L, Pesenti A, Grasselli G (2017) Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med 43(10):1453–1463. https://doi.org/10.1007/s00134-017-4890-1
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, Angus DC (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801. https://doi.org/10.1001/jama.2016.0287
Acknowledgements
None.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.R.C, M.I., and A.C; methodology, F.R.C, M.I., A.M., and A.C; data curation, F.R.C, M.I., A.M., G.C., M.M., A.G., and A.C.; writing—original draft preparation, F.R.C, M.I., A.M., G.C., M.M., A.G., and A.C.; writing—review and editing, F.R.C, M.I., A.M., G.C, M.M., and A.C.; supervision, A.C.; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Material 1. Full search strategy.
Additional file 2:
Supplementary Material 2. PRISMA checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Catalanotto, F.R., Ippolito, M., Mirasola, A. et al. Hyperoxia in critically ill patients with sepsis and septic shock: a systematic review. J Anesth Analg Crit Care 3, 12 (2023). https://doi.org/10.1186/s44158-023-00096-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44158-023-00096-5